Submitted:
18 March 2024
Posted:
19 March 2024
You are already at the latest version
Abstract
Keywords:
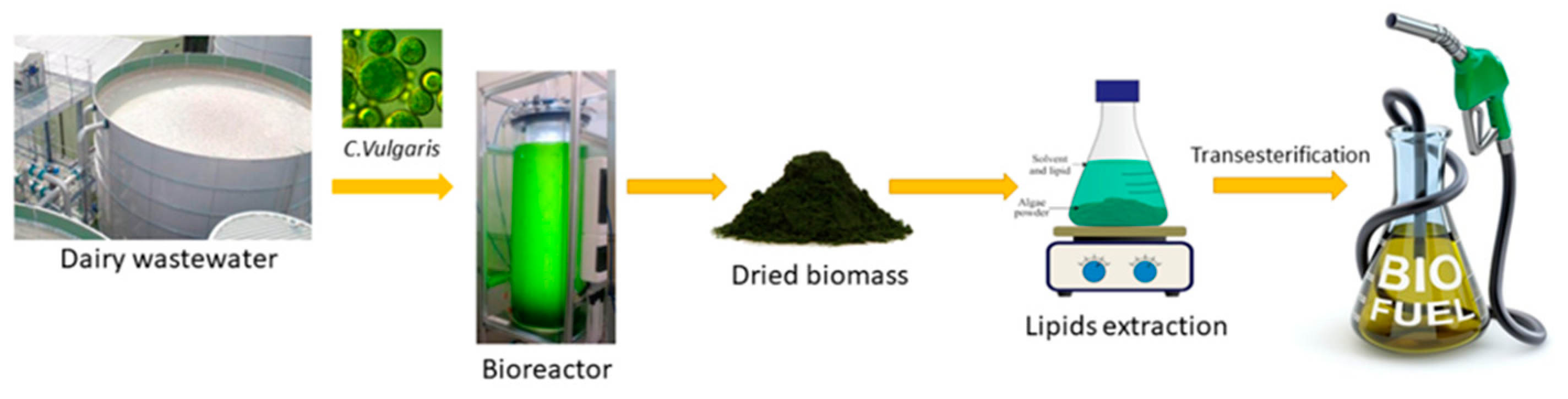
1. Introduction
2. Materials and Methods
2.2. Physicochemical Characteristics of Wastewater
2.3. Microalgae Strain and Cultivation in Dairy Wastewater
2.4. Nutrient Content in the Wastewater
