Submitted:
18 March 2024
Posted:
19 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Synthesis of Co3O4 Nanoparticles by Solution Combustion Method
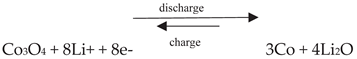

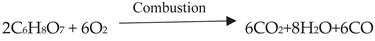

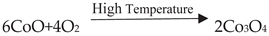

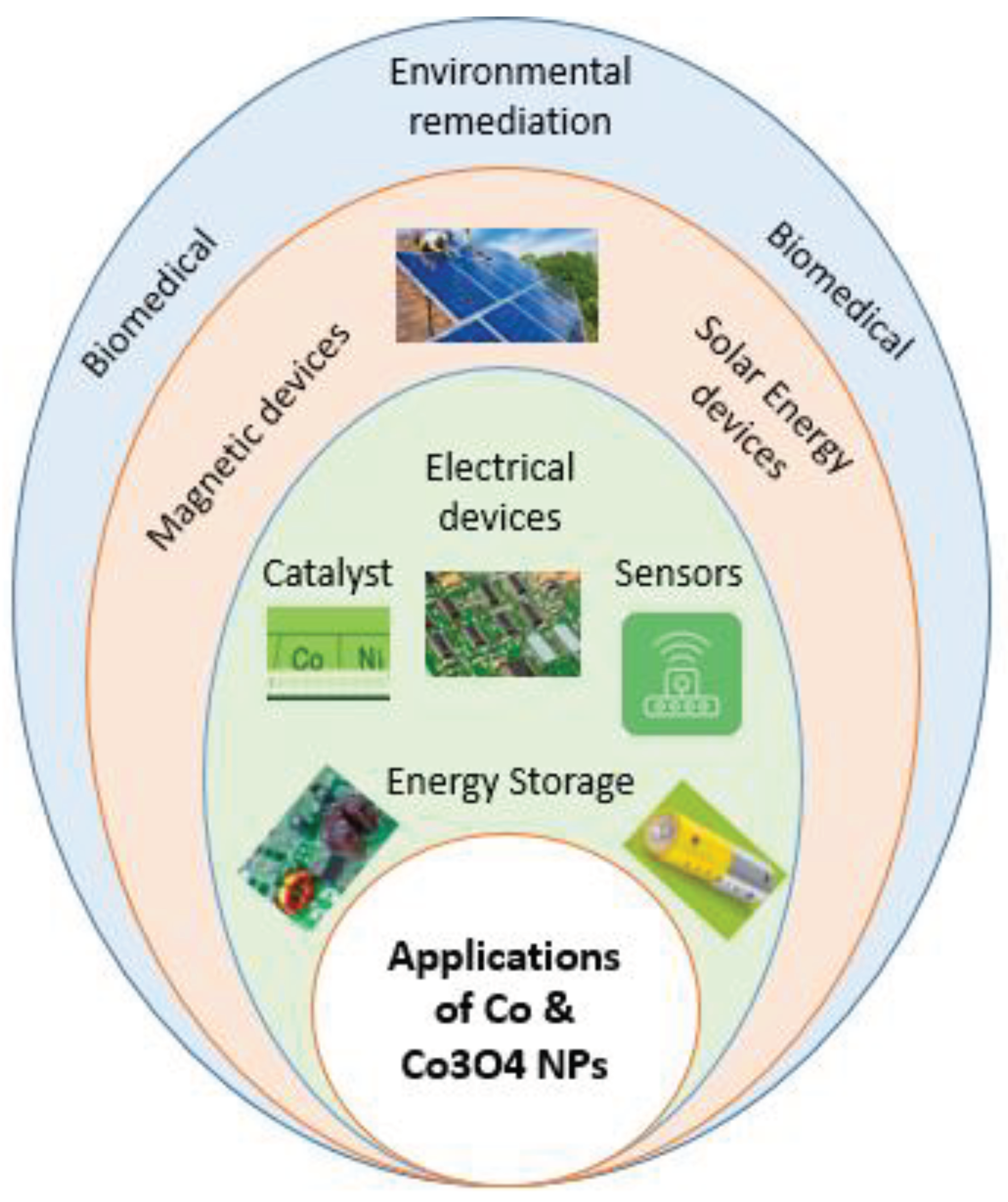
3. Application
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Abu-Zied, B.M.; Alam, M.M.; Asiri, A.M.; Ahmed, J.; Rahman, M.M. Efficient hydroquinone sensor development based on Co3O4 nanoparticle. Microchem. J. 2020, 157, 104972. [Google Scholar] [CrossRef]
- Acedera, R.A.E.; Gupta, G.; Mamlouk, M.; Balela, M.D.L. Solution combustion synthesis of porous Co3O4 nanoparticles as oxygen evolution reaction (OER) electrocatalysts in alkaline medium. J. Alloys Compd. 2020, 836, 154919. [Google Scholar] [CrossRef]
- Afrooze, A.; Shaik, D. P. Porous Co3O4 nanospheres synthesized via solution combustion method for supercapacitors. Chem. Papers 2023, 77, 1201–1211. [Google Scholar]
- Afrooze, A.; Shaik, D.P.M.D.; Xu, J. Marigold flower-like Co3O4 nanoparticles as high-performance electrodes for supercapacitors. Inorg. Chem. Commun. 2024, 159, 111663. [Google Scholar] [CrossRef]
- Al Jahdaly, B.A.; Abu-Rayyan, A.; Taher, M.M.; Shoueir, K. Phytosynthesis of Co3O4 nanoparticles as the high-energy storage material of an activated carbon/Co3O4 symmetric supercapacitor device with excellent cyclic stability based on a Na2SO4 aqueous electrolyte. ACS Omega 2022, 7, 23673–23684. [Google Scholar] [CrossRef]
- Aldashukurova, G.B.; Mironenko, A.V.; Mansurov, Z.A.; Shikina, N.V.; Yashnik, S.A.; Kuznetsov, V.V.; Ismagilov, Z.R. Synthesis gas production on glass cloth catalysts modified by Ni and Co oxides. J. Energy Chem. 2013, 22, 811–818. [Google Scholar] [CrossRef]
- Alem, A.F.; Worku, A.K.; Ayele, D.W.; Wubieneh, T.A.; Teshager, A.A.; Admasu, B.T.; Teshager, M.A.; Asege, A.A.; Ambaw, M.D.; Zeleke, M.A.; Shibesh, A.K. Ag-doped Co3O4 nanoparticles for high-performance supercapacitor application. Heliyon 2023, 9, e13286. [Google Scholar] [PubMed]
- Anele, A.; Obare, S.; Wei, J. Recent trends and advances of Co3O4 nanoparticles in the environmental remediation of bacteria in wastewater. Nanomaterials 2022, 12, 1129. [Google Scholar] [CrossRef] [PubMed]
- Ashok, A.; Kumar, A.; Tarlochan, F. Surface Alloying in Silver-Cobalt through a Second Wave Solution Combustion Synthesis Technique. Nanomaterials 2018, 8, 604. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Kang, L.; Bai, G.; Li, Y.; Li, P.; Liu, X.; Yang, Y.; Gao, F.; Liang, W. Solution combustion synthesis of cobalt oxides (Co3O4 and Co3O4/CoO) nanoparticles as supercapacitor electrode materials. Electrochim. Acta 2014, 132, 127–135. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Sethuraman, M.G.; Lee, Y.R. Supercapacitor Performance of Carbon Supported Co3O4 nanoparticles Synthesized Using Terminalia Chebula Fruit. J. Taiwan Inst. Chem. Eng. 2016, 68, 489–495. [Google Scholar] [CrossRef]
- El-Shafie, A.S.; Ahsan, I.; Radhwani, M.; Al-Khangi, M.A.; El-Azazy, M. Synthesis and application of cobalt oxide (Co3O4)-impregnated olive stones biochar for the removal of rifampicin and tigecycline: multivariate controlled performance. Nanomater. 2022, 12, 379. [Google Scholar] [CrossRef]
- Farhadi, S.; Javanmard, M.; Nadri, G. Characterization of cobalt oxide nanoparticles prepared by the thermal decomposition of Co(NH3)5(H2O)3 complex and study of their photocatalytic activity. ACS Sustainable Chem. Eng. 2016, 4, 5915–5923. [Google Scholar]
- Gopalakrishnan, M.; Srikesh, G.; Mohan, A.; Arivazhagan, V. In-Situ Synthesis of Co3O4/Graphite nanocomposite for High-Performance Supercapacitor Electrode Applications. Appl. Surf. Sci. 2017, 403, 578–583. [Google Scholar] [CrossRef]
