1. Introduction
Abdominal-based flaps, particularly the deep inferior epigastric perforator (DIEP) flap, have become the standard approach for autologous breast reconstruction following Nipple Sparing Mastectomy (NSME) or Skin Sparing Mastectomy (SSME) procedures [
1,
2,
3]. This technique offers several notable advantages, including an enhanced quality of life and greater patient satisfaction when compared to implant-based approaches [
4,
5,
6]. However, the trade-offs are longer surgical duration, extended hospital stay and the necessity to sacrifice an additional donor site. Considering this additional effort, our objective should be to minimize the risks associated with the additional surgery for the patients and continuously strive to enhance the surgical outcome.
Abdominal flap harvesting is performed with electrosurgical devices, using high-frequency electrical current for tissue dissection and simultaneous hemostasis [
7]. Different modalities are available, the conventional monopolar electrocautery (MPE) uses a continuous waveform of radiofrequency energy via an uninsulated metal electrode for tissue cutting through thermal ablation, operating at temperatures between 180 and 240°C [
8]. In contrast, the PEAK PlasmaBlade (PPB) employs short (40 µs) high-frequency pulses of radiofrequency energy to generate electrical plasma along an insulated electrode’s edge and it maintains a lower operating temperature around 45 °C [
8,
9]. Previous investigations suggest that the PlasmaBlade may offer advantages over electrocautery, demonstrating reduced thermal injury depth and inflammatory responses [
8,
10,
11]. Inflammatory processes and trauma to the lymphatic network during surgical dissection are known factors contributing to postoperative seroma formation, a common complication after DIEP flap harvesting, with reported incidences ranging from 1.4% to 16.2% [
12,
13,
14,
15,
16]. Prolonged drainage duration to prevent seroma formation poses disadvantages such as extended hospitalization and the risk of ascending infections through the drain tube. Previous research has generated conflicting and inconclusive findings regarding whether the choice of the surgical dissection device significantly impacts clinical outcomes. While some studies suggest benefits associated with using the PPB, such as reduced seroma rates and shorter drain dwelling times, these studies have limitations, notably small sample sizes and none have evaluated patient-specific risk factors in this context [
11,
17,
18,
19,
20,
21,
22].
Our study seeks to evaluate the influence of the dissection device utilized during abdominal flap harvesting on clinical outcomes, while considering risk factors, such as Body Mass Index (BMI), smoking status and previous chemotherapy.
2. Materials and Methods
This retrospective, single-center cohort study was approved by the Institutional Ethics Committee of Medical University Innsbruck (protocol code 1082/2021).
2.1. Patients
We included a total of 128 patients who underwent autologous DIEP flap breast reconstruction at our Department between 2013 and 2020. Among these, 56 patients underwent abdominal flap harvesting using the PEAK PlasmaBlade (Medtronic, Dublin, Ireland) (PPB), while 72 patients underwent the procedure using monopolar electrocautery (MPE). Inclusion criteria were defined as age over 18 years, uni- or bilateral NSME or SSME and immediate or staged DIEP-based autologous breast reconstruction by or under the supervision of one single surgeon. One patient was excluded due to the development of extreme seroma formation (1730 ml) to prevent bias.
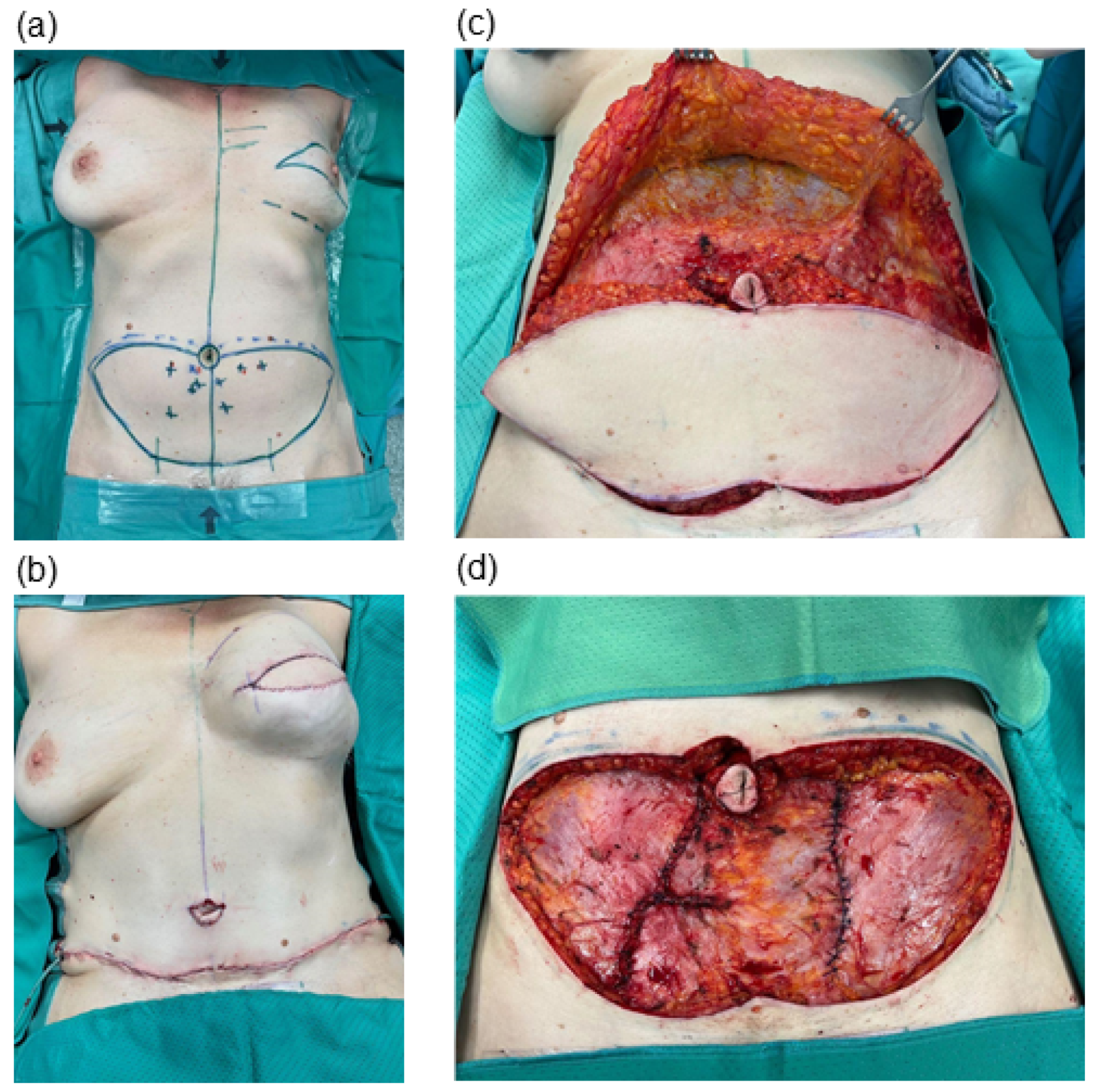
We conducted a retrospective chart analysis to assess patient demographics, complications and clinical outcomes. All patients included in this study underwent abdominal flap harvesting and received postoperative care following our institutional protocol. Redon drains were placed at the end of surgery, just before wound closure, and were placed under suction (
Figure 1). Drain removal was undertaken when the output was less than 30 ml per 24 hours. The volume of drainage fluid was recorded at 24-hour intervals. All patients were required to wear an abdominal binder for a duration of six weeks following surgery.