2.5. Determination of Algal Biomass
2.6. Extraction of Algal Oil from C. Vulgaris
2.7. Biodiesel Production and FAME Profile
2.8. Biodiesel Physical-Chemical Evaluation
2.8.1. Kinematic Viscosity
2.8.2. Calorific Value
2.8.3. Flash Point
2.8.4. Density
2.8.5. Cetane Number
2.8.6. Elemental Analysis
2.9. Generator Performance Test
2.10. Statistical Analysis
3. Results and Discussion
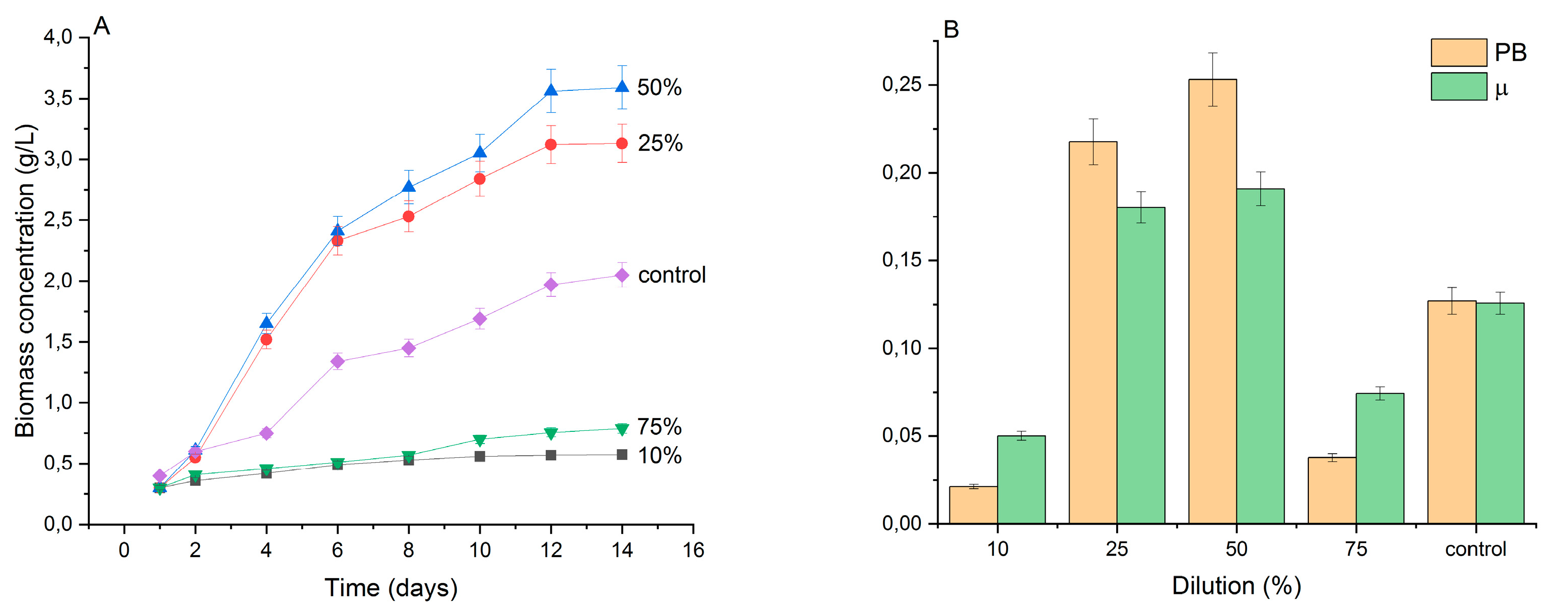
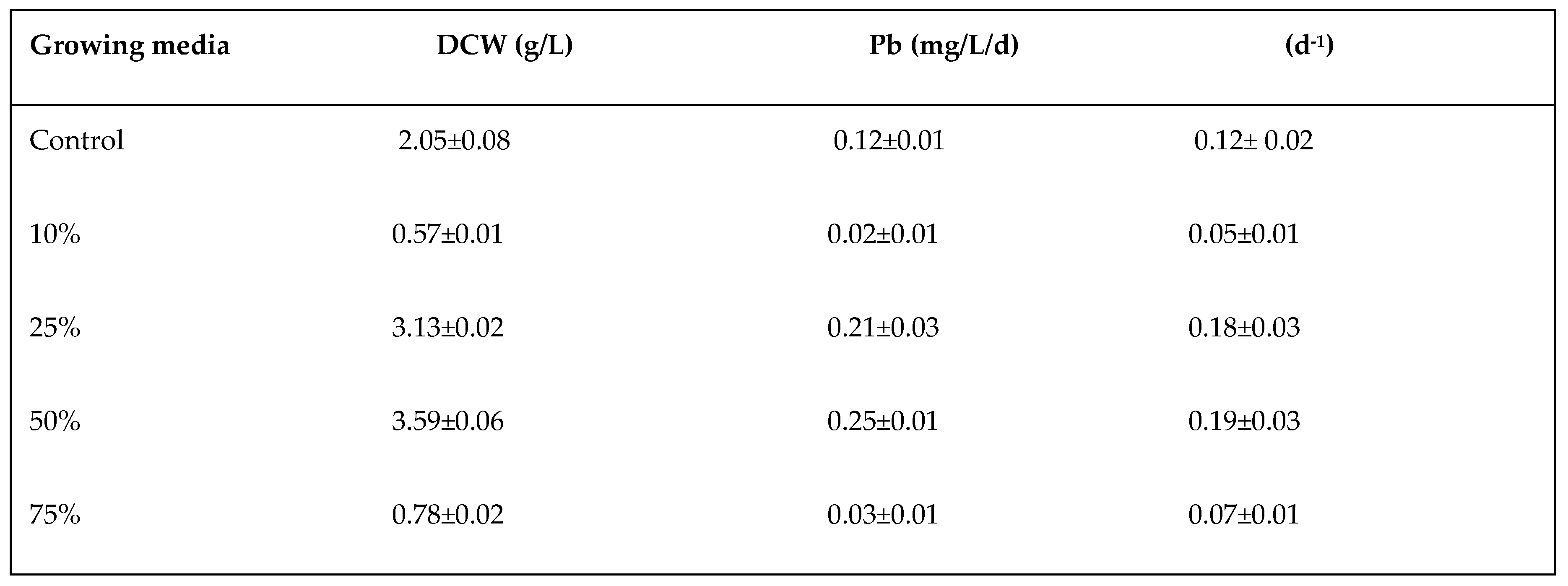
3.1. Effect of Wastewater Dilution on C.vulgaris Biomass
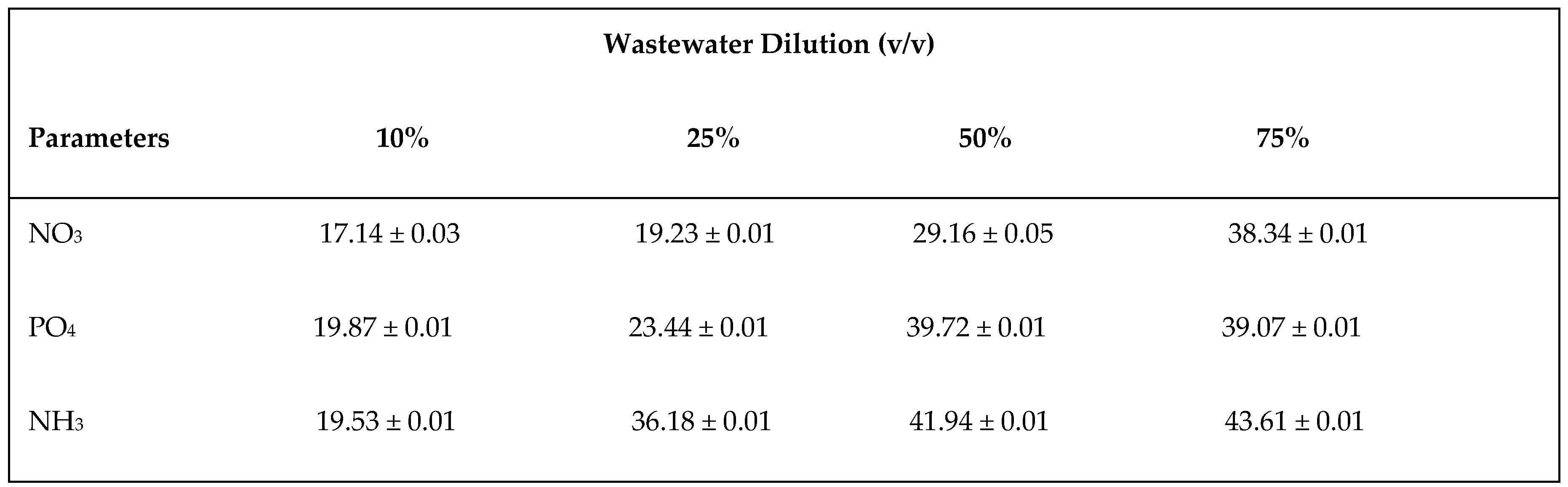
3.2. Effect of Different Dilutions of Wastewater on Nutrient Removal
