1. Introduction
Monitoring cerebral oxygenation (rSO2) by NIRS was introduced at the turn of the 1980s, and many clinicians believed it was a valuable method for assessing cerebral perfusion and subsequent measures to optimize cerebral flow in patients with brain damage [
1,
2,
3]. The problem is extremely important because the assessment of cerebral perfusion is based on very invasive and sophisticated diagnostic methods, hence the limitation in their application. The most commonly used invasive ones include measurements of intracranial pressure (ICP), brain temperature (BT), brain oxygen tension (PbtO2), neurochemistry via micro dialysis (MD), cerebral blood flow (CBF), and jugular oxygen saturation (SjvO2) [
1,
2].
The methods described allow monitoring of cerebral oxygenation and perfusion continuously. Non-invasive examination of cerebral vascular blood flow by transcranial Doppler limits observations to the time when measurements are taken in the patient. Therefore, the introduction of cerebral tissue saturation monitoring by non-invasive continuous observation has become an optimal and easy tool in the diagnosis of patients with TBI [
1]. However, the initial interest in the method and the peak of the resulting medical research and publications ended in 2014- 2015 [
2].
The problem includes serious questions about the validation of the method, the dependence of the result on the presence of chromophores in intermediate structures. A very important argument for this statement is based on observations published repeatedly, but based on studies conducted on small groups of patients [