Postoperative pain levels were assessed at various time intervals within a 24-hour period using the Numerical Analog Scale, which utilizes a scale ranging from 0 (indicating the absence of pain) to 10 (representing the most severe imaginable pain). For our evaluation, we recorded the highest daily pain score.
2.2. Statistical Analysis
Statistical analysis was performed using Prism Software 10 for macOS (GraphPad, USA) and Google Sheets (Google LLC,
https://www.google.com/sheets/about/, accessed October 2023). To assess significant differences between the two groups, we employed the independent sample Student’s t-test for continuous data. For categorical data, Fisher’s exact and Chi-Square tests were utilized to determine statistical significance. A Two-Way Analysis of Variance (ANOVA) test was conducted to assess interactions between the types of surgical devices used (PPB versus MPE) and their individual effects on postoperative pain. Statistical significance of simple linear regression has been determined by comparison of slopes and intercepts with a confidence interval of 95%. A p-value of < 0.05 was considered significant. The significance threshold was set at p < 0.05. The level for statistical significance was set at pns ≥ 0.05, p* < 0.05, p** < 0.02, p*** < 0.001, p**** < 0.0001 for all statistical tests.
3. Results
All 128 patients included in the study were categorized into two distinct cohorts based on the method employed for abdominal flap preparation: either utilizing the PEAK PlasmaBlade (PPB; n = 56) or the monopolar electrocautery (MPE; n = 72). Analysis of patient characteristics revealed no statistically significant distinctions between the two cohorts in terms of age, BMI, smoking status, neoadjuvant oncologic therapy, diabetes mellitus and the indication for mastectomy (
Table 1).
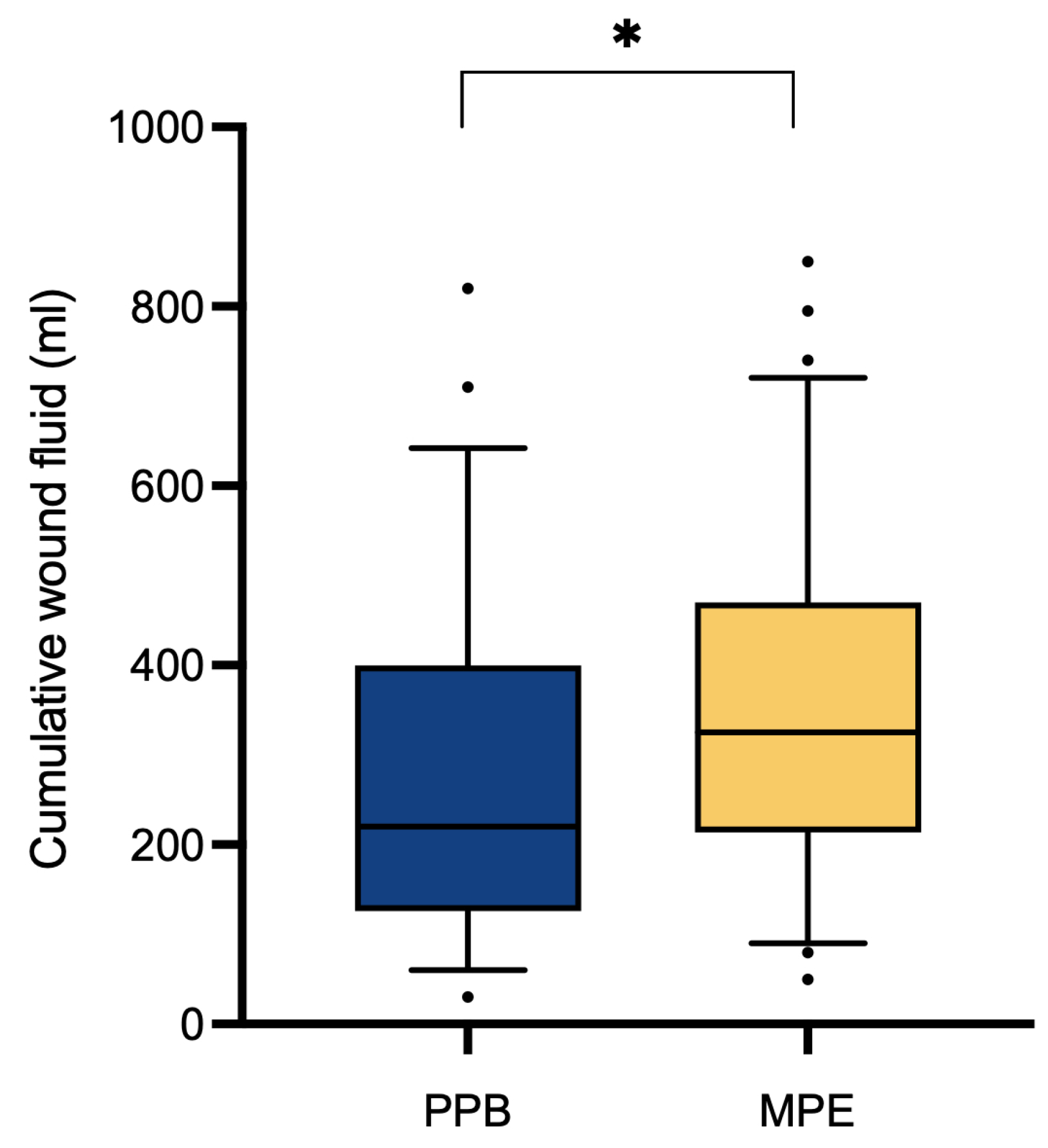
Assessment of clinical outcomes through a comparative analysis between the two patient cohorts regarding the overall abdominal wound fluid production revealed a significantly (p* = 0.0324) higher quantity within the monopolar electrocautery (MPE) group (351.11 ± 185.96 ml) in contrast to the PEAK PlasmaBlade (PPB) group (279.38 ± 183.38 ml) (
Table 2,
Figure 2). Analysis of hospitalization duration (10.86 ± 2.29 days vs. 11.13 ± 3.00 days, pns = 0.5828) and drain dwell time (5.63 ± 1.54 days vs. 5.53 vs. 1.73 days, pns = 0.6133) demonstrated no significant discrepancies between the two patient cohorts.
