1. Introduction
Alzheimer's Disease (AD) is a multifaceted neurodegenerative disorder that plagues millions across the globe[
1]. It is characterized by a relentless decline in cognitive function, debilitating memory impairment, and a significant component of neuroinflammation [
2]. The complex interplay of these factors compounds the challenges in developing effective treatments. Despite many reports, the quest for pharmacological interventions that can decisively treat AD remains unfulfilled. The limitations of existing pharmacological treatments, which often carry side effects, have stimulated a shift towards non-pharmacological protocols. In response to this impasse, the study needs to turn its sight toward innovative non-pharmacological approaches.
The onset of AD is influenced by an extensive variety of parameters, and many pathogenic processes have been identified. According to the Aβ hypothesis, the Aβ-protein is perceived as the primary factor influencing the occurrence of AD [
3]. According to prior research, Aβ impacts the brain tissue of individuals with initial AD to a heightened extent in contrast to people with optimal health conditions. This has a damaging impact on brain neurons, resulting in their degeneration or death [
4]. According to the tau protein abnormal modification hypothesis, atypical alteration of tau proteins induces the loss of tubulin's structural functioning, which subsequently causes microtubules to disintegrate, thus inhibiting the usual transport activities of nerve terminals and neuronal cells. This may eventually lead to degenerative changes in the synaptic and neuronal systems [
5]. The core of AD patients' cholinergic system displays evident degradation and abnormalities, as described by the cholinergic system damage hypothesis. These findings are compatible with the medical signs of AD patients [
6]. The disrupted equilibrium between the body's peroxide and antioxidant systems, instigating excessive synthesis of peroxides and oxygen-free radicals, is referred to as "oxidative stress," which harms the brain cells [
7]. The primary reason for nerve impairment in AD patients can be attributed to the death of brain neuronal cells, which is regarded as the ultimate step in many hypotheses pertaining to the pathophysiology of AD. Corresponding research indicates that neuronal cell death in AD patients is mostly caused by Aβ accumulation, oxidative stress, and malfunctioning mitochondria [
8]. There is currently no effective therapy for AD as a result of its diverse and complex processes, thus initiating for further exploration of significant medical protocols.
Recent studies have increasingly demonstrated alterations in immune system functionality in Alzheimer's Disease (AD) cases [
9]. Notably, AD patients exhibit significant chronic neuroinflammation, contributing to immune system dysregulation [
10]. Toll-like receptors (TLRs), a group of highly conserved receptors governing innate immune responses [
11], have increased attention in neurodegenerative disease research. Among the 13 mammalian TLRs, Toll-like receptor 4 (TLR4) has been extensively investigated in this context. TLR4 can initiate downstream signaling via both MyD88-dependent and MyD88-independent pathways [
12]. The nuclear factor kappa B (NFκB) promoter, responsible for transcribing inflammatory mediators such as TNF-α, interleukin 1β (IL-1β), and interleukin 6 (IL-6), is a downstream effector of TLR4 [
13]. In the central nervous system, TLR4 is predominantly expressed in glial cells, with minimal expression in neurons [
14]. Upregulation of TLR4 has been observed in the brains of both AD patients and AD model mice [
15]. The presence of toxic Aβ activates glial cells and enhances their phagocytic activity via TLR4 [
16]. Given the observed alterations in TLR4 during AD progression, targeting this receptor represents a promising method for overcoming AD. Indeed, studies have shown that TLR4 suppression exerts a protective effect in AD pathology through its anti-inflammatory mechanism [
17,
18].
We reported that our research highlights the potential of microcurrent therapy as an alternative non-pharmacological intervention for mitigating cognitive decline and neuroinflammation in Alzheimer's Disease. Microcurrent stimulation therapy is a treatment method that uses a current of less than 1000 μA, which can hardly be felt by the human body, and is measured in mA units [
19]. This therapy holds promising the nonpharmacological therapeutic modalities in the treatment to AD, shedding light on the value of developing unconventional therapeutic modalities. Thus, we investigated that determining the clinical point balance between maximizing therapeutic effects and minimizing potential neuronal damage is crucial.
2. Materials and Methods
2.1. Chemicals and Antibodies
Here is the information regarding the chemicals and antibodies utilized in the procedure: Cresyl violet (C5042, Sigma Aldrich, St. Louis, MO, USA), Thioflavin T (T3516, Sigma Aldrich), Amyloid-β (sc-53822, 1:500, Santa Cruz, Dallas, TX, USA), β-actin (sc-8432, 1:1000, Santa Cruz), NeuN (MAB-377, 1:100, Merck, Darmstadt, Hessen, Germary), Caspase-3 (cs-9661, 1:500, Cell signaling, Danvers, MA, USA), TNF-α (sc-52746, 1:1000, Santa Cruz), IL-1β (sc-7884, 1:1000, Santa Cruz), TLR4 (sc-293072, 1:1000, Santa Cruz), TRAF6 (sc-8409, 1:1000, Santa Cruz), MyD88 (sc-74532, 1:1000, Santa Cruz), p-NF-Kb (MA5-15160, ThermoFisher, Waltham, MA, USA), Anti-goat mouse (ADI-SAB-100-J, 1:4000, Enzo Life Sciences, Farmingdale, NY, USA), Anti-goat rabbit (ADI-SAB-300-J, 1:4000, Enzo Life Sciences), ABC kit (PK-6100, Vector, Newark, CA, USA), DAB kit (SK-4100, Vector).
2.2. Microcurrent Therapy
Microcurrent was administered at the designated intervals in accordance with the particular time frame established for every group in order to assess its potential medicinal benefits. With this configuration, the electricity passed through the mice's feet that were in touch with the cage surface, thus allowing the flow of current to their brains. Corresponding to the application of microcurrent in humans during the day when they are active, the passage of current in mice was done during the night hours to coincide with their active nocturnal routines. Besides the existence of different waveforms, Kim et al.'s research [
20] pointed out that despite the effectiveness of each waveform, the stepform waveform had a particularly notable impact on clinical measures including cognition and Alzheimer's-linked protein synthesis among mice. Resultantly, we opted for the microcurrent featuring wave superposition and a stepform waveform of 0, 1.5, 3, and 5 V. The base frequency of 7 Hz with an extra 44 kHz frequency superimposition was set, along with the 5V voltage and the microcurrent with a magnitude of 1 μA (250 ohm).
