Submitted:
18 March 2024
Posted:
19 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
- To analyze the spectral weight of fluorescence mediated by plasmonic resonance behavior and its angular dispersion;
- To define the enhancement factor for each measurement configuration.
2. Materials and Methods
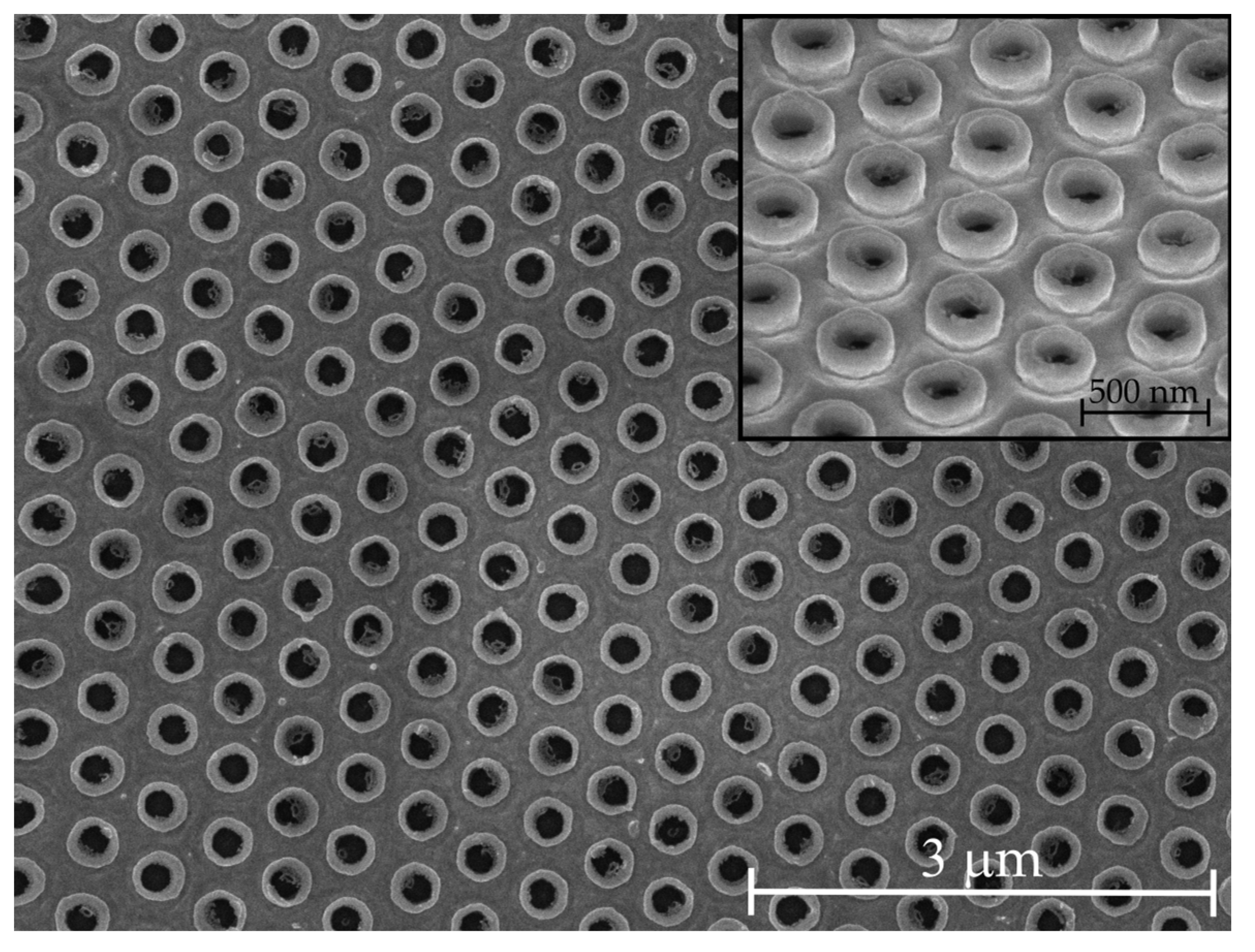
2.1. Nanofabrication of the Plasmonic Metasurface