3.1. Risk Factors
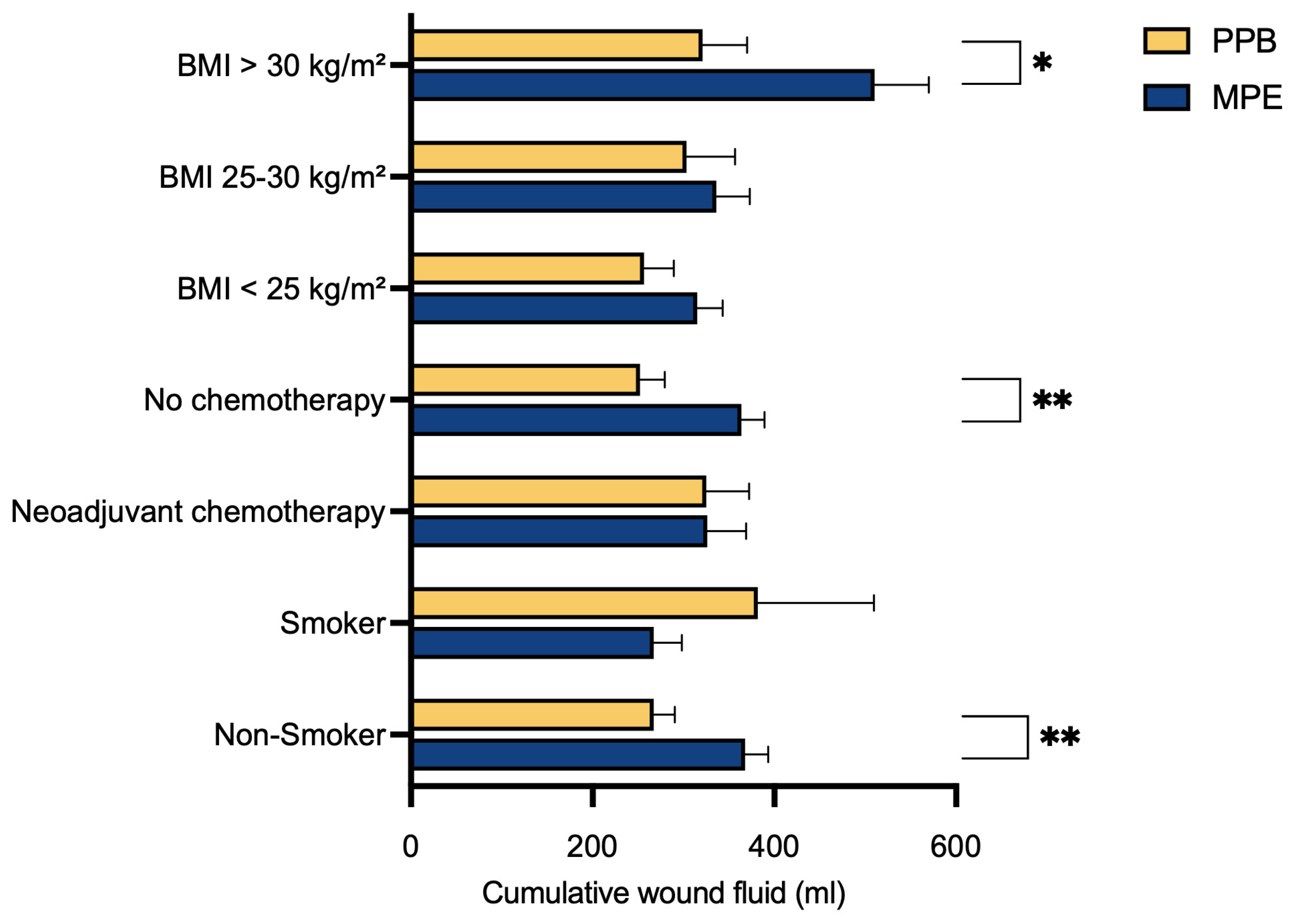
To evaluate the potential impact of various risk factors, patients were subdivided into specific groups based on their individual characteristics, followed by a comparison. Notably, a significant reduction in postoperative abdominal wound fluid production after surgery with the PPB was observed for non-smokers (p** = 0.0046), those without prior neoadjuvant chemotherapy (p** = 0.0041) and those with a BMI exceeding 30 kg/m² (p* = 0.0284) (
Table 2,
Figure 3).