- Gyulasaryan, H.; Kuzanyan, A.; Manukyan, A.; Mukasyan, A.S. Combustion Synthesis of Magnetic Nanomaterials for Biomedical Applications. Nanomater. 2023, 13, 1902. [Google Scholar] [CrossRef]
- Hou, C.; Wang, B.; Murugadoss, V.; Vupputuri, S.; Chao, Y.; Guo, Z.; Wang, C.; Du, W. Recent advances in Co3O4 as anode materials for high-performance lithium-ion batteries. Eng. Sci. 2020, 11, 19–30. [Google Scholar] [CrossRef]
- Jahani, M.; Khavari-Nejad, R.A.; Mahmoodzadeh, H.; Saadatmand, S. Effects of cobalt oxide nanoparticles (Co3O4 NPs) on ion leakage, total phenol, antioxidant enzymes activities, and cobalt accumulation in Brassica napus L. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1260–1275. [Google Scholar] [CrossRef]
- Kalyani, M.; Emerson, R.N. High-performance supercapacitor cobalt oxide thin films by the influence of copper substrate. Int. J. Pure Appl. Phys. 2018, 14, 115–124. [Google Scholar]
- Kazemi, S.H.; Asghari, A.; Kiani, M.A. High-Performance Supercapacitors Based on the Electrodeposited Co3O4 nanoflakes on Electro-Etched Carbon Fibers. Electrochim. Acta 2014, 138, 9–14. [Google Scholar] [CrossRef]
- Keneshbekova, А.; Imash, A.; Kaidar, B.; Yensep, E.; Ilyanov, A.; Artykbayeva, M.; Prikhodko, N.; Smagulova, G. Morphological features of Co3O4 nanoparticles obtained by solution combustion method.
- Kumar, G.P.; Jawahar, I.N.; Biju, V. Synthesis of nanocrystalline Co3O4 through solution combustion method: effect of fuel to oxidizer ratio on structural and physical properties. J. Mater. Sci.: Mater. Electron. 2021, 32, 14919–14931. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.; Shioyama, H.; Mukai, T.; Sakai, T.; Xu, Q. Converting cobalt oxide subunits in cobalt metal-organic framework into agglomerated Co3O4 nanoparticles as an electrode material for lithium-ion battery. J. Power Sources 2010, 195, 857–861. [Google Scholar] [CrossRef]
- Liu, F.; Su, H.; Jin, L.; Zhang, H.; Chu, X.; Yang, W. Facile synthesis of ultrafine cobalt oxide nanoparticles for high-performance supercapacitors. J. Colloid Interface Sci. 2017, 505, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, Z.H.; Jarosova, M.; Kzar, H.H.; Machek, P.; Zaidi, M.; Dehno Khalaji, A.; Khlewee, I.H.; Altimari, U.S.; Mustafa, Y.F.; Kadhim, M.M. Synthesis and characterization of Co3O4 nanoparticles: application as performing anode in Li-ion batteries. J. Chin. Chem. Soc. 2022, 69, 657–662. [Google Scholar] [CrossRef]
- Manteghi, F.; Kazemi, S.H.; Peyvandipour, M.; Asghari, A. Preparation and application of cobalt oxide nanostructures as electrode materials for electrochemical supercapacitors. RSC Adv. 2015, 5, 76458–76463. [Google Scholar] [CrossRef]
- Maurya, M.P.; Raitani, K.; Gyanprakash, M.; Rastogi, C.K.; Krishna, R.H.; Manjunatha, C. The copper interlayer tunes the reactivity of cobalt oxide towards the oxygen evolution reaction. Mater. Today: Proc. 2023, 79, 193–197. [Google Scholar] [CrossRef]
- Mironenko, A.V.; Mansurov, Z.A.; Shikina, N.V.; Yashnik, S.A.; Kuznetsov, V.V.; Ismagilov, Z.R.; Aldashukurova, G.B. Synthesis gas production on glass cloth catalysts modified by Ni and Co oxides. Adv. Powder Technol. 2014.
- Michalska, H.; Xu, Q.; Shan, Q.; Zhang, S.; Dall'Agnese, Y.; Gao, Y.; Jain, A.; Krajewski, M. Solution combustion synthesis of a nanometer-scale Co3O4 anode material for Li-ion batteries. Beilstein J. Nanotechnol. 2021, 12, 424–431. [Google Scholar] [CrossRef]
- Murayama, M.; Kobayashi, N.; Matsushima, Y.; Unuma, H. Low-temperature synthesis of porous Co3O4 thin films through two approaches: metal-organic framework-decomposition and solution combustion methods.