3.3. Lipid Extraction from Chlorella vulgaris Biomass
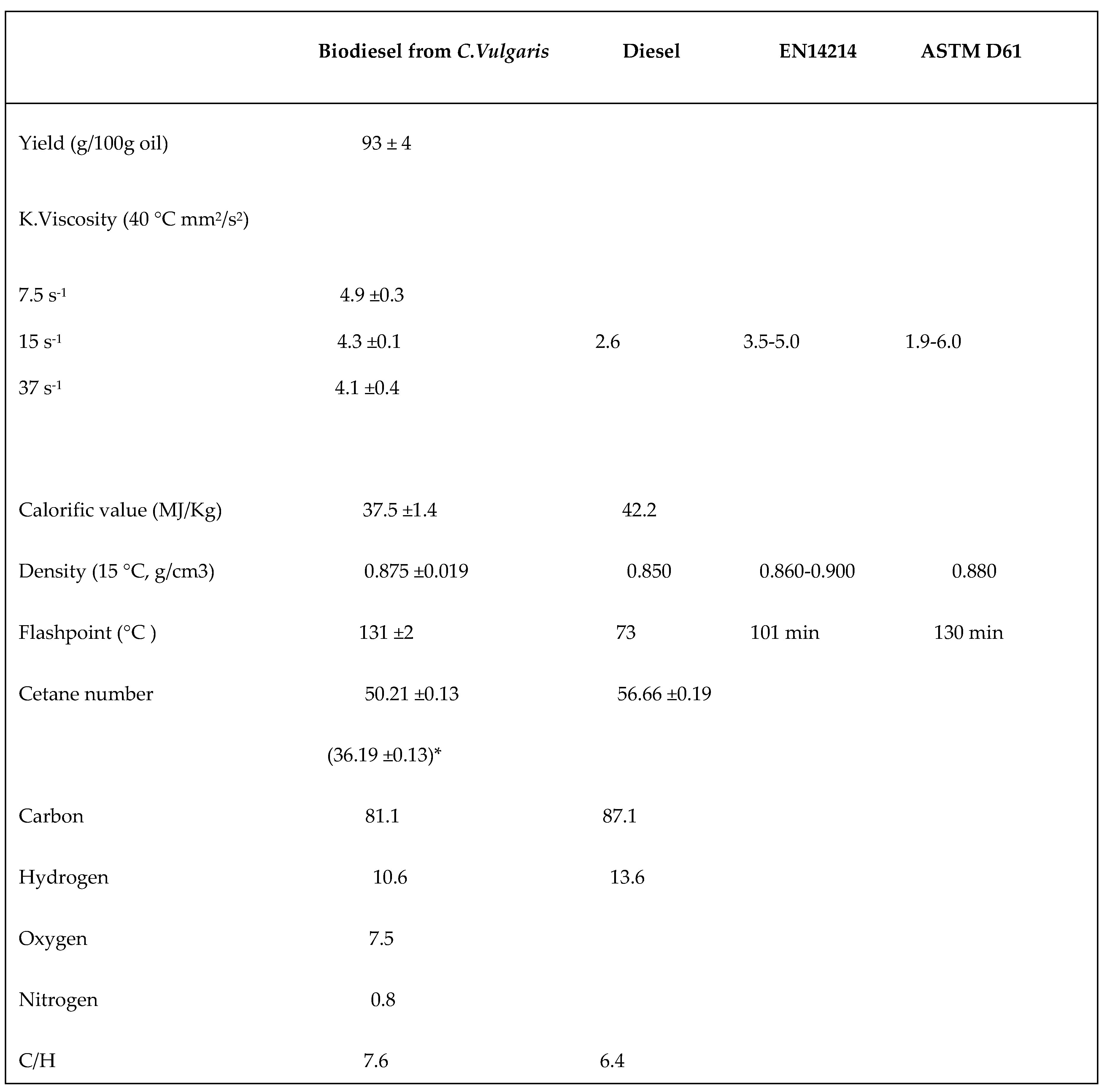
3.4. Physical-Chemical Characterization
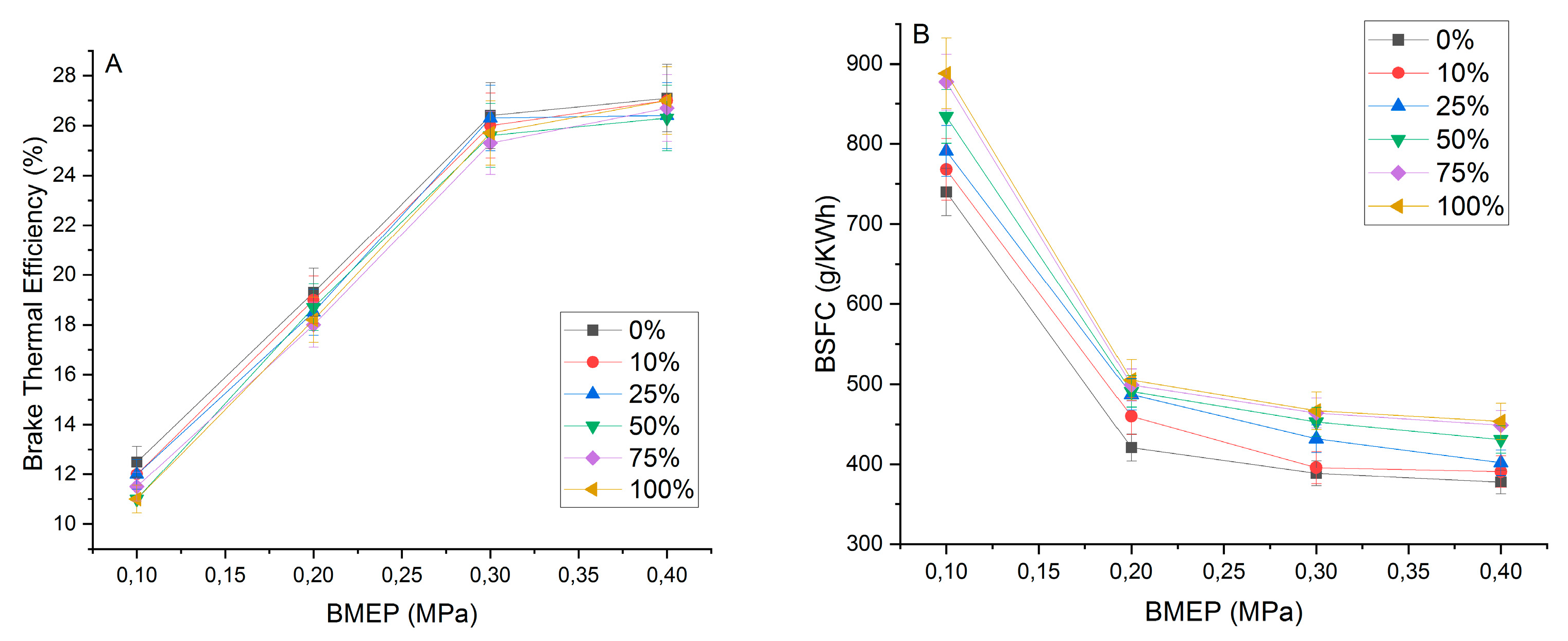
3.4.1. Biodiesel Yield, Kinematic Viscosity, Density, and Calorific Value
3.4.2. Flash Point
3.4.3. FAME Composition
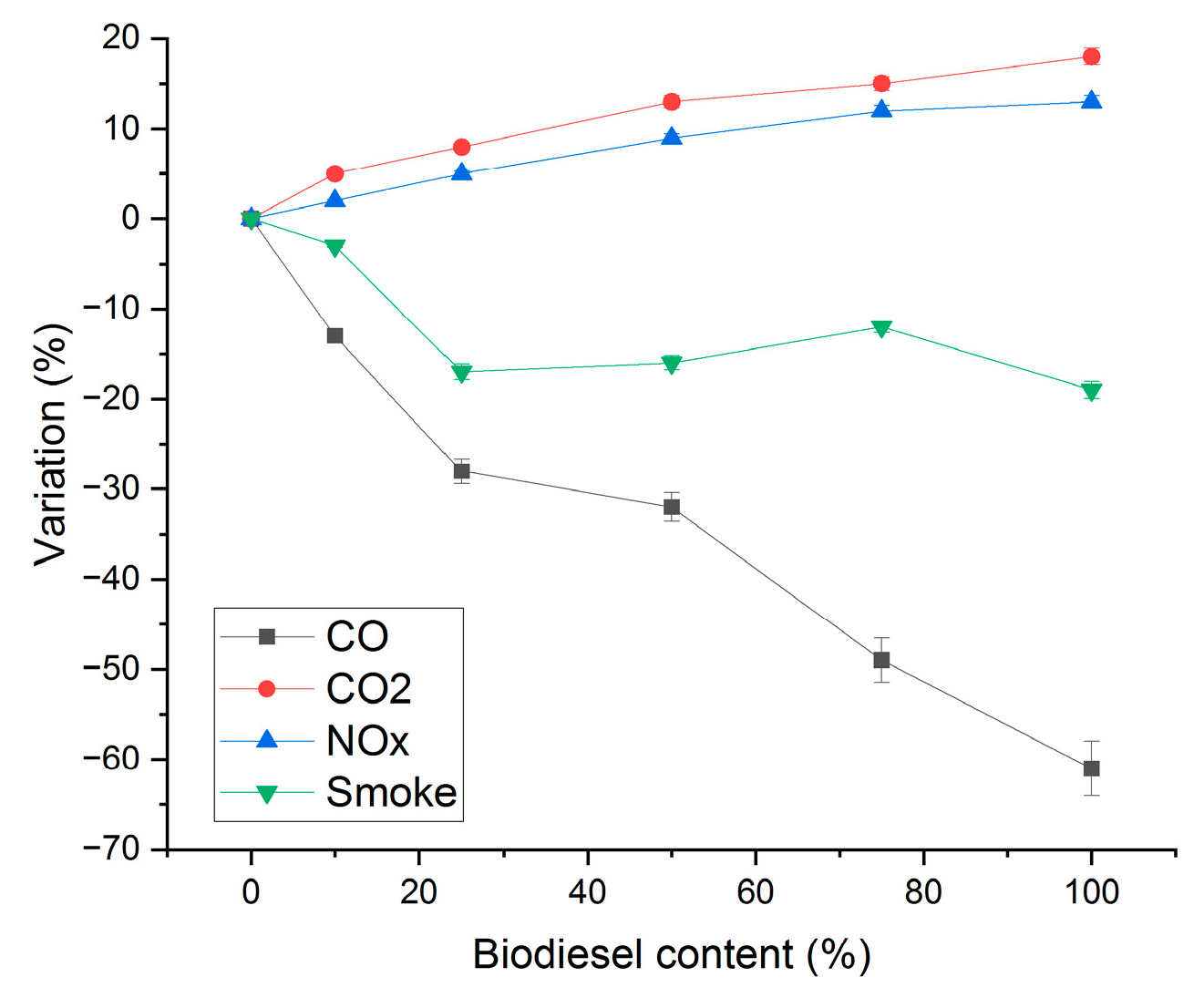
3.5. Generator Performance Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Raghunath, B.V.; Punnagaiarasi, A.; Rajarajan, G.; Irshad, A.; Elango, A.; Mahesh Kumar, G. Impact of dairy effluent on environment—a review. Integrated Waste Management in India: Status and Future Prospects for Environmental Sustainability 2016, 239–249.