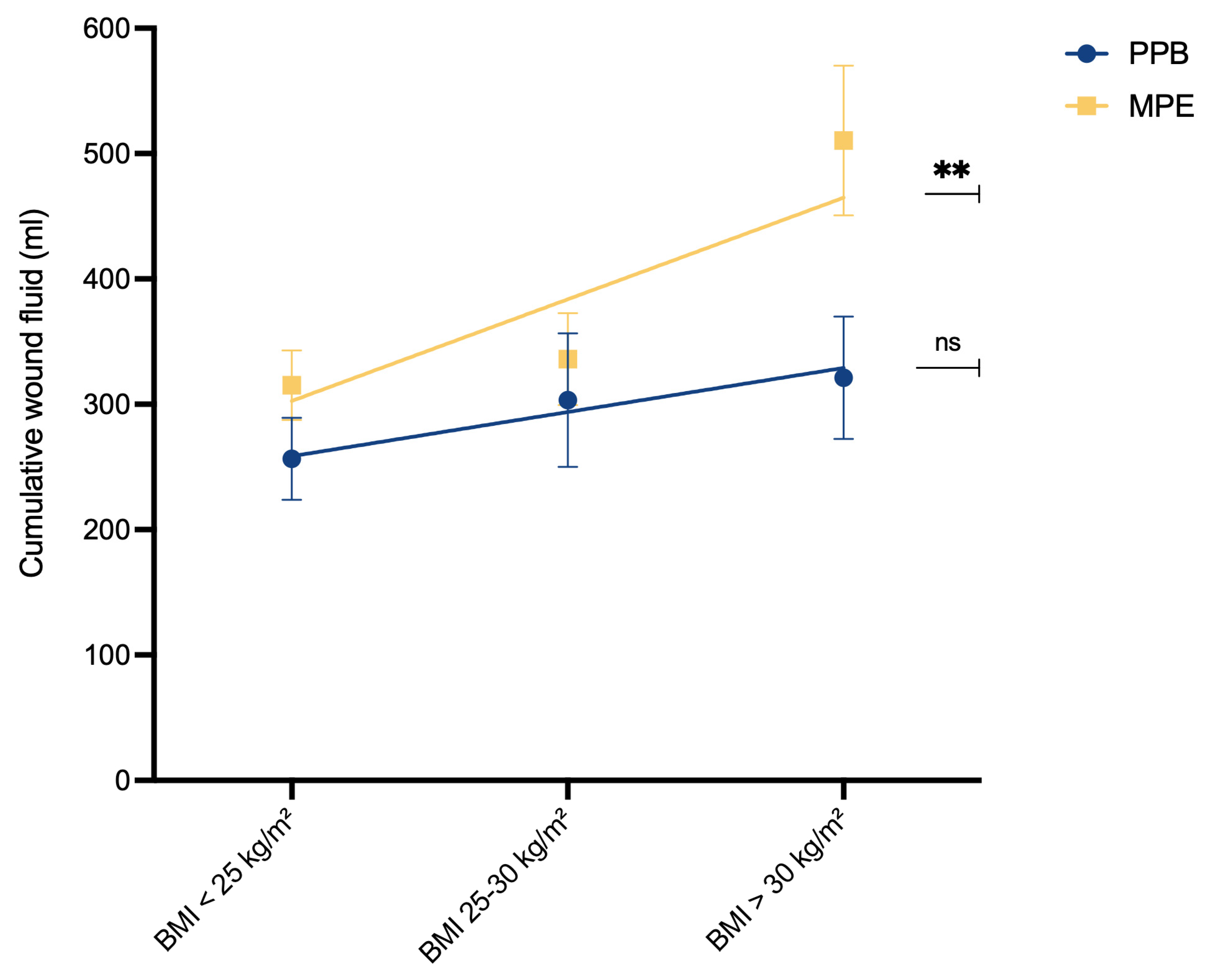
To assess the relationship between BMI and wound fluid production, simple linear regression analysis was conducted (
Figure 4). In the MPE cohort, a significant association emerged between higher BMI and increased postoperative wound fluid production (p** = 0.0058). However, such a correlation was not observed within the PPB cohort (pns = 0.2895).
Within all subgroups that exhibited favorable outcomes associated with the use of PEAK PlasmaBlade (PPB) in the context of the volume of postoperative drainage fluid, an examination of both drain dwell time and the duration of hospitalization was performed. Consistent with the observed reduction in wound fluid volume, a tendency towards decreased drain dwell time and shortened hospital stays was observed, although these trends did not achieve statistical significance (
Table 3).
3.2. Postoperative Complications and Pain
An analysis of postoperative complications did not yield any statistically significant distinctions between the two patient cohorts, both in terms of overall complications (pns = 0.2758) and in the specific assessment of bleeding or hematoma (pns = 0.7128) (
Table 4). All postoperative complications, as per the Clavien-Dindo [
23] classification, are outlined in
Table 4.