- Niveditha, C.V.; Aswini, R.; Jabeen Fatima, M.J.; Ramanarayan, R.; Pullanjiyot, N.; Swaminathan, S. Feather-like highly active Co3O4 electrode for supercapacitor application: A potentiodynamic approach. Mater. Res. Express 2018, 5, 11–18. [Google Scholar] [CrossRef]
- Pagar, T.; Ghotekar, S.; Pagar, K.; Pansambal, S.; Oza, R. A review on bio-synthesized Co3O4 nanoparticles using plant extracts and their diverse applications. J. Chem. Rev. 2019, 1, 260–270. [Google Scholar]
- Sahoo, P.; Djieutedjeu, H.; Poudeu, P.F.P. Co3O4 nanostructures: The effect of synthesis conditions on particle size, magnetism, and transport properties. J. Mater. Chem. A 2013, 1, 15022–15030. [Google Scholar] [CrossRef]
- Singhal, A.; Bisht, A.; Kumar, A.; Sharma, S. One-pot, rapid synthesis of Co3O4 by solution combustion method and its electrochemical properties in different electrolytes. J. Electroanal. Chem. 2016, 776, 152–161. [Google Scholar] [CrossRef]
- Sisakyan, N.; Chilingaryan, G.; Manukyan, A.; Mukasyan, A.S. Combustion Synthesis of Materials for Application in Supercapacitors: A Review. Nanomaterials 2023, 13, 3030. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Chen, M.D.; Li, G.Q.; Teng, Y.; Xu, T.G.; Hang, Y.C.; Zhu, Y.F. High combustion activity of CH4 and catalluminescence properties of CO oxidation over porous Co3O4 nanorods. Appl. Catal. B: Environ. 2011, 110, 133–140. [Google Scholar] [CrossRef]
- Trivedi, S.; Prasad, R. Reactive calcination route for synthesis of active Mn- Co3O4 spinel catalysts for abatement of CO-CH4 emissions from CNG vehicles. J. Environ. Chem. Eng. 2016, 4, 1017–1028. [Google Scholar] [CrossRef]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution combustion synthesis of nanoscale materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef]