2.2. Dye Deposition
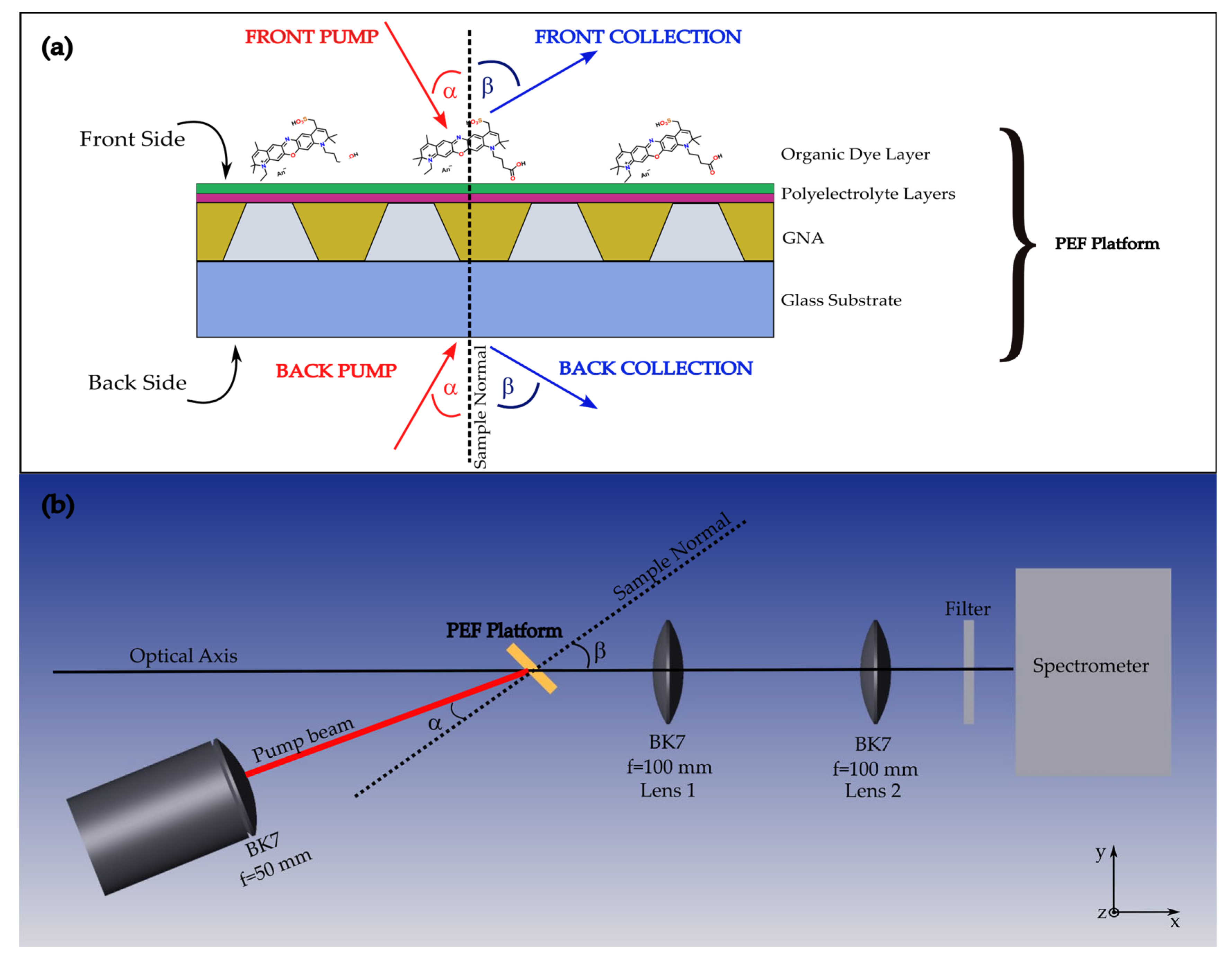
2.3. Experimental Optical Setups
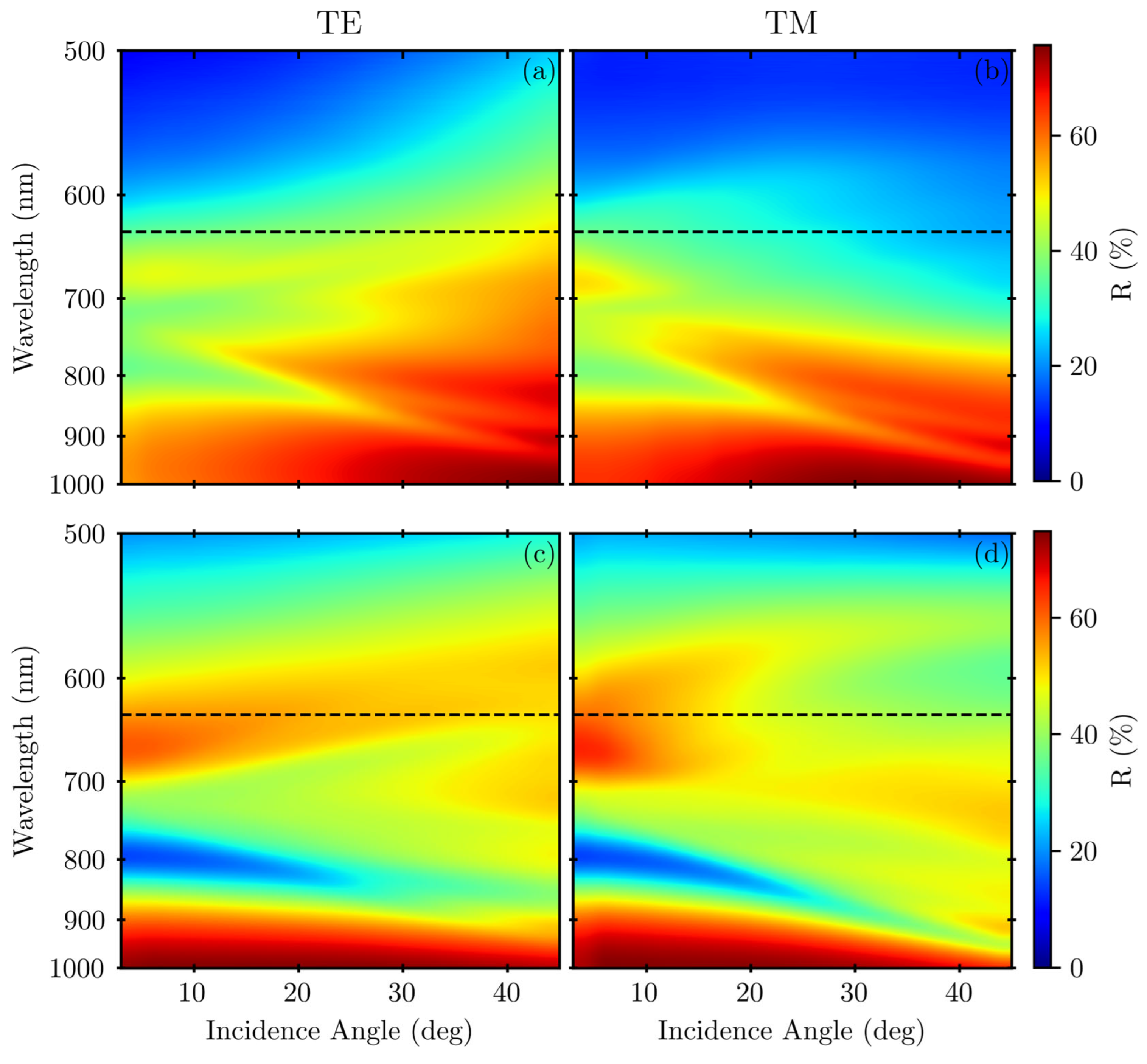
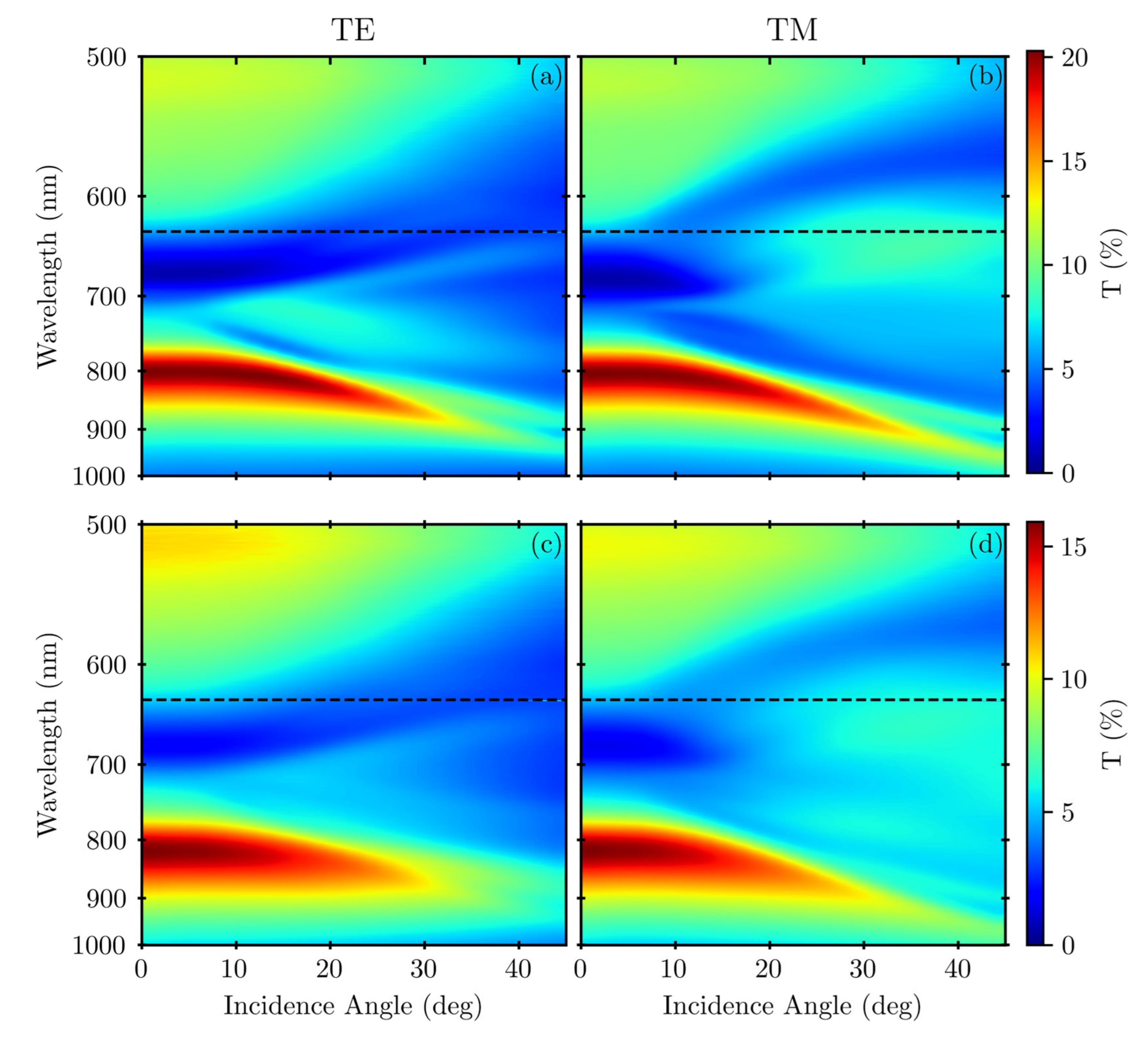
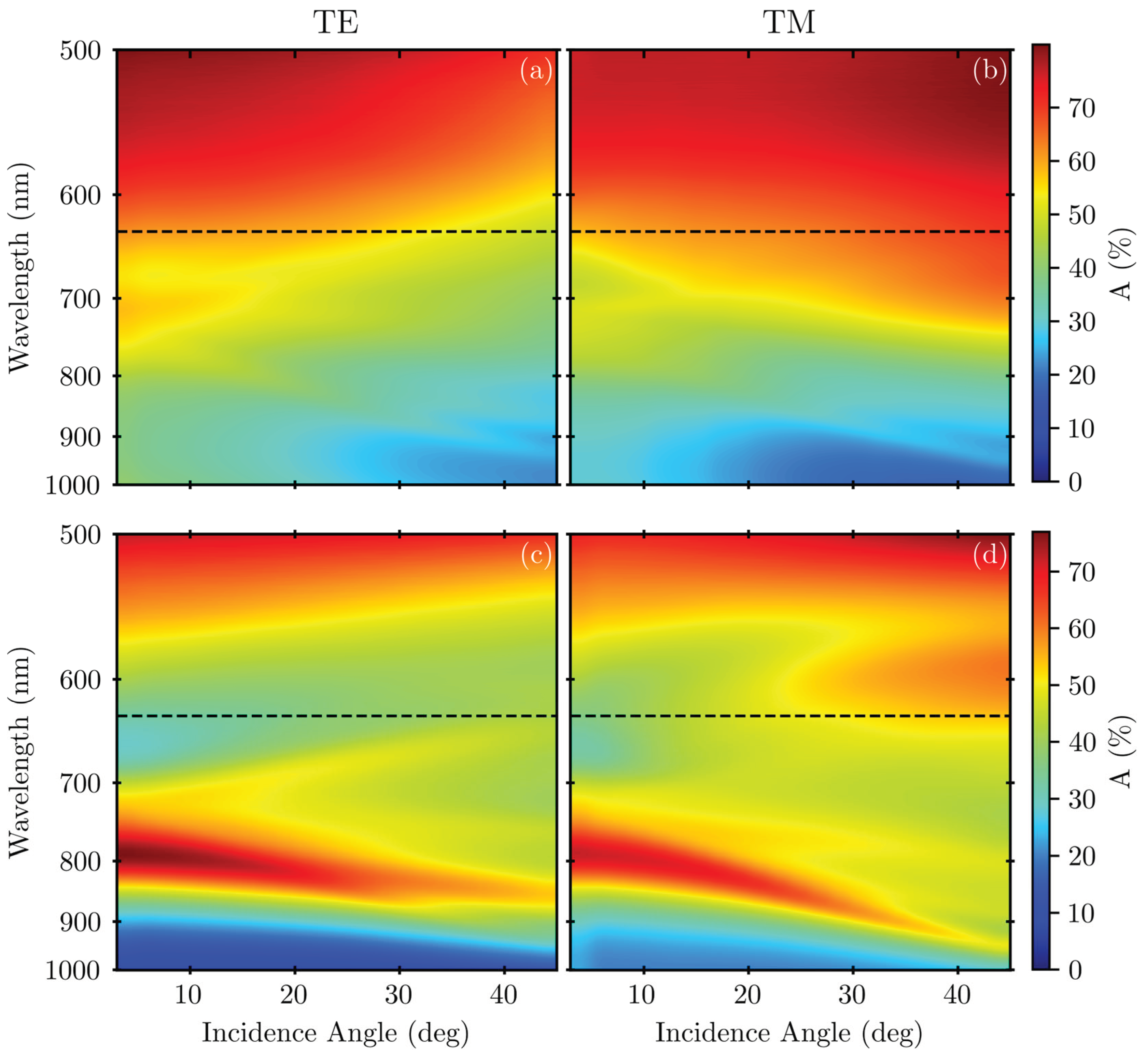
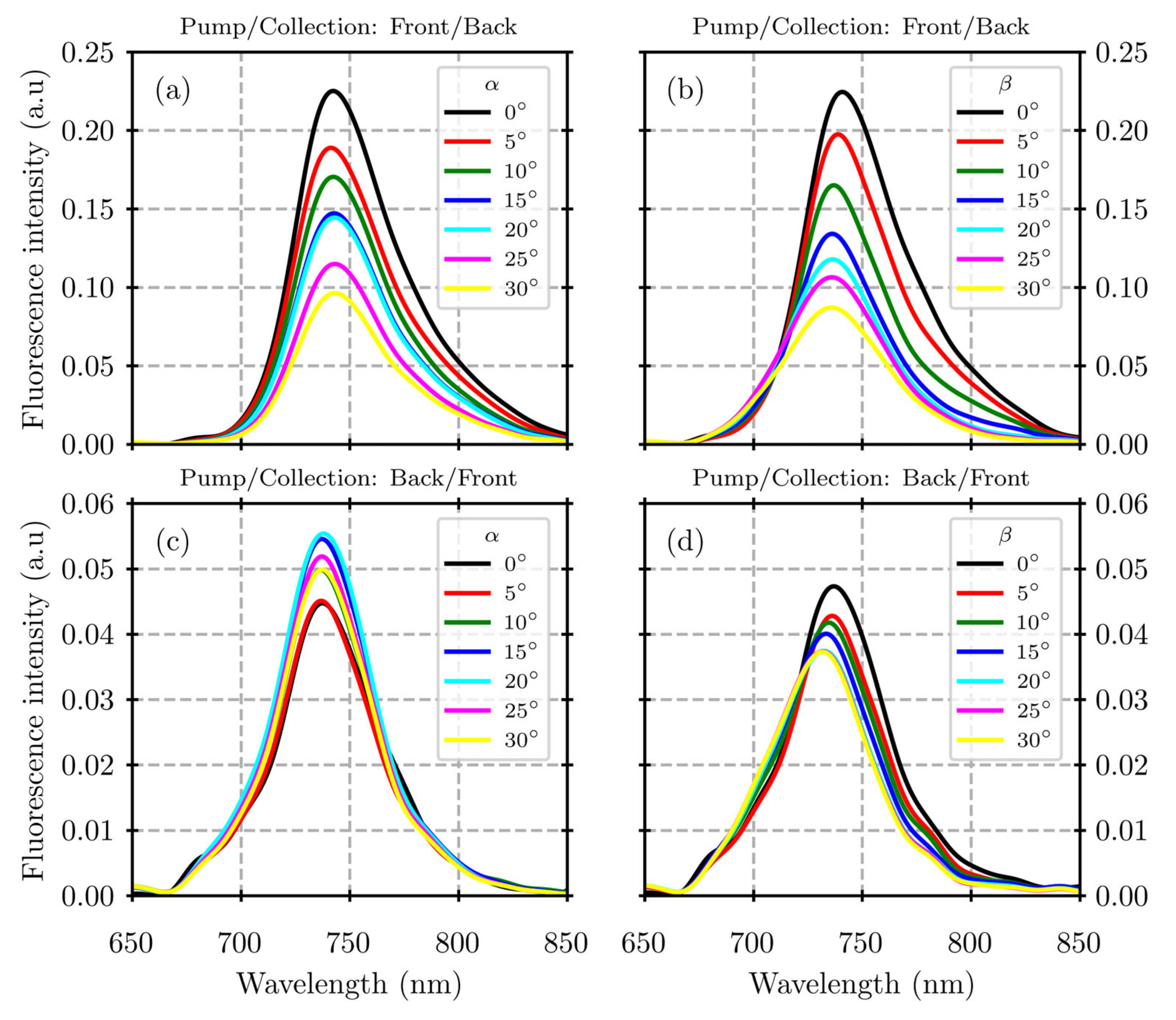
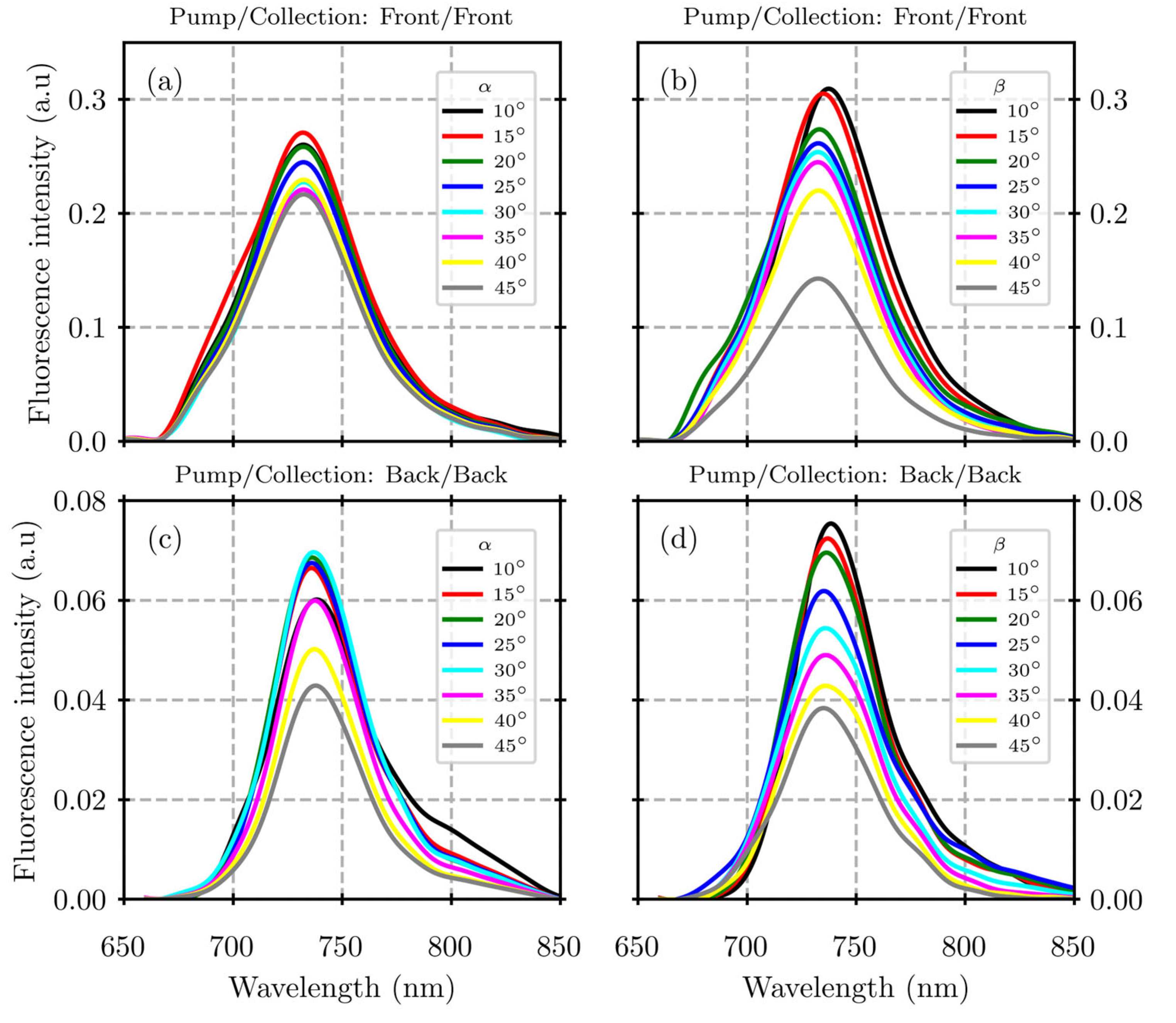
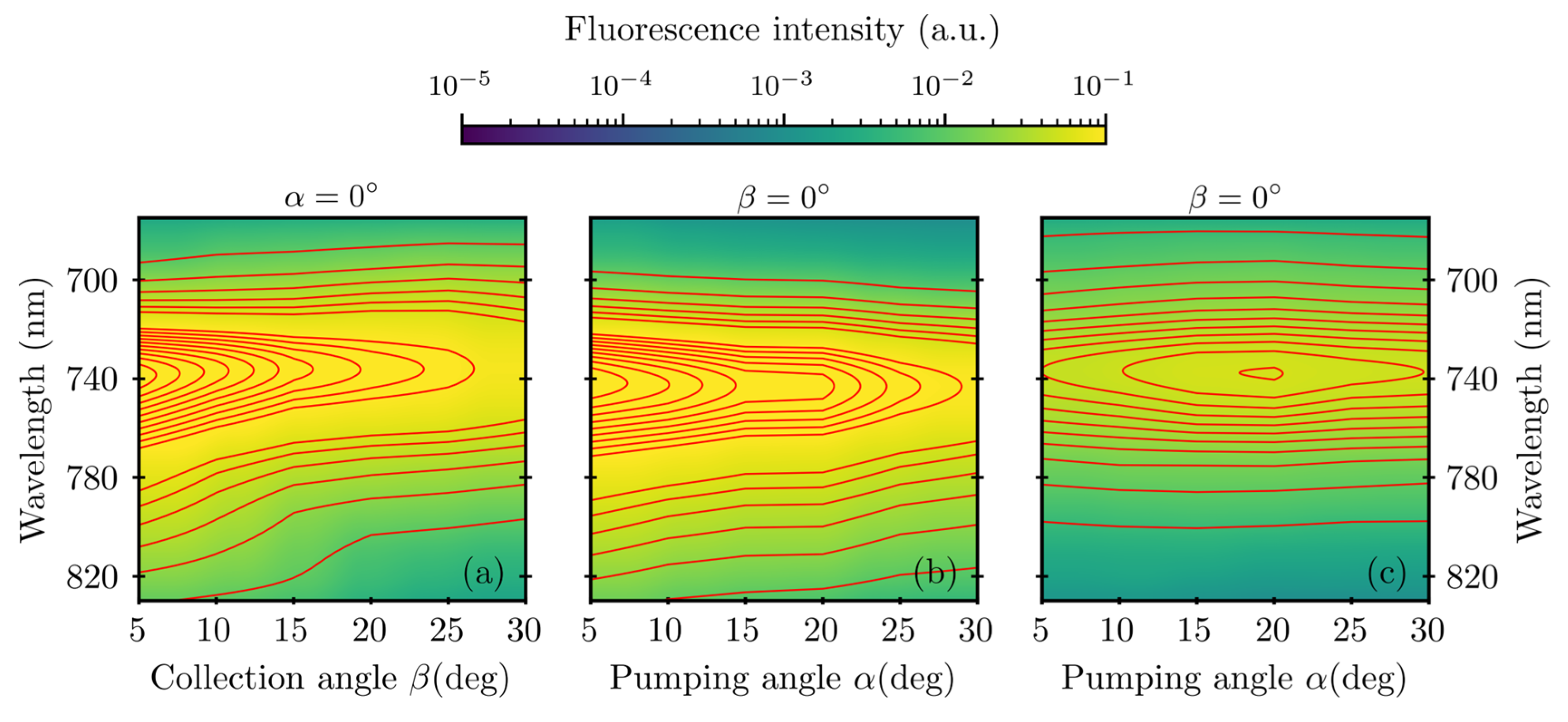
