Submitted:
15 March 2024
Posted:
18 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Obtaining Dental Pulp Mesenchymal Stem Cells (DP-MSC)
2.2. Cell Culture and Expansion
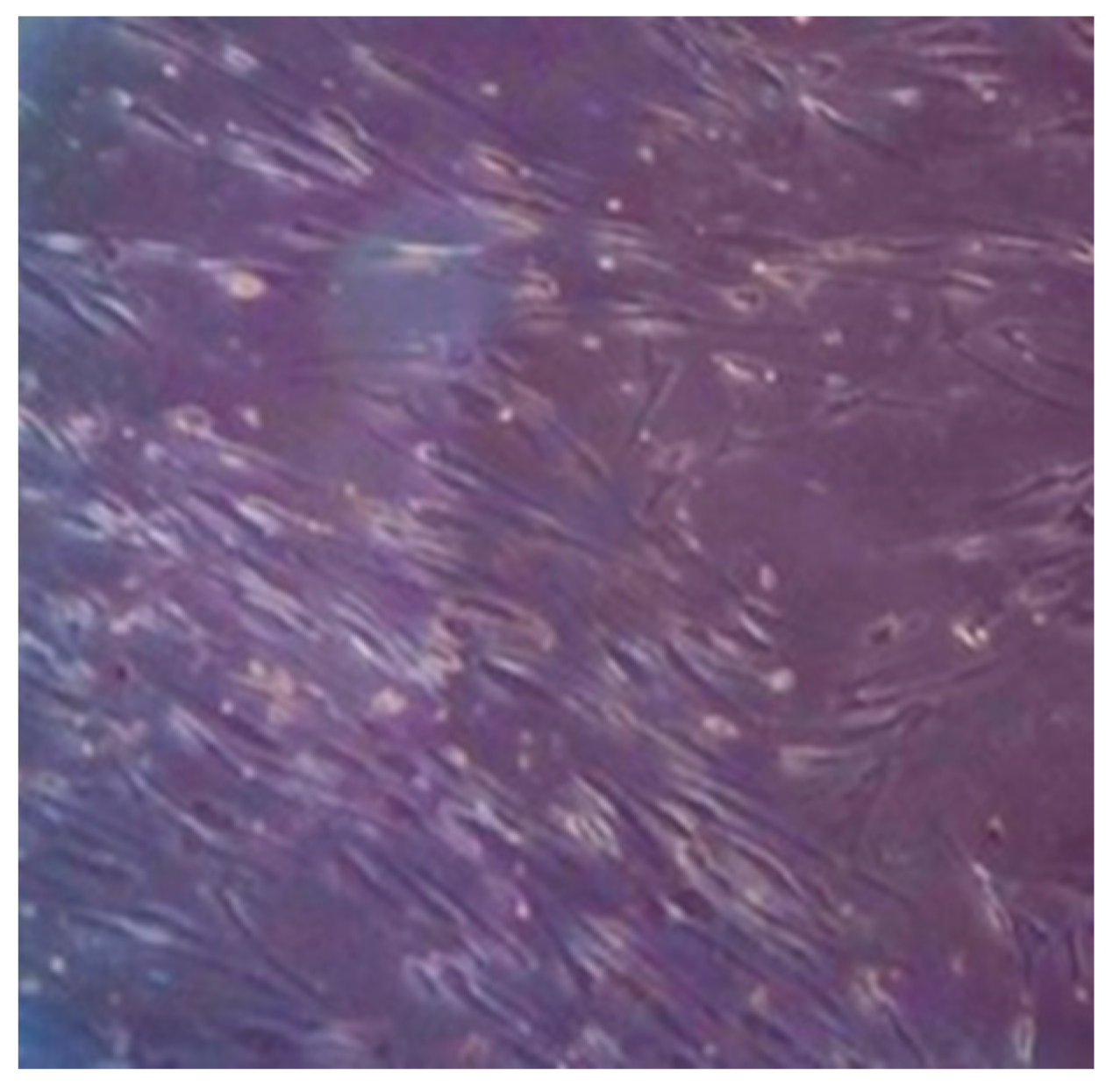
2.3. Characterisation. Flow Cytometry
2.4. Verifying the Cell Viability of the Implants
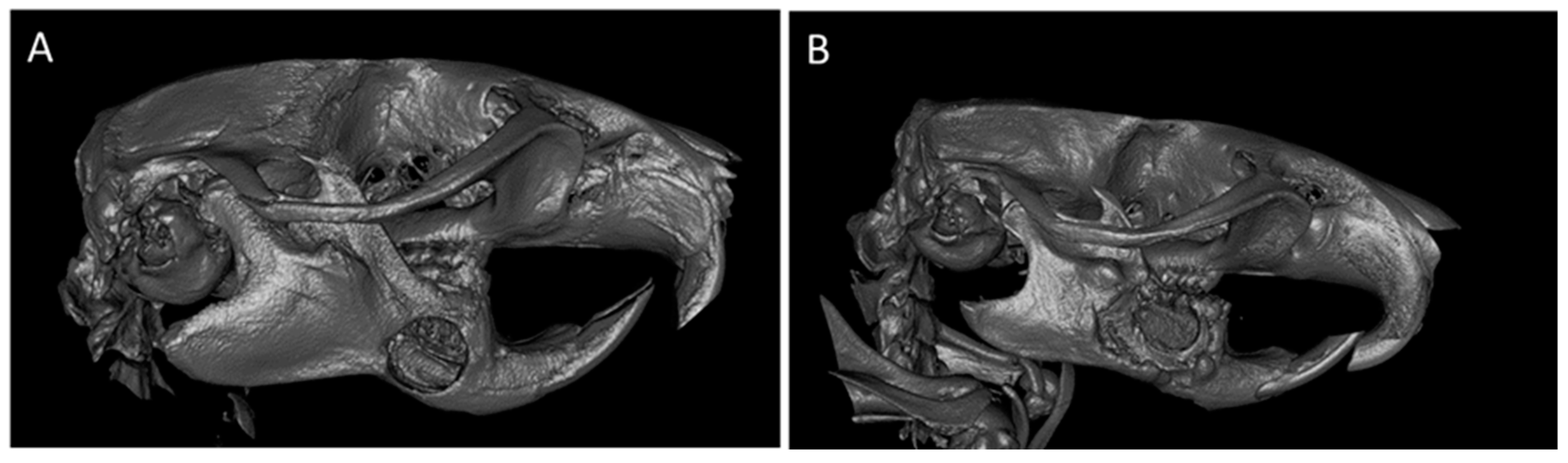
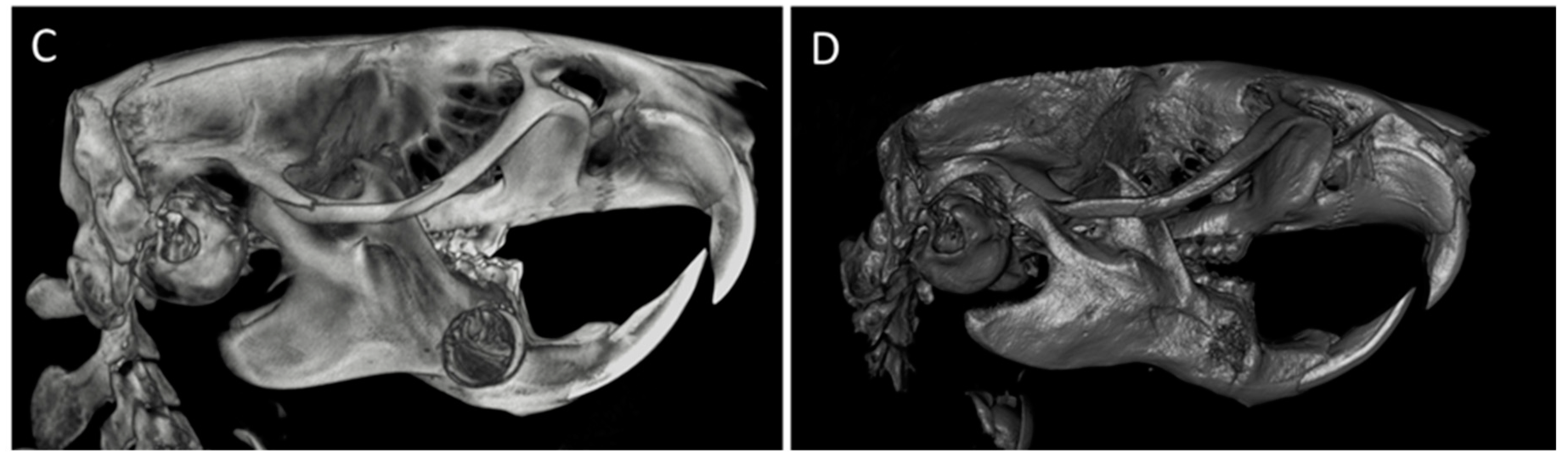
2.5. In Vivo Results in the Experimental Model
2.5.1. CONTROL Group
