Submitted:
05 April 2024
Posted:
09 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Methods of the Scoping Review
2.2. Recruitment of Scientific Board Members and Panelists
2.3. Conduction of the Delphi Survey
2.4. Data Analysis and Consensus Definition
3. Results
3.1. Vitamin B12 Deficiency in the Medical Literature
3.2. Results of the Delphi Survey
3.2.1. Characteristics of the Survey Panelists
3.2.2. Delphi Survey Rounds
3.2.3. Consensus on the Clinical Practice of Diagnosing Vitamin B12 Deficiency
3.2.4. Consensus for Clinical Practices of Treatment, Prophylaxis, and Long-Term Management of Vitamin B12 Deficiency
4. Discussion
4.1. Delphi Consensus
4.2. Additional Points Raised in the Board Discussion
4.3. Strengths and Limitations
4.4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
The Vitamin B12 Consensus Panelists Group included
- Agata Sobczyńska-Malefora, Nutristasis Unit, Haemostasis & Thrombosis, St. Thomas’ Hospital, London, UK.
- Aleksandra Araszkiewicz, Poznan University of Medical Sciences, Poland.
- Andrew McCaddon, Visiting Professor at Faculty of Social and Life Sciences, Wrexham University, Wrexham, UK.
- Anne M Molloy, Trinity College Dublin, Ireland.
- Bruce H.R. Wolffenbuttel, Department of Endocrinology, University Medical Center Groningen, 9700 RB Groningen, The Netherlands.
- Bruno Annibale, Department of Medical Surgical and Translational, Medicine Sapienza University, Rome, Italy.
- Christine A.F. von Arnim, Department of Geriatrics, University Medical Center Göttingen, Germany.
- Christy C Tangney, PhD, FACN, CNS, Departments of Clinical Nutrition & Preventive Medicine, Rush University Medical Center, 600 S Paulina St, Room 716 ACC, Chicago, USA.
- David Smith, Department of Pharmacology, University of Oxford, Oxford, UK.
- Dinh Tung Do, Hanoi Saint Paul Hospital, Vietnam.
- Dongming Zheng, Department of Neurology, Shengjing Hospital of China Medical University, China.
- Edith Lahner, Sapienza University of Rome, Department of Medical-surgical sciences and translational medicine, Italy.
- Gabriela Spulber, Clinical geriatrics, Karolinska Institutet, Sweden.
- Georgeta Daniela Georgescu, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania.
- Francesca Mangialasche, Karolinska Institutet, Center for Alzheimer Research, Sweden.
- Hendrika (H.J.M.) Smelt, Obesity Center, Catharina Hospital Eindhoven, The Netherlands.
- Janet B McGill, Washington University School of Medicine, 660 S. Euclid, Campus Box 8127, St. Louis, MO 63110, USA.
- J. David Spence, Professor Emeritus of Neurology & Clinical Pharmacology, Western University, and Director, Stroke Prevention & Atherosclerosis Research Centre, Robarts Research Institute, London, ON, Canada.
- John Killen, Macquarie Medical School Sydney, Australia.
- P Julian Owen, Department of Trauma and Orthopaedics, Addenbrooke’s, Cambridge University Hospitals NHS Trust, Cambridge, UK.
- Lisette CPGM de Groot, Wageningen University, The Netherlands.
- Michelle Murphy, Faculty of Medicine & Health Sciences, Universitat Rovira i Virgili, Spain.
- G Bhanuprakash Reddy, ICMR-National Institute of Nutrition, Hyderabad-500007, India.
- Pradeepa Rajendra, Madras Diabetes Research Foundation, Chennai, India.
- Ralph Green, University of California, Davis Medical Center.
- Sadanand Naik, K.E.M. Hospital, Pune, India.
- Tsvetalina Tankova, Medical University, Sofia, Bulgaria.
- William Huynh, Randwick Clinical Campus, UNSW Medicine and Health; FMH Translation Research Collective, Faculty of Medicine and Health, University of Sydney; and Prince of Wales Hospital, Southern Neurology, Kogarah NSW, , Sydney, Australia.
- Wolfgang N. Löscher, Department of Neurology, Medical University Innsbruck, Austria.
- Zyta Beata Wojszel, Department of Geriatrics, Medical University of Bialystok, Poland.
- The remaining 16 panelists chose not to disclose their names in the article.