4,
5,
6].
The main problem put forward is the presence of the described intermediate structures-skin, skull, meninges, cerebrospinal fluid-and their influence on the outcome of the test. In order to exclude the influence of chromatophores in superficial structures, two or more light detectors are used, which allow separate data to be obtained from shallow and deep optical signals. This process is referred to as spatial resolution. [
7] There are a number of publications in the literature that show a significant effect on the result of NIRS measurement through intermediate structures. These publications use the phenomenon of orthostatic changes in blood flow in craniofacial structures, the effect of the Valsalva maneuver [
8]. The effects of vasoconstrictors and even mechanical restriction of flow through the skin by pressure bands [
9]. We present a case in which the described dilemma of NIRS reading sensitivity is directly related to the diversification of CNS and craniofacial blood flow. NIRS assessment performed on a patient during brain death attempts, in such a clinical situation in which cerebral flow is stopped, can give an idea of the magnitude of the influence of these intermediate structures on the monitoring result.
2. Case
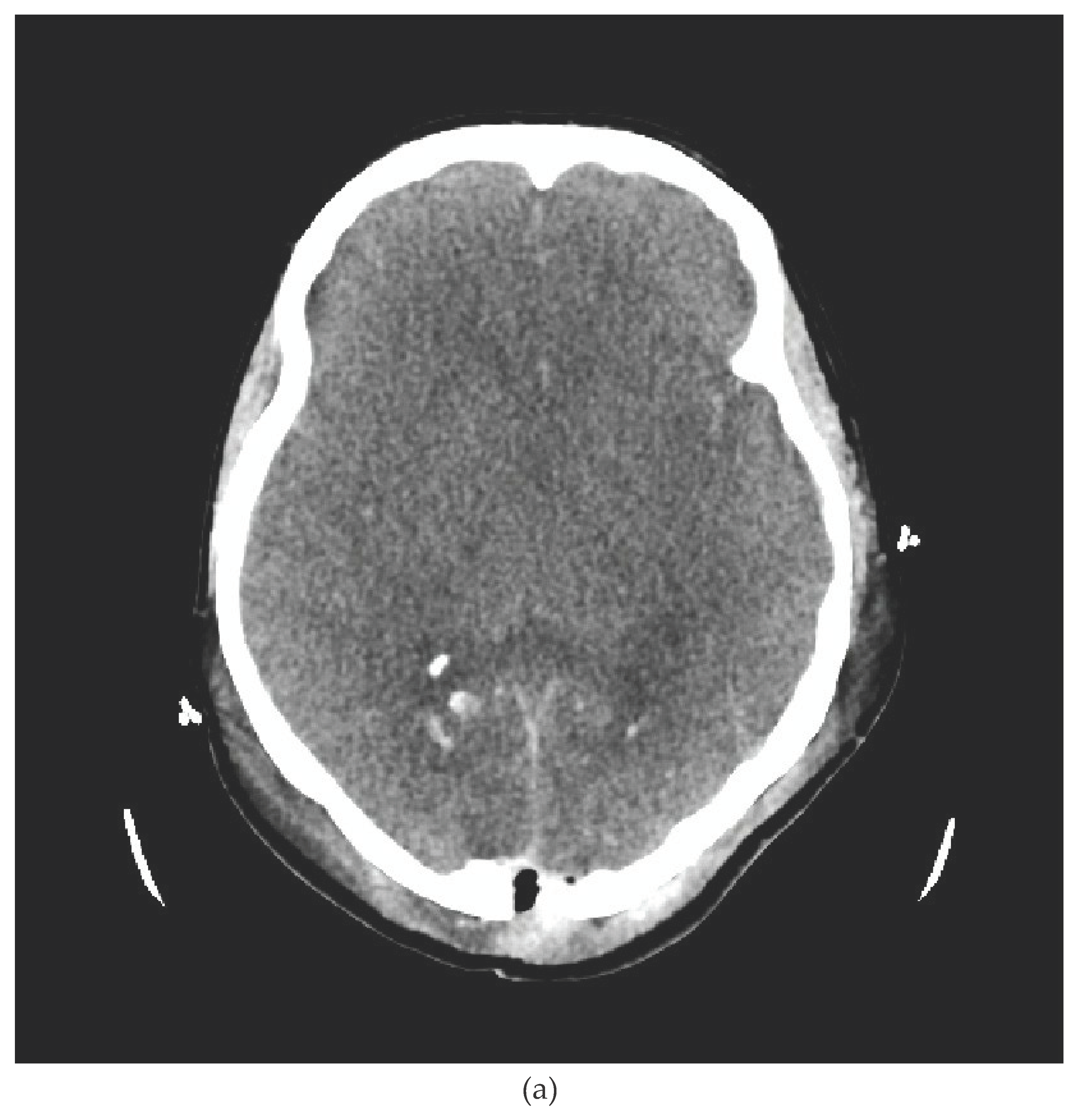
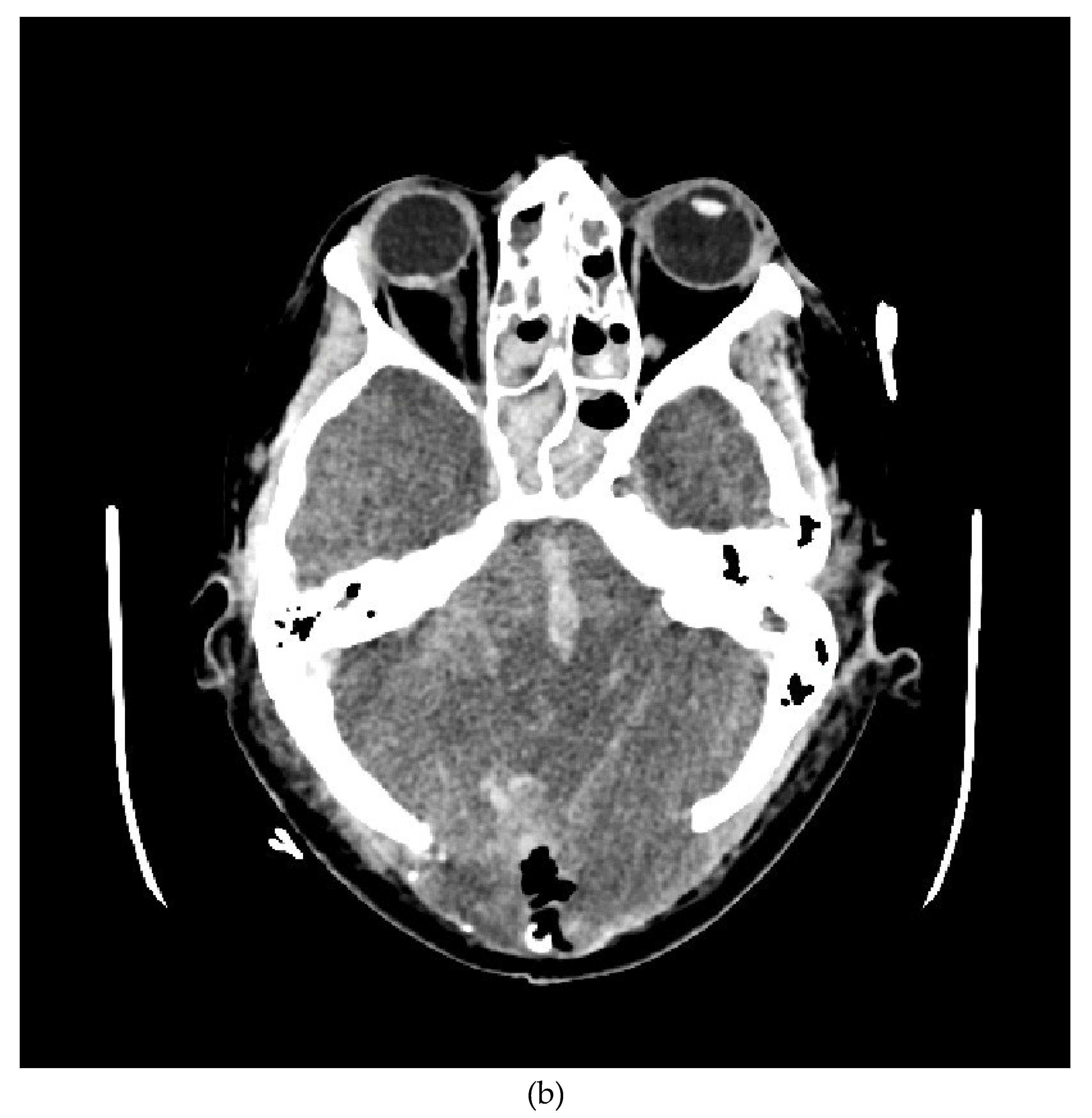
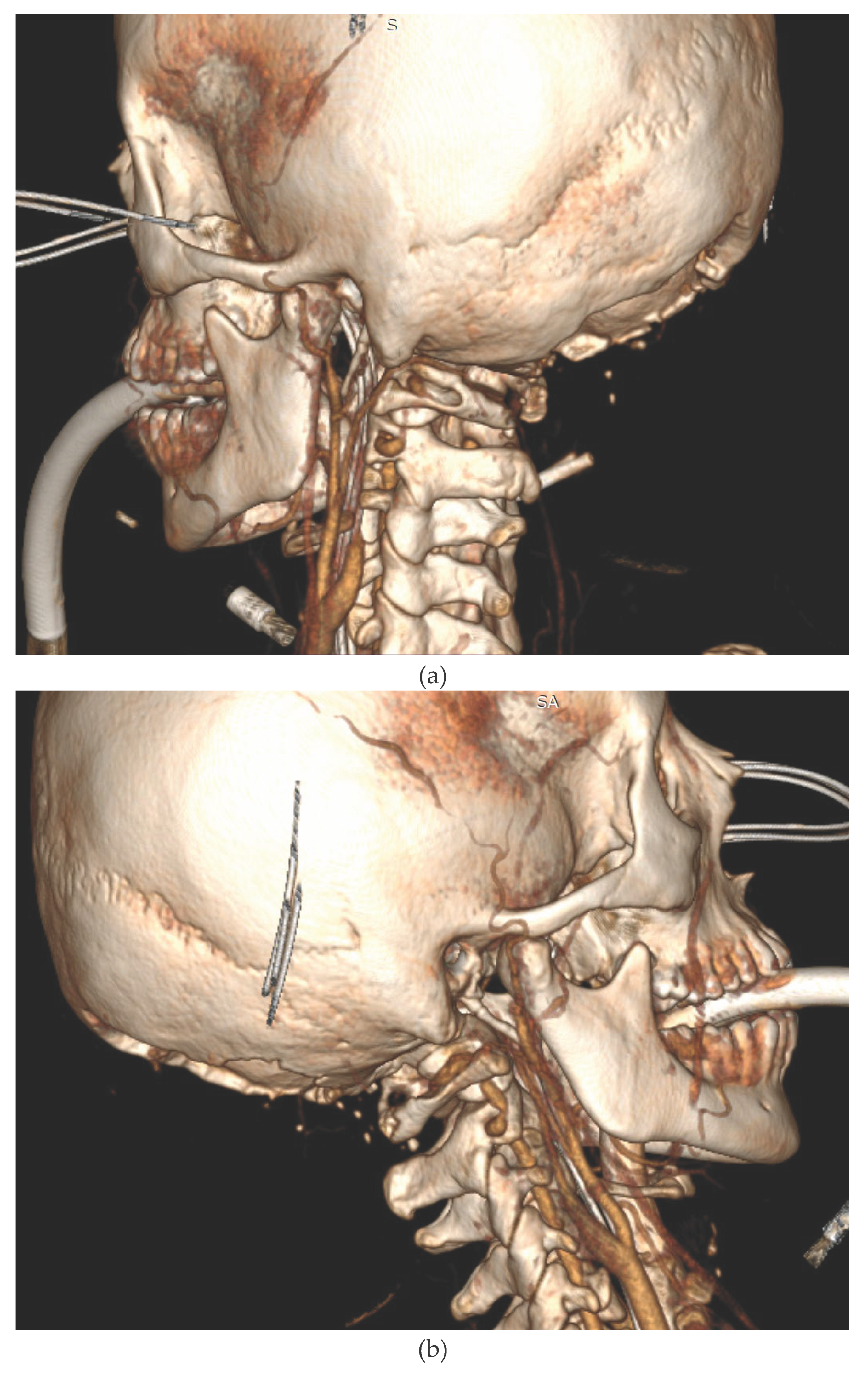