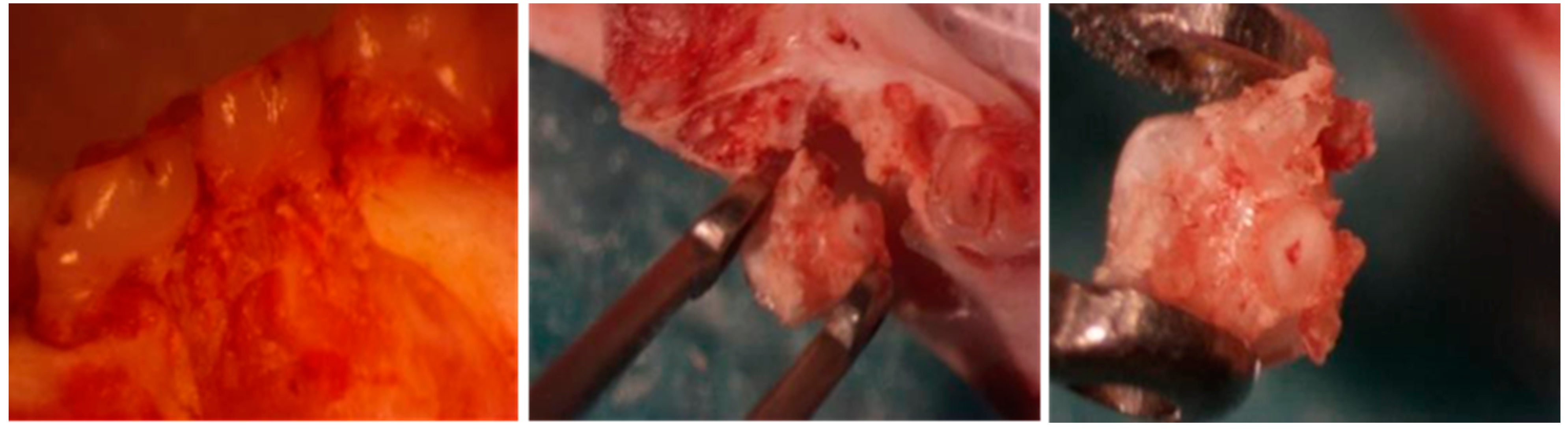
2.5.2. G+M Group
2.5.3. G+M+S Group
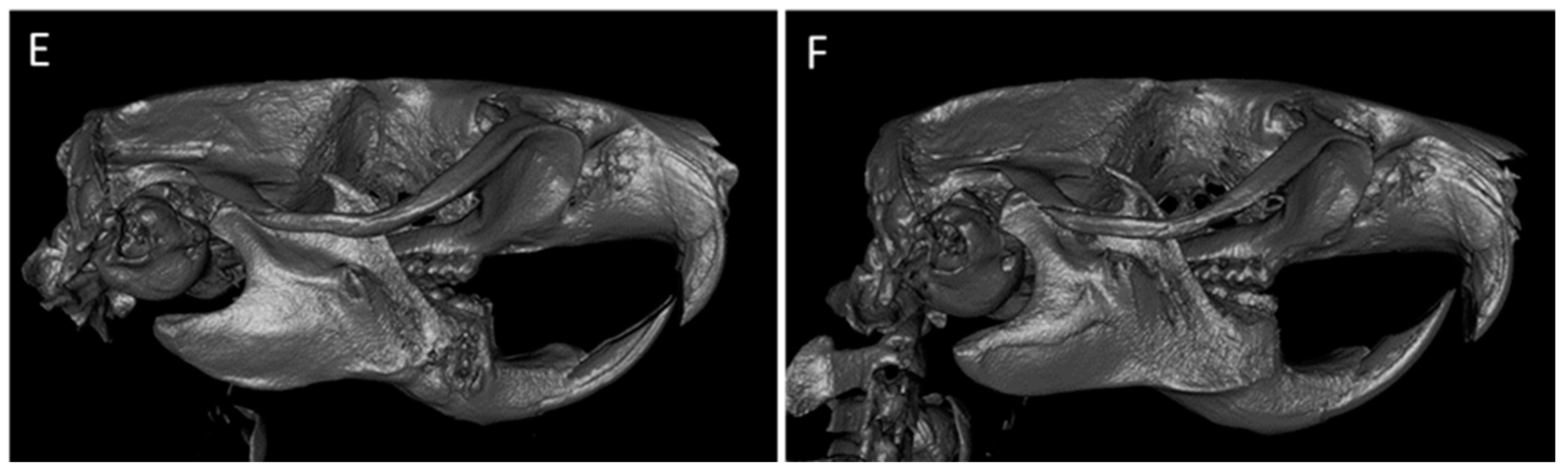
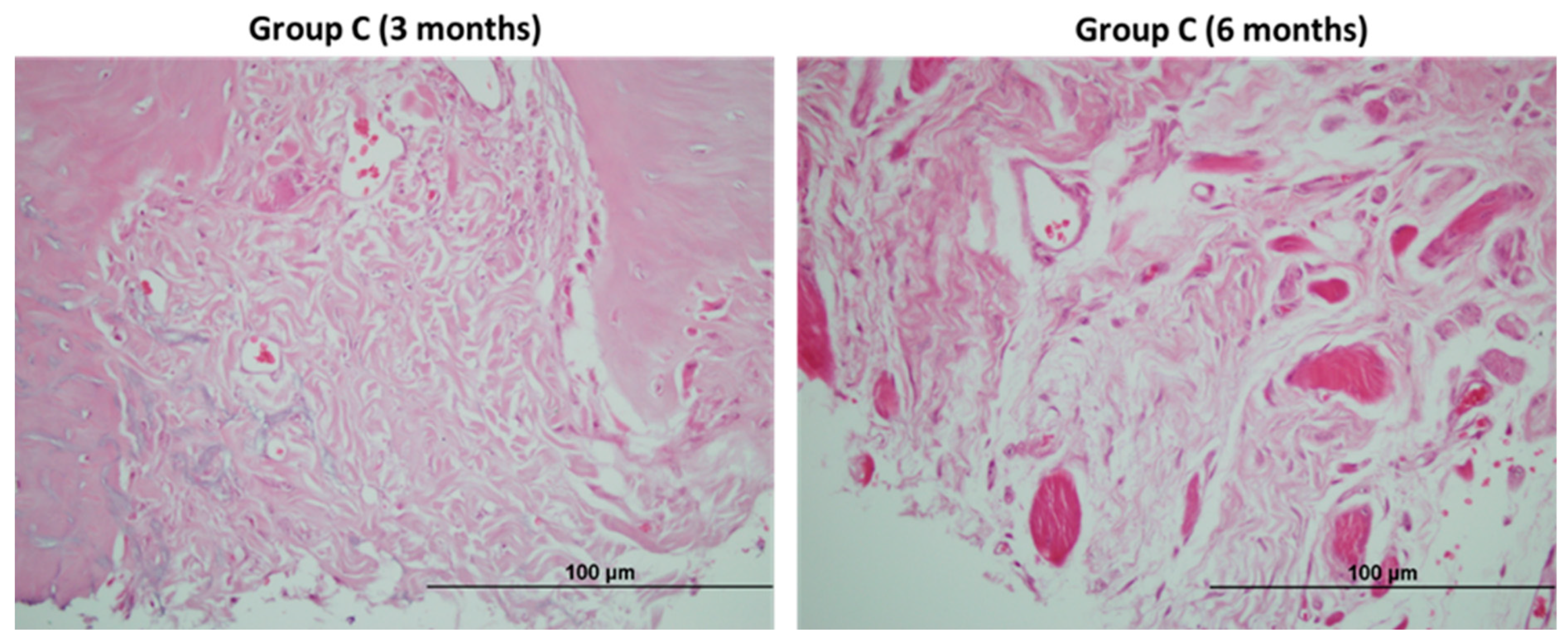
2.6. Descriptive Histological Analysis
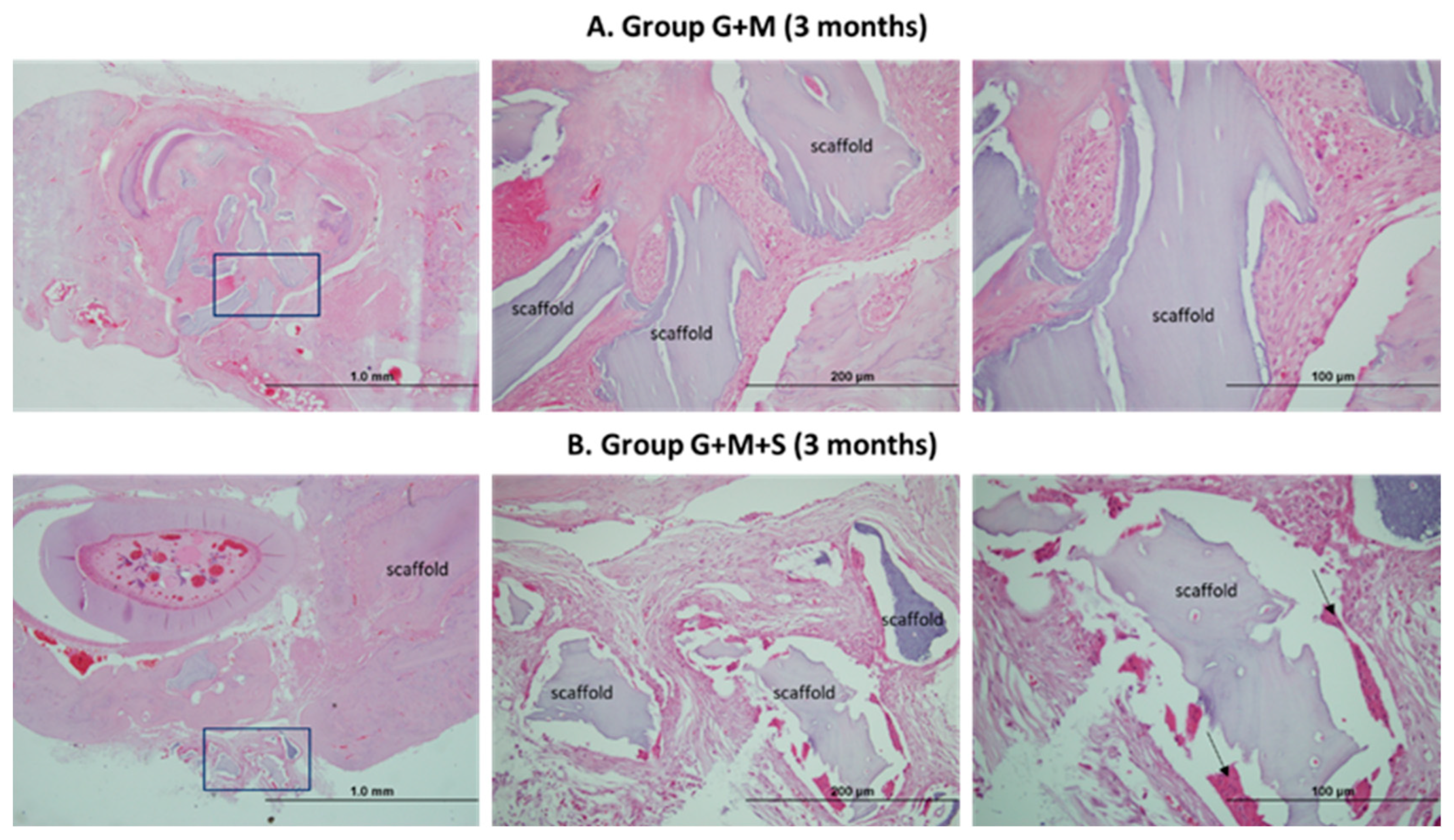
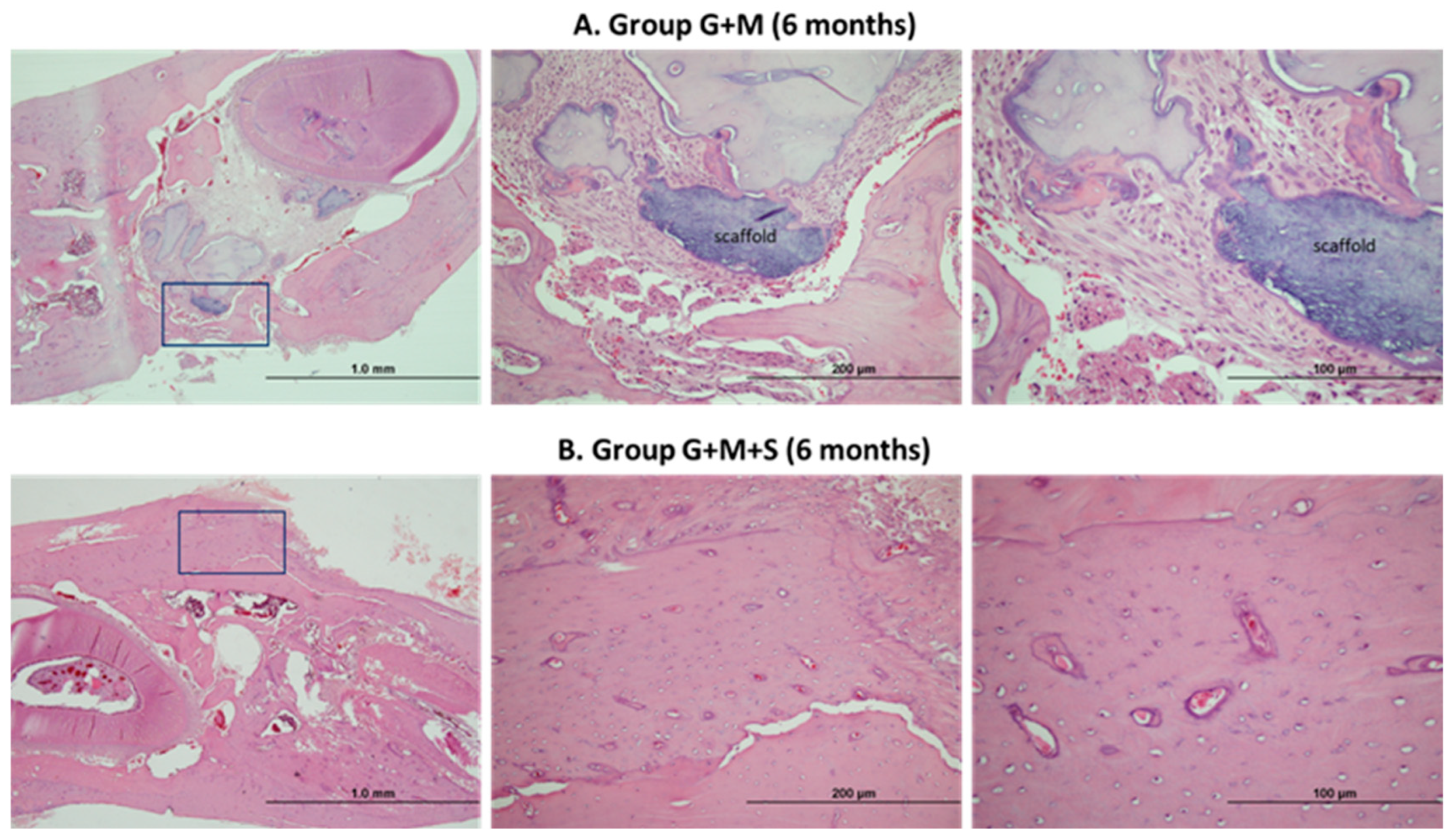
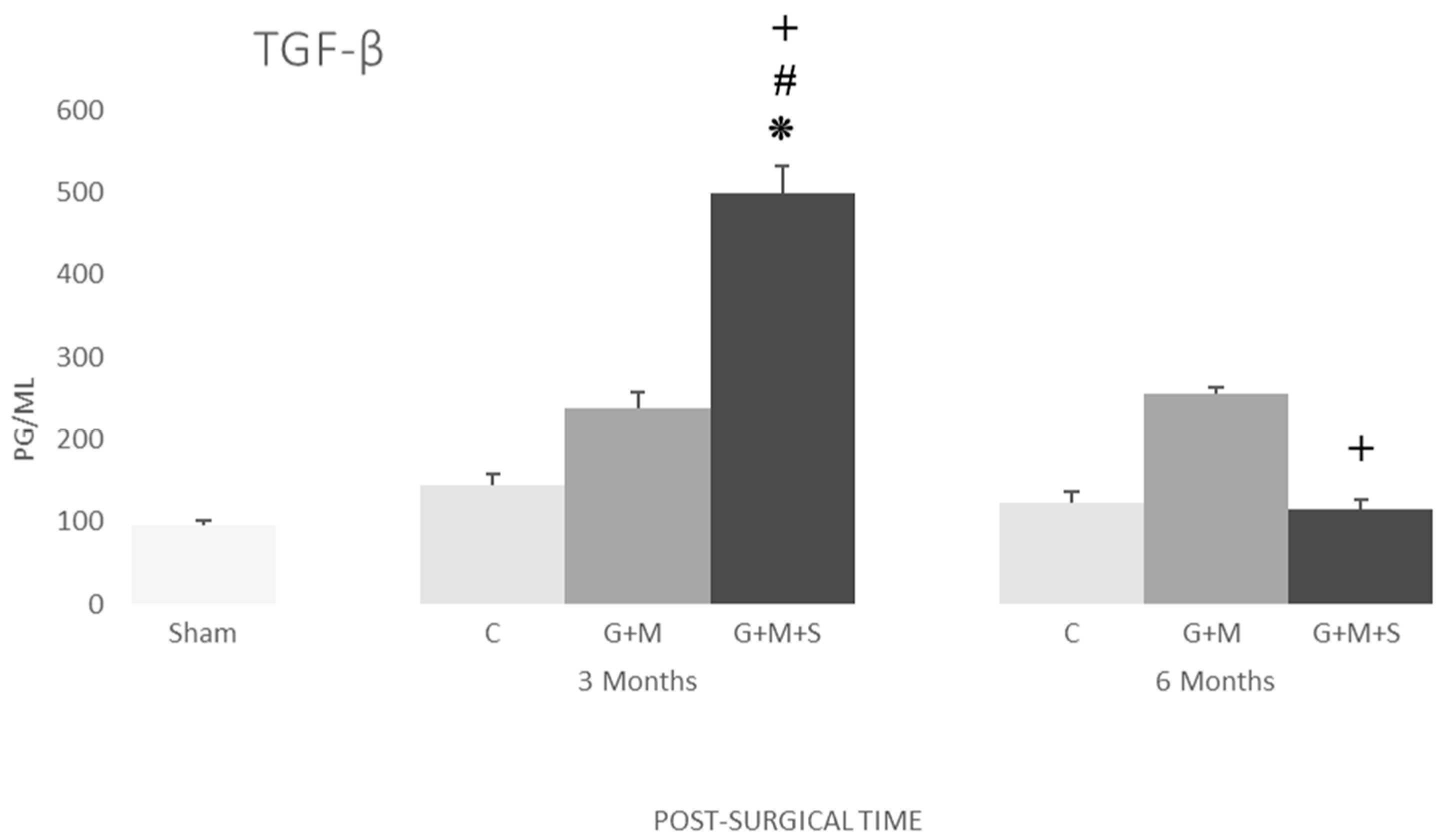
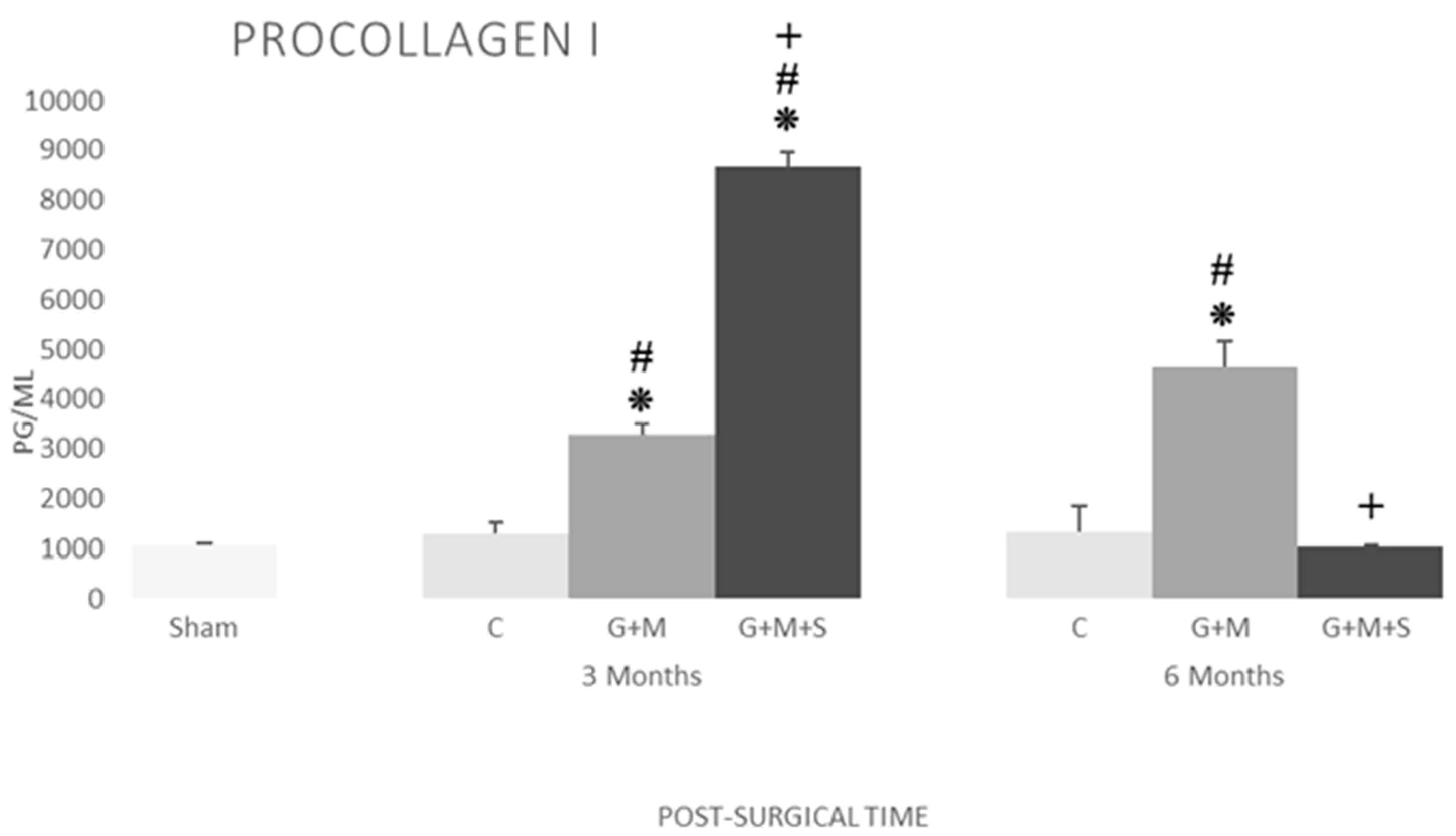
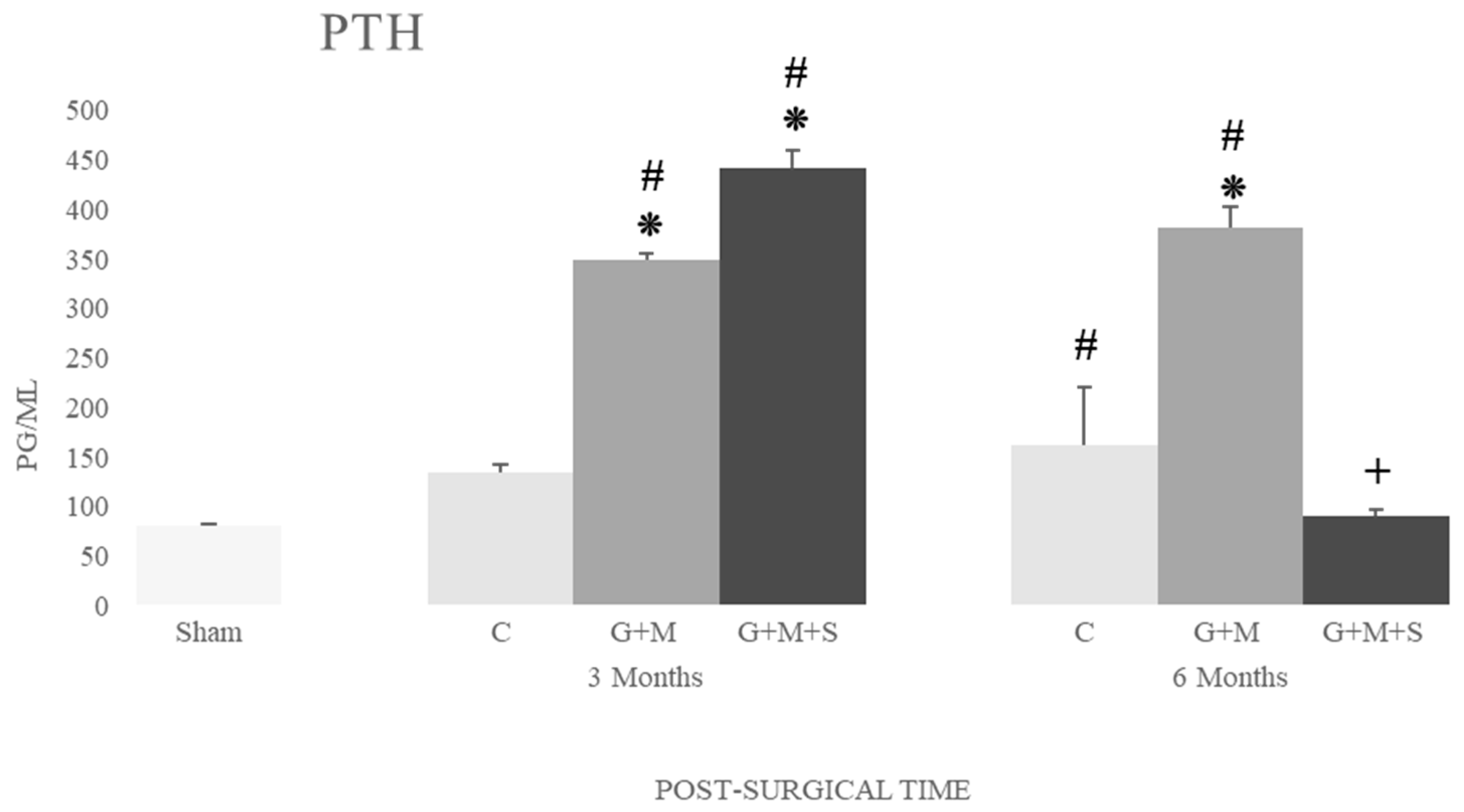
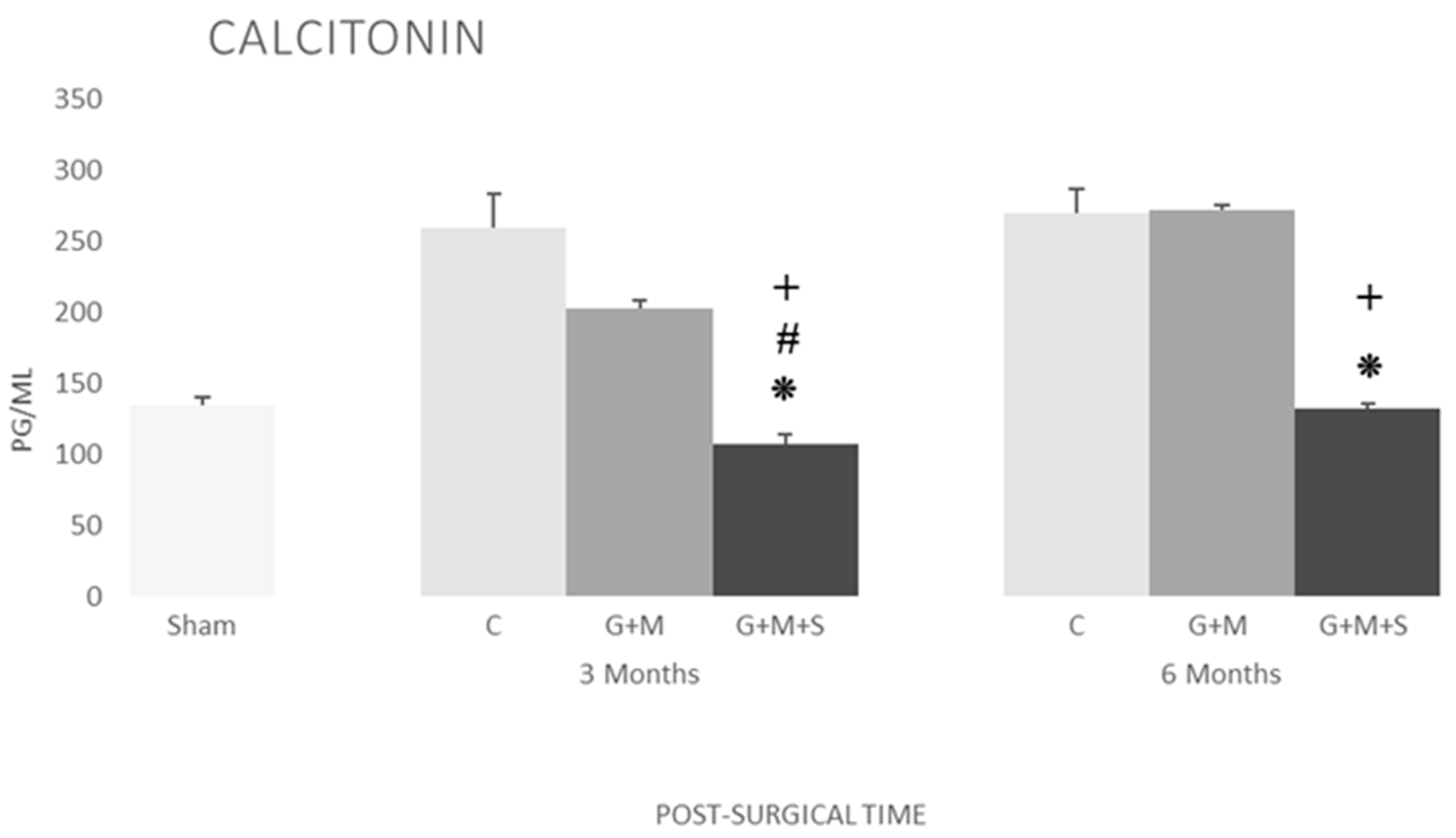
2.7. Molecular Studies
