Submitted:
19 March 2024
Posted:
19 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Structure of (1) Oxo-Complex
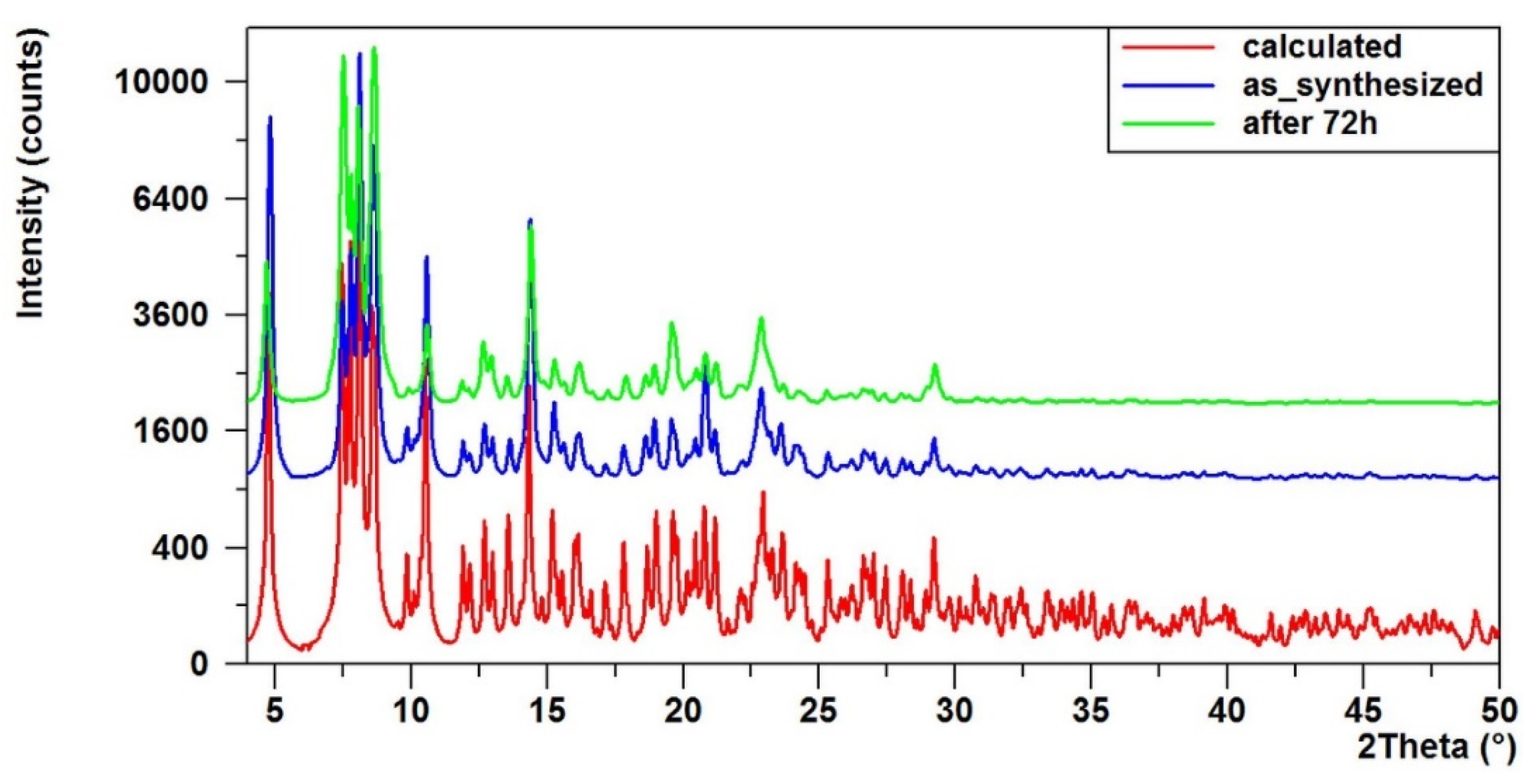