2.3. Animals
We acquired transgenic mice bearing the B6SJL Tg (APPSwFlLon, PSEN1*M146L* L286V)6799Vas/Mmjax strain from Jackson Laboratory located in Bar Harbor, ME, USA. Both female WT and Tg-5xFAD AD mice were employed for this study and were assigned different groups. The mice were exposed to microcurrents starting at 1.5 months of age, implying the underdeveloped condition of their brains. The initiation of genetic advancement of Aβ aggregation in Tg-5xfaD AD mice renders it a critical phase. The Institutional Animal Care and Use Committee accorded permission for conducting these experiments (IRB no.: DCIAFCR-230329-06-YR). We adhered to every official and global standard concerning the treatment of animals. After 7 days of adaptation, the sample group comprising Tg-5xFAD mice underwent random segregation as the AD model group or the microcurrent plus AD model group (n=5 in each group). Additionally, mice were randomized into the microcurrent + NC group (n = 5 per group) or the normal control (NC) group. The therapy was followed by behavioral and biochemical analysis.
2.4. Novel Object Recognition Test (NOR)
Prior to the training; the mice were kept in a testing chamber at 23±1°C and 50%–60% humidity with food and water available at all times for an entire night, and were subjected to a twelve-hour light-dark cycle. Two circular filter units with identical heights and diameters (27 and 33 mm, respectively) were placed within the chamber throughout the training session. Subsequently, the mice were left for 10 minutes to explore the chamber. Next day; a 30 mm long plastic cone with a diameter of 25 mm was used to replace an originally placed item. The object recognition was defined on the basis of the duration required to touch or smell the newly placed item in a span of five minutes [
21]. The recording and analysis of the training and the assessment of the trials was done through EthoVision XT8.5 [
22].
2.5. Radial Arm Maze Test (RAM)
The microcurrent treatment was preceded as well as succeeded by a neurocognitive RAM assessment of mice groups, comprising normal and non-Tg wild-type samples. As previously mentioned, an eight-arm radial maze was utilized to assess spatial operational memory [
23]. The distal end of each arm was furnished with a single reward cup on top of a platform. Initially, for a span of three days, all the mice were subjected to habituation training sessions for 10 minutes' duration on the radial arm maze. Throughout the experiment, the mice were given access to open arms after being acclimated with their two arms baited and placed 135 degrees apart for being searched. Only the visit of the mice and their subsequent consumption of the baited food cup were counted as an accurate response. The revisit of the mouse to an arm that was either unbaited or was baited before was deemed a visiting mistake, which implies an inaccuracy of spatial functional memory [
24]. These performance metrics were collected prior to as well as after the microcurrent therapy, and the data were evaluated through ANOVA to identify the deviations.
2.6. Tissue Preparation
The mice were sacrificed for brain tissue retrieval following two months of behavioral screening subsequent to microcurrent therapy. Regarding the histology study, the right hemispheres of three mice (per group) were immersed in RNA at 4°C overnight, while the left hemispheres were kept in a 4% paraformaldehyde solution. The hippocampal tissue was removed from the brain prior to its freezing at -80°C for subsequent PCR and microarray evaluation. Three mice per group were chosen for dissection of their hippocampal and entorhinal cortex for Western blotting assessment, in accordance with Paxinos and Franklin mapping [
25].
2.7. Nissl Staining
All mice were sacrificed under anesthesia at the end of the experimental period, and the hippocampal and cortical tissues were immediately isolated on ice and stored at -80°C for further analysis. Three mouse brains from each group were perfused intracardially with PBS (pH 7.4) and fixed in 4% paraformaldehyde. Brain paraffin blocks (5-μm thick) were prepared. The section slides were deparaffinized in xylene and hydrated with a series of graded ethanol. To quench endogenous peroxidase activity, slides were treated with 0.3% hydrogen peroxide in methanol for 25 min and briefly rinsed in PBS. For Nissl staining, slides were immersed in 1% cresyl violet acetate solution, washed with water, and dehydrated through 90% and 100% ethanol for 5 min before mounting in xylene.
2.8. Thioflavin-T Staining
The protocol involves treating tissue slides with xylene and graded ethanol to deparaffinize and hydrate them. Then, incubate them in filtered Thioflavin-T (25 uM) for 8 minutes, protecting from light. After washing, coverslip with mounting media and allow drying overnight. Seal with nail polish the next day. Analyze within days-weeks as staining fades over time. Store slides in the dark at 4°C [
26].
2.9. Immunohistochemistry
Sections’ immunostaining was carried out using a Vectastain Elite ABC kit purchased from US-based Vector Laboratories Inc. To retrieve the antigen, the first step entailed placing these sections inside citrate buffer before being boiled inside the water for half an hour. In terms of immunoperoxidase labeling, 0.3% H2O2 was used to block endogenous peroxidase in methanol at room temperature for around fifteen minutes. Regarding immunohistochemistry, the blocking of sections was done using horse serum before being incubated at 4°C for one night with antibodies. After being incubated with mouse IgG or biotinylated goat anti-human at RT for 30 minutes. This was followed by immunoreaction with a peroxidase complex avidin-biotin-based) at RT for half an hour. The DAB kit was used to develop the peroxidase reaction. In all experiments, it was seen that the primary antibody would be omitted for some sections counterstained using the hematoxylin of Harris before being mounted. Each positive cells counting were quantified by using the Image J program (Java-based image processing program, NIH, Bethesda, MD, USA).
2.10. Analysis of Western Blot
Total proteins from cells were extracted in RIPA buffer (50 mM Tris-Cl, pH 7.4; 1% NP-40; 150 mM NaCl, and 1 mM EDTA) before being supplemented with protease inhibitors (1 mM PMSF, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 mM Na
3VO
4) and then quantified using the Bradford method. Protein samples (30 μg) were separated by SDS/polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane used as described previously [
27].