- Choudhury, P.; Ray, R.N.; Bandyopadhyay, T.K.; Basak, B.; Muthuraj, M.; Bhunia, B. Process Engineering for Stable Power Recovery from Dairy Wastewater Using Microbial Fuel Cell. Int. J. Hydrogen Energy 2021, 46, 3171–3182. [Google Scholar] [CrossRef]
- Khalaji, M.; Hosseini, S.A.; Ghorbani, R.; Agh, N.; Rezaei, H.; Kornaros, M.; Koutra, E. Treatment of dairy wastewater by microalgae Chlorella vulgaris for biofuels production. Biomass Conversion and Biorefinery 2021, 1–7. [Google Scholar] [CrossRef]
- Dongre, A.; Sogani, M.; Sonu, K.; Syed, Z.; Sharma, G. Treatment of Dairy Wastewaters: Evaluating Microbial Fuel Cell Tools and Mechanism. In Environmental Issues and Sustainable Development; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- You, X.; Yang, L.; Zhou, X.; Zhang, Y. Sustainability and carbon neutrality trends for microalgae-based wastewater treatment: A review. Environmental Research 2022, 209, 112860. [Google Scholar] [CrossRef] [PubMed]
- Gramegna, G.; Scortica, A.; Scafati, V.; Ferella, F.; Gurrieri, L.; Giovannoni, M.; Benedetti, M. Exploring the potential of microalgae in the recycling of dairy wastes. Bioresource Technology Reports 2020, 12, 100604. [Google Scholar] [CrossRef]
- Silva, C.K.D.; Almeida, A.C.A.D.; Costa, J.A.V.; Morais, M.G.D. Cyanobacterial biomass by reuse of wastewater-containing hypochlorite. Ind Biotechnol 2018, 14, 265–269. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—areview of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energ Rev 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl Biochem Biotechnol 2010, 162, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Biodiesel from microalgae. Biotechnol Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Ratomski, P.; Hawrot-Paw, M. Influence of nutrient-stress conditions on Chlorella vulgaris biomass production and lipid content. Catalysts. 2021, 11, 573. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: a review. Renewable and sustainable energy reviews 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Dong, W.; Zhang, X.; Tyagi, R.D.; Drogui, P.; Su-rampalli, R.Y. The potential of microalgae in biodiesel production. Renewable and Sustainable Energy Reviews 2018, 90, 336–346. [Google Scholar] [CrossRef]
- Lee, R.A.; Lavoie, J.-M. From first- to third-generation biofuels: Challenges of producing a commodity from a biomass of increasing complexity. Animal Frontiers 2013, 3, 6–11. [Google Scholar] [CrossRef]
- Hosseini Tafreshi, A.; Shariati, M. Dunaliella biotechnology: methods and applications. J Appl Microbiol 2009, 107, 14–35. [Google Scholar] [CrossRef]
- Tao, R.; Kinnunen, V.; Praveenkumar, R.; Lakaniemi, A.-M.; Rintala, J.A. Comparison of Scenedesmus acuminatus and Chlorella vulgaris cultivation in liquid digestates from anaerobic digestion of pulp and paper industry and municipal wastewater treatment sludge. J Appl Phycol 2017, 29, 2845–2856. [Google Scholar] [CrossRef]
- Gouveia, L.; Batista, A.P.; Miranda, A.; Empis, J.; Raymundo, A. Chlorella vulgaris biomass used as colouring source in traditional butter cookies. Innovative food science & emerging technologies 2007, 8, 433–436. [Google Scholar]
- Martins, C.F.; Pestana, J.M.; Alfaia, C.M.; Costa, M.; Ribeiro, D.M.; Coelho, D.; Prates, J.A. Effects of Chlorella vul-garis as a feed ingredient on the quality and nutritional value of weaned piglets’ meat. Foods 2021, 10, 1155. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kwon, S.; Kim, M.K. Effect of Chlorella vul-garis intake on cadmium detoxification in rats fed cadmium. Nutrition Research and Practice 2009, 3, 89–94. [Google Scholar] [CrossRef]
- Galasso, C.; Gentile, A.; Orefice, I.; Ianora, A.; Bruno, A.; Noonan, D.M.; Sansone, C.; Albini, A.; Brunet, C. Microalgal derivatives as potential nutraceutical and food supplements for human health: A focus on cancer prevention. 2019.