References
- Healton, E.B.; Savage, D.G.; Brust, J.C.; Garrett, T.J.; Lindenbaum, J. Neurologic aspects of cobalamin deficiency. Medicine (Baltimore) 1991, 70, 229–45. [Google Scholar] [CrossRef] [PubMed]
- Miceli, E.; Lenti, M.V.; Padula, D.; Luinetti, O.; Vattiato, C.; Monti, C.M.; et al. Common features of patients with autoimmune atrophic gastritis. Clin Gastroenterol Hepatol 2012, 10, 812–4. [Google Scholar] [CrossRef] [PubMed]
- Misra, U.K.; Kalita, J. Comparison of clinical and electrodiagnostic features in B12 deficiency neurological syndromes with and without antiparietal cell antibodies. Postgrad Med J 2007, 83, 124–7. [Google Scholar] [CrossRef] [PubMed]
- Divate, P.G.; Patanwala, R. Neurological manifestations of B(12) deficiency with emphasis on its aetiology. J Assoc Physicians India 2014, 62, 400–5. [Google Scholar] [PubMed]
- Aaron, S.; Kumar, S.; Vijayan, J.; Jacob, J.; Alexander, M.; Gnanamuthu, C. Clinical and laboratory features and response to treatment in patients presenting with vitamin B12 deficiency-related neurological syndromes. Neurol India 2005, 53, 55–8. [Google Scholar]
- Lachner, C.; Martin, C.; John, D.; Nekkalapu, S.; Sasan, A.; Steinle, N.; et al. Older adult psychiatric inpatients with non-cognitive disorders should be screened for vitamin B12 deficiency. J Nutr Health Aging 2014, 18, 209–12. [Google Scholar] [CrossRef]
- Jain, K.K.; Malhotra, H.S.; Garg, R.K.; Gupta, P.K.; Roy, B.; Gupta, R.K. Prevalence of MR imaging abnormalities in vitamin B12 deficiency patients presenting with clinical features of subacute combined degeneration of the spinal cord. J Neurol Sci 2014, 342, 162–6. [Google Scholar] [CrossRef] [PubMed]
- Stabler, S.P.; Allen, R.H.; Savage, D.G.; Lindenbaum, J. Clinical spectrum and diagnosis of cobalamin deficiency. Blood 1990, 76, 871–81. [Google Scholar] [CrossRef]
- Lindenbaum, J.; Healton, E.B.; Savage, D.G.; Brust, J.C.; Garrett, T.J.; Podell, E.R.; et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med 1988, 318, 1720–8. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.; Obeid, R. Utility and limitations of biochemical markers of vitamin B12 deficiency. Eur J Clin Invest 2013, 43, 231–7. [Google Scholar] [CrossRef]
- Green, R.; Allen, L.H.; Bjorke-Monsen, A.L.; Brito, A.; Gueant, J.L.; Miller, J.W.; et al. Vitamin B(12) deficiency. Nat Rev Dis Primers 2017, 3, 17040. [Google Scholar] [CrossRef] [PubMed]
- Andres, E.; Zulfiqar, A.A.; Vogel, T. State of the art review: oral and nasal vitamin B12 therapy in the elderly. QJM 2020, 113, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, O.A.; Abdelaziz, A.; Diab, S.; Khazragy, A.; Elboraay, T.; Fayad, T.; et al. Efficacy of different routes of vitamin B12 supplementation for the treatment of patients with vitamin B12 deficiency: A systematic review and network meta-analysis. Ir J Med Sci 2024. [CrossRef] [PubMed]
- Butler, C.C.; Vidal-Alaball, J.; Cannings-John, R.; McCaddon, A.; Hood, K.; Papaioannou, A.; et al. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency: a systematic review of randomized controlled trials. Fam Pract 2006, 23, 279–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, L.; Qin, L.L.; Song, Y.; Vidal-Alaball, J.; Liu, T.H. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency. Cochrane Database Syst Rev 2018, 3, CD004655. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence, NICE. Vitamin B12 deficiency in over 16s: diagnosis and management (NG239), www.nice.org.uk/guidance/ng239 (Accessed on 6-3-2024).
- Beiderbeck, D.; Frevel, N.; von der Gracht, H.A.; Schmidt, S.L.; Schweitzer, V.M. Preparing, conducting, and analyzing Delphi surveys: Cross-disciplinary practices, new directions, and advancements. MethodsX 2021, 8, 101401. [Google Scholar] [CrossRef] [PubMed]