2.11. Statistical Analysis
Statistical significance was determined using Tukey's method with one-way ANOVA. Differences were deemed statistically important in case the P value was lower than 0.001 or0.05. (*p<0.05; **P < 0.01; ***P < 0.001).
3. Results
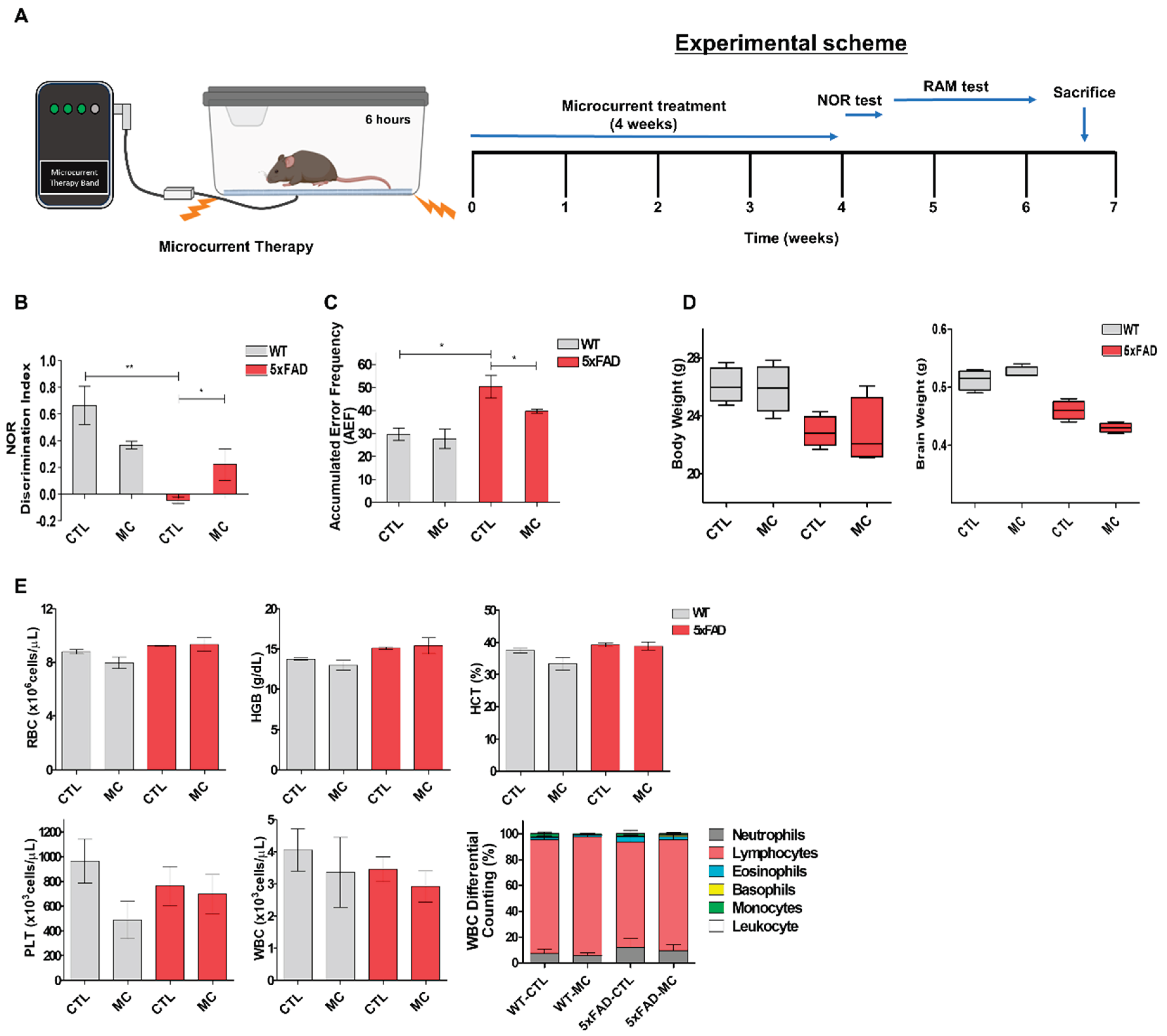
3.1. Ameliorating Effects of MC Therapy on Memory Impairment in an Aβ-Induced Mouse Model of AD
5xFAD AD-mice were used to examine whether MC was capable of enhancing impaired memory.
Figure 1A presents a brief outline of the research methodology. To compute the percentage recognition index, the ratio of the time spent examining the novel object to the time when 5xFAD AD could identify familiar objects during the testing period was used. In comparison to the control group, the percentage recognition index increased substantially in the MC group. When the 5xFAD started recalling the familiar objects more often, they became more intrigued to examine the novel object. According to the findings, the greatest improvement in recognition memory performance was shown by the group treated with MC (
Figure 1B,C).
Figure 1E demonstrates the hematologic profiles after a single MC treatment was performed on 5xFAD mice. There was no change in any other hematological factor and the body weight gain was found to be the same throughout the entire life of WT mice undergoing MC treatment in comparison to control WT mice (
Figure 1D,E).
