Submitted:
12 March 2024
Posted:
18 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
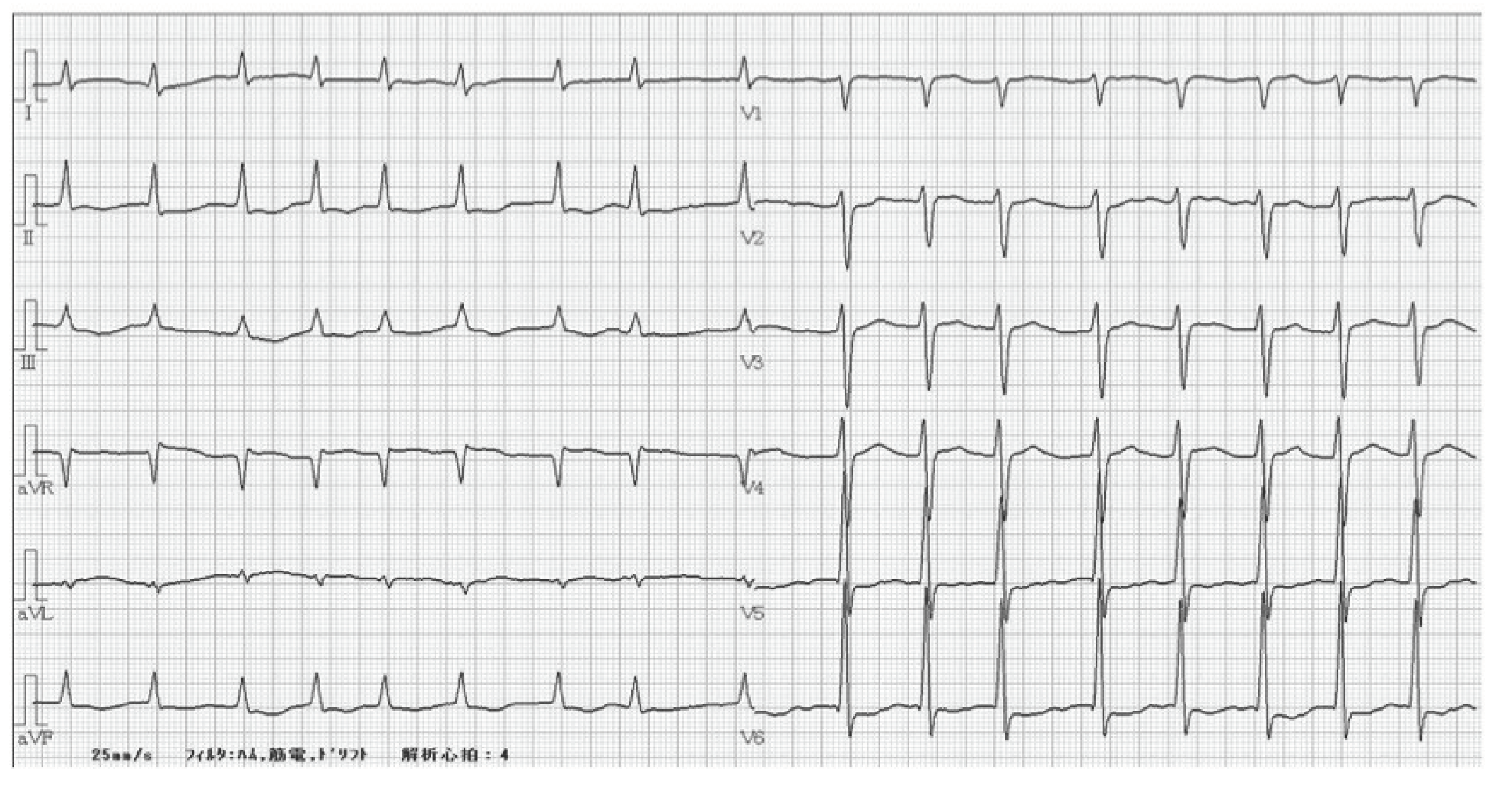
2. Case Report
3. Discussion
4. Conclusions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Harron, D.W.G.; Brogden, R.N.; Faulds, D.; Fitton, A. Cibenzoline. Drugs 1992, 43, 734–759. [Google Scholar] [CrossRef] [PubMed]
- Ono K, Iwasaki YK, Akao M, et al. JCS/JHRS 2020 Guideline on Pharmacotherapy of Cardiac Arrhythmias. Circ J. 2022 ;86(11):1790-1924.