- Vennela, A.B.; Mangalaraj, D.; Muthukumarasamy, N.; Agilan, S.; Hemalatha, K.V. Structural and optical properties of Co3O4 nanoparticles prepared by sol-gel technique for photocatalytic application. Int. J. Electrochem. Sci. 2019, 14, 3535–3552. [Google Scholar] [CrossRef]
- Wang, S.B.; Zhao, C.C.; Li, S.G.; Sun, Y.H. First principles prediction of CH4 reactivities with Co3O4 nanocatalysts of different morphologies. Phys. Chem. Chem. Phys. 2017, 19, 30874–30882. [Google Scholar] [CrossRef]
- Wang, X.; Mou, H.; Zhao, Q.; Sun, Y.; Zeng, Z.; Zhao, H.; An, B.; Xu, J. Carbon Monoxide Combustion on Metal Oxide Supported Au@CuxO Catalysts at Low Temperature. Combust. Sci. Technol. 2023, 193, 2505–2513. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, X.; Shen, J.; Li, T. Supported cobalt oxide nanocrystals: Morphology control and catalytic performance for styrene oxidation. RSC Adv. 2016, 6, 89503–89509. [Google Scholar] [CrossRef]
- Waris, A.; Din, M.; Ali, A.; Afridi, S.; Baset, A.; Khan, A.U.; Ali, M. Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review. Open Life Sci. 2021, 16, 14–30. [Google Scholar] [CrossRef]
- Wei, X.; Kang, J.; Gan, L.; Wang, W.; Yang, L.; Wang, D.; Zhong, R.; Qi, J. Recent Advances in Co3O4-Based Composites: Synthesis and Application in Combustion of Methane. Nanomaterials 2023, 13, 1917. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, S.; Ercolino, G.; Gajewska, M.; Moncada Quintero, C.W.; Specchia, S.; Kotarba, A. Robust Co3O4|α-Al2O3|cordierite structured catalyst for N2O abatement – Validation of the SCS method for active phase synthesis and deposition. Chem. Eng. J. 2019, 377, 120088. [Google Scholar] [CrossRef]