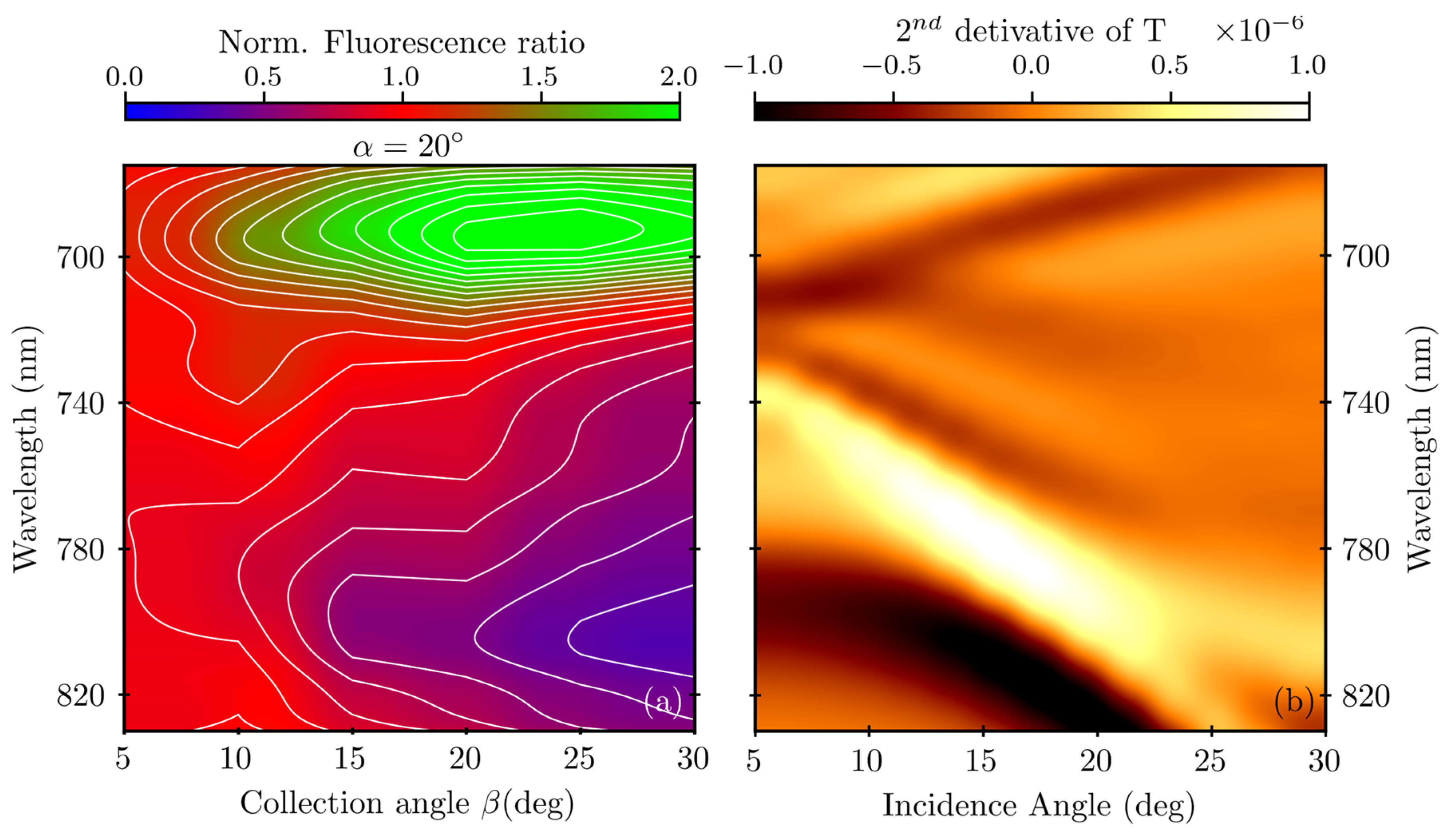
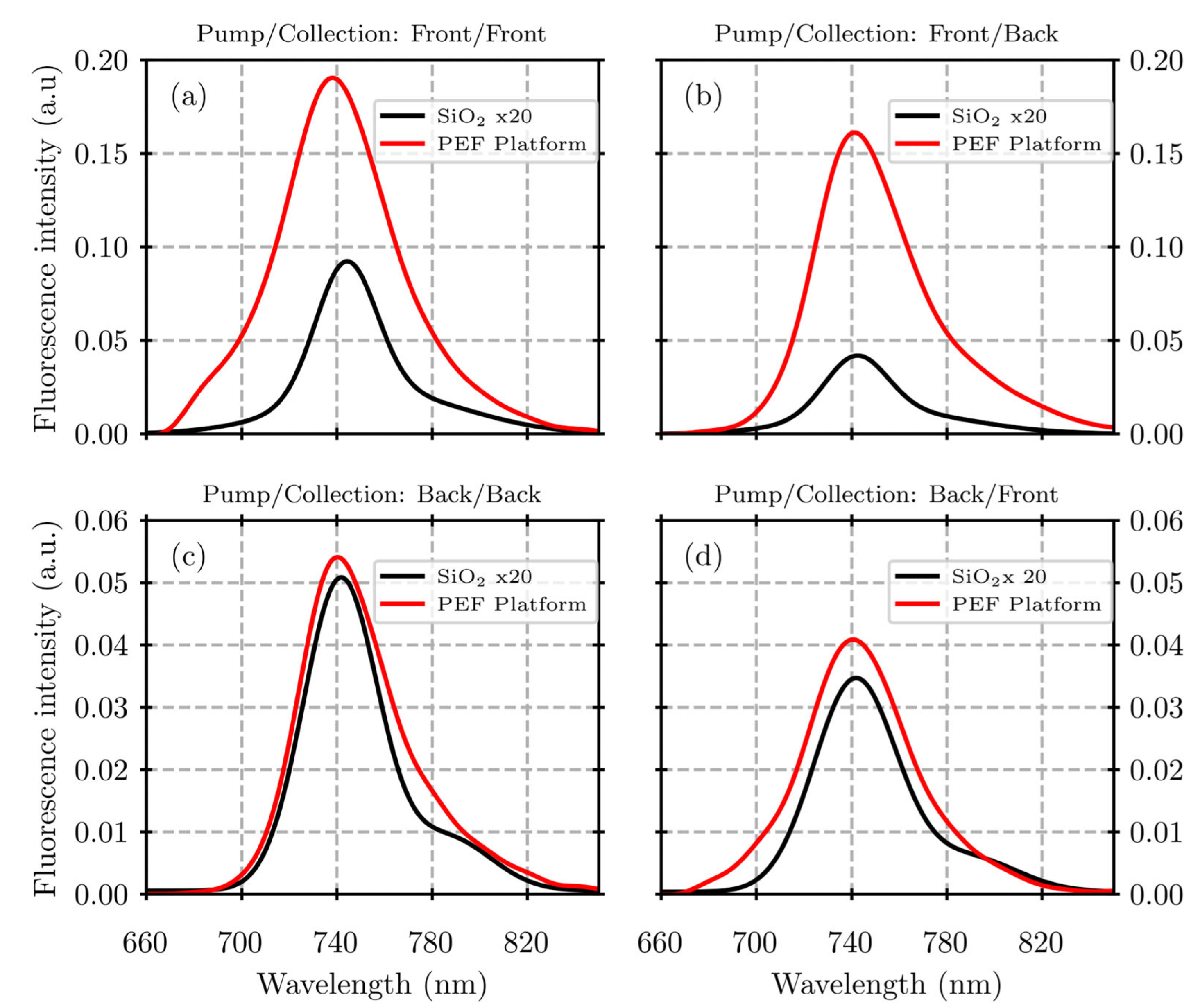
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
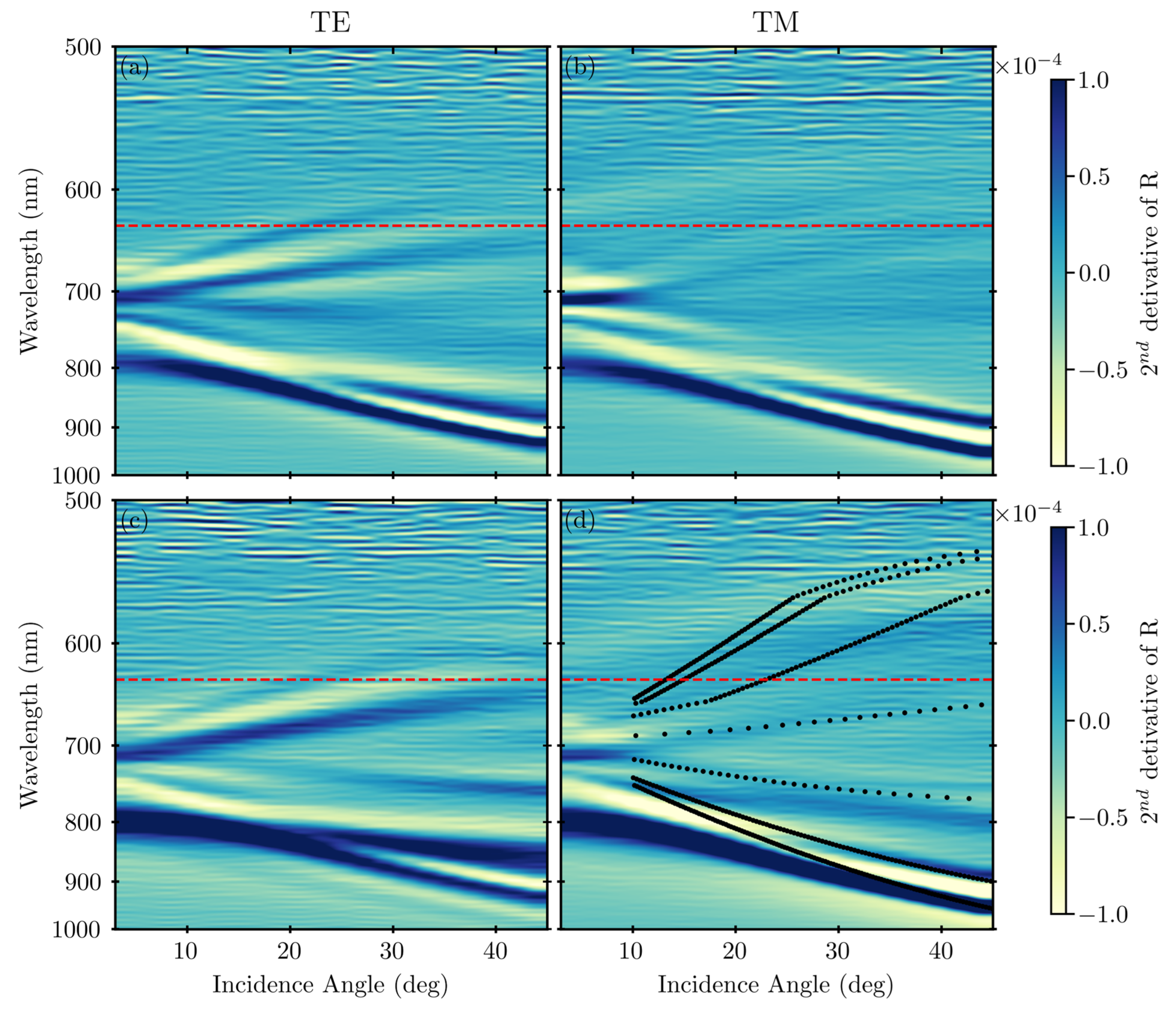
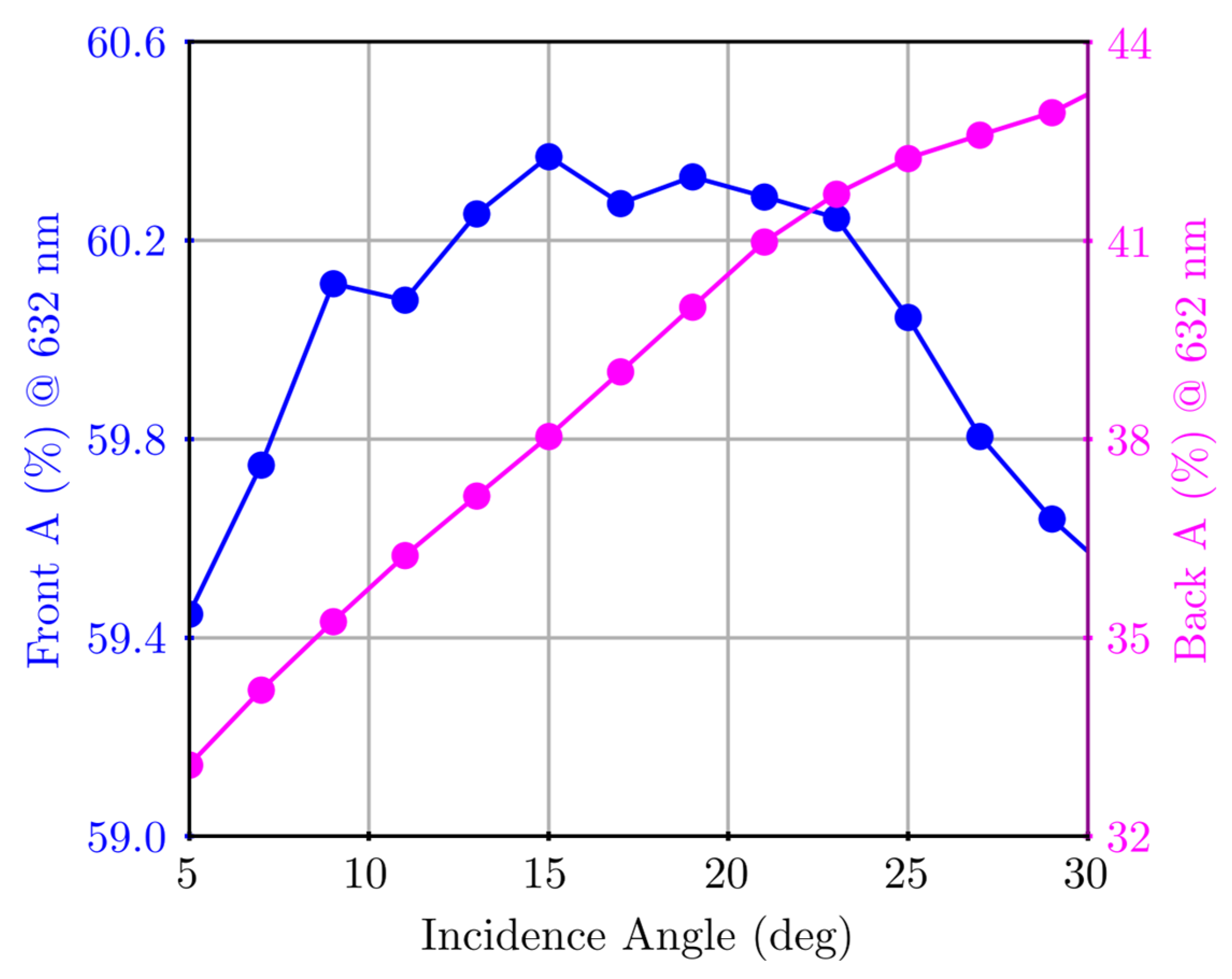
Appendix A.1. Surface Plasmon Polariton Dispersion
Appendix A.2. Variable-Angle Spectra
References
- Jack George Calvert; James N. Pitts Photochemistry; Wiley, 1966; Vol. 378;