- Stabler, S.P. Clinical practice. Vitamin B12 deficiency. N Engl J Med 2013, 368, 149–60. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Su, Z.Y.; Xu, S.B.; Liu, C.C. Subacute combined degeneration: A retrospective study of 68 cases with short-term follow-up. Eur Neurol 2018, 79, 247–55. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ren, M.; Dong, A.; Wu, Y.; Han, N.; Deng, B.; et al. A retrospective study of 23 cases with subacute combined degeneration. Int J Neurosci 2016, 126, 872–7. [Google Scholar] [CrossRef] [PubMed]
- Linazi, G.; Abudureyimu, S.; Zhang, J.; Wulamu, A.; Maimaitiaili, M.; Wang, B.; et al. Clinical features of different stage subacute combined degeneration of the spinal cord. Medicine (Baltimore) 2022, 101, e30420. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Cao, J.; Shang, K.; Su, Z.; Xu, S.; Liu, C. Correlation between anemia and clinical severity in subacute combined degeneration patients. J Clin Neurosci 2020, 80, 11–5. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Xu, S.; Liu, C. Is serum vitamin B12 decrease a necessity for the diagnosis of subacute combined degeneration? : A meta-analysis. Medicine (Baltimore) 2020, 99, e19700. [Google Scholar] [CrossRef] [PubMed]
- Franques, J.; Chiche, L.; De Paula, A.M.; Grapperon, A.M.; Attarian, S.; Pouget, J.; et al. Characteristics of patients with vitamin B12-responsive neuropathy: a case series with systematic repeated electrophysiological assessment. Neurol Res 2019, 41, 569–76. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, P.; Kulkarni, J.D.; Pai, S.A. Vitamin B12 deficiency in India: mean corpuscular volume is an unreliable screening parameter. Natl Med J India 2012, 25, 336–8. [Google Scholar] [PubMed]
- Sanz-Cuesta, T.; Escortell-Mayor, E.; Cura-Gonzalez, I.; Martin-Fernandez, J.; Riesgo-Fuertes, R.; Garrido-Elustondo, S.; et al. Oral versus intramuscular administration of vitamin B12 for vitamin B12 deficiency in primary care: a pragmatic, randomised, non-inferiority clinical trial (OB12). BMJ Open 2020, 10, e033687. [Google Scholar] [CrossRef] [PubMed]
- Soykan, I.; Yakut, M.; Keskin, O.; Bektas, M. Clinical profiles, endoscopic and laboratory features and associated factors in patients with autoimmune gastritis. Digestion 2012, 86, 20–6. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Hyung, W.J.; Song, K.J.; Choi, S.H.; Kim, C.B.; Noh, S.H. Oral vitamin B12 replacement: an effective treatment for vitamin B12 deficiency after total gastrectomy in gastric cancer patients. Ann Surg Oncol 2011, 18, 3711–7. [Google Scholar] [CrossRef] [PubMed]
- Saperstein, D.S.; Wolfe, G.I.; Gronseth, G.S.; Nations, S.P.; Herbelin, L.L.; Bryan, W.W.; et al. Challenges in the identification of cobalamin-deficiency polyneuropathy. Arch Neurol 2003, 60, 1296–301. [Google Scholar] [CrossRef] [PubMed]
- Adali, Y.; Binnetoglu, K. Evaluation of the response to vitamin B12 supplementation in patients with atrophy in sleeve gastrectomy materials. Cir Cir 2022, 90, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Rajabally, Y.A.; Martey, J. Neuropathy in Parkinson disease: prevalence and determinants. Neurology 2011, 77, 1947–50. [Google Scholar] [CrossRef] [PubMed]
- Siswanto, O.; Smeall, K.; Watson, T.; Donnelly-Vanderloo, M.; O’Connor, C.; Foley, N.; et al. Examining the association between vitamin B12 deficiency and dementia in high-risk hospitalized patients. J Nutr Health Aging 2015, 19, 1003–8. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.C.; Lo, C.P.; Chen, C.Y.; Huang, C.F. Correlation of Tc-99 m ethyl cysteinate dimer single-photon emission computed tomography and clinical presentations in patients with low cobalamin status. BMC Neurol 2015, 15, 251. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Gupta, R.K.; Garg, R.K.; Rai, Y.; Roy, B.; Pandey, C.M.; et al. DTI correlates of cognition in conventional MRI of normal-appearing brain in patients with clinical features of subacute combined degeneration and biochemically proven vitamin B(12) deficiency. AJNR Am J Neuroradiol 2014, 35, 872–7. [Google Scholar] [CrossRef] [PubMed]