3. Discussion
4. Materials and Methods
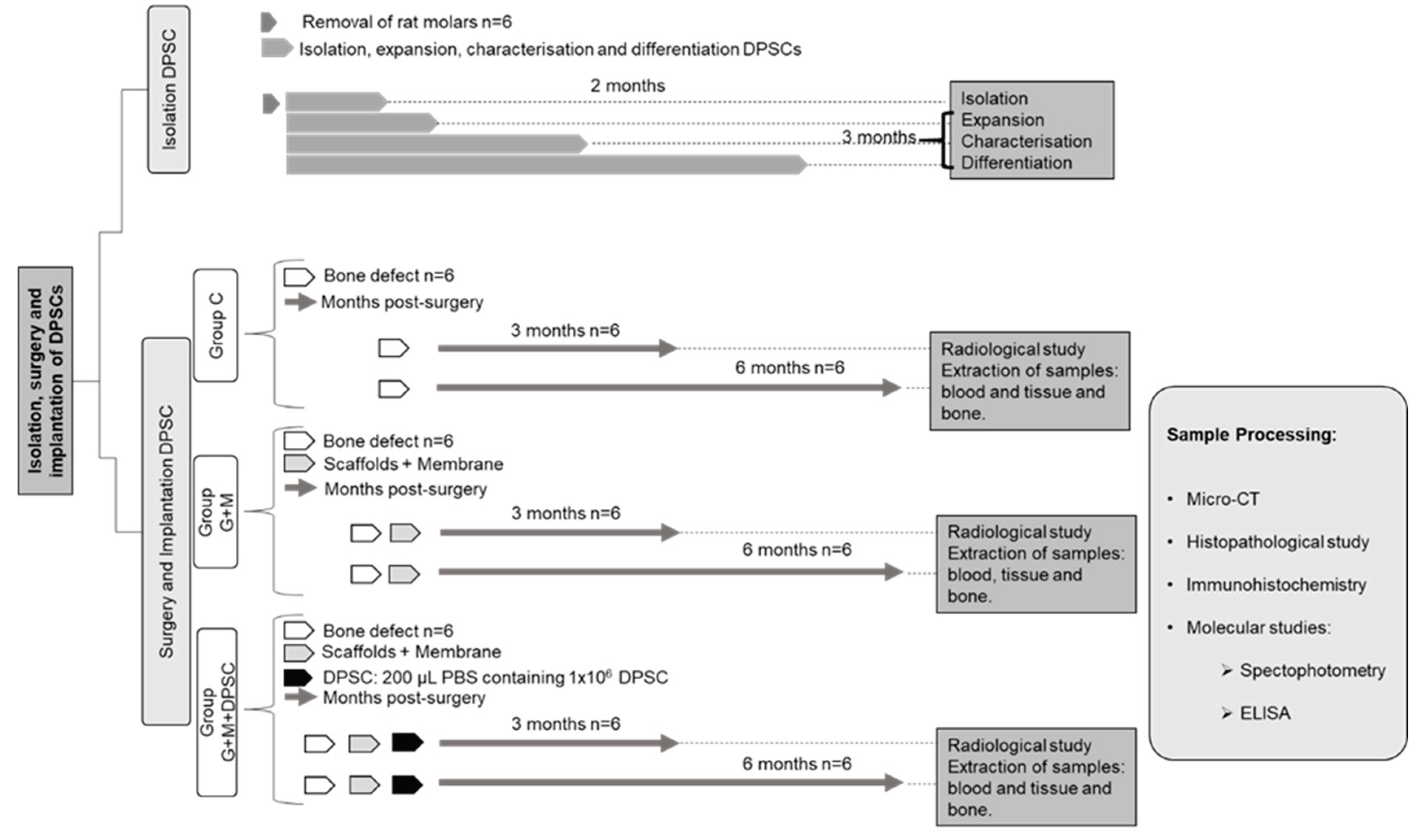
4.1. Experimental Design, Specimens, and Groups (Figure 7)
- Sham (Sham): Subject to the same anesthetic/surgical technique as the other groups except for the bone lesion and its treatment, as indicated below. The specimens remained caged for three months, whereupon the samples were gathered and they were euthanized (N = 4).