- Chance, R.R.; Prock, A.; Silbey, R. Molecular Fluorescence and Energy Transfer Near Interfaces. In Advances in Chemical Physics; Prigogine, I., Rice, S.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1978. [Google Scholar]
- Barnes, W.L. Fluorescence near Interfaces: The Role of Photonic Mode Density. 39.
- Ford, G.W.; Weber, W.H. Electromagnetic Interactions of Molecules with Metal Surfaces. Physics Reports 1984, 113, 195–287. [Google Scholar] [CrossRef]
- Kitson, S.C.; Barnes, W.L.; Sambles, J.R. Surface-Plasmon Energy Gaps and Photoluminescence. Phys. Rev. B 1995, 52, 11441–11445. [Google Scholar] [CrossRef]
- Liebermann, T.; Knoll, W. Surface-Plasmon Field-Enhanced Fluorescence Spectroscopy. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2000, 171, 115–130. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Radiative Decay Engineering: Biophysical and Biomedical Applications. Analytical Biochemistry 2001, 298, 1–24. [Google Scholar] [CrossRef]
- Geddes, C.D.; Lakowicz, R. Metal-Enhanced Fluorescence. J. Fluoresc. 2002, 15, 124–129. [Google Scholar]
- Lakowicz, J.R. Radiative Decay Engineering 5: Metal-Enhanced Fluorescence and Plasmon Emission. Analytical Biochemistry 2005, 337, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Fort, E.; Grésillon, S. Surface Enhanced Fluorescence. J. Phys. D: Appl. Phys. 2008, 41, 013001. [Google Scholar] [CrossRef]
- Bauch, M.; Toma, K.; Toma, M.; Zhang, Q.; Dostalek, J. Plasmon-Enhanced Fluorescence Biosensors: A Review. 2014, 19.