- Warendorf, J.K.; van Doormaal, P.T.C.; Vrancken, A.F.J.E.; Verhoeven-Duif, N.M.; van Eijk, R.P.A.; van den Berg, L.H.; et al. Clinical relevance of testing for metabolic vitamin B12 deficiency in patients with polyneuropathy. Nutr Neurosci 2022, 25, 2536–46. [Google Scholar] [CrossRef] [PubMed]
- Tangney, C.C.; Aggarwal, N.T.; Li, H.; Wilson, R.S.; DeCarli, C.; Evans, D.A.; et al. Vitamin B12, cognition, and brain MRI measures: A cross-sectional examination. Neurology 2011, 77, 1276–82. [Google Scholar] [CrossRef] [PubMed]
- Tangney, C.C.; Tang, Y.; Evans, D.A.; Morris, M.C. Biochemical indicators of vitamin B12 and folate insufficiency and cognitive decline. Neurology 2009, 72, 361–7. [Google Scholar] [CrossRef] [PubMed]
- Leishear, K.; Boudreau, R.M.; Studenski, S.A.; Ferrucci, L.; Rosano, C.; de, R.N.; et al. Relationship between vitamin B12 and sensory and motor peripheral nerve function in older adults. J Am Geriatr Soc 2012, 60, 1057–63. [Google Scholar] [CrossRef] [PubMed]
- Solomon, L.R. Vitamin B12-responsive neuropathies: A case series. Nutr Neurosci 2016, 19, 162–8. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Jain, A.; Rohatgi, A. An observational study of vitamin b12 levels and peripheral neuropathy profile in patients of diabetes mellitus on metformin therapy. Diabetes Metab Syndr 2018, 12, 51–8. [Google Scholar] [CrossRef] [PubMed]
- Kancherla, V.; Elliott, J.L.; Jr Patel, B.B.; Holland, N.W.; Johnson, T.M.; Khakharia, A.; et al. Long-term metformin therapy and monitoring for vitamin B12 deficiency among older veterans. J Am Geriatr Soc 2017, 65, 1061–6. [Google Scholar] [CrossRef] [PubMed]
- Kos, E.; Liszek, M.J.; Emanuele, M.A.; Durazo-Arvizu, R.; Camacho, P. Effect of metformin therapy on vitamin D and vitamin B(1)(2) levels in patients with type 2 diabetes mellitus. Endocr Pract 2012, 18, 179–84. [Google Scholar] [CrossRef] [PubMed]
- Jayashri, R.; Venkatesan, U.; Rohan, M.; Gokulakrishnan, K.; Shanthi Rani, C.S.; Deepa, M.; et al. Prevalence of vitamin B(12) deficiency in South Indians with different grades of glucose tolerance. Acta Diabetol 2018, 55, 1283–93. [Google Scholar] [CrossRef] [PubMed]
- Bherwani, S.; Ahirwar, A.K.; Saumya, A.S.; Sandhya, A.S.; Prajapat, B.; Patel, S.; et al. The study of association of Vitamin B(12) deficiency in type 2 diabetes mellitus with and without diabetic nephropathy in North Indian Population. Diabetes Metab Syndr 2017, 11 (Suppl 1), S365–S368. [Google Scholar] [CrossRef]
- Kang, D.; Yun, J.S.; Ko, S.H.; Lim, T.S.; Ahn, Y.B.; Park, Y.M.; et al. Higher prevalence of metformin-induced vitamin B12 deficiency in sulfonylurea combination compared with insulin combination in patients with type 2 diabetes: a cross-sectional study. PLoS ONE 2014, 9, e109878. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Kumar, A.; Karmakar, D.; Jha, R.K. Association of B12 deficiency and clinical neuropathy with metformin use in type 2 diabetes patients. J Postgrad Med 2013, 59, 253–7. [Google Scholar] [CrossRef] [PubMed]
- Chapman, L.E.; Darling, A.L.; Brown, J.E. Association between metformin and vitamin B(12) deficiency in patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab 2016, 42, 316–27. [Google Scholar] [CrossRef] [PubMed]
- de Jager, J.; Kooy, A.; Lehert, P.; Wulffele, M.G.; van der Kolk, J.; Bets, D.; et al. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. BMJ 2010, 340, c2181. [Google Scholar] [CrossRef]
- Yang, R.; Yu, H.; Wu, J.; Chen, H.; Wang, M.; Wang, S.; et al. Metformin treatment and risk of diabetic peripheral neuropathy in patients with type 2 diabetes mellitus in Beijing, China. Front Endocrinol (Lausanne) 2023, 14, 1082720. [Google Scholar] [CrossRef] [PubMed]
- Aroda, V.R.; Edelstein, S.L.; Goldberg, R.B.; Knowler, W.C.; Marcovina, S.M.; Orchard, T.J.; et al. Long-term metformin use and vitamin B12 deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab 2016, 101, 1754–61. [Google Scholar] [CrossRef] [PubMed]