- Control (C): The bone lesion was effected but not treated, as indicated below. The specimens remained caged for three and six months (N = 6 in each case), whereupon the samples were gathered and they were euthanised (N = 12).
- Gen-Os® + Evolution® (G+M): The bone lesion was effected and then treated with the substitute bone biomaterial and covered with resorbable membrane, as indicated below The specimens remained caged for three and six months (N = 6 in each case), whereupon the samples were gathered and they were euthanized (N = 12).
- Gen-Os® + Evolution® + DPSC (G+M+S): The bone lesion was effected and then treated with the substitute bone biomaterial plus DP-MSC and covered with resorbable membrane, as indicated below. The specimens remained caged for three and six months (N = 6 in each case), whereupon the samples were gathered and they were euthanized (N = 12).
4.2. Biomaterials
4.3. Obtaining the DP-MSC from Pulp Tissue
4.4. Surgical Procedure
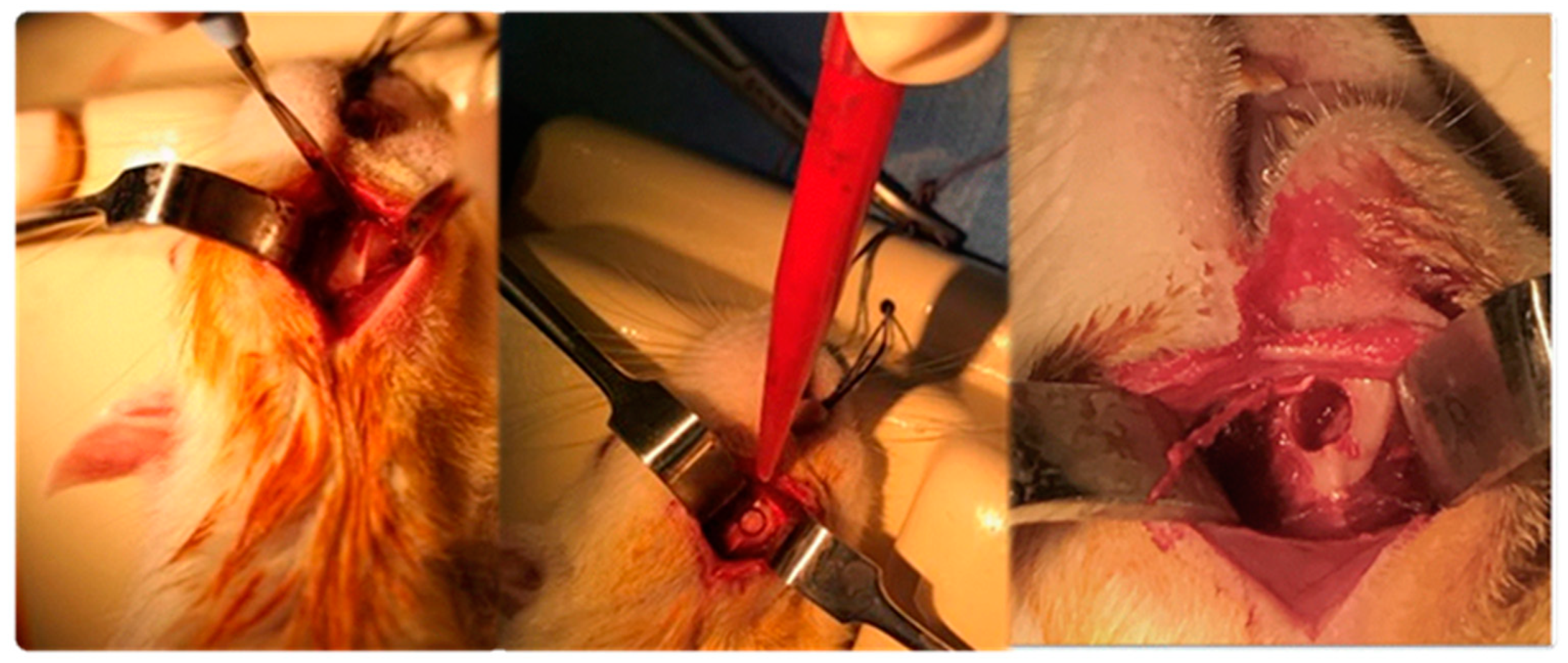
- Total blood by aortic puncture, centrifuged (20 minutes at 4500 rpm and 4ºC), extraction of plasma, aliquoted, and frozen at -80ºC.
- Perilesional tissue (bone and muscle) placed in liquid nitrogen and stored at -80ºC.
- Affected mandibulae removed in bloc, removal of the tissue on the bone surface, with some being immersed in formaldehyde 3.7-4.0% w/v buffered to pH = 7, and others stored at -80°C.