- Yu, J.; Ni, Y.; Zhai, M. Highly selective non-enzyme glucose detection based on Co-CoO- Co3O4 nanocomposites prepared via a solution-combustion and subsequent heat-treating route. J. Alloys Compd. 2017, 723, 904–911. [Google Scholar] [CrossRef]
- Zallouz, S. Co3O4 nanoparticles embedded in mesoporous carbon for supercapacitor applications. Master's Thesis, Université de Haute-Alsace, Amiens Cedex, France, 2021. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Li, L.; Shi, S.J.; Xiong, Q.Q.; Zhao, X.Y.; Wang, X.L.; Gu, C.D.; Tu, J.P. Synthesis of porous Co3O4 nanoflake array and its temperature behavior as pseudo-capacitor electrode. J. Power Sources 2014, 256, 200–205. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, C.; Gao, J.; Gui, J.; Deng, L.; Wang, Y.; Xu, R. Co3O4 nanoparticles embedded in laser-induced graphene for a flexible and highly sensitive enzyme-free glucose biosensor. Sens. Actuators B: Chem. 2021, 347, 130653. [Google Scholar] [CrossRef]
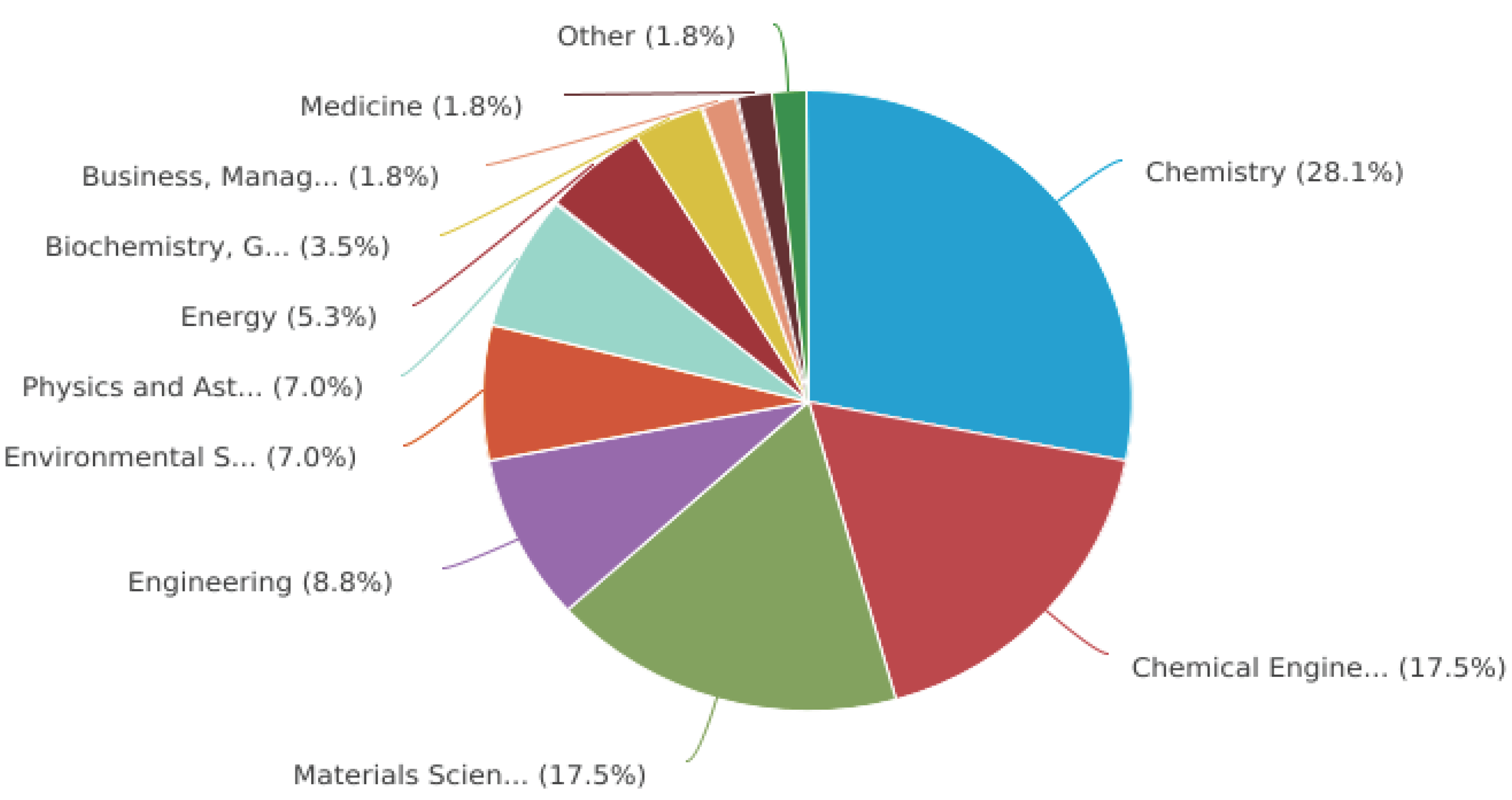
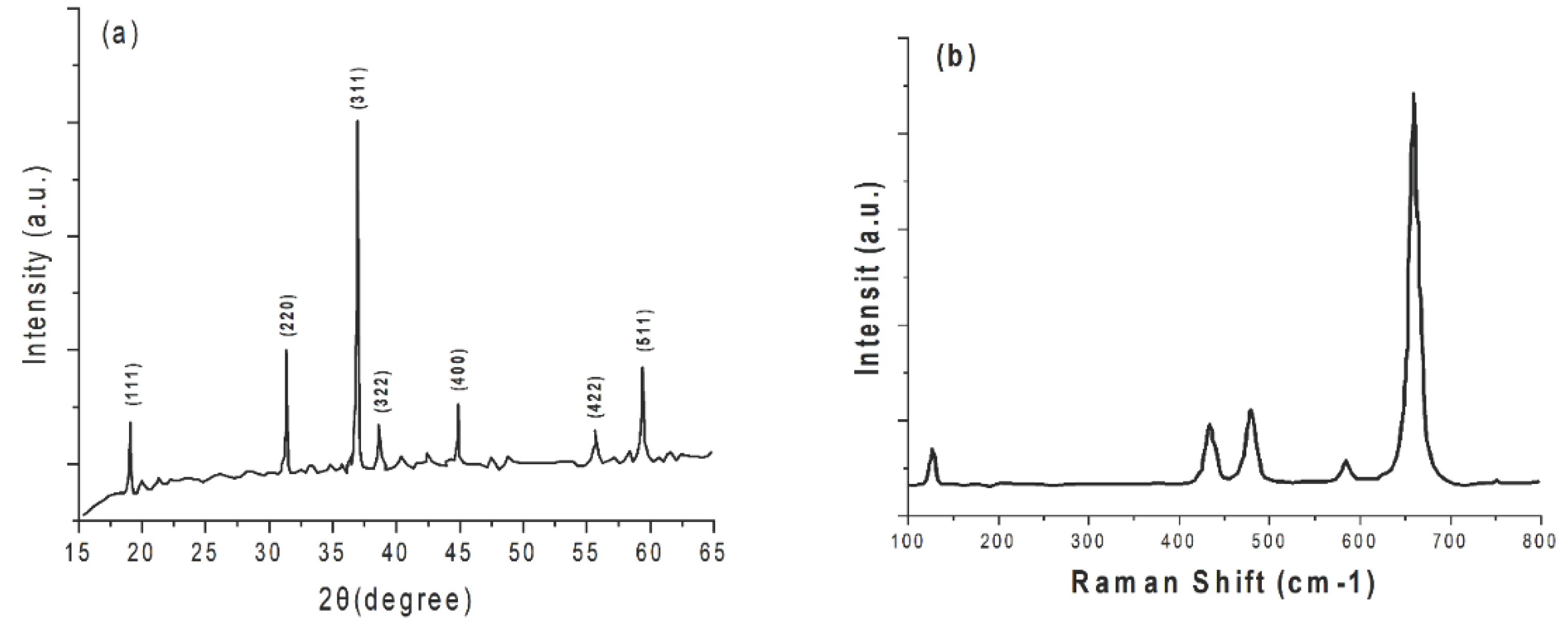
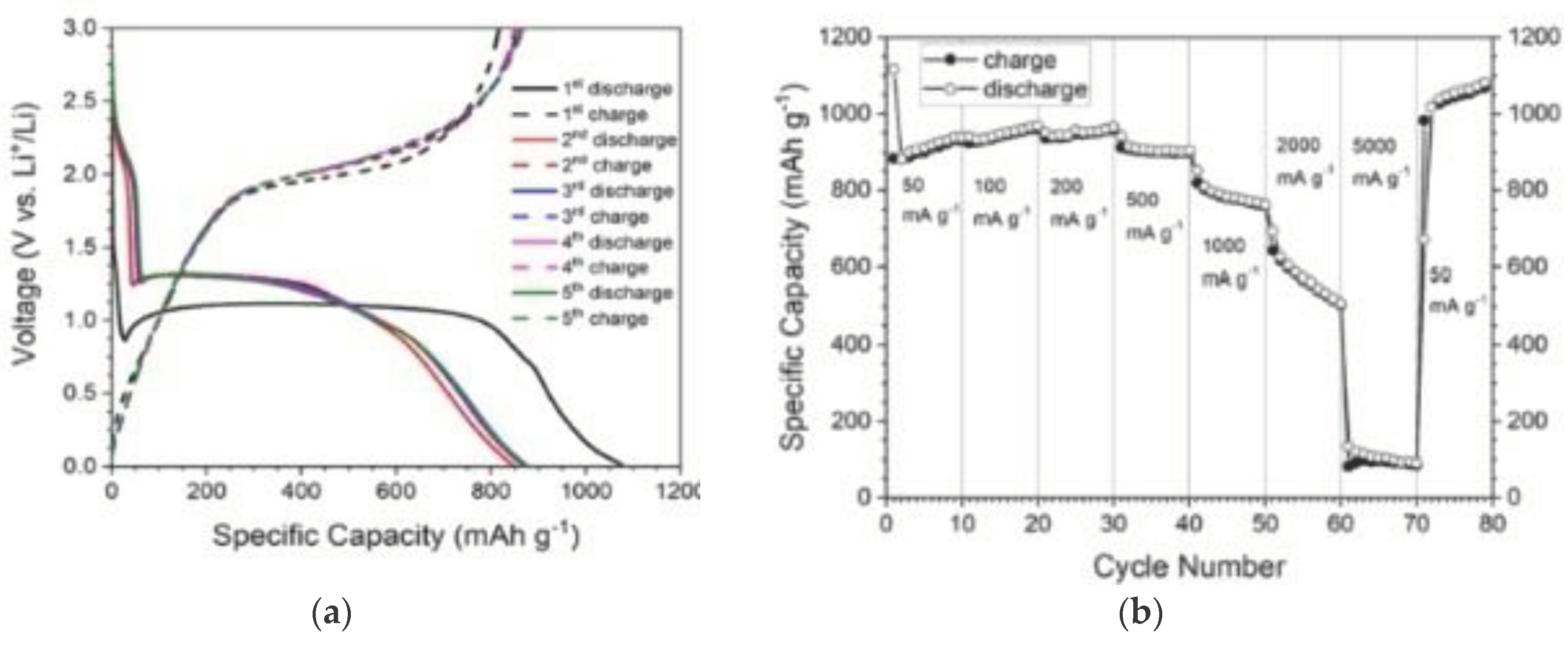
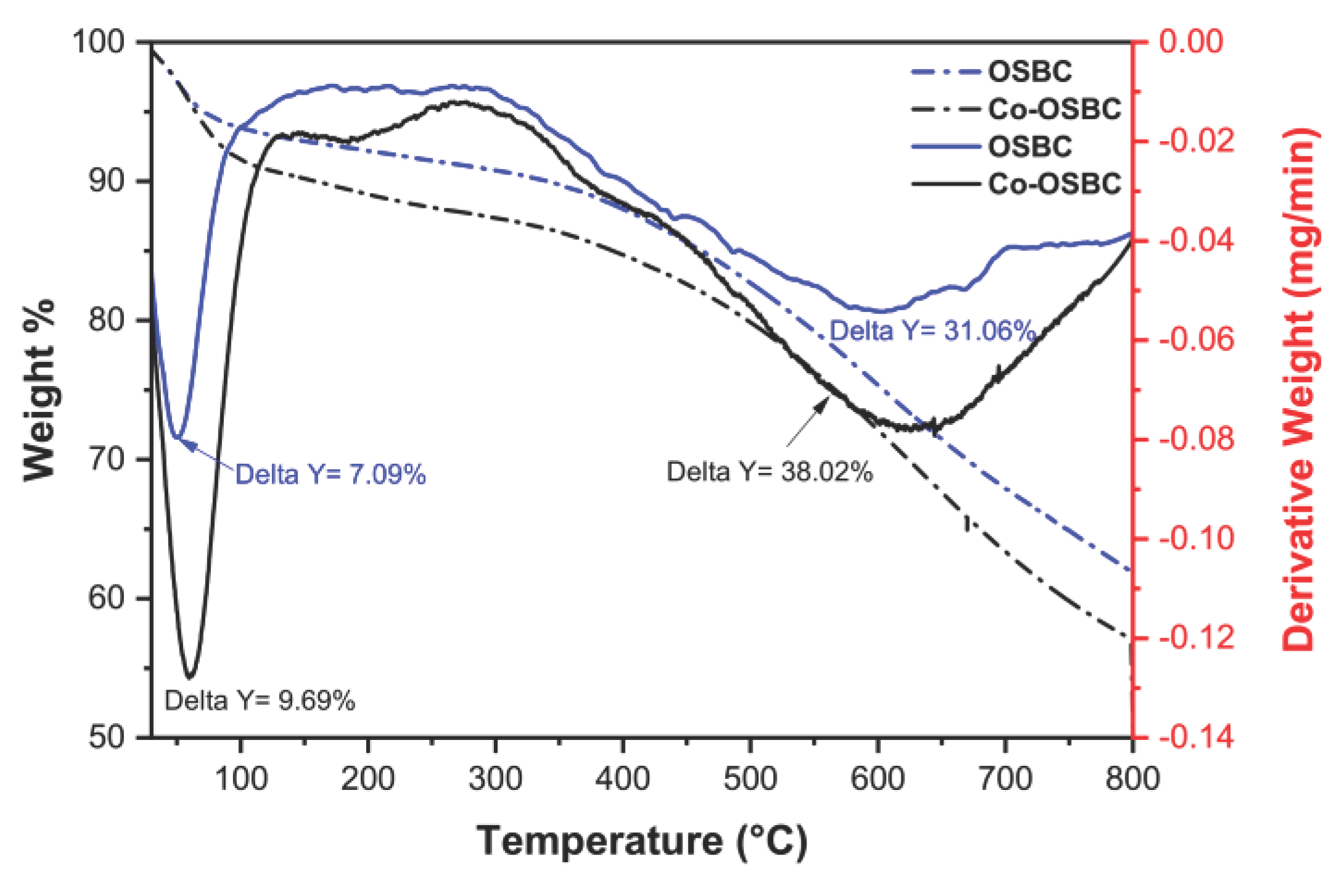
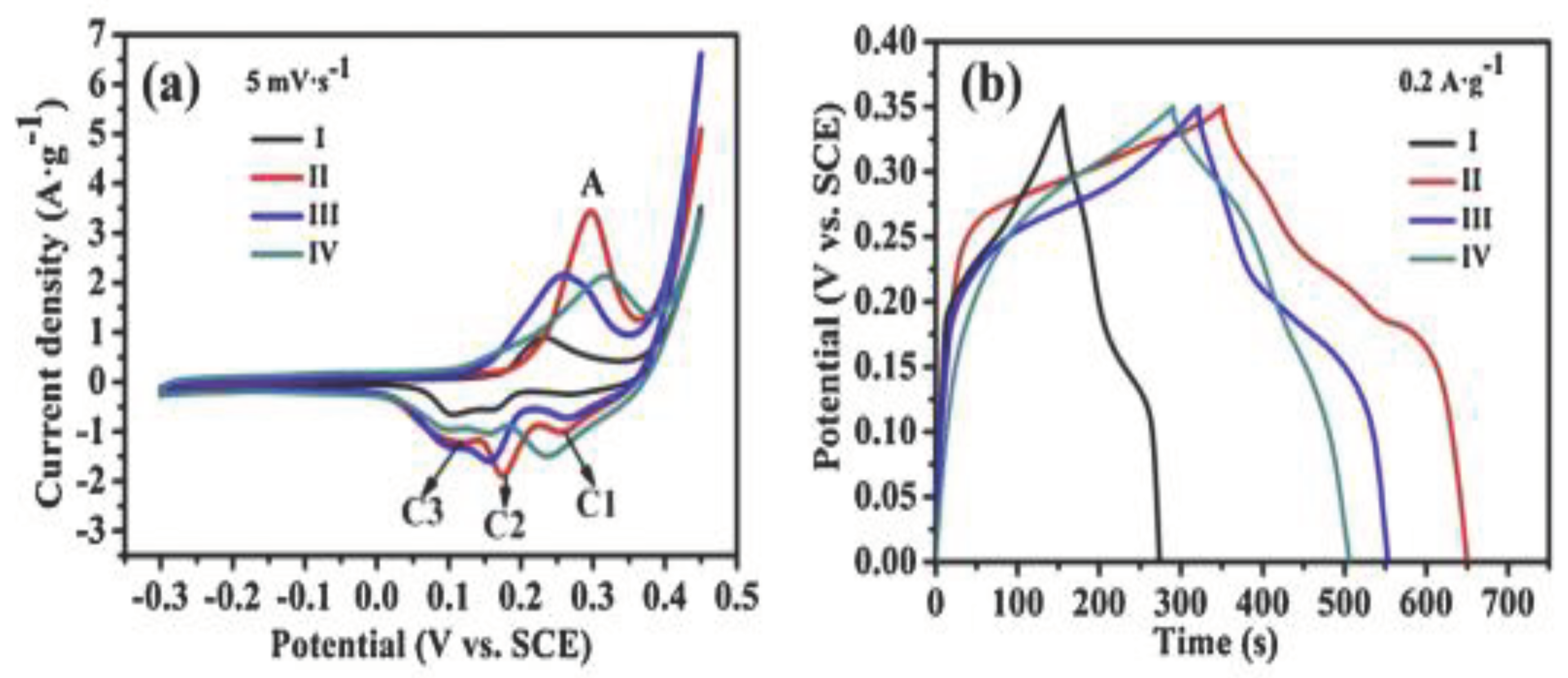
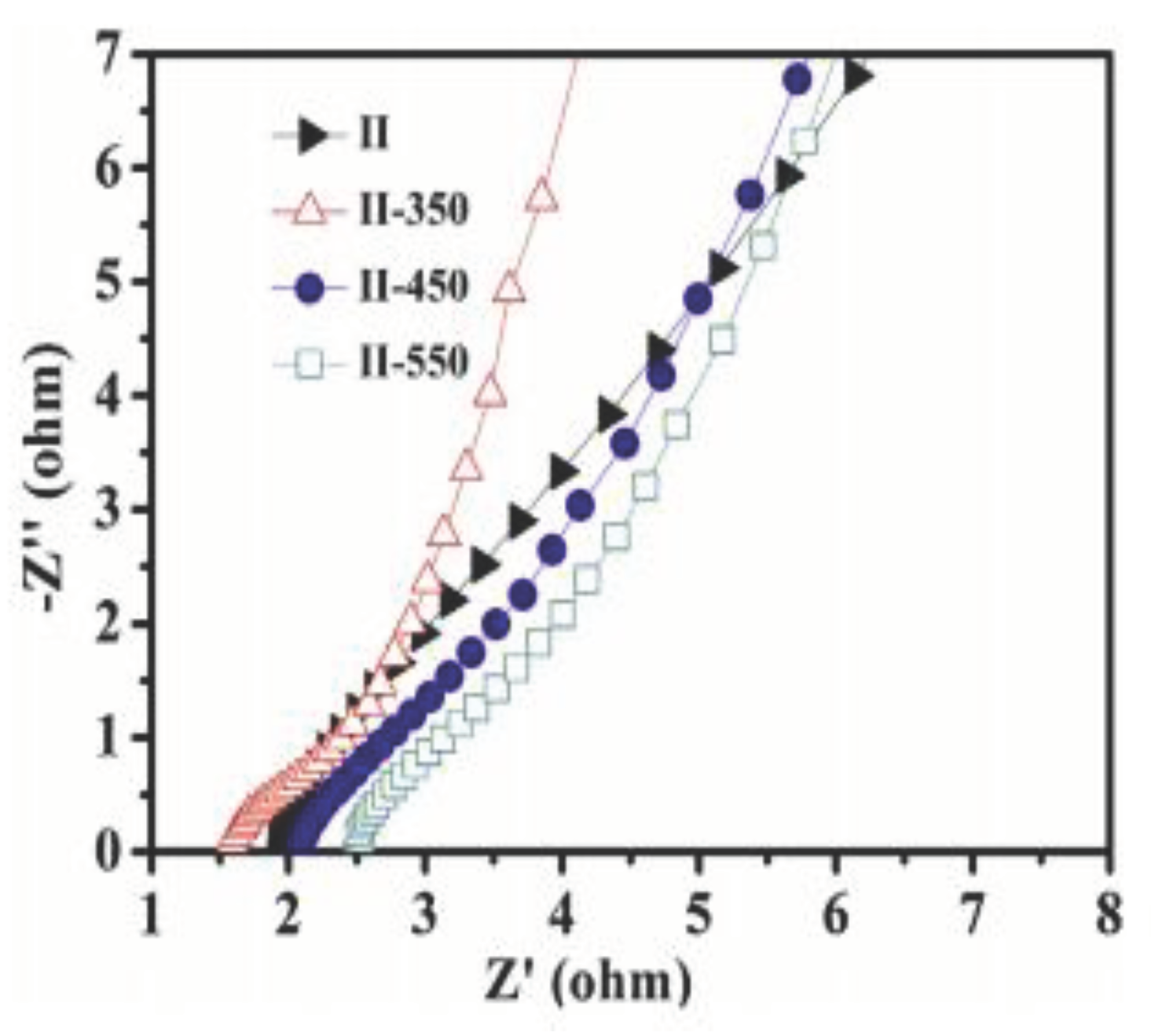
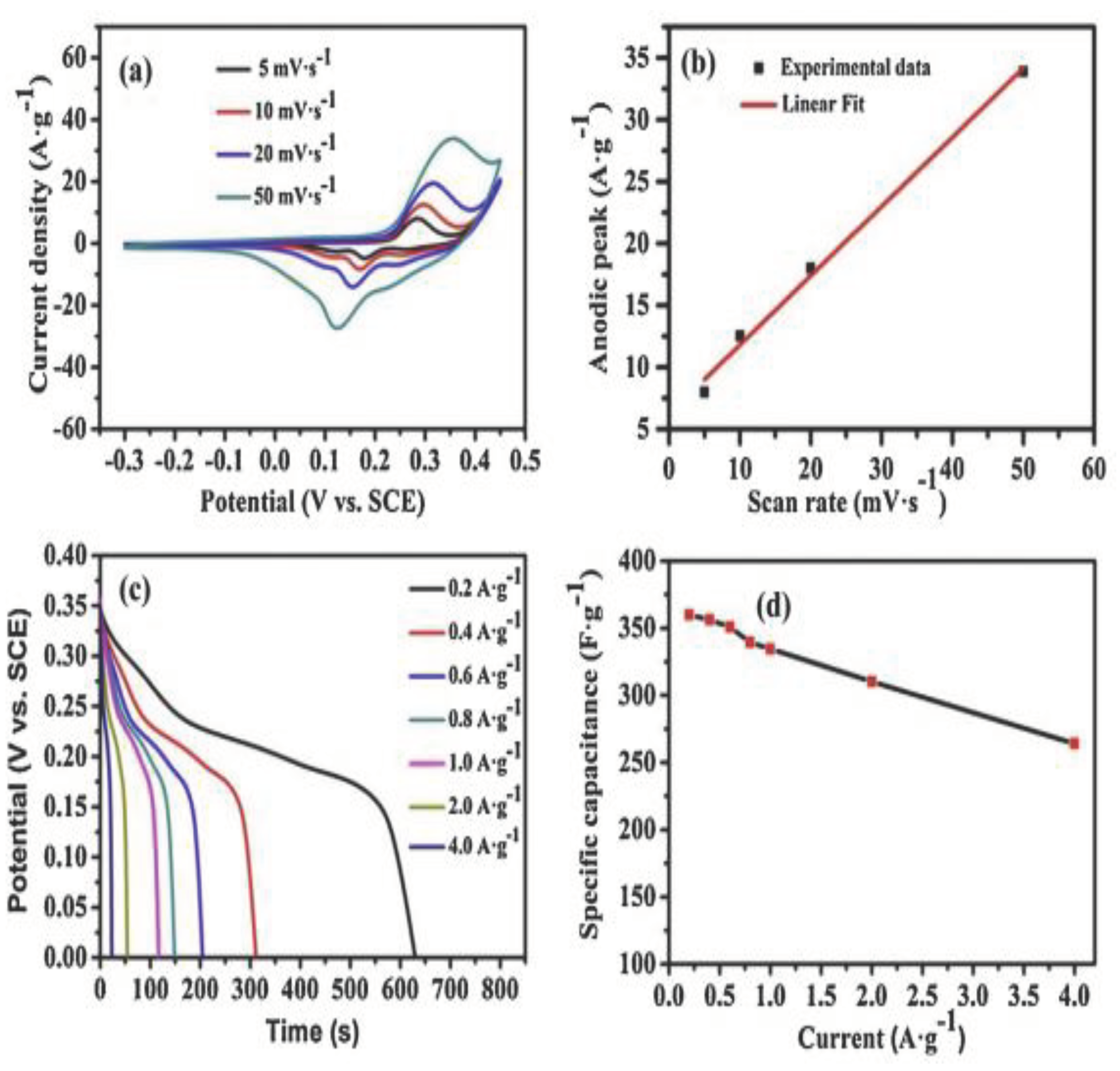
| No | Used precursors and fuel solution | Electrolyte | Specific capacitance, Fg-1 | Surface area/m2g-1 | Pure volume/cm3g-1 | TA, oC | Reaction T, oC | Particle size/diameter, nm | Proposed applications | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cobalt nitrate hexahydrate and 2-imidazolidinone hemihydrate (ethylenurea) |