- Miyan, Z.; Waris, N. Association of vitamin B(12) deficiency in people with type 2 diabetes on metformin and without metformin: a multicenter study, Karachi, Pakistan. BMJ Open Diabetes Res Care 2020, 8, e001151. [Google Scholar] [CrossRef]
- Parry-Strong, A.; Langdana, F.; Haeusler, S.; Weatherall, M.; Krebs, J. Sublingual vitamin B12 compared to intramuscular injection in patients with type 2 diabetes treated with metformin: a randomised trial. N Z Med J 2016, 129, 67–75. [Google Scholar] [PubMed]
- Mirkazemi, C.; Peterson, G.M.; Tenni, P.C.; Jackson, S.L. Vitamin B12 deficiency in Australian residential aged care facilities. J Nutr Health Aging 2012, 16, 277–80. [Google Scholar] [CrossRef] [PubMed]
- Soh, Y.; Won, C.W. Association between frailty and vitamin B12 in the older Korean population. Medicine (Baltimore) 2020, 99, e22327. [Google Scholar] [CrossRef] [PubMed]
- Couderc, A.L.; Camalet, J.; Schneider, S.; Turpin, J.M.; Bereder, I.; Boulahssass, R.; et al. Cobalamin deficiency in the elderly: aetiology and management: a study of 125 patients in a geriatric hospital. J Nutr Health Aging 2015, 19, 234–9. [Google Scholar] [CrossRef] [PubMed]
- Andres, E.; Affenberger, S.; Vinzio, S.; Kurtz, J.E.; Noel, E.; Kaltenbach, G.; et al. Food-cobalamin malabsorption in elderly patients: clinical manifestations and treatment. Am J Med 2005, 118, 1154–9. [Google Scholar] [CrossRef] [PubMed]
- Ates, B.E.; Soysal, P.; Aydin, A.E.; Dokuzlar, O.; Kocyigit, S.E.; Isik, A.T. Vitamin B12 deficiency might be related to sarcopenia in older adults. Exp Gerontol 2017, 95, 136–40. [Google Scholar] [CrossRef] [PubMed]
- Junca, J.; de Soria, P.L.; Granada, M.L.; Flores, A.; Marquez, E. Detection of early abnormalities in gastric function in first-degree relatives of patients with pernicious anemia. Eur J Haematol 2006, 77, 518–22. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.W.; Muegge, B.D.; Tobin, G.S.; Litvin, M.; Sun, L.; Saenz, J.B.; et al. High-risk gastric pathology and prevalent autoimmune diseases in patients with pernicious anemia. Endocr Pract 2017, 23, 1297–303. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.T.; Zhao, H.Y.; Kong, Y.; Sun, N.N.; Dong, A.Q. Correlation between serum vitamin B12 level and peripheral neuropathy in atrophic gastritis. World J Gastroenterol 2018, 24, 1343–52. [Google Scholar] [CrossRef] [PubMed]
- Ao, M.; Tsuji, H.; Shide, K.; Kosaka, Y.; Noda, A.; Inagaki, N.; et al. High prevalence of vitamin B-12 insufficiency in patients with Crohn’s disease. Asia Pac J Clin Nutr 2017, 26, 1076–81. [Google Scholar] [PubMed]
- Madanchi, M.; Fagagnini, S.; Fournier, N.; Biedermann, L.; Zeitz, J.; Battegay, E.; et al. The relevance of vitamin and iron deficiency in patients with inflammatory bowel diseases in patients of the Swiss IBD Cohort. Inflamm Bowel Dis 2018, 24, 1768–79. [Google Scholar] [CrossRef]
- Yakut, M.; Ustun, Y.; Kabacam, G.; Soykan, I. Serum vitamin B12 and folate status in patients with inflammatory bowel diseases. Eur J Intern Med 2010, 21, 320–3. [Google Scholar] [CrossRef] [PubMed]
- Schosler, L.; Christensen, L.A.; Hvas, C.L. Symptoms and findings in adult-onset celiac disease in a historical Danish patient cohort. Scand J Gastroenterol 2016, 51, 288–94. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; van Oijen, M.G.; Janssen, M.J.; Laheij, R.J.; Jansen, J.B.; van, A.H. Vitamin B12 deficiency in patients with upper gastrointestinal symptoms in the Mekong Delta, Vietnam. Dig Liver Dis 2006, 38, 438–9. [Google Scholar] [CrossRef]
- Schiavon, C.A.; Bhatt, D.L.; Ikeoka, D.; Santucci, E.V.; Santos, R.N.; Damiani, L.P.; et al. Three-year outcomes of bariatric surgery in patients with obesity and hypertension: A randomized clinical trial. Ann Intern Med 2020, 173, 685–93. [Google Scholar] [CrossRef] [PubMed]