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae : current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 1–21. [Google Scholar] [CrossRef]
- Peter, A.P.; Chew, K.W.; Koyande, A.K.; Yuk-Heng, S.; Ting, H.Y.; Rajendran, S.; Show, P.L. Cultivation of Chlorella vulgaris on dairy waste using vision imaging for biomass growth monitoring. Bioresource technology 2021, 341, 125892. [Google Scholar] [CrossRef]
- Goh BH, H.; Ong, H.C.; Cheah, M.Y.; Chen, W.H.; Yu, K.L.; Mahlia TM, I. Sustainability of direct biodiesel synthesis from microalgae biomass: A critical review. Renewable and Sustainable Energy Reviews 2019, 107, 59–74. [Google Scholar] [CrossRef]
- Davoodbasha, M.; Pugazhendhi, A.; Kim, J.W.; Lee, S.Y.; Nooruddin, T. Biodiesel production through transesterification of Chlorella vulgaris: Synthesis and characterization of CaO nanocatalyst. Fuel 2021, 300, 121018. [Google Scholar] [CrossRef]
- Khan, E.; Ozaltin, K.; Spagnuolo, D.; Bernal-Ballen, A.; Piskunov, M.V.; Di Martino, A. Biodiesel from Rapeseed and Sunflower Oil: Effect of the Transesterification Conditions and Oxidation Stability. Energies 2023, 16, 657. [Google Scholar] [CrossRef]
- Roosta, A.; Bardool, R. A predictive correlation for dynamic viscosity of fatty acid methyl esters and biodiesel. Journal of the American Oil Chemists' Society 2019, 96, 741–750. [Google Scholar] [CrossRef]
- Hossain, A.K.; Davies, P.A. Plant oils as fuels for compression ignition engines: A technical review and life-cycle analysis. Renewable energy 2010, 35, 1–13. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Pimerzin, A.A.; Glotov, A.P.; Vutolkina, A.V. Biofuels energetics: Measurements and evaluation of calorific values of triglycerides. Fuel 2022, 326, 125101. [Google Scholar] [CrossRef]
- Giakoumis, E.G. A statistical investigation of biodiesel physical and chemical properties, and their correlation with the degree of unsaturation. Renewable Energy 2013, 50, 858–878. [Google Scholar] [CrossRef]
- Tesfa, B. , Mishra, R., Gu, F., & Powles, N. Prediction models for density and viscosity of biodiesel and their effects on fuel supply system in CI engines. Renewable energy 2010, 35, 2752–2760. [Google Scholar]
- Demirbas, A. Progress and recent trends in biofuels. Progress in energy and combustion science 2007, 33, 1–18. [Google Scholar] [CrossRef]
- Nabi, M.N.; Rahman, M.M.; Akhter, M.S. Biodiesel from cotton seed oil and its effect on engine performance and exhaust emissions. Applied thermal engineering 2009, 29, 2265–2270. [Google Scholar] [CrossRef]
- Chalatlon, V.; Roy, M.M.; Dutta, A.; Kumar, S. Jatropha oil production and an experimental investigation of its use as an alternative fuel in a DI diesel engine. Journal of Petroleum Technology and Alternative Fuels 2011, 2, 76–85. [Google Scholar]
- Emaish, H.; Abualnaja, K.M.; Kandil, E.E.; Abdelsalam, N.R. Evaluation of the performance and gas emissions of a tractor diesel engine using blended fuel diesel and biodiesel to determine the best loading stages. Scientific Reports 2021, 11, 9811. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.W.; Chia, S.R.; Show, P.L.; Ling, T.C.; Arya, S.S.; Chang, J.S. Food waste compost as an organic nutrient source for the cultivation of Chlorella vulgaris. Bioresource technology 2018, 267, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Pereira, H.; Costa, M.; Santos, T.; Carvalho, B.; Soares, M.; Silva, J. Development of an organic culture medium for autotrophic production of Chlorella vulgaris biomass. Applied Sciences 2020, 10, 2156. [Google Scholar] [CrossRef]
- Eloka-Eboka, A.C.; Inambao, F.L. Effects of CO2 sequestration on lipid and biomass productivity in microalgal biomass production. Applied Energy 2017, 195, 1100–1111. [Google Scholar] [CrossRef]
- Kothari, R.; Prasad, R.; Kumar, V.; Singh, D. Production of biodiesel from microalgae Chlamydomonas polypyrenoideum grown on dairy industry wastewater. Bioresour Technol 2013, 144, 499–503. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Algae: anatomy, biochemistry, and biotechnology. CRC press, 2014; p. 295.