500 | 26.0 | Sensitive sensors for the safety of environmental and healthcare | [1] | |||||
| 2 | (Co(NO3)2·6H2O) and glycine, NH2CH2COOH |
1M KOH | 10.45 | 25 | 300 | 13.1 | Best-performing electrode obtaining | [2] | ||
| 3 | Co(NO3)2⋅6H2O and urea, NH2CONH2 with 100 mL deionized water | 3M KOH | 212 | 69.34 | 0.0431 | 600 | 13.64 | High performance electrodes for supercapacitors | [4] | |
| 4 | (CoCl2. 6H2O), (AgNO3) and (NH3), in deionized water | 0.1M KOH | 992.7 | 407.33 | 0.1155 | 12.98 | Super capacitores applications | [7] | ||
| 5 | (CoCl2. 6H2O), (AgNO3) and (NH3), in deionized water | 0.1M KOH | 53.06 | 0.07425 | 19.37 | Super capacitores applications | [7] | |||
| 6 | (Co(CH3-CO2)2_6H2O) and citric acid monohydrate(C6H8O7·H2O),and ammonium nitrate (NH4NO3) were used as fuel, | 362.8 | 17.9 | 0.095 | 350 | 550 | 26.1 | Supercapacitors electrode materials | [10] | |
| 7 | 3M(Co(NO3)2∙6H2O), 6M glycine (C2H5NO2), 10% by weight of cobalt nitrate (nitric acid) and 50ml deionized water | 700 | 90 | 292.66 | 260 | 1200 | 20-65 | Gas sensors | [20] | |
| 8 | (Co(CH3-CO2)2 4H2O) and urea (CH4N2O) As fuel. |
500 | 70 | Catalysis and energy storage applications |
[21] | |||||
| 9 | (Co(NO3)2·6H2O) and (CO(NH2)2) as fuel | Alkaline | 5000 | 3 | 0.02 | 600 | 36 | As the anode material for Li-ion batteries | [28] | |
| 10 | (Co(NO3)2·6H2O) and methanol as feul | 1M KOH | 3560 | 500 | electrode for electrochemical applications | [29] | ||||
| 11 | 5g(Co(CH3- CO2)2_6H2O) and 1.72g urea (CH4N2O) As fuel. And 15 ml deionized water |
KOH | 400 | 900 | 50 | Active for oxygen evolution reaction (OER) |
[33] | |||
| 12 | Cobalt nitrate, urea as fuel and deionized water | 1.4 | 0.016 | 400 | 200 | In catalysts as coatings | [44] | |||