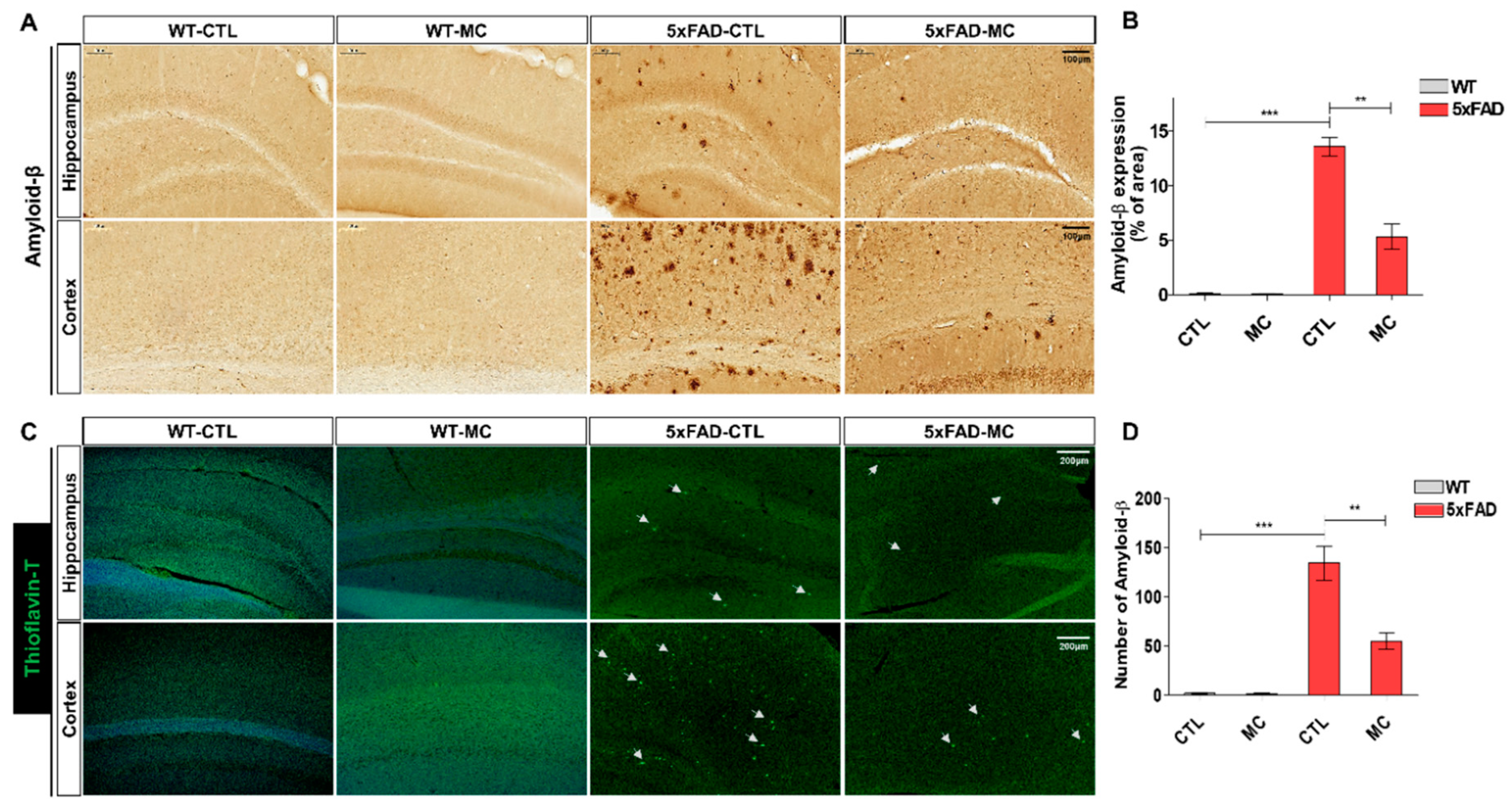
3.2. MC Therapy Decreased Aβ Levels and Amyloid Burden in the Cortex and the Hippocampus of 5xFAD AD Mice
The impact of MC therapy on Aβ pathology in the brain of the 5xFAD AD mice was studied by using the immunohistochemical analysis (
Figure 2A,B) and thioflavin-T staining (
Figure 2C,D) to examine the Aβ plaque. It was seen that in the frontal cortex and the hippocampus regions, there was a low expression of Aβ-positive proteins in the NC group, while it was upregulated in the AD model group and inhibited in the group undergoing MC treatment. The findings of the quantification analysis demonstrated that there was a substantial decrease in the amount of Aβ immunolabeled cells in the cortex and the hippocampus of the MC-treated group in comparison to the AD model group. The regions covered with Aβ also exhibited a decrease of around 68% in the cortex and hippocampus of the MC treatment group in contrast to the AD model group. Thioflavin-T staining, a fluorescein that particularly attaches to amyloid deposits and can be excited to generate green fluorescence, was used to show that the group treated with MC brings about a considerable decrease in the amount of thioflavin-T positive plaques in the cortex as well as the hippocampus regions in comparison to the AD model group. Compared to the AD model group, the MC-treated group decreased the plaque load by 59% in the cortex and the hippocampus region, which showed that it was possible to bring about a significant decline in Aβ deposition in AD brains through MC treatment.
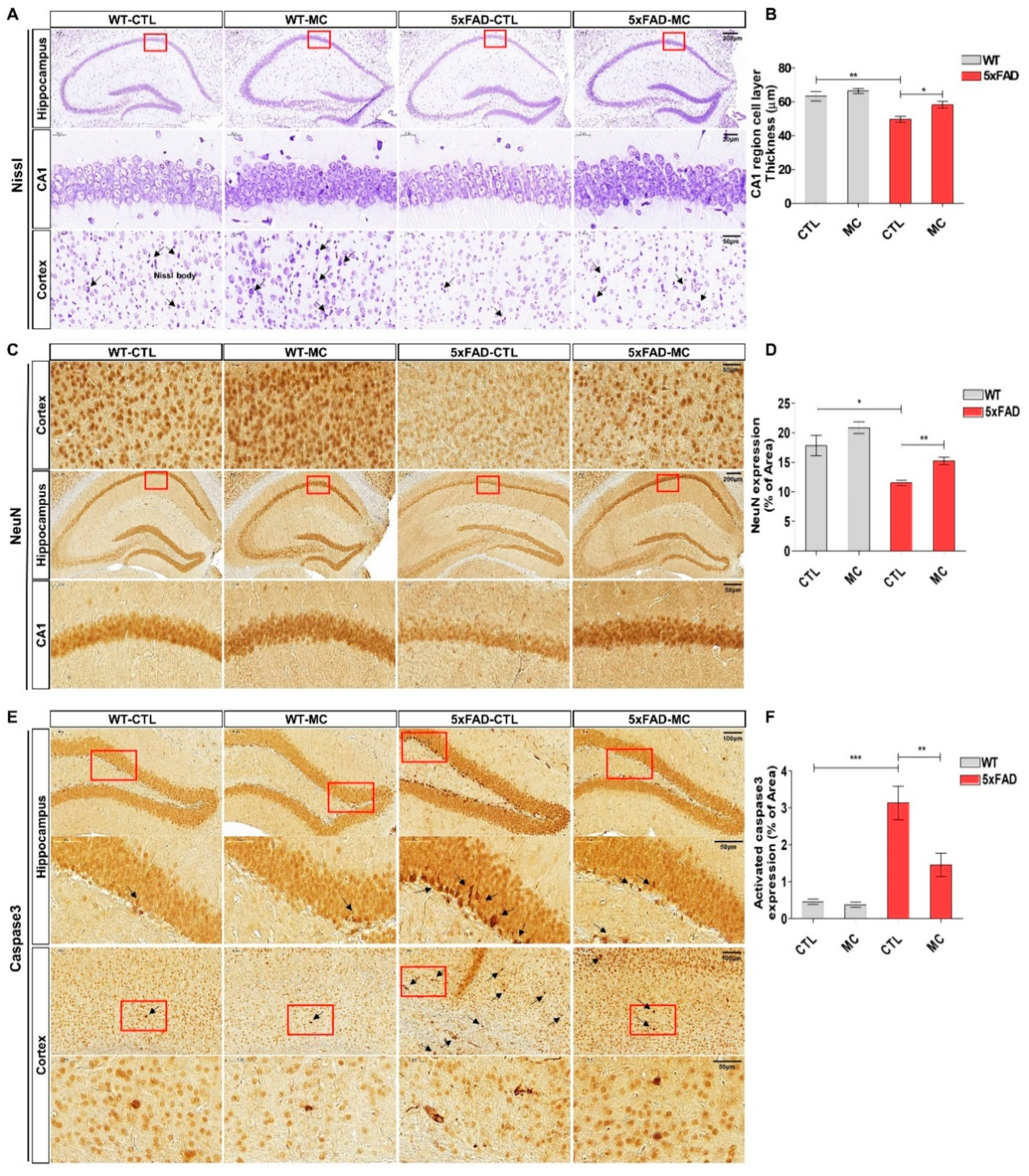
3.3. MC Therapy Reduced Neuronal Loss and Inhibited Apoptosis in 5xFAD AD Mice
The impact of MC treatment on neuronal loss in the AD brain was examined by carrying out nissl staining. The figure shows that in comparison to the NC group, a standard Alzheimer’s pathology was exhibited by the 5xFAD AD mice, which involved loss of neurons, nucleus contraction, and loss of Nissl bodies within the cortex and the hippocampus (