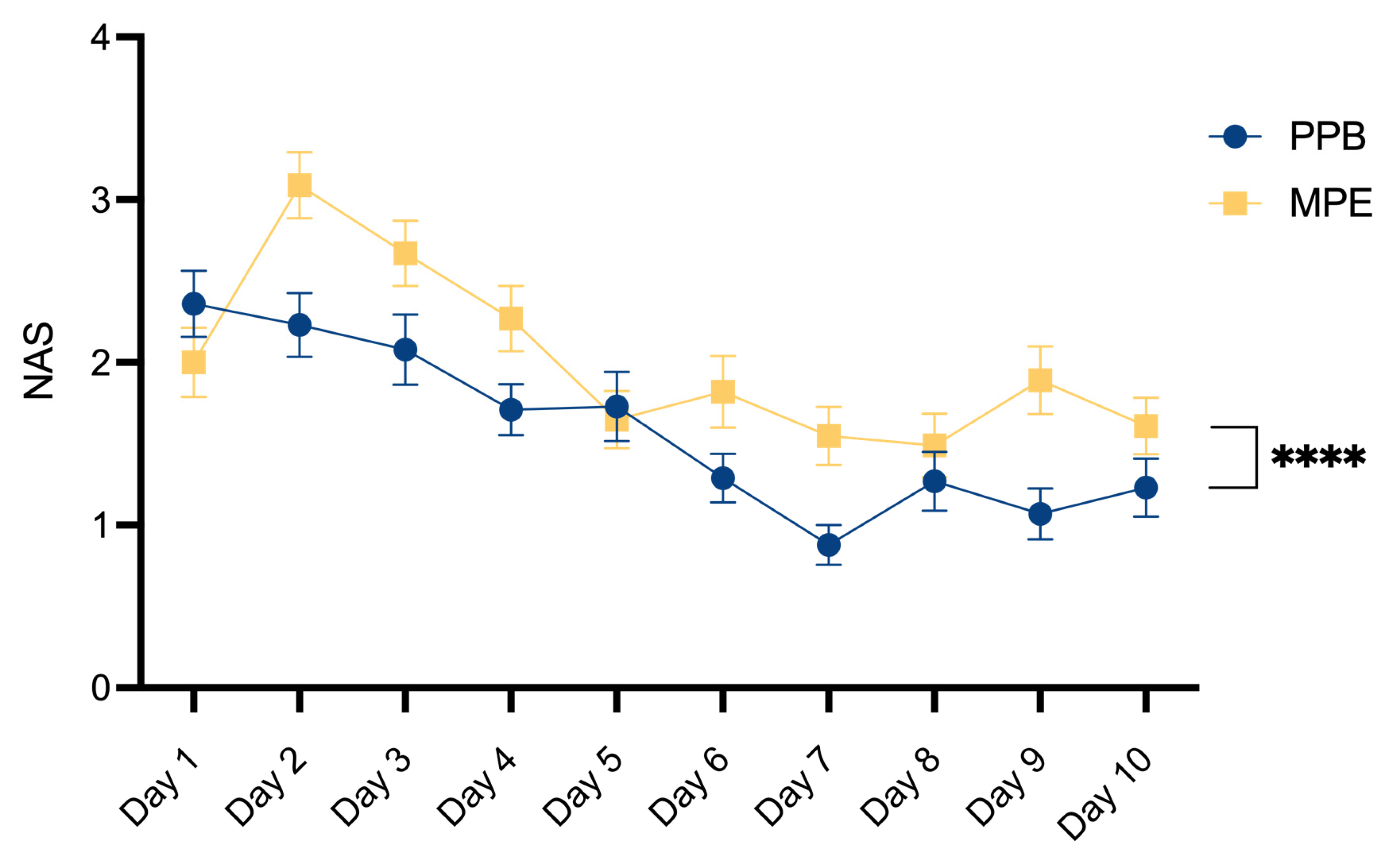
Postoperative pain assessment was conducted using a Numerical Analog Scale, graded on a scale ranging from 0 (indicating no pain) to 10 (reflecting the most severe imaginable pain). As outlined in
Table 5 and
Figure 5, a comparative investigation between the two patient cohorts unveiled a statistically significant decrease in postoperative pain within the PPB group (p**** < 0.0001).
4. Discussion
Autologous DIEP based breast reconstruction involves extensive undermining at the abdominal donor site, resulting in sizable wound areas. Typically, flap dissection is undertaken with electrosurgical devices to allow simultaneous hemostasis and efficient operating times [
24]. We evaluated two specific dissection devices – the standard monopolar electrocautery and the newer PEAK PlasmaBlade - in the context of abdominal flap harvesting, aiming to discern their impacts on clinical outcomes.
Previous research has yielded conflicting and inconclusive results regarding whether the selection of the dissection device can genuinely lead to better clinical outcomes.
Studies assessing outcomes in extensive wound areas, like those involved in autologous breast reconstruction using the abdominal donor site, have often been limited by small sample sizes [
11,
17,
18,
19,
20,
21,
22,
25,
26]. While more extensive investigations have been undertaken in distinct surgical contexts, such as tonsillectomy [
27,
28,
29] and surgical implant replacement [
30], these findings may not be directly applicable to the specific circumstances of autologous breast reconstruction.
Our retrospective analysis sought to broaden the scope of this research by including a total of 128 patients in our study. Of these patients, 72 underwent abdominal flap preparation using conventional electrocautery, while 56 patients underwent the procedure with the PEAK PlasmaBlade. Comparison of these two patient cohorts revealed no statistically significant differences in age, body mass index, smoking status, history of neoadjuvant oncologic therapy, diabetes status and the primary indication for mastectomy. This comparability ensures the reliability and robustness of our observed outcomes.
Our analysis yielded the significant (p* = 0.0324) finding of a higher cumulative wound fluid quantity in the monopolar electrocautery group (351.11 ± 185.96 ml) compared to the PEAK PlasmaBlade group (279.38 ± 183.38 ml). While previous research on this topic has provided inconclusive data, some studies support our finding by reporting lower total drain output following the use of the PEAK PlasmaBlade [
11,
17,
18,
19,
20,
22], whereas other authors found no difference between the two dissection devices [
21,
25,
26]. It’s worth noting that, to the best of our knowledge, no prior study has reported an increase in seroma rates after utilizing the PEAK Plasma Blade. It has previously been suggested that the reduced working temperature of the PEAK PlasmaBlade leads to lower tissue damage [
8,
10], which contributes to reduced seroma formation. One study in gender-affirming mastectomy patients has investigated histologic samples, showing a 22% reduction of thermal injury depth with the PlasmaBlade compared to conventional monopolar cautery [
18].
Similarly, the existing literature on the evaluation of hospitalization and drain dwell time yields inconsistent results. While Schlosshauer, Dogan, and Sowa reported positive outcomes in the PlasmaBlade cohorts [
11,
17,
19,
20], other studies did not identify variations in terms of hospitalization and drain dwell time [
21,
25,
26]. Our data did not reveal a difference between both devices for these aspects. There is no previous work indicating inferiority of the PlasmaBlade in these regards.