| 13 | (Cocl2 6H2O), D-glucose, fructose, maltose, sucrose. |
1M KOH | 600 | Non-enzyme glucose detection | [45] |
| No | Synthesis method | Raw materials | Catalyst | Temp, oC | BET, surface area m2g-1 | References |
|---|---|---|---|---|---|---|
| 1 | Co-precipitation method | (CoCl2. 6H2O), (AgNO3) and (NH3), in deionized water | single-cubic Co3O4 nanostructure Ag doped. | 407.33 | [7] | |
| 2 | Solution combustion | (Co(NO3)2·6H2O and urea ((NH2)2CO) | Co3O4 nanoparticles | 300-800 | 39-2 | [32] |
| 3 | Solution combustion | (C4H6O4Co·4H2O) and ᴅ-(+)(C6H12O6) |
Spinel-structured Co3O4 powder | 700 | 3 | [28] |
| 4 | Solution combustion | (Co(NO3)2·6H2O) and urea (CO(NH2)2) in deionized water | Nano-crystalline Co3O4 | 600 | 10 | [33] |
| 5 | Sol-gel method | (Co(NO3)2·6H2O) and PEG in deionized water | Co3O4 nanorod | 90-350-700 | 170.2-48-20.9 | [35] |
| 6 | Reactive calcination route | (Co(NO3)2·6H2O) and (Mn(NO3)2·4H2O) in deionized water | Mn promoted Co3O4 spinel (Cat-R) | 340-380-420 | 127.94-94.5-57.43 | [36] |
| 7 | Hydro thermal method | (Co(NO3)2·6H2O) and urea (CO(NH2)2) in deionized water | Co3O4 nanoplate | 325 | 45.5 | [39] |
| 8 | Hydro thermal method | (Co(NO3)2·6H2O) and urea (CO(NH2)2) in deionized water | Co3O4 nanorod | 325 | 111.4 | [39] |
| 9 | Hydro thermal method | (Co(NO3)2·6H2O) and urea (CO(NH2)2) in deionized water | Co3O4 NPs | 325 | 112.6 | [39] |
| 10 | Sol-gel method | (Co(NO3)2·6H2O) and (C2H5-OH) | Co3O4 NPs | 150 | 15 | [41] |
| 11 | Sol-gel method | (Co(NO3)2·6H2O) and ethanol (C2H5-OH) | Co3O4 NPs | 550-650 | 46-42 | [41] |