Figure 3A,B). There was a remarkable improvement in the cell organization in the MC treatment group, where the MC treatment offered significant protection of 17% against neuron loss in the hippocampal CA1 region in comparison to the AD model group. It is indicated by these findings that MC treatment is capable of increasing the number of neurons and enhancing neuronal structures in the AD brain. Neuronal marker NeuN IHC staining was carried out in the cortex and hippocampus to examine whether MC has an impact on the number of neurons in 5xFAD mice (
Figure 3C,D). A substantial decrease in NeuN-positive cell number in the cortex and hippocampus of 5xFAD mice was observed in comparison to WT mice. These findings were corroborated by evaluating the expression level of stimulated caspase-3 in the brain of each group using immunohistochemistry. There was a substantial increase in the number of activated caspase-3 immunolabeled cells and in the covered parts of activated caspase-3 in the cortex and hippocampus of the AD model in contrast to the NC group (
Figure 3E). On the other hand, there was a significant decline in the number of activated caspase-3 immunolabeled cells in the cortex and hippocampus of the MC treatment group in comparison to the AD model group. In contrast to the AD model group, there was a decrease of around 61% in the covered regions of activated caspase-3 in the cortex and hippocampus of the MC treatment group (
p < 0.01), which suggested that neuronal apoptosis can be suppressed by MC treatment.
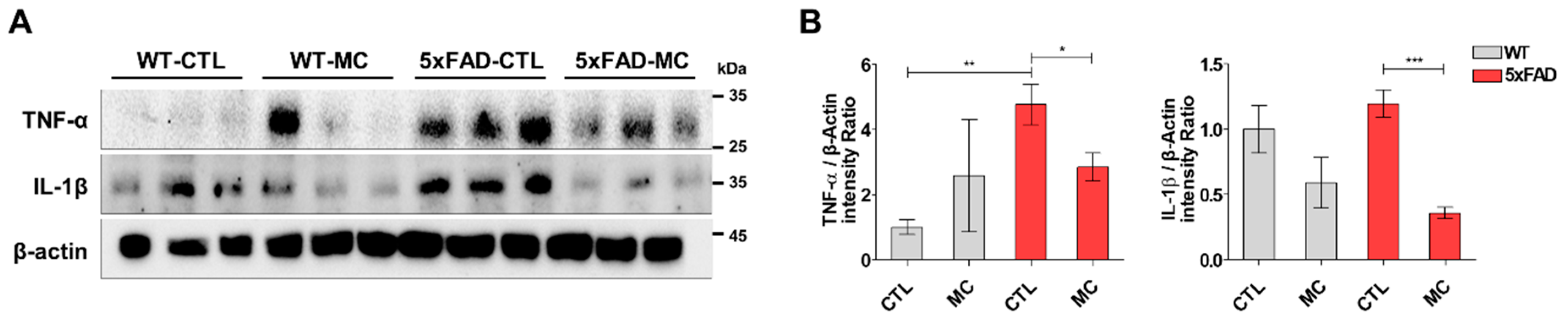
3.4. Treatment with MC Exerts Anti-Inflammatory Effects on the Brains of 5xFAD AD Mice
The inflammatory response in the transgenic mice brain was quantitatively examined in the cortex and hippocampus by evaluating the amount of the proinflammatory markers tumor necrosis factor α (TNFα) and interleukin-1β (IL-1β). It was shown by Westen blotting that there was a substantial increase in the levels of TNFα and IL-1β in the cortex and hippocampus of the transgenic mice, and this increase was further enhanced by MC treatment (
Figure 4A,B).