We intentionally selected abdominal-based autologous breast reconstruction as our research focus. This patient group not only represents a highly standardized approach to large-scale wound preparation but also encompasses a spectrum of patient-specific risk factors, including those related to oncologic treatments, which are pertinent to our analysis. Extensive cohort studies conducted so far have failed to establish a definitive link between neoadjuvant chemotherapy and the occurrence of surgical complications during breast reconstruction [
31,
32,
33,
34,
35]. Nevertheless, we were determined to assess the role of dissection instruments in relation to neoadjuvant chemotherapy and other risk factors and its impact on surgical outcomes.
In our multivariate data analysis, we identified a substantial reduction in postoperative abdominal wound fluid production in the PEAK PlasmaBlade cohort compared to the electrocautery cohort across three specific subgroups. These groups comprised individuals without prior neoadjuvant chemotherapy (p** = 0.0041), non-smokers (p** = 0.0046) and those with a BMI exceeding 30 kg/m² (p* = 0.0284).
It is noteworthy that these groups typically do not share the same risk profile. In standard practice, non-smokers and patients without previous neoadjuvant chemotherapy are typically regarded as low-risk individuals for surgical complications. Conversely, patients with a BMI exceeding 30 kg/m² are commonly perceived as a high-risk population for adverse events in the surgical context [
36]. Mani et al. previously investigated the association between BMI and the occurrence of donor-site seroma following the harvesting of DIEP flaps. Their findings revealed that obese patients (BMI > 30 kg/m²) had the highest incidence of postoperative seroma formation, reaching a rate of 16% [
16]. Our results revealed a significant association between higher BMI and increased postoperative wound fluid production (p** = 0.0058) within the MPE cohort (
Figure 4). In contrast, no such correlation was evident within the PPB cohort (pns = 0.2895), suggesting that the utilization of PPB might mitigate the risks associated with higher BMI.
Although not statistically significant, all subgroups that displayed positive outcomes with the use of the PEAK PlasmaBlade regarding postoperative drainage volume also demonstrated a trend toward reduced drain dwell time and shorter hospital stays.
The assessment of hospitalization duration may be subject to some inaccuracies, as our Department typically retains patients in the hospital until histopathological results become accessible, and the removal of the monitoring-skin-island is performed. Nevertheless, it is noteworthy that a tendency towards reduced drainage catheter dwell time in the PPB group, may potentially be linked to our observed outcomes of a significant decrease in post-operative pain levels around day 7 and day 9 in the PPB group, attributable to the earlier removal of drainage devices.
Our data reveal a significant decrease in post-operative pain within the PEAK PlasmaBlade cohort. To our knowledge, only Friebel et al. has previously compared postoperative pain levels between both dissection devices, reporting an increase in the PPB cohort [
21]. This was attributed to the potential impact of tighter abdominal closure due to lower flap weights in this group. Additionally, there have been studies examining postoperative pain following surgical skin incisions made with electrocautery versus steel scalpel, which reported reduced pain levels and decreased use of analgetics in the electrocautery group [
37,
38]. Chrysos explains this by highlighting that the vaporization of cells resulting from the application of pure sinusoidal current leads to immediate tissue and nerve necrosis without significantly affecting nearby structures [
38]. The varying degrees of nerve damage between electrocautery and the PEAK PlasmaBlade may also contribute to our significant finding of reduced postoperative pain in the PPB group, but such evaluations should be a focus of future studies.
Our study demonstrates also several limitations. Due to retrospective data analysis, we cannot offer detailed information concerning the adjustments of the used devices. But we tried to overcome this lack of information by only including patients that were operated under the lead of one single surgeon, so it may be assumed that the same preferred settings were used. Furthermore, as a single surgeon study, we can minimize the risk of performance bias. Moreover, the similarity in patient demographic characteristics across both cohorts indicates a low risk of selection bias, despite the study’s retrospective nature.
5. Conclusions
In conclusion, our findings align with previous research, indicating that the PEAK PlasmaBlade is not inferior to conventional electrocautery. Furthermore, patients may experience advantages such as reduced drainage volume and lower postoperative pain levels following wound preparation with the PlasmaBlade. Notably, our analysis identified patient subgroups that could derive even greater benefits from the use of this device. In these specific patient groups, utilizing the PEAK PlasmaBlade could result in better clinical outcomes. Therefore, the choice to use this device may be based on the potential benefits for patients, rather than solely on the surgeon’s subjective preferences.
Author Contributions
Conceptualization, D.W. and T.B.; methodology, T.B.; software, A.A. and I.S.; validation, A.A., J.K., I.S. and S.U.; formal analysis, A.A.; investigation, T.B.; data curation, J.K. and S.U.; writing—original draft preparation, A.A.; writing—review and editing, I.S and D.W.; visualization, A.A.; supervision, D.W.; project administration, D.W., I.S. and T.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of MEDICAL UNIVERSITY INNSBRUCK (protocol code 1082/2021, 23 June 2021).