- Osorio-Román, I.O.; Guerrero, A.R.; Albella, P.; Aroca, R.F. Plasmon Enhanced Fluorescence with Aggregated Shell-Isolated Nanoparticles. Anal. Chem. 2014, 86, 10246–10251. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhang, Z.; Zheng, H.; Sun, M. Recent Progress on Plasmon-Enhanced Fluorescence. Nanophotonics 2015, 4, 472–490. [Google Scholar] [CrossRef]
- Li, J.-F.; Li, C.-Y.; Aroca, R.F. Plasmon-Enhanced Fluorescence Spectroscopy. Chem. Soc. Rev. 2017, 46, 3962–3979. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Kook, Y.-M.; Lee, K.; Koh, W.-G. Metal Enhanced Fluorescence (MEF) for Biosensors: General Approaches and a Review of Recent Developments. Biosensors and Bioelectronics 2018, 111, 102–116. [Google Scholar] [CrossRef]
- Liu, Y.; Blair, S. Fluorescence Enhancement from an Array of Subwavelength Metal Apertures. Opt. Lett. 2003, 28, 507. [Google Scholar] [CrossRef]
- Andrew, P.; Barnes, W.L. Molecular Fluorescence above Metallic Gratings. Phys. Rev. B 2001, 64, 125405. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, H.-Y.; Wang, H.; Gao, B.-R.; Hao, Y.; Jin, Y.; Chen, Q.-D.; Sun, H.-B. Surface Plasmon Enhanced Fluorescence of Dye Molecules on Metal Grating Films. J. Phys. Chem. C 2011, 115, 12636–12642. [Google Scholar] [CrossRef]
- Garrett *, S.H.; Smith, L.H.; Barnes, W.L. Fluorescence in the Presence of Metallic Hole Arrays. Journal of Modern Optics 2005, 52, 1105–1122. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Ray, K.; Chowdhury, M.; Szmacinski, H.; Fu, Y.; Zhang, J.; Nowaczyk, K. Plasmon-Controlled Fluorescence: A New Paradigm in Fluorescence Spectroscopy. Analyst 2008, 133, 1308. [Google Scholar] [CrossRef]
- Cui, X.; Tawa, K.; Hori, H.; Nishii, J. Tailored Plasmonic Gratings for Enhanced Fluorescence Detection and Microscopic Imaging. Adv. Funct. Mater. 2010, 20, 546–553. [Google Scholar] [CrossRef]
- Cui, X.; Tawa, K.; Kintaka, K.; Nishii, J. Enhanced Fluorescence Microscopic Imaging by Plasmonic Nanostructures: From a 1D Grating to a 2D Nanohole Array. Adv Funct Materials 2010, 20, 945–950. [Google Scholar] [CrossRef]
- Langguth, L.; Punj, D.; Wenger, J.; Koenderink, A.F. Plasmonic Band Structure Controls Single-Molecule Fluorescence. ACS Nano 2013, 7, 8840–8848. [Google Scholar] [CrossRef]
- Chan, K.F.; Hui, K.C.; Li, J.; Fok, C.H.; Ong, H.C. Roles of Surface Plasmon Polaritons in Fluorescence Enhancement. In Surface Plasmon Enhanced, Coupled and Controlled Fluorescence; Geddes, C.D., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 91–109. ISBN 978-1-119-32516-1. [Google Scholar]
- Min, Jouha; Son, Taehwang; Hong, Jae-Sang; Cheah, Pike See; Wegemann, Andreas; Murlidharan, Koushik; Weissleder, Ralph; Lee, Hakho; Im, Hyungsoon Plasmon-Enhanced Biosensing for Multiplexed Profiling of Extracellular Vesicles. Adv. Biosys. 2020, 4, 2000003. [CrossRef]
- Izumi, S.; Yamamura, S.; Hayashi, N.; Toma, M.; Tawa, K. Dual-Color Fluorescence Imaging of EpCAM and EGFR in Breast Cancer Cells with a Bull’s Eye-Type Plasmonic Chip. 2017, 10.
- Zhang, Q.; Wu, L.; Wong, T.I.; Zhang, J.; Liu, X.; Zhou, X.; Bai, P.; Liedberg, B.; Wang, Y. Surface Plasmon-Enhanced Fluorescence on Au Nanohole Array for Prostate-Specific Antigen Detection. IJN 2017, Volume 12, 2307–2314. [Google Scholar] [CrossRef]
- Semeniak, D.; Cruz, D.F.; Chilkoti, A.; Mikkelsen, M.H. Plasmonic Fluorescence Enhancement in Diagnostics for Clinical Tests at Point-of-Care: A Review of Recent Technologies. Advanced Materials 2023, 35, 2107986. [Google Scholar] [CrossRef]
- Giudicatti, S.; Valsesia, A.; Marabelli, F.; Colpo, P.; Rossi, F. Plasmonic Resonances in Nanostructured Gold/Polymer Surfaces by Colloidal Lithography: Plasmonic Resonances in Nanostructured Gold/Polymer Surfaces. phys. stat. sol. (a) 2010, 207, 935–942. [Google Scholar] [CrossRef]
- Giudicatti, S.; Marabelli, F.; Valsesia, A.; Pellacani, P.; Colpo, P.; Rossi, F. Interaction among Plasmonic Resonances in a Gold Film Embedding a Two-Dimensional Array of Polymeric Nanopillars. J. Opt. Soc. Am. B 2012, 29, 1641. [Google Scholar] [CrossRef]
- Bottazzi, B.; Fornasari, L.; Frangolho, A.; Giudicatti, S.; Mantovani, A.; Marabelli, F.; Marchesini, G.; Pellacani, P.; Therisod, R.; Valsesia, A. Multiplexed Label-Free Optical Biosensor for Medical Diagnostics. J. Biomed. Opt 2014, 19, 017006. [Google Scholar] [CrossRef] [PubMed]
- Available online : Https://H-Alo.Eu/ (accessed on 30 January 2024).
- Angelini, M.; Manobianco, E.; Pellacani, P.; Floris, F.; Marabelli, F. Plasmonic Modes and Fluorescence Enhancement Coupling Mechanism: A Case with a Nanostructured Grating. Nanomaterials 2022, 12, 4339. [Google Scholar] [CrossRef] [PubMed]
- Angelini, M.; Manobianco, E.; Pellacani, P.; Floris, F.; Marabelli, F. Refractive Index Dependence of Fluorescence Enhancement in a Nanostructured Plasmonic Grating. Materials 2023, 16, 1289. [Google Scholar] [CrossRef] [PubMed]
- Glinel, K.; Moussa, A.; Jonas, A.M.; Laschewsky, A. Influence of Polyelectrolyte Charge Density on the Formation of Multilayers of Strong Polyelectrolytes at Low Ionic Strength. Langmuir 2002, 18, 1408–1412. [Google Scholar] [CrossRef]
- Nicol, E.; Habib-Jiwan, J.-L.; Jonas, A.M. Polyelectrolyte Multilayers as Nanocontainers for Functional Hydrophilic Molecules. Langmuir 2003, 19, 6178–6186. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