We present the case of a 33-year-old female patient with a diagnosis of AML (Acute Myeloblastic Leucaemia). The patient, was transferred from Haematology Unit to ICU following neurological deterioration. On the basis of the imaging studies performed, the diagnosis was an acute intracranial haemorrhage. Immediately after the CT scan, the patient underwent neurosurgical treatment. After the surgical procedure, he was readmitted to the ICU. Despite the therapy, the patient was diagnosed with the absence of brain reflexes. Among the imaging tests performed, there was a CT scan of the CNS (
Figure 1a,b) and CTA (
Figure 2a,b), which showed catastrophic brain damage. Then, according to institutional brain death guidelines clinical evaluations and apnoea tests were performed at certain intervals (Current AAN brain death guidelines do not require multiple exams) [
10].
We present a retrospective analysis of cerebral oxygenation measurements using the INVOS™ Regional Oximeter in the presented case of a patient undergoing a brain death diagnostic procedure. A clinical situation where the cerebral blood flow is stopped can give an idea of the specificity of the method, particularly the impact of the influence of intermediate structures on the monitoring outcome. The brain death determination was done according to accepted international protocols and included a clinical exam and apnoea test.
In detail, the brain death protocol consisted of a consecutive series of tests to determine the irreversible damage to the brain stem, including apnoea tests on two occasions. The respiratory centre areactivity test is considered positive if there is a lack of respiratory activity during the 5-minute apnoea observation of the patient with normal baseline gasometry during apnoea observation, and there is an increase in CO2 level to a value above 60 mmHg, by at least 20% from baseline. The diagnosis is made according to a protocol monitored by the Brain Death Committee [
10].
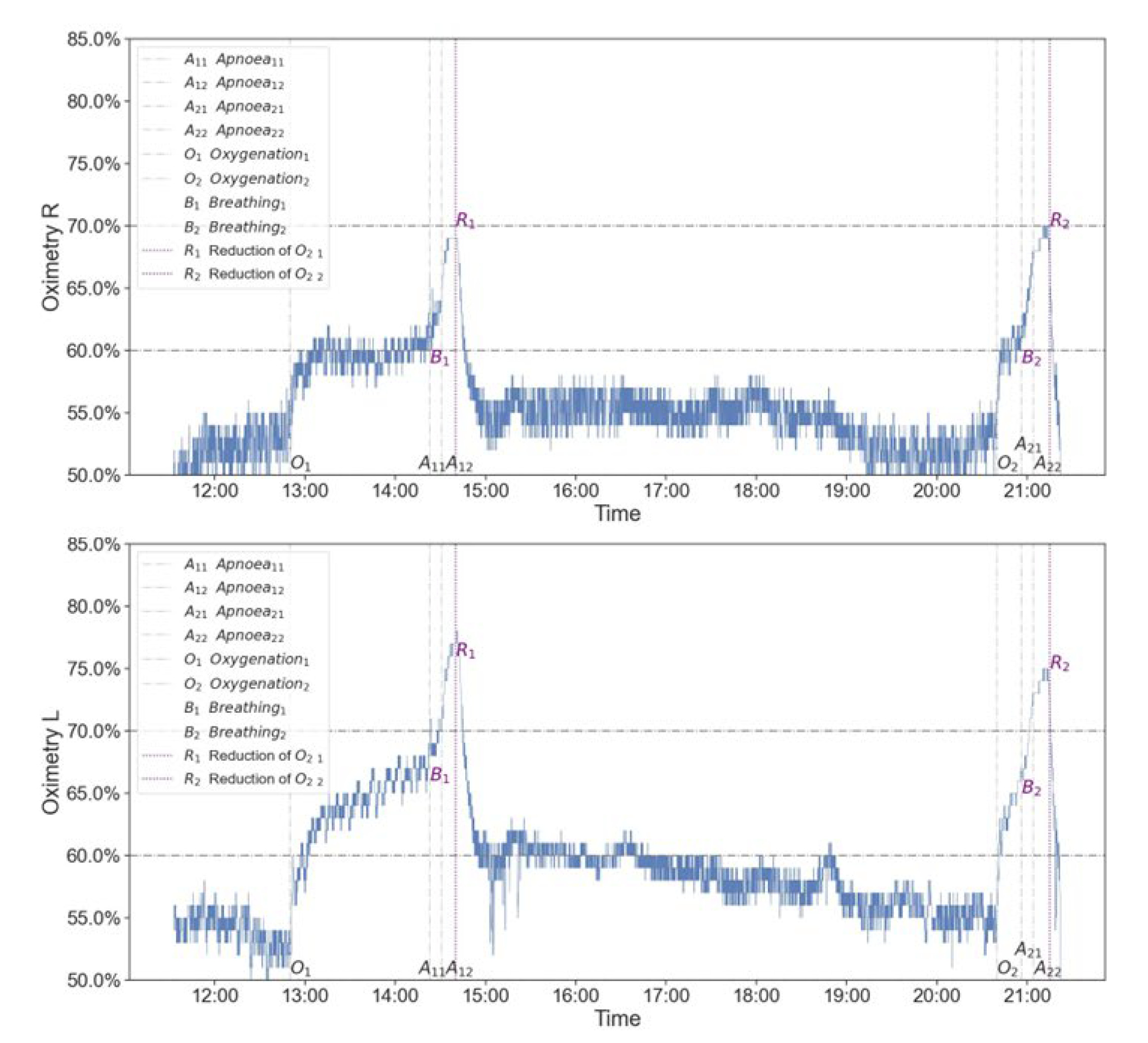
Throughout the procedure, from the diagnosis of areflexia until the patient was pronounced dead, the patient was monitored with NIRS (INVOS™ Regional Oximeter).
As can be seen, the level of cerebral oxygenation was relatively constant, symmetrical from both cerebral hemispheres, and reached values close to 50%, both before and between the tests performed (
Figure 3). Prior to testing respiratory center drive, the patient was first provided with 100% FiO2. On NIRS examination, a significant increase in rSO2 was observed with rSO2 reaching 60-70% (improvement up to 20%). The increased saturation to a value close to rSO2 70% is maintained throughout the whole apnoea trial and then, after the patient is ventilated again, a further increase in the NIRS result is observed. Subsequently, a reduction in FiO2 to a baseline of 50% is followed by a return of NIRS to baseline values. A similar pattern was seen in both apnoea tests (
Figure 3).
3. Discussion
In this case, the NIRS result is increased by the oxygenation of the patient before the apnoea test. The influence of chromophores in the tissues surrounding the CNS, reflections, and scattering of the light wave spectrum has a very significant effect on the final result of cerebral saturation measurement. A very important argument for this statement is based on observations that have been repeatedly published from studies conducted on small groups of patients [
4,
5,
6,
7,
8,
9]. This is exemplified by the publication by Tatli and colleagues, who confirmed the lack of sensitivity of NIRS diagnosis for confirming brain death in patients [
5]. Attempts to explain these observations have already been described in publications, but the magnitude of the changes in oxygenation in NIRS measurements in our study, i.e., an increase of more than 20% from baseline, shows the importance of the measurement artifacts described. The problem of the variability of cerebral oximetry measurements and its dependence on the ratio of intracranial and extracranial arterio- venous flow is complex. In case the following increase in NIRS measurements, which is evidently initiated when pressure ventilation with PEEP is switched on, is related to the phenomenon of venous outflow resistance. The breathing can lead to a redistribution of venous blood, a predominance of the arterial component, and an increase in the level of oxygenation in the measurements. The described dependency additionally shows possible bias in the interpretation of measurements with NIRS [
11].