- Schijns, W.; Schuurman, L.T.; Melse-Boonstra, A.; van Laarhoven, C.J.H.M.; Berends, F.J.; Aarts, E.O. Do specialized bariatric multivitamins lower deficiencies after RYGB? Surg Obes Relat Dis 2018, 14, 1005–12. [Google Scholar] [CrossRef] [PubMed]
- Vilarrasa, N.; Fabregat, A.; Toro, S.; Gordejuela, A.G.; Casajoana, A.; Montserrat, M.; et al. Nutritional deficiencies and bone metabolism after endobarrier in obese type 2 patients with diabetes. Eur J Clin Nutr 2018, 72, 1447–50. [Google Scholar] [CrossRef] [PubMed]
- Bilici, A.; Sonkaya, A.; Ercan, S.; Ustaalioglu, B.B.; Seker, M.; Aliustaoglu, M.; et al. The changing of serum vitamin B12 and homocysteine levels after gastrectomy in patients with gastric cancer: do they associate with clinicopathological factors? Tumour Biol 2015, 36, 823–8. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Kim, H.I.; Hyung, W.J.; Song, K.J.; Lee, J.H.; Kim, Y.M.; et al. Vitamin B(12) deficiency after gastrectomy for gastric cancer: an analysis of clinical patterns and risk factors. Ann Surg 2013, 258, 970–5. [Google Scholar] [CrossRef]
- Rozgony, N.R.; Fang, C.; Kuczmarski, M.F.; Bob, H. Vitamin B(12) deficiency is linked with long-term use of proton pump inhibitors in institutionalized older adults: could a cyanocobalamin nasal spray be beneficial? J Nutr Elder 2010, 29, 87–99. [Google Scholar] [CrossRef]
- Aoyama, T.; Hara, K.; Maezawa, Y.; Kazama, K.; Hashimoto, I.; Sawazaki, S.; et al. Clinical course of vitamin B12 deficiency and associated risk factors in patients after total gastrectomy for gastric cancer. Anticancer Res 2023, 43, 689–94. [Google Scholar] [CrossRef]
- Vargas-Ruiz, A.G.; Hernandez-Rivera, G.; Herrera, M.F. Prevalence of iron, folate, and vitamin B12 deficiency anemia after laparoscopic Roux-en-Y gastric bypass. Obes Surg 2008, 18, 288–93. [Google Scholar] [CrossRef]
- Aoyama, T.; Maezawa, Y.; Cho, H.; Saigusa, Y.; Tamura, J.; Tsuchida, K.; et al. Phase II study of a multi-center randomized controlled trial to evaluate oral vitamin B12 treatment for vitamin B12 deficiency after total gastrectomy in gastric cancer patients. Anticancer Res 2022, 42, 3963–70. [Google Scholar] [CrossRef] [PubMed]
- Mahawar, K.K.; Reid, A.; Graham, Y.; Callejas-Diaz, L.; Parmar, C.; Carr, W.R.; et al. Oral vitamin B(12) supplementation after Roux-en-Y gastric bypass: a systematic review. Obes Surg 2018, 28, 1916–23. [Google Scholar] [CrossRef] [PubMed]
- Smelt, H.J.; Pouwels, S.; Smulders, J.F. Different supplementation regimes to treat perioperative vitamin B12 deficiencies in bariatric surgery: a systematic review. Obes Surg 2017, 27, 254–62. [Google Scholar] [CrossRef]
- Majumder, S.; Soriano, J.; Louie, C.A.; Dasanu, C.A. Vitamin B12 deficiency in patients undergoing bariatric surgery: preventive strategies and key recommendations. Surg Obes Relat Dis 2013, 9, 1013–9. [Google Scholar] [CrossRef] [PubMed]
- Engebretsen, K.V.; Blom-Hogestol, I.K.; Hewitt, S.; Risstad, H.; Moum, B.; Kristinsson, J.A.; et al. Anemia following Roux-en-Y gastric bypass for morbid obesity; a 5-year follow-up study. Scand J Gastroenterol 2018, 53, 917–22. [Google Scholar] [CrossRef] [PubMed]
- Antoine, D.; Li, Z.; Quilliot, D.; Sirveaux, M.A.; Meyre, D.; Mangeon, A.; et al. Medium term post-bariatric surgery deficit of vitamin B12 is predicted by deficit at time of surgery. Clin Nutr 2021, 40, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Didangelos, T.; Karlafti, E.; Kotzakioulafi, E.; Margariti, E.; Giannoulaki, P.; Batanis, G.; et al. Vitamin B12 supplementation in diabetic neuropathy: A 1-year, randomized, double-blind, placebo-controlled trial. Nutrients 2021, 13, 395. [Google Scholar] [CrossRef]
- Namikawa, T.; Maeda, M.; Yokota, K.; Iwabu, J.; Munekage, M.; Uemura, S.; et al. Enteral vitamin B12 supplementation is effective for improving anemia in patients who underwent total gastrectomy. Oncology 2021, 99, 225–33. [Google Scholar] [CrossRef] [PubMed]