| № | Material | Method of preparation | Electrolyte | Specific capacitance, Fg-1 | Scan rate/ Current density, A g−1 |
Retention | Cycles | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Co3O4 nanospheres | Solution combustion | 1 M KOH | 182 | 0.5 | 71% | 2000 | [3] |
| 2 | Marigold 3D flower like Co3O4 nanoparticles | Solution combustion | 3 M KOH | 603 | 97.6% | 5000 | [4] | |
| 3 | Co3O4 nanospheres | One pot hydrothermal | 182 | 1 | 93.75% | 8000 | [5] | |
| 4 | Co3O4@C NPs | Simple thermolysis | 2 MKOH | 642 | 1 | [11] | ||
| 5 | Pure Co3O4 nanoparticles and Co3O4 /graphite nanocomposite | Co-precipitation method | 6M KOH | 239.5 for pure 395.04 for Co3O4/ graphite |
0.5 | 2.68% | 1000 | [14] |
| 6 | Cobalt oxide | Electrodeposition | PH 12 | 504 | 600 | [18] | ||
| 7 | Co3O4 nanoflakes | Cathodic potential step method | 598.9 | 6.25 | [19] | |||
| 8 | Hexagonal Co3O4 | Solution combustion | 6 M KOH | 227 | 1 | 95% | 1000 | [22] |
| 9 | Co3O4 thin films | Electrodeposition | KOH | 235 | [23] | |||
| 10 | Co3O4 nanoparticles | Solid-state calcination | 100 | 1.1 | 50 | [24] | ||
| 11 | Cobalt oxide | Solution combustion | 2 M KOH | 351 | 0.85 | 98.6% | 1000 | [25] |
| 12 | Spinel-nanostructured Co3O4 powder | Solution combustion | 100 | 0.05-5 | 75% | 100 | [28] | |
| 13 | Cobalt oxide flakes | Potentiodynamic approach | 0.1 M Na2SO4 | 396.67 | better cyclic retention | 1600 | [30] | |
| 14 | Cobalt oxide thin film | Heating of an alkaline bath of cobalt salt | KOH 0.25 to 2.0 M | 118 | [33] | |||
| 15 | Cobalt oxide | Solution combustion | 2 M KOH | 54 | 10 | 82% | 10000 | [46] |
| 16 | Co3O4 nanoflake | Hydrothermal | 2 M KOH | 351 | good | 4000 | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