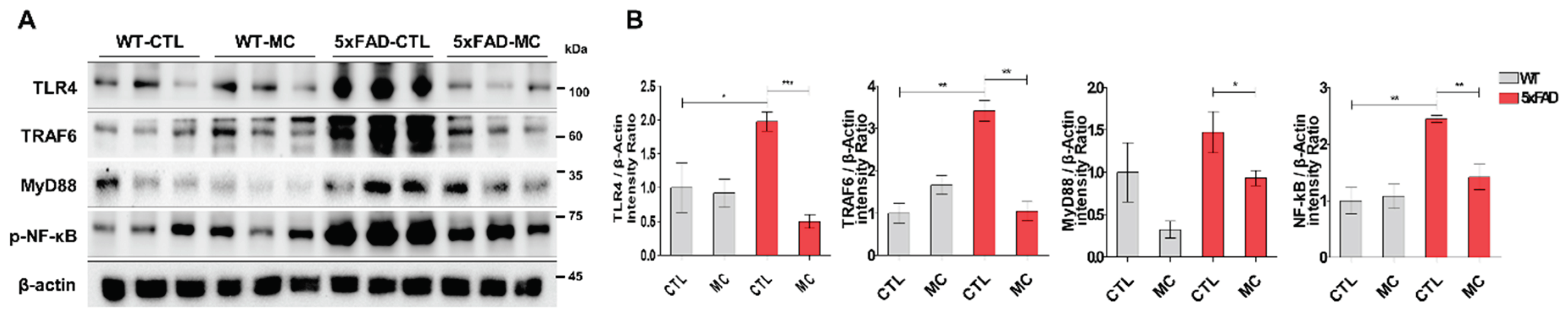
3.5. MC Therapy Ameliorated Neuroinflammation through Reducing TLR4 in AD Mice Model
The TLR4 signaling pathway proteins were examined to further evaluate the possible method forming the basis of the impact of MC treatment on AD pathology-related neuroinflammation. It was determined that in 5xFAD mice, there were higher concentrations of TLR4, MyD88, TRAF6, and p-NFκB in comparison to the WT mice (
Figure 5A,B). The levels of TLR4, MyD88, TRAF6, and p-NFκB decreased following MC treatment in comparison to the vehicle-treated 5xFAD mice. A significant variation was also noted between the MC-treated mice and the control group.
4. Discussion
Alzheimer's disease (AD) is a complex neurodegenerative disorder marked by cognitive decline and neuropathological alterations, including abnormal protein accumulation, neuronal loss, and alterations in brain chemistry [
28]. These results showed AD difficult to treat comprehensively.
The evidences from various studies suggest that MC therapy has in mitigating neuronal damage associated with neurodegenerative disorders such as AD. Our research indicates that MC therapy effectively preserves neuronal integrity, reduces apoptosis, reduces oxidative stress, and potentially modulates neuronal excitotoxicity. These findings underscore MC therapy's potential as a therapeutic intervention for protecting neurons from damage or degeneration. However, a deeper understanding of the precise mechanisms behind these effects and validation of its efficacy in clinical settings are crucial aspects that require further investigation.
The positive outcomes of our study suggest that MC therapy could verify a multifaceted approach to directing the complex challenges posed by AD. Instead of targeting a single aspect of AD pathology, our findings imply that MC therapy may simultaneously address multiple facets, making it a versatile solution. The term "multifaceted intervention" aligns with the necessity of tackling various aspects of AD, such as memory impairment, neuronal damage, Aβ plaque accumulation, and oxidative stress, for effective treatment.
Our study's positive results with MC therapy suggest its potential to impact various elements of AD pathology concurrently, presenting it as a variable prospect for further investigation and potential application in managing Alzheimer's disease.
A significant finding in our study is the remarkable improvement in memory impairment following MC treatment. The increased percentage recognition index in the MC-treated groups signifies enhanced recognition memory ability, crucial for addressing cognitive deficiencies linked to AD. This improvement aligns with prior research indicating the cognitive benefits of MC therapy in various neurological disorders. Importantly, the reduction of histological abnormalities and observed structural changes in the prefrontal cortex underscore the neuroprotective effects of MC therapy, with Nissl staining showing a partial reversal of neurodegeneration, suggesting the potential of MC to maintain neuronal integrity.
Amyloid-beta (Aβ) plaque buildup is a key indicator of AD pathogenesis. Our study demonstrates a significant decrease in Aβ levels and amyloid burden in the cortex and hippocampus after MC therapy. This reduction in Aβ-positive proteins and amyloid plaque load highlights the potential of MC therapy in targeting crucial aspects of AD treatment. Moreover, MC treatment exhibited notable benefits in halting apoptosis and decreasing neuronal loss in the AD brain, suggesting its ability to preserve neurons and reduce neuronal apoptosis—critical factors in AD progression.
The potential of MC treatment to prevent synapse loss and its effects on synaptic degeneration are particularly promising. The increase in Nissl-positive and NeuN-positive cells in MC-treated AD animals indicates the preservation of neuronal and synaptic integrity, essential for neural communication and cognitive function.
The pathogenesis of AD is complex, and as such, the inhibition of Aβ production and aggregation should not be the only intervention target. Alternatives, including anti-inflammatory and anti-oxidative drugs, have also been developed as therapeutic strategies for AD [
29,
30]. The present study revealed that MC suppresses glial activation, oxidative stress and inhibits the release of pro-inflammatory factors, including TNFα and IL-1β, consistent with our previous in vitro findings [
31].
While our research revealed that MC therapy can inhibit reactive gliosis in AD mouse brains, the precise molecular mechanisms underlying this inhibition were unknown. Prior investigations have indicated that the anti-inflammatory properties of MC are linked to the inhibition of NFκB phosphorylation both in vivo and in vitro [
32]. Expounding on this observation, we examined the proteins involved in the TLR4-MyD88-NFκB pathway. Consistent with our expectations, we observed overexpression of TLR4-MyD88 pathway proteins and phosphorylation of NFκB in the brains of 5xFAD mice. However, treatment with MC reversed these changes in this AD mouse model, suggesting that the anti-inflammatory effects of MC might be predicated on the TLR4-MyD88-NFκB pathway. Furthermore, our investigations demonstrated that MC diminished the levels of inflammatory factors regulated by NFκB phosphorylation in correlation with this reference [
32].