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Stephan Sigl is acknowledged for providing clinical guidance and administrative support. The authors thank Karin Langert, Angelika Feichter and Theresa Zagrajsek for photo documentation of our patients.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Allen, R. J.; Treece, P. Deep Inferior Epigastric Perforator Flap for Breast Reconstruction. Ann. Plast. Surg. 1994, 32 (1), 32–38. [CrossRef]
- Healy, C.; Allen, R. J. The Evolution of Perforator Flap Breast Reconstruction: Twenty Years after the First DIEP Flap. J. Reconstr. Microsurg. 2014, 30 (2), 121–126. [CrossRef]
- Granzow, J. W.; Levine, J. L.; Chiu, E. S.; Allen, R. J. Breast Reconstruction with the Deep Inferior Epigastric Perforator Flap: History and an Update on Current Technique. J. Plast. Reconstr. Aesthetic Surg. 2006, 59 (6), 571–579. [CrossRef]
- Santosa, K. B.; Qi, J.; Kim, H. M.; Hamill, J. B.; Wilkins, E. G.; Pusic, A. L. Long-Term Patient-Reported Outcomes in Postmastectomy Breast Reconstruction. JAMA Surg. 2018, 153 (10), 891. [CrossRef]
- Nelson, J. A.; Allen, R. J.; Polanco, T.; Shamsunder, M.; Patel, A. R.; McCarthy, C. M.; Matros, E.; Dayan, J. H.; Disa, J. J.; Cordeiro, P. G.; Mehrara, B. J.; Pusic, A. L. Long-Term Patient-Reported Outcomes Following Postmastectomy Breast Reconstruction: An 8-Year Examination of 3268 Patients. Ann. Surg. 2019, 270 (3), 473–483. [CrossRef]
- Miseré, R. M.; van Kuijk, S. M.; Claassens, E. L.; Heuts, E. M.; Piatkowski, A. A.; van der Hulst, R. R. Breast-Related and Body-Related Quality of Life Following Autologous Breast Reconstruction Is Superior to Implant-Based Breast Reconstruction - A Long-Term Follow-up Study. Breast 2021, 59, 176–182. [CrossRef]
- Messenger, D.; Carter, F.; Noble, E.; Francis, N. Electrosurgery and Energized Dissection. Surg. 2020, 38 (3), 133–138. [CrossRef]
- Loh, S. A.; Carlson, G. A.; Chang, E. I.; Huang, E.; Palanker, D.; Gurtner, G. C. Comparative Healing of Surgical Incisions Created by the PEAK PlasmaBlade, Conventional Electrosurgery, and a Scalpel. Plast. Reconstr. Surg. 2009, 124 (6), 1849–1859. [CrossRef]
- Palanker, D. V.; Vankov, A.; Huie, P. Electrosurgery With Cellular Precision. IEEE Trans. Biomed. Eng. 2008, 55 (2), 838–841. [CrossRef]
- Ruidiaz, M. E.; Messmer, D.; Atmodjo, D. Y.; Vose, J. G.; Huang, E. J.; Kummel, A. C.; Rosenberg, H. L.; Gurtner, G. C. Comparative Healing of Human Cutaneous Surgical Incisions Created by the PEAK PlasmaBlade, Conventional Electrosurgery, and a Standard Scalpel. Plast. Reconstr. Surg. 2011, 128 (1), 104–111. [CrossRef]
- Schlosshauer, T.; Kiehlmann, M.; Riener, M. O.; Rothenberger, J.; Sader, R.; Rieger, U. M. Effect of Low-Thermal Dissection Device versus Conventional Electrocautery in Mastectomy for Female-to-Male Transgender Patients. Int. Wound J. 2020, 17 (5), 1239–1245. [CrossRef]
- Xiao, X.; Ye, L. Efficacy and Safety of Scarpa Fascia Preservation During Abdominoplasty: A Systematic Review and Meta-Analysis. Aesthetic Plast. Surg. 2017, 41 (3), 585–590. [CrossRef]
- Wormald, J. C. R.; Wade, R. G.; Figus, A. The Increased Risk of Adverse Outcomes in Bilateral Deep Inferior Epigastric Artery Perforator Flap Breast Reconstruction Compared to Unilateral Reconstruction: A Systematic Review and Meta-Analysis. J. Plast. Reconstr. Aesthetic Surg. 2014, 67 (2), 143–156. [CrossRef]
- Salgarello, M.; Tambasco, D.; Farallo, E. DIEP Flap Donor Site Versus Elective Abdominoplasty Short-Term Complication Rates: A Meta-Analysis. Aesthetic Plast. Surg. 2012, 36 (2), 363–369. [CrossRef]
- Tan, M. Y. L.; Onggo, J.; Serag, S.; Phan, K.; Dusseldorp, J. R. Deep Inferior Epigastric Perforator (DIEP) Flap Safety Profile in Slim versus Non-Slim BMI Patients: A Systematic Review and Meta-Analysis. J. Plast. Reconstr. Aesthet. Surg. 2022, 75 (7), 2180–2189. [CrossRef]
- Mani, M.; Wang, T.; Harris, P.; JAMES, S. Breast Reconstruction with the Deep Inferior Epigastric Perforator Flap Is a Reliable Alternative in Slim Patients. Microsurgery 2016, 36 (7), 552–558. [CrossRef]
- Schlosshauer, T.; Kiehlmann, M.; Rothenberger, J.; Sader, R.; Rieger, U. M. Bilateral Reduction Mammaplasty with Pulsed Electron Avalanche Knife PlasmaBladeTM and Conventional Electrosurgical Surgery: A Retrospective, Randomised Controlled Clinical Trial. Int. Wound J. 2020, 17 (6), 1695–1701. [CrossRef]
- Schlosshauer, T.; Kiehlmann, M.; Riener, M. O.; Sader, R.; Rieger, U. M. Comparative Analysis on the Effect of Low-Thermal Plasma Dissection Device (PEAK PlasmaBlade) vs Conventional Electrosurgery in Post-Bariatric Abdominoplasty: A Retrospective Randomised Clinical Study. Int. Wound J. 2019, 16 (6), 1494–1502. [CrossRef]
- Sowa, Y.; Inafuku, N.; Kodama, T.; Morita, D.; Numajiri, T. Preventive Effect on Seroma of Use of Peak Plasmablade after Latissimus Dorsi Breast Reconstruction. Plast. Reconstr. Surg. - Glob. Open 2018, 6 (12). [CrossRef]