4.5. Verification of Cell Viability
4.6. Macroscopic Study
4.7. Scanning with Micro-Computed Tomography (micro-CT)
4.8. Histomorphometric Evaluation
4.9. ELISA
4.10. Statistical Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diana, A.; Carlino, F.; Giunta, E.F.; Franzese, E.; Guerrera, L.P.; Di Lauro, V.; Ciardiello, F.; Daniele, B.; Orditura, M. Cancer Treatment-Induced Bone Loss (CTIBL): State of the Art and Proper Management in Breast Cancer Patients on Endocrine Therapy. Curr Treat Options Oncol 2021, 22, 45. [CrossRef]
- Stumpf, U.; Kostev, K.; Kyvernitakis, J.; Böcker, W.; Hadji, P. Incidence of fractures in young women with breast cancer - a retrospective cohort study. J Bone Oncol 2019, 18, 100254. [CrossRef]
- Vestergaard, P. Drugs Causing Bone Loss. Handb Exp Pharmacol 2020, 262, 475-497. [CrossRef]
- Osipov, B.; Emami, A.J.; Christiansen, B.A. Systemic Bone Loss After Fracture. Clin Rev Bone Miner Metab 2018, 16, 116-130. [CrossRef]
- Pluskiewicz, W.; Adamczyk, P.; Drozdzowska, B. Fracture risk and fracture prevalence in women from outpatient osteoporosis clinic and subjects from population-based sample: A comparison between GO Study and RAC-OST-POL cohorts. Adv Clin Exp Med 2022. [CrossRef]
- Ruchlemer, R.; Amit-Kohn, M.; Tvito, A.; Sindelovsky, I.; Zimran, A.; Raveh-Brawer, D. Bone loss and hematological malignancies in adults: a pilot study. Support Care Cancer 2018, 26, 3013-3020. [CrossRef]
- Aguado, E.; Mabilleau, G.; Goyenvalle, E.; Chappard, D. Hypodynamia Alters Bone Quality and Trabecular Microarchitecture. Calcif Tissue Int 2017, 100, 332-340. [CrossRef]
- Arthur, A.; Gronthos, S. Clinical application of bone marrow mesenchymal stem/stromal cells to repair skeletal tissue. International journal of molecular sciences 2020, 21, 9759. [CrossRef]
- Zhang, Z.C.; He, Q.F.; Zhu, J.H.; Lin, X.X.; Yang, Y.; Chen, H.C.; Huang, X.Q.; Xu, R.G.; Deng, F.L. Optimizing the combined soft tissue repair and osteogenesis using double surfaces of crosslinked collagen scaffolds. Journal of Biomedical Materials Research Part B-Applied Biomaterials 2023, 111, 1271-1285. [CrossRef]
- Sugiaman, V.K.; Jeffrey; Naliani, S.; Pranata, N.; Djuanda, R.; Saputri, R.I. Polymeric Scaffolds Used in Dental Pulp Regeneration by Tissue Engineering Approach. Polymers 2023, 15. [CrossRef]
- Guo, T.; Yuan, X.; Li, X.; Liu, Y.; Zhou, J. Bone regeneration of mouse critical-sized calvarial defects with human mesenchymal stem cell sheets co-expressing BMP2 and VEGF. J Dent Sci 2023, 18, 135-144. [CrossRef]
- Ohori-Morita, Y.; Niibe, K.; Limraksasin, P.; Nattasit, P.; Miao, X.C.; Yamada, M.; Mabuchi, Y.; Matsuzaki, Y.; Egusa, H. Novel Mesenchymal Stem Cell Spheroids with Enhanced Stem Cell Characteristics and Bone Regeneration Ability. STEM CELLS TRANSLATIONAL MEDICINE 2022, 11, 434-449. [CrossRef]
- Zha, K.K.; Tian, Y.; Panayi, A.C.; Mi, B.B.; Liu, G.H. Recent Advances in Enhancement Strategies for Osteogenic Differentiation of Mesenchymal Stem Cells in Bone Tissue Engineering. Frontiers in Cell and Developmental Biology 2022, 10. [CrossRef]
- Costela-Ruiz, V.J.; Melguizo-Rodriguez, L.; Bellotti, C.; Illescas-Montes, R.; Stanco, D.; Arciola, C.R.; Lucarelli, E. Different Sources of Mesenchymal Stem Cells for Tissue Regeneration: A Guide to Identifying the Most Favorable One in Orthopedics and Dentistry Applications. INTERNATIONAL JOURNAL OF MOLECULAR SCIENCES 2022, 23. [CrossRef]
- Chen, X.; Gao, C.Y.; Chu, X.Y.; Zheng, C.Y.; Luan, Y.Y.; He, X.; Yang, K.; Zhang, D.L. VEGF-Loaded Heparinised Gelatine-Hydroxyapatite-Tricalcium Phosphate Scaffold Accelerates Bone Regeneration via Enhancing Osteogenesis-Angiogenesis Coupling. Frontiers in Bioengineering and Biotechnology 2022, 10. [CrossRef]
- Camacho-Alonso, F.; Tudela-Mulero, M.R.; Navarro, J.A.; Buendia, A.J.; Mercado-Diaz, A.M. Use of buccal fat pad-derived stem cells cultured on bioceramics for repair of critical-sized mandibular defects in healthy and osteoporotic rats. Clinical Oral Investigations 2022, 26, 5389-5408. [CrossRef]
- Su, X.; Wang, T.; Guo, S. Applications of 3D printed bone tissue engineering scaffolds in the stem cell field. Regen Ther 2021, 16, 63-72. [CrossRef]
- Hollinger, J.O.; Kleinschmidt, J.C. The critical size defect as an experimental model to test bone repair materials. J Craniofac Surg 1990, 1, 60-68. [CrossRef]
- Furuhata, M.; Takayama, T.; Yamamoto, T.; Ozawa, Y.; Senoo, M.; Ozaki, M.; Yamano, S.; Sato, S. Real-time assessment of guided bone regeneration in critical size mandibular bone defects in rats using collagen membranes with adjunct fibroblast growth factor-2. J Dent Sci 2021, 16, 1170-1181. [CrossRef]
- Peña, G.; Gallego, L.; Redondo, L.M.; Junquera, L.; Doval, J.F.; Meana, Á. Comparative analysis of plasma-derived albumin scaffold, alveolar osteoblasts and synthetic membrane in critical mandibular bone defects: An experimental study on rats. J Biomater Appl 2021, 36, 481-491. [CrossRef]
- Xu, L.J.; Yuan, H.; Ye, Q.; Li, J.Y. [Repair of mandibular defects with hydrogel loaded with nano-hydroxyapatite in rats]. Shanghai Kou Qiang Yi Xue 2022, 31, 449-453.