In congruence with our findings, previous studies have revealed that loss of function or inhibition of TLR4 attenuates AD progression in mouse models [
33,
34]. Additionally, research has indicated that inhibiting the TLR4 pathway ameliorates motor impairment and prevents dopaminergic neuron death in Parkinson’s disease mouse models [
35]. Moreover, TLR4 antagonists have been shown to mitigate the production of proinflammatory factors and motor dysfunction in experimental autoimmune encephalomyelitis mouse models [
36]. Collectively, these studies suggest that TLR4 represents a promising target for neuroinflammation treatment.
Given that inflammatory factors contribute to neuronal and synaptic loss as well as behavioral impairments in AD progression, we propose that the TLR4-MyD88-NFκB-dependent anti-inflammatory effects of MC therapy may underlie its neuroprotective effects. Taken together, our findings highlight the broad potential of MC therapy in addressing various aspects of AD pathophysiology, including memory loss, neuronal damage, Aβ accumulation, and synaptic degeneration.
While the results are encouraging, it is essential to consider certain factors. Firstly, variations in brain complexity and disease manifestation between animal models and human clinical situations warrant cautious interpretation of results. Secondly, additional research is required to determine the optimal waveform, intensity, and duration for MC therapy to maximize therapeutic success. Practical utility depends on understanding the precise mechanisms underlying the observed effects of MC treatment on AD pathogenesis.
Author Contributions
(I) Conception and design: K.D.R, K.E.H, (II) Administrative support: K.D.R, (III) Provision of study materials or patients: K.D.R, K.E.H. (IV) Collection and assembly of data: K.D.R, K.E.H, and L.W.S, (V) Data analysis and interpretation: All authors., (VI) Manuscript writing: All authors., (VII) Final approval of manuscript: All authors.
Funding
The authors disclosed receipt of the following financial support for the research, au-thorship, and/or publication of this article: This work was supported by the grant of Daegu Catholic University Medical Center(RD-23-0030).
Institutional Review Board Statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and re-solved. The study was conducted according to the guidelines of the IACUC, and approved by the Catholic University of Daegu School of Medicine Animal Care and Use Committee (IRB no.: DCIAFCR-230329-06-YR).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
All authors have completed the ICMJE uniform disclosure form. All authors have no conflict of interest to declare.
References
- Knopman, D. S.; Amieva, H.; Petersen, R. C.; Chetelat, G.; Holtzman, D. M.; Hyman, B. T.; Nixon, R. A.; Jones, D. T. , Alzheimer disease. Nat Rev Dis Primers 2021, 7, 33. [Google Scholar] [CrossRef]
- DeTure, M. A.; Dickson, D. W. , The neuropathological diagnosis of Alzheimer's disease. Mol Neurodegener 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. , Nanomedicine in cancer therapy. Signal Transduct Target Ther 2023, 8, 293. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S. H.; Villemagne, V. L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; Masters, C. L.; Cho, M.; Lannfelt, L.; Cummings, J. L.; Vergallo, A. , The Amyloid-beta Pathway in Alzheimer's Disease. Mol Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, J. L.; Tank, D. W. , A Dedicated Population for Reward Coding in the Hippocampus. Neuron 2018, 99, 179–193 e7. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Fu, M.; Wang, S.; Chen, W.; Wang, J.; Zhang, N. , Naringin ameliorates memory deficits and exerts neuroprotective effects in a mouse model of Alzheimer's disease by regulating multiple metabolic pathways. Mol Med Rep 2021, 23. [Google Scholar] [CrossRef] [PubMed]
- Olufunmilayo, E. O.; Gerke-Duncan, M. B.; Holsinger, R. M. D. , Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Misrani, A.; Tabassum, S.; Yang, L. , Mitochondrial Dysfunction and Oxidative Stress in Alzheimer's Disease. Front Aging Neurosci 2021, 13, 617588. [Google Scholar] [CrossRef]
- Wu, K. M.; Zhang, Y. R.; Huang, Y. Y.; Dong, Q.; Tan, L.; Yu, J. T. , The role of the immune system in Alzheimer's disease. Ageing Res Rev 2021, 70, 101409. [Google Scholar] [CrossRef]
- Lutshumba, J.; Nikolajczyk, B. S.; Bachstetter, A. D. , Dysregulation of Systemic Immunity in Aging and Dementia. Front Cell Neurosci 2021, 15, 652111. [Google Scholar] [CrossRef]
- O'Donnell, T.; Vabret, N. , Repeat elements amplify TLR signaling. Nat Rev Immunol 2021, 21, 760. [Google Scholar] [CrossRef]
- Kollmann, T. R.; Levy, O.; Montgomery, R. R.; Goriely, S. , Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity 2012, 37, 771–83. [Google Scholar] [CrossRef]
- Saleh, H. A.; Yousef, M. H.; Abdelnaser, A. , The Anti-Inflammatory Properties of Phytochemicals and Their Effects on Epigenetic Mechanisms Involved in TLR4/NF-kappaB-Mediated Inflammation. Front Immunol 2021, 12, 606069. [Google Scholar] [CrossRef]
- Kerfoot, S. M.; Long, E. M.; Hickey, M. J.; Andonegui, G.; Lapointe, B. M.; Zanardo, R. C.; Bonder, C.; James, W. G.; Robbins, S. M.; Kubes, P. , TLR4 contributes to disease-inducing mechanisms resulting in central nervous system autoimmune disease. J Immunol 2004, 173, 7070–7. [Google Scholar] [CrossRef]