The majority of research observations describing changes in cerebral perfusion or its optimization may be burdened by the problem described, i.e., the significant influence of measured oxygenation from intermediate structures. Despite the described dilemmas, publications continue to confirm the usefulness of the method without considering the presented pitfalls [
12,
13]. Numerous examples of the dilemma described can be found in works using NIRS monitoring in cardiac surgery. Often the issue of autoregulation of cerebral flow is not taken into account, and the increase in arterial pressure and improvement in surface saturation are translated into a positive effect of the applied hemodynamic maneuvers [
12,
13,
14,
15]. The publication by Takegawa et al. provides an example of the ambiguity of the problem [
16]. A significant decrease in cerebral oximetry was observed in patients with induced hypothermia. According to the authors, this contradicts the logic of reduced CNS oxygen consumption. On the other hand, the authors do not link the possibility of the result of reduced rSO2 with reduced blood flow through the described superficial structures, which depends precisely on the external temperature. They explain this phenomenon by an increase in cerebral vascular resistance and a decrease in brain blood flow, which is in particular the opposite of the intended effects of hypothermia.
The specificity of NIRS to assess cerebral perfusion requires careful analysis. Despite the use of spatial resolution, multiple light emitters and detectors, commercially available spectroscopes do not deliver a pure cerebral oxygenation signal [
9]. The therapeutic implications of monitoring cerebral oxygenation with NIRS are of great importance and, from the example presented and the literature provided, the method should be used with caution. It has been shown that in a patient with brain death, the result of NIRS oxygenation measurements depends on the surrounding structures of the brain. Their influence can be so significant that, after oxygenation of the patient, it is possible to obtain NIRS measurements of rSO2 in a patient without cerebral flow that are comparable to measurements in a healthy person. The example given vividly confirms the data from the literature and how large the margin of error of the measurements and the subsequent misinterpretation of the results obtained can be.
In a review published by Nature Publishing Groupe in 2022, which described the experience with NIRS to date, we did not encounter clear conclusions [
17]. Perhaps solving all the problems that limit the method and taking into account artefacts that can affect the measurement result will increase its specificity.
4. Summary
The described case of a patient undergoing the brain death protocol and the recording of the obtained NIRS measurements confirm the limitations of the method in diagnosing brain death. We show the extent to which the results are contaminated by intermediate structures. The extent of measurement contamination can be related to other cases of monitored patients. This problem is reflected in numerous publications in which the NIRS monitoring does not lead to improved patient outcomes. The described example of continuous monitoring of a patient during attempts at brainstem death is very illustrative and also gives an insight into the limitations of infrared spectrophotometry in its broad application
Author Contributions
The role of the authors: ML- 1, 2, 3, 4, NK- 1, 2, 3, 4. 1. Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; 2. Drafting the article or revising it critically for important intellectual content;3. Final approval of the version to be published; 4. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study has been approved by the Bioethics Committee of the Alfred Sokolowski Specialist Hospital in Walbrzych, Oncology Support Centre for Clinical Trials, 58-309 Walbrzych, Poland. Details page: 1) We confirm that manuscript complies with all instructions to authors. 2) We confirm that the authorship requirements have been met and that the final manuscript has been approved by all authors. 3) We confirm that this manuscript has not been published elsewhere and is not under consideration by another journal UPDATED June 1, 2023 P a g e | 8. 4) We confirm adherence to ethical guidelines and indicate IRB approval and use of informed consent where appropriate. 5) We disclose Conflicts of Interest for all authors. 6) There are no funding sources for the study. 7) No checklist required for publication.
References
- Vinciguerra, L.; Bösel, J. Noninvasive Neuromonitoring: Current Utility in Subarachnoid Hemorrhage, Traumatic Brain Injury, and Stroke. Neurocritical Care 2016, 27, 122–140. [Google Scholar] [CrossRef] [PubMed]
- Roldán, M.; Kyriacou, P.A. Near-Infrared Spectroscopy (NIRS) in Traumatic Brain Injury (TBI). Sensors 2021, 21, 1586. [Google Scholar] [CrossRef] [PubMed]
- Sekhon MS, Griesdale DE. Individualised perfusion targets for hypoxic ischaemic brain injury after cardiac arrest. Crit Care. 2: 2017 Oct 24;21(1), 2017.