- Kalita, J.; Agarwal, R.; Chandra, S.; Misra, U.K. A study of neurobehavioral, clinical psychometric, and P3 changes in vitamin B12 deficiency neurological syndrome. Nutr Neurosci 2013, 16, 39–46. [Google Scholar] [CrossRef]
- Maghsoudlou, P.; Varlamova, J.; Pandit, J. Patients with unexplained neurological symptoms and signs should be screened for vitamin B12 deficiency regardless of haemoglobin levels. Eye (Lond) 2022, 36, 1124–5. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Garg, R.K.; Gupta, R.K.; Malhotra, H.S.; Paliwal, V.K.; Rathore, R.K.; et al. Diffusion tensor tractography and neuropsychological assessment in patients with vitamin B12 deficiency. Neuroradiology 2014, 56, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol 2006, 5, 949–60. [Google Scholar] [CrossRef] [PubMed]
- Puri, V.; Chaudhry, N.; Goel, S.; Gulati, P.; Nehru, R.; Chowdhury, D. Vitamin B12 deficiency: a clinical and electrophysiological profile. Electromyogr Clin Neurophysiol 2005, 45, 273–84. [Google Scholar] [PubMed]
- Misra, U.K.; Kalita, J.; Das, A. Vitamin B12 deficiency neurological syndromes: a clinical, MRI and electrodiagnostic study. Electromyogr Clin Neurophysiol 2003, 43, 57–64. [Google Scholar] [PubMed]
- Bolaman, Z.; Kadikoylu, G.; Yukselen, V.; Yavasoglu, I.; Barutca, S.; Senturk, T. Oral versus intramuscular cobalamin treatment in megaloblastic anemia: a single-center, prospective, randomized, open-label study. Clin Ther 2003, 25, 3124–34. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.P.; Ren, C.P.; Cheng, J.L.; Zhang, Y.; Li, Y.; Li, B.B.; et al. Conventional MRI for diagnosis of subacute combined degeneration (SCD) of the spinal cord due to vitamin B-12 deficiency. Asia Pac J Clin Nutr 2016, 25, 34–8. [Google Scholar] [PubMed]
- Roy, B.; Trivedi, R.; Garg, R.K.; Gupta, P.K.; Tyagi, R.; Gupta, R.K. Assessment of functional and structural damage in brain parenchyma in patients with vitamin B12 deficiency: A longitudinal perfusion and diffusion tensor imaging study. Magn Reson Imaging 2015, 33, 537–43. [Google Scholar] [CrossRef] [PubMed]
- Urbanski, G.; Hamel, J.F.; Prouveur, B.; Annweiler, C.; Ghali, A.; Cassereau, J.; et al. Strength of the association of elevated vitamin B12 and solid cancers: An adjusted case-control study. J Clin Med 2020, 9, 474. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R. High plasma vitamin B12 and cancer in human studies: A scoping review to judge causality and alternative explanations. Nutrients 2022, 14, 4476. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cai, X.; Wu, H.; Ji, L. Associations between metformin use and vitamin B(12) levels, anemia, and neuropathy in patients with diabetes: a meta-analysis. J Diabetes 2019, 11, 729–43. [Google Scholar] [CrossRef]
- Ceravolo, R.; Cossu, G.; Bandettini di, P.M.; Santoro, L.; Barone, P.; Zibetti, M.; et al. Neuropathy and levodopa in Parkinson’s disease: evidence from a multicenter study. Mov Disord 2013, 28, 1391–7. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Park, D.; Ko, P.W.; Kang, K.; Lee, H.W. Serum methylmalonic acid correlates with neuropathic pain in idiopathic Parkinson’s disease. Neurol Sci 2017, 38, 1799–804. [Google Scholar] [CrossRef] [PubMed]
- Jhunjhnuwala, D.; Tanglay, O.; Briggs, N.E.; Yuen, M.T.Y.; Huynh, W. Prognostic indicators of subacute combined degeneration from B12 deficiency: A systematic review. PM R 2022, 14, 504–14. [Google Scholar] [CrossRef] [PubMed]

| Questions | n (panelists)1 | Mean (95%CI)2 | |
| Identification of vitamin B12 deficiency: Challenges, barriers and opportunities | |||
| 1. | The delay in diagnosing B12 deficiency in a significant number of patients may be due to the following factors:
|
42 | 0.95 (0.84 − 0.99) |
|
42 | 0.93 (0.81 − 0.99) | |
|
41 | 0.85 (0.71 − 0.94) | |
|
41 | 0.68 (0.52 − 0.82) | |
| 2. | The following initiatives can reduce the burden of unidentified B12 deficiency:
|
42 | 100% |
|
41 | 0.83 (0.68 − 0.93) | |