- Gambuzza, M. E.; Sofo, V.; Salmeri, F. M.; Soraci, L.; Marino, S.; Bramanti, P. , Toll-like receptors in Alzheimer's disease: a therapeutic perspective. CNS Neurol Disord Drug Targets 2014, 13, 1542–58. [Google Scholar] [CrossRef]
- Doyle, S. E.; O'Connell, R. M.; Miranda, G. A.; Vaidya, S. A.; Chow, E. K.; Liu, P. T.; Suzuki, S.; Suzuki, N.; Modlin, R. L.; Yeh, W. C.; Lane, T. F.; Cheng, G. , Toll-like receptors induce a phagocytic gene program through p38. J Exp Med 2004, 199, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Rahimifard, M.; Maqbool, F.; Moeini-Nodeh, S.; Niaz, K.; Abdollahi, M.; Braidy, N.; Nabavi, S. M.; Nabavi, S. F. , Targeting the TLR4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflammation. Ageing Res Rev 2017, 36, 11–19. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Z.; Zhi, Z.; Tian, J.; Zhao, Y.; Sun, J. , Targeting the TLR4/NF-kappaB pathway in beta-amyloid-stimulated microglial cells: A possible mechanism that oxysophoridine exerts anti-oxidative and anti-inflammatory effects in an in vitro model of Alzheimer's disease. Brain Res Bull 2021, 175, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Wu, A. D.; Walter, B. L.; Brooks, A.; Buetow, E.; Amodeo, K.; Richard, I.; Mundth, K.; Azmi, H. , Standardizing default electronic health record tools to improve safety for hospitalized patients with Parkinson's disease. Front Aging Neurosci 2023, 15, 1278322. [Google Scholar] [CrossRef]
- Kim, M. J. L., A. Y; Cho, D. S; Cho, E. J, Effect of Microcurrent Wave Superposition on Cognitive Improvement in Alzheimer’s Disease Mice Model. Journal of the Korea Academia-Industrial cooperation Society 2019, 20, 241–251. [Google Scholar]
- Antunes, M.; Biala, G. , The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 2012, 13, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, N. J.; Gaskin, S.; Squire, L. R.; Clark, R. E. , Object recognition memory and the rodent hippocampus. Learn Mem 2010, 17, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Clark, J. K.; Furgerson, M.; Crystal, J. D.; Fechheimer, M.; Furukawa, R.; Wagner, J. J. , Alterations in synaptic plasticity coincide with deficits in spatial working memory in presymptomatic 3xTg-AD mice. Neurobiol Learn Mem 2015, 125, 152–162. [Google Scholar] [CrossRef]
- Penley, S. C.; Gaudet, C. M.; Threlkeld, S. W. , Use of an eight-arm radial water maze to assess working and reference memory following neonatal brain injury. J Vis Exp 2013, (82), 50940. [Google Scholar]
- y Keith, B.J. Franklin MA, G. P. A., Paxinos and Franklin's the Mouse Brain in Stereotaxic Coordinates, Compact: The Coronal Plates and Diagrams 5th edition. Academic Press: 2019.
- Xue, C.; Lin, T. Y.; Chang, D.; Guo, Z. , Thioflavin T as an amyloid dye: fibril quantification, optimal concentration and effect on aggregation. R Soc Open Sci 2017, 4, 160696. [Google Scholar] [CrossRef] [PubMed]
- Kim, E. H.; Jo, Y.; Sai, S.; Park, M. J.; Kim, J. Y.; Kim, J. S.; Lee, Y. J.; Cho, J. M.; Kwak, S. Y.; Baek, J. H.; Jeong, Y. K.; Song, J. Y.; Yoon, M.; Hwang, S. G. , Tumor-treating fields induce autophagy by blocking the Akt2/miR29b axis in glioblastoma cells. Oncogene 2019, 38, 6630–6646. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. , Comprehensive Review on Alzheimer's Disease: Causes and Treatment. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Mangialasche, F.; Solomon, A.; Winblad, B.; Mecocci, P.; Kivipelto, M. , Alzheimer's disease: clinical trials and drug development. Lancet Neurol 2010, 9, 702–16. [Google Scholar] [CrossRef]
- Ballard, C.; Gauthier, S.; Corbett, A.; Brayne, C.; Aarsland, D.; Jones, E. , Alzheimer's disease. Lancet 2011, 377, 1019–31. [Google Scholar] [CrossRef]
- Jiao, J.; Xue, B.; Zhang, L.; Gong, Y.; Li, K.; Wang, H.; Jing, L.; Xie, J.; Wang, X. , Triptolide inhibits amyloid-beta1-42-induced TNF-alpha and IL-1beta production in cultured rat microglia. J Neuroimmunol 2008, 205, 32–36. [Google Scholar] [CrossRef]
- Lee, H.; Hwang, D.; Lee, M.; Lee, J.; Cho, S.; Kim, T. J.; Kim, H. S. , Micro-Current Stimulation Suppresses Inflammatory Responses in Peptidoglycan-Treated Raw 264.7 Macrophages and Propionibacterium acnes-Induced Skin Inflammation via TLR2/NF-kappaB Signaling Pathway. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Cameron, B.; Tse, W.; Lamb, R.; Li, X.; Lamb, B. T.; Landreth, G. E. , Loss of interleukin receptor-associated kinase 4 signaling suppresses amyloid pathology and alters microglial phenotype in a mouse model of Alzheimer's disease. J Neurosci 2012, 32, 15112–23. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wise, L.; Fukuchi, K. I. , TLR4 Cross-Talk With NLRP3 Inflammasome and Complement Signaling Pathways in Alzheimer's Disease. Front Immunol 2020, 11, 724. [Google Scholar] [CrossRef]
- Hann, H. W.; Stahlhut, M. W.; Evans, A. E. , Source of increased ferritin in neuroblastoma: studies with concanavalin A-sepharose binding. J Natl Cancer Inst 1986, 76, 1031–3. [Google Scholar]
- Kwilasz, A. J.; Green Fulgham, S. M.; Duran-Malle, J. C.; Schrama, A. E. W.; Mitten, E. H.; Todd, L. S.; Patel, H. P.; Larson, T. A.; Clements, M. A.; Harris, K. M.; Litwiler, S. T.; Harvey, L. O., Jr.; Maier, S. F.; Chavez, R. A.; Rice, K. C.; Van Dam, A. M.; Watkins, L. R. , Toll-like receptor 2 and 4 antagonism for the treatment of experimental autoimmune encephalomyelitis (EAE)-related pain. Brain Behav Immun 2021, 93, 80–95. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).