- Aoyama, N.; Sasaki, T.; Yoshida, M.; Suzuki, K.; Matsuyama, K.; Aizaki, T.; Izumi, T.; Kondo, R.; Kamijo, Y.; Soma, K.; et al. Effect of charcoal hemoperfusion on clearance of cibenzoline succinate (cifenline) poisoning. J. Toxicol. Clin. Toxicol. 1999, 37, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Fujita, S.; Katayama, Y.; Harano, Y.; Shibakawa, M. The relationship between risk of hypoglycemia and use of cibenzoline and disopyramide. Eur. J. Clin. Pharmacol. 2000, 56, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Kyan, R.; Uemura, S.; Toyo, Y.; Wada, K.; Nomura, K.; Bunya, N.; Narimatsu, E. Usefulness of venoarterial extracorporeal membranous oxygenation for fatal cibenzoline succinate poisoning. Acute Med. Surg. 2020, 7, e507. [Google Scholar] [CrossRef]
- Wang, D.W.; Kiyosue, T.; Sato, T.; Arita, M. Comparison of the Effects of Class I Anti-arrhythmic Drugs, Cibenzoline, Mexiletine and Flecainide, on the Delayed Rectifier K+Current of Guinea-pig Ventricular Myocytes. J. Mol. Cell. Cardiol. 1996, 28, 893–903. [Google Scholar] [CrossRef]
- Nakamura Takuya, Seki Masahiko, et al.A case where percutaneous cardiopulmonary support device was used to save a life from cardiac arrest due to Sibenadet overdose.Japanese Journal of Emergency Medicine in 2019, Vol. 30 (2), pp. 50-56.
- Wang, G.S.; on Behalf of the Toxicology Investigators Consortium. ; Levitan, R.; Wiegand, T.J.; Lowry, J.; Schult, R.F.; Yin, S. Extracorporeal Membrane Oxygenation (ECMO) for Severe Toxicological Exposures: Review of the Toxicology Investigators Consortium (ToxIC). J. Med Toxicol. 2015, 12, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Dresser, G.K.; Spence, J.D.; Bailey, D.G. Pharmacokinetic-Pharmacodynamic Consequences and Clinical Relevance of Cytochrome P450 3A4 Inhibition. Clin. Pharmacokinet. 2000, 38, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Grymonprez, M.; Vanspranghe, K.; Capiau, A.; Boussery, K.; Steurbaut, S.; Lahousse, L. Impact of P-glycoprotein and/or CYP3A4-interacting drugs on effectiveness and safety of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: A meta-analysis. Br. J. Clin. Pharmacol. 2022, 88, 3039–3051. [Google Scholar] [CrossRef]
| <CBC> | <Chemistry> | <Arterial Blood Gas> | ||||||
| WBC | 5900 | /μL | AST | 61 | U/L | pH | 7.303 | |
| RBC | 363 | *4 / μL | ALT | 37 | U/L | pCO2 | 39.1 | mmHg |
| Hb | 13.1 | g/dL | LDH | 347 | U/L | pO2 | 120 | mmHg |
| Hct | 39.3 | % | ALP | 105 | U/L | HCO3- | 19.5 | mmol/L |
| MCV | 108.3 | fl | CK | 87 | U/L | Lactate | 4.5 | mmol/L |
| PLT | 9.7 | *4 /μL | T-Bil | 1.3 | mg/dL | Glucose | 90 | mg/dL |
| <Coagulation> | Alb | 3.7 | g/dL | |||||
| PT-INR | 4.17 | BUN | 18 | U/L | ||||
| APTT | 36.4 | sec | Cr | 1.43 | mg/dL | |||
| Fib | 232 | mg/dL | Na | 141 | mmol/L | |||
| K | 3.7 | mmol/L | ||||||
| Cl | 107 | mmol/L | ||||||
| CRP | 0.28 | mg/dL | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