- dos Santos, R.R.; Moreira, D.M.; Kunigami, C.N.; Aranda, D.A.G.; Teixeira, C.M.L.L. Comparison between several methods of total lipid extraction from Chlorella vulgaris biomass. Ultrasonics sonochemistry 2015, 22, 95–99. [Google Scholar] [CrossRef]
- Kumar, R.R.; Rao, P.H.; Arumugam, M. Lipid extraction methods from microalgae: a comprehensive review. Front Energy Res 2014, 2, 61–69. [Google Scholar]
- Couto, D.; Melo, T.; Conde, T.A.; Moreira, A.S.; Ferreira, P.; Costa, M.; Domingues, P. Food grade extraction of Chlorella vulgaris polar lipids: A comparative lipidomic study. Food Chemistry 2022, 375, 131685. [Google Scholar] [CrossRef]
- Nguyen TD, P.; Nguyen, D.H.; Lim, J.W.; Chang, C.K.; Leong, H.Y.; Tran TN, T.; Show, P.L. Investigation of the relationship between bacteria growth and lipid production cultivating of microalgae Chlorella vulgaris in seafood wastewater. Energies 2019, 12, 2282. [Google Scholar] [CrossRef]
- Asadi, P.; Rad, H.A.; Qaderi, F. Lipid and biodiesel production by cultivation isolated strain Chlorella sorokiniana pa. 91 and Chlorella vulgaris in dairy wastewater treatment plant effluents. Journal of Environmental Health Science and Engineering 2020, 18, 573–585. [Google Scholar] [CrossRef]
- Scarponi, P.; Izzo, F.C.; Bravi, M.; Cavinato, C.C. vulgaris growth batch tests using winery waste digestate as promising raw material for biodiesel and stearin production. Waste Management 2021, 136, 266–272. [Google Scholar]
- Katırcıoğlu Sınmaz, G.; Erden, B.; Şengil, I.A. Cultivation of Chlorella vulgaris in alkaline condition for biodiesel feedstock after biological treatment of poultry slaughterhouse wastewater. International Journal of Environmental Science and Technology 2023, 20, 3237–3246. [Google Scholar] [CrossRef]
- Sharma, A.; Kodgire, P.; Kachhwaha, S.S. Investigation of ultrasound-assisted KOH and CaO catalyzed transesterification for biodiesel production from waste cotton-seed cooking oil: Process optimization and conversion rate evaluation. Journal of Cleaner Production 2020, 259, 120982. [Google Scholar] [CrossRef]
- Aydın, S. Comprehensive analysis of combustion, performance and emissions of power generator diesel engine fueled with different source of biodiesel blends. Energy 2020, 205, 118074. [Google Scholar] [CrossRef]
- Dey, P.; Ray, S.; Newar, A. Defining a waste vegetable oil-biodiesel based diesel substitute blend fuel by response surface optimization of density and calorific value. Fuel 2021, 283, 118978. [Google Scholar] [CrossRef]
- Santos, S.M.; Nascimento, D.C.; Costa, M.C.; Neto, A.M.; Fregolente, L.V. Flash point prediction: Reviewing em-pirical models for hydrocarbons, petroleum fraction, biodiesel, and blends. Fuel 2020, 263, 116375. [Google Scholar] [CrossRef]

| Parameters | NH3 | NO3 | PO4 | pH |
|---|---|---|---|---|
| 52 | 69 | 600 | 8.0 |
| FAME (Fatty Acid Methyl Ester) | (%) |
| Tridecanoic acid (13:0) | 4.02 |
| Myristoleic acid (14:1) | 7.95 |
| Palmitic acid (16:0) | 52.4 |
| Stearic acid | 17.3 |
| Linolelaidic acid (18:2) | 6.83 |
| Linoleic acid (18:3) | 7.90 |
| Oleic acid | 5.21 |
| cis, 11, 14-Eicosadienoic acid (20:2) | 4.70 |
| Arachidonic acid (20:4) | 3.09 |
| Erucic acid (22:1) | 4.46 |
| Tricosanoid acid (23:0) | 3.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