| 3. | Signs and symptoms of B12 deficiency may affect multiple organ systems at variable frequency. The crude order of affected systems (highest to lowest prevalence) is shown in Figure S4. | 41 | 0.71 (0.54 − 0.84) |
| 4. | The most difficult symptoms to link to clinically manifested B12 deficiency are (as ordered from most to least difficult) as shown in Figure S3. | 40 | 0.80 (0.64 − 0.91) |
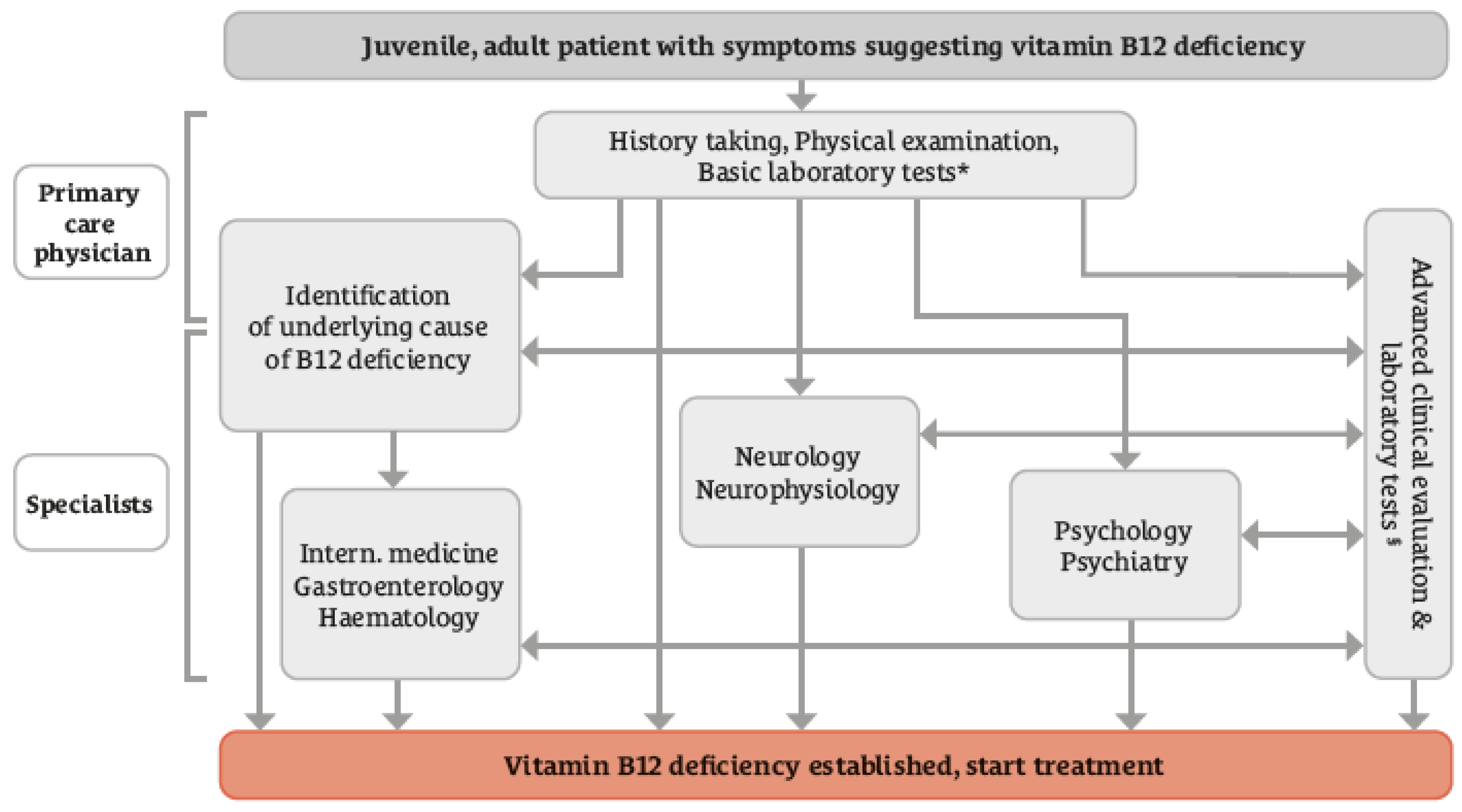
| 5. | Clinically manifested B12 deficiency is commonly first identified in primary medical care. Some patients may require referral to a specialist. Referral of patients to gastroenterologists is least frequent compared to referral to neurologists/psychiatrists and hematologists | 38 | 0.71 (0.54 − 0.85) |
| 6. | Concordance with the diagnostic pathway shown in Figure 2. | 42 | 0.76 (0.61 − 0.88) |
| Biomarkers and their utility in clinical practice | |||
| 7. | Considering the cost‒benefit and the added value of advanced laboratory tests beyond plasma B12 concentrations and blood cell count:
|
41 | 0.88 (0.74 − 0.96) |
|
41 | 0.76 (0.60 − 0.88) | |
|
39 | 0.69 (0.52 − 0.83) | |
|
41 | 0.83 (0.68 − 0.93) | |
|
41 | 0.88 (0.74 − 0.96) | |
| 8. | Because chronic use of metformin in patients with diabetes is associated with lower plasma concentrations of B12 and linked to the frequency and severity of neuropathy, measurement of B12 status once per year in this group of patients can help detecting a deficiency prior to clinical manifestation. | 40 | 0.83 (0.67 − 0.93) |
| 9. | If plasma B12 concentrations far above the reference range are encountered in a person without specific medical conditions:
|
41 | 0.98 (0.87− 0.999) |
|
40 | 0.70 (0.53 − 0.83) | |
|
39 | 0.85 (0.69 − 0.94) | |
| Identifying the cause of vitamin B12 deficiency | |||
| 10. | A holistic approach is deemed necessary for diagnosing B12 deficiency and identifying the cause(s). This includes:
|
42 | 0.93 (0.81 − 0.99) |
|
42 | 0.93 (0.81 − 0.99) | |
|
42 | 0.95 (0.84 − 0.99) | |
|
42 | 0.98 (0.87 − 0.999) | |
|
40 | 0.70 (0.53 − 0.83) | |
| 11. | The following conditions may provide clues for B12 deficiency being due to B12 malabsorption:
|
39 | 0.87 (0.73 − 0.96) |
|
42 | 0.98 (0.87 − 0.999) | |
|
42 | 100% | |
|
41 | 0.93 (0.80 − 0.98) | |
|
42 | 0.88 (0.74 − 0.96) | |
| In context of the B12 diagnostic work-up, folate and iron status should also be assessed. | 42 | 0.95 (0.84 − 0.99) | |
| Question | n (panelists)1 | Mean (95%CI)2 | |
|---|---|---|---|
| 1. | At present, it is unclear whether different forms of B12 differ in their effectiveness or safety. Clinical trials comparing the safety and effectiveness of the commercially available forms are needed. | 42 | 0.88 (0.74 − 0.96) |
| 2. | Regarding the use of prophylactic B12 supplementation:
|
41 | 0.85 (0.71− 0.94) |
|
41 | 0.85 (0.71 − 0.94) | |
|
41 | 0.90 (0.77 − 0.97) | |
|
42 | 0.83 (0.69 − 0.93) | |
|
39 | 0.85 (0.69 − 0.94) | |
| 3. | There is no one-size-fits-all regarding the dose of B12, the frequency and the route of B12 therapy in people with B12 deficiency. Regarding the decision on the route of B12 administration:
|
38 | 0.87 (0.72 − 0.96) |
|
32 | 0.75 (0.57 − 0.89) | |
|
40 | 0.78 (0.62 − 0.89) | |
| 4. | If B12 treatment fails in symptomatic patients, one or more of the following measures are recommended:
|
40 | 0.98 (0.87 − 0.999) |
|
39 | 0.95 (0.83 − 0.99) | |
|
38 | 0.87 (0.72 − 0.96) | |
| 5. | B12 deficiency during pregnancy, lactation and in infancy needs to be detected and treated as early as possible because of the serious effects of B12 deficiency on fetal and infant development. | 38 | 0.89 (0.75 − 0.97) |
| 6. | Women with a previously diagnosed B12 deficiency or dietary restriction of animal foods should take prophylactic B12 supplementation from pre-pregnancy to the end of the lactation period. | 38 | 0.92 (0.79 − 0.98) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