- Bexell, D.; Gunnarsson, S.; Tormin, A.; Darabi, A.; Gisselsson, D.; Roybon, L.; Scheding, S.; Bengzon, J. Bone Marrow Multipotent Mesenchymal Stroma Cells Act as Pericyte-like Migratory Vehicles in Experimental Gliomas. Molecular Therapy 2009, 17, 183-190. [CrossRef]
- Barzilay, R.; Sadan, O.; Melamed, E.; Offen, D. Comparative characterization of bone marrow-derived mesenchymal stromal cells from four different rat strains. Cytotherapy 2009, 11, 435-442. [CrossRef]
- Karaoz, E.; Aksoy, A.; Ayhan, S.; Sariboyaci, A.; Kaymaz, F.; Kasap, M. Characterization of mesenchymal stem cells from rat bone marrow: ultrastructural properties, differentiation potential and immunophenotypic markers. Histochemistry and Cell Biology 2009, 132, 533-546. [CrossRef]
- Harting, M.; Jimenez, F.; Pati, S.; Baumgartner, J.; Cox, C. Immunophenotype characterization of rat mesenchyrnal stromal cells. Cytotherapy 2008, 10, 243-253. [CrossRef]
- Boxall, S.; Jones, E. Markers for Characterization of Bone Marrow Multipotential Stromal Cells. Stem Cells International 2012, 2012. [CrossRef]
- Song, K.; Huang, M.; Shi, Q.; Du, T.; Cao, Y. Cultivation and identification of rat bone marrow-derived mesenchymal stem cells. Molecular Medicine Reports 2014, 10, 755-760. [CrossRef]
- HEMLER, M. VLA PROTEINS IN THE INTEGRIN FAMILY - STRUCTURES, FUNCTIONS, AND THEIR ROLE ON LEUKOCYTES. Annual Review of Immunology 1990, 8, 365-400. [CrossRef]
- AKIYAMA, S.; HASEGAWA, E.; HASEGAWA, T.; YAMADA, K. THE INTERACTION OF FIBRONECTIN FRAGMENTS WITH FIBROBLASTIC CELLS. Journal of Biological Chemistry 1985, 260, 3256-3260. [CrossRef]
- Brown, M.A.; Wallace, C.S.; Anamelechi, C.C.; Clermont, E.; Reichert, W.M.; Truskey, G.A. The use of mild trypsinization conditions in the detachment of endothelial cells to promote subsequent endothelialization on synthetic surfaces. Biomaterials 2007, 28, 3928-3935. [CrossRef]
- D'Aquino, R.; De Rosa, A.; Laino, G.; Caruso, F.; Guida, L.; Rullo, R.; Checchi, V.; Laino, L.; Tirino, V.; Papaccio, G. Human dental pulp stem cells: from biology to clinical applications. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 2009, 312B, 408-415. [CrossRef]
- Heitzer, M.; Modabber, A.; Zhang, X.; Winnand, P.; Zhao, Q.; Bläsius, F.M.; Buhl, E.M.; Wolf, M.; Neuss, S.; Hölzle, F.; et al. In vitro comparison of the osteogenic capability of human pulp stem cells on alloplastic, allogeneic, and xenogeneic bone scaffolds. [CrossRef]
- Merckx, G.; Hosseinkhani, B.; Kuypers, S.; Deville, S.; Irobi, J.; Nelissen, I.; Michiels, L.; Lambrichts, I.; Bronckaers, A. Angiogenic Effects of Human Dental Pulp and Bone Marrow-Derived Mesenchymal Stromal Cells and their Extracellular Vesicles. LID. [CrossRef]
- Rombouts, C.; Jeanneau C Fau - Camilleri, J.; Camilleri J Fau - Laurent, P.; Laurent P Fau - About, I.; About, I. Characterization and angiogenic potential of xenogeneic bone grafting materials: Role of periodontal ligament cells.
- Luzuriaga, J.; Polo, Y.; Pastor-Alonso, O.; Pardo-Rodríguez, B.; Larrañaga, A.; Unda, F.; Sarasua, J.-R.; Pineda, J.R.; Ibarretxe, G. Advances and perspectives in dental pulp stem cell based neuroregeneration therapies. International journal of molecular sciences 2021, 22, 3546. [CrossRef]
- El Moshy, S.; Radwan, I.A.; Rady, D.; Abbass, M.M.; El-Rashidy, A.A.; Sadek, K.M.; Dörfer, C.E.; El-Sayed, K.M.F. Dental stem cell-derived secretome/conditioned medium: the future for regenerative therapeutic applications. Stem Cells International 2020, 2020. [CrossRef]
- Sultan, N.; Amin, L.E.; Zaher, A.R.; Scheven, B.A.; Grawish, M.E. Dental pulp stem cells: Novel cell-based and cell-free therapy for peripheral nerve repair. World Journal of Stomatology 2019, 7, 1-19. [CrossRef]
- Lan, X.; Sun, Z.; Chu, C.; Boltze, J.; Li, S. Dental pulp stem cells: an attractive alternative for cell therapy in ischemic stroke. Frontiers in neurology 2019, 10, 824. [CrossRef]
- Man, R.C.; Sulaiman, N.; Idrus, R.B.H.; Ariffin, S.H.Z.; Wahab, R.M.A.; Yazid, M.D. Insights into the effects of the dental stem cell secretome on nerve regeneration: towards cell-free treatment. Stem cells international 2019, 2019. [CrossRef]
- Bar, J.K.; Lis-Nawara, A.; Grelewski, P.G. Dental Pulp Stem Cell-Derived Secretome and Its Regenerative Potential. International Journal of Molecular Sciences 2021, 22, 12018. [CrossRef]
- Valluru, M.; Staton Ca Fau - Reed, M.W.R.; Reed Mw Fau - Brown, N.J.; Brown, N.J. Transforming Growth Factor-β and Endoglin Signaling Orchestrate Wound Healing.
- Patil, A.S.; Sable Rb Fau - Kothari, R.M.; Kothari, R.M. An update on transforming growth factor-β (TGF-β): sources, types, functions and clinical applicability for cartilage/bone healing.
- Boskey, A.L.; Coleman, R. Aging and Bone. Journal of Dental Research 2010, 89, 1333-1348. [CrossRef]
- Wu, M.; Chen, G.; Li, Y.P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease.
- Chen, G.; Deng C Fau - Li, Y.-P.; Li, Y.P. TGF-β and BMP signaling in osteoblast differentiation and bone formation.
- Phillips, A.M. Overview of the fracture healing cascade. [CrossRef]
- Henriksen, K.; Karsdal, M.A.; Martin, T.J. Osteoclast-derived coupling factors in bone remodeling. Calcif Tissue Int 2014, 94, 88-97. [CrossRef]
- Huang, J.; Lin, D.; Wei, Z.; Li, Q.; Zheng, J.; Zheng, Q.; Cai, L.; Li, X.; Yuan, Y.; Li, J. Parathyroid Hormone Derivative with Reduced Osteoclastic Activity Promoted Bone Regeneration via Synergistic Bone Remodeling and Angiogenesis. Small 2020, 16, e1905876. [CrossRef]
- Chen, T.; Wang, Y.; Hao, Z.; Hu, Y.; Li, J. Parathyroid hormone and its related peptides in bone metabolism. [CrossRef]
- Fan, Y.; Hanai, J.-i.; Le, P.T.; Bi, R.; Maridas, D.; DeMambro, V.; Figueroa, C.A.; Kir, S.; Zhou, X.; Mannstadt, M. Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell metabolism 2017, 25, 661-672. [CrossRef]
- Yang, M.; Arai, A.; Udagawa, N.; Zhao, L.; Nishida, D.; Murakami, K.; Hiraga, T.; Takao-Kawabata, R.; Matsuo, K.; Komori, T. Parathyroid hormone shifts cell fate of a leptin receptor-marked stromal population from adipogenic to osteoblastic lineage. Journal of Bone and Mineral Research 2019, 34, 1952-1963. [CrossRef]
- Larsson, S.; Jones, H.A.; Göransson, O.; Degerman, E.; Holm, C. Parathyroid hormone induces adipocyte lipolysis via PKA-mediated phosphorylation of hormone-sensitive lipase. Cellular signalling 2016, 28, 204-213. [CrossRef]
- Jiang, L.; Zhang, W.; Wei, L.; Zhou, Q.; Yang, G.; Qian, N.; Tang, Y.; Gao, Y.; Jiang, X. Early effects of parathyroid hormone on vascularized bone regeneration and implant osseointegration in aged rats. Biomaterials 2018, 179, 15-28. [CrossRef]
- Keller, J.; Catala-Lehnen, P.; Huebner, A.K.; Jeschke, A.; Heckt, T.; Lueth, A.; Krause, M.; Koehne, T.; Albers, J.; Schulze, J.; et al. Calcitonin controls bone formation by inhibiting the release of sphingosine 1-phosphate from osteoclasts. [CrossRef]
- Yuan, G.; Lu, H.; Yin, Z.; Dai, S.; Jia, R.; Xu, J.; Song, X.; Li, L. Effects of mixed subchronic lead acetate and cadmium chloride on bone metabolism in rats. Int J Clin Exp Med 2014, 7, 1378-1385.
- Jeanneau, C.; Le Fournis, C.; About, I. Xenogeneic bone filling materials modulate mesenchymal stem cell recruitment: role of the Complement C5a. [CrossRef]
- Figueiredo, A.; Coimbra P Fau - Cabrita, A.; Cabrita A Fau - Guerra, F.; Guerra F Fau - Figueiredo, M.; Figueiredo, M. Comparison of a xenogeneic and an alloplastic material used in dental implants in terms of physico-chemical characteristics and in vivo inflammatory response.


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