- Dogan, L.; Gulcelik, M. A.; Yuksel, M.; Uyar, O.; Erdogan, O.; Reis, E. The Effect of Plasmakinetic Cautery on Wound Healing and Complications in Mastectomy. J. Breast Cancer 2013, 16 (2), 198–201. [CrossRef]
- Friebel, T. R.; Narayan, N.; Ramakrishnan, V.; Morgan, M.; Cellek, S.; Griffiths, M. Comparison of PEAK PlasmaBladeTM to Conventional Diathermy in Abdominal-Based Free-Flap Breast Reconstruction Surgery—A Single-Centre Double-Blinded Randomised Controlled Trial. J. Plast. Reconstr. Aesthetic Surg. 2021, 74 (8), 1731–1742. [CrossRef]
- Chiappa, C.; Fachinetti, A.; Boeri, C.; Arlant, V.; Rausei, S.; Dionigi, G.; Rovera, F. Wound Healing and Postsurgical Complications in Breast Cancer Surgery: A Comparison between PEAK PlasmaBlade and Conventional Electrosurgery – A Preliminary Report of a Case Series. Ann. Surg. Treat. Res. 2018, 95 (3), 129–134. [CrossRef]
- Clavien, P. A.; Barkun, J.; De Oliveira, M. L.; Vauthey, J. N.; Dindo, D.; Schulick, R. D.; De Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; Graf, R.; Vonlanthen, R.; Padbury, R.; Cameron, J. L.; Makuuchi, M. The Clavien-Dindo Classification of Surgical Complications: Five-Year Experience. Ann. Surg. 2009, 250 (2), 187–196. [CrossRef]
- Yilmaz, K. B.; Dogan, L.; Nalbant, H.; Akinci, M.; Karaman, N.; Ozaslan, C.; Kulacoglu, H. Comparing Scalpel, Electrocautery and Ultrasonic Dissector Effects: The Impact on Wound Complications and Pro-Inflammatory Cytokine Levels in Wound Fluid from Mastectomy Patients. J. Breast Cancer 2011, 14 (1), 58. [CrossRef]
- Duscher, D.; Aitzetmüller, M. M.; Shan, J. J.; Wenny, R.; Brett, E. A.; Staud, C. J.; Kiesl, D.; Huemer, G. M. Comparison of Energy-Based Tissue Dissection Techniques in Abdominoplasty: A Randomized, Open-Label Study Including Economic Aspects. Aesthetic Surg. J. 2019, 39 (5), 536–543. [CrossRef]
- Chow, W. T. H.; Oni, G.; Ramakrishnan, V. V.; Griffiths, M. The Use of Plasmakinetic Cautery Compared to Conventional Electrocautery for Dissection of Abdominal Free Flap for Breast Reconstruction: Single-Centre, Randomized Controlled Study. Gland Surg. 2019, 8 (3), 242–248. [CrossRef]
- Chen, A. W. G.; Chen, M. K. Comparison of Post-Tonsillectomy Hemorrhage between Monopolar and Plasma Blade Techniques. J. Clin. Med. 2021, 10 (10). [CrossRef]
- Lane, J. C.; Dworkin-Valenti, J.; Chiodo, L.; Haupert, M. Postoperative Tonsillectomy Bleeding Complications in Children: A Comparison of Three Surgical Techniques. Int. J. Pediatr. Otorhinolaryngol. 2016, 88, 184–188. [CrossRef]
- Thottam, P. J.; Christenson, J. R.; Cohen, D. S.; Metz, C. M.; Saraiya, S. S.; Haupert, M. S. The Utility of Common Surgical Instruments for Pediatric Adenotonsillectomy. Laryngoscope 2015, 125 (2), 475–479. [CrossRef]
- Kypta, A.; Blessberger, H.; Saleh, K.; Hönig, S.; Kammler, J.; Neeser, K.; Steinwender, C. An Electrical Plasma Surgery Tool for Device Replacement—Retrospective Evaluation of Complications and Economic Evaluation of Costs and Resource Use. Pacing Clin. Electrophysiol. 2015, 38 (1), 28–34. [CrossRef]
- Decker, M. R.; Greenblatt, D. Y.; Havlena, J.; Wilke, L. G.; Greenberg, C. C.; Neuman, H. B. Impact of Neoadjuvant Chemotherapy on Wound Complications after Breast Surgery. Surgery 2012, 152 (3), 382–388. [CrossRef]
- Azzawi, K.; Ismail, A.; Earl, H.; Forouhi, P.; Malata, C. M. Influence of Neoadjuvant Chemotherapy on Outcomes of Immediate Breast Reconstruction. Plast. Reconstr. Surg. 2010, 126 (1), 1–11. [CrossRef]
- Bowen, M. E.; Mone, M. C.; Buys, S. S.; Sheng, X.; Nelson, E. W. Surgical Outcomes for Mastectomy Patients Receiving Neoadjuvant Chemotherapy. Ann. Surg. 2017, 265 (3), 448–456. [CrossRef]
- Varghese, J.; Gohari, S. S.; Rizki, H.; Faheem, I.; Langridge, B.; Kümmel, S.; Johnson, L.; Schmid, P. A Systematic Review and Meta-Analysis on the Effect of Neoadjuvant Chemotherapy on Complications Following Immediate Breast Reconstruction. Breast. Churchill Livingstone February 1, 2021, pp 55–62. [CrossRef]
- Song, J.; Zhang, X.; Liu, Q.; Peng, J.; Liang, X.; Shen, Y.; Liu, H.; Li, H. Impact of Neoadjuvant Chemotherapy on Immediate Breast Reconstruction: A Meta-Analysis. PLoS One 2014, 9 (5). [CrossRef]
- Heidekrueger, P. I.; Fritschen, U.; Moellhoff, N.; Germann, G.; Giunta, R. E.; Zeman, F.; Prantl, L. Impact of Body Mass Index on Free DIEP Flap Breast Reconstruction: A Multicenter Cohort Study. J. Plast. Reconstr. Aesthetic Surg. 2021, 74 (8), 1718–1724. [CrossRef]
- Kearns, S. R.; Connolly, E. M.; Mcnally, S.; Mcnamara, D. A.; Deasy, J. Randomized Clinical Trial of Diathermy versus Scalpel Incision in Elective Midline Laparotomy; 2000; Vol. 88.
- Chrysos, E.; Athanasakis, E.; Antonakakis, S.; Zoras, O. A Prospective Study Comparing Diathermy and Scalpel Incisions in Tension-Free Inguinal Hernioplasty.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).