- Kato, S.; Yoshitani, K.; Kubota, Y.; Inatomi, Y.; Ohnishi, Y. Effect of posture and extracranial contamination on results of cerebral oximetry by near-infrared spectroscopy. J. Anesthesia 2016, 31, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Tatli, O.; Bekar, O.; Imamoglu, M.; Cekic, O.G.; Aygun, A.; Eryigit, U.; Karaca, Y.; Sahin, A.; Turkmen, S.; Turedi, S. Cerebral Oximetry as an Auxiliary Diagnostic Tool in the Diagnosis of Brain Death. Transplant. Proc. 2017, 49, 1702–1707. [Google Scholar] [CrossRef] [PubMed]
- Kyttä J, Ohman J, Tanskanen P, Randell T. Extracranial contribution to cerebral oximetry in brain dead patients: a report of six cases. J Neurosurg Anesthesiol. 1999 Oct;11(4):252-4Wijdicks EF, Varelas PN, Gronseth GS, Greer DM; American Academy of Neurology. Evidence-based guideline update: determining brain death in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 1: 2010 Jun 8;74(23), 2010.
- Moerman, A.T.; Vandenheuvel, M.; Tuybens, P.-J.; Van Gompel, C.; De Hert, S.G. Incongruous effect of phenylephrine on changes in cerebral blood volume measured by near-infrared spectroscopy (NIRS) indicating extracranial contamination. J. Clin. Monit. Comput. 2021, 36, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Canova D, Roatta S, Bosone D, Micieli G. Inconsistent detection of changes in cerebral blood volume by near infrared spectroscopyin standard clinical tests. J Appl Physiol. 1: 2011;110, 2011.
- Davie SN, Grocott HP. Impact of extracranial contamination on regional cerebral oxygen saturation: a comparison of three cerebral oximetry technologies. Anesthesiology. 2012;116:834–40.
- Spears, W.; Mian, A.; Greer, D. Brain death: a clinical overview. J. Intensiv. Care 2022, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gisolf, J.; Van Lieshout, J.J.; Van Heusden, K.; Pott, F.; Stok, W.J.; Karemaker, J.M. Human cerebral venous outflow pathway depends on posture and central venous pressure. J. Physiol. 2004, 560, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Lanning, K.M.; Ylikauma, L.A.; Erkinaro, T.M.; Ohtonen, P.P.; Vakkala, M.A.; Kaakinen, T.I. Changes in transcranial near-infrared spectroscopy values reflect changes in cardiac index during cardiac surgery. Acta Anaesthesiol. Scand. 2023, 67, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Schraag, S. Combined Monitoring—Brain Function Monitoring and Cerebral Oximetry. J. Cardiothorac. Vasc. Anesthesia 2019, 33, S53–S57. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Cardim, D.; Ball, L.; Battaglini, D.; Dabrowski, W.; Bassetti, M.; Giacobbe, D.R.; Czosnyka, M.; Badenes, R.; Pelosi, P.; et al. The Use of Different Components of Brain Oxygenation for the Assessment of Cerebral Haemodynamics: A Prospective Observational Study on COVID-19 Patients. Front. Neurol. 2021, 12, 735469. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Lara L, Geocadin R, Zorrilla-Vaca A, at al. Optimizing Mean Arterial Pressure in Acutely Comatose Patients Using Cerebral Autoregulation Multimodal Monitoring With Near-Infrared Spectroscopy. Crit Care Med. 1: 2019 Oct;47(10), 2019.
- Takegawa, R.; Hayashida, K.; Rolston, D.M.; Li, T.; Miyara, S.J.; Ohnishi, M.; Shiozaki, T.; Becker, L.B. Near-Infrared Spectroscopy Assessments of Regional Cerebral Oxygen Saturation for the Prediction of Clinical Outcomes in Patients With Cardiac Arrest: A Review of Clinical Impact, Evolution, and Future Directions. Front. Med. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.L.; Hyttel-Sørensen, S.; Jakobsen, J.C.; Gluud, C.; Kooi, E.M.W.; Mintzer, J.; de Boode, W.P.; Fumagalli, M.; Alarcon, A.; Alderliesten, T.; et al. Cerebral near-infrared spectroscopy monitoring (NIRS) in children and adults: a systematic review with meta-analysis. Pediatr. Res. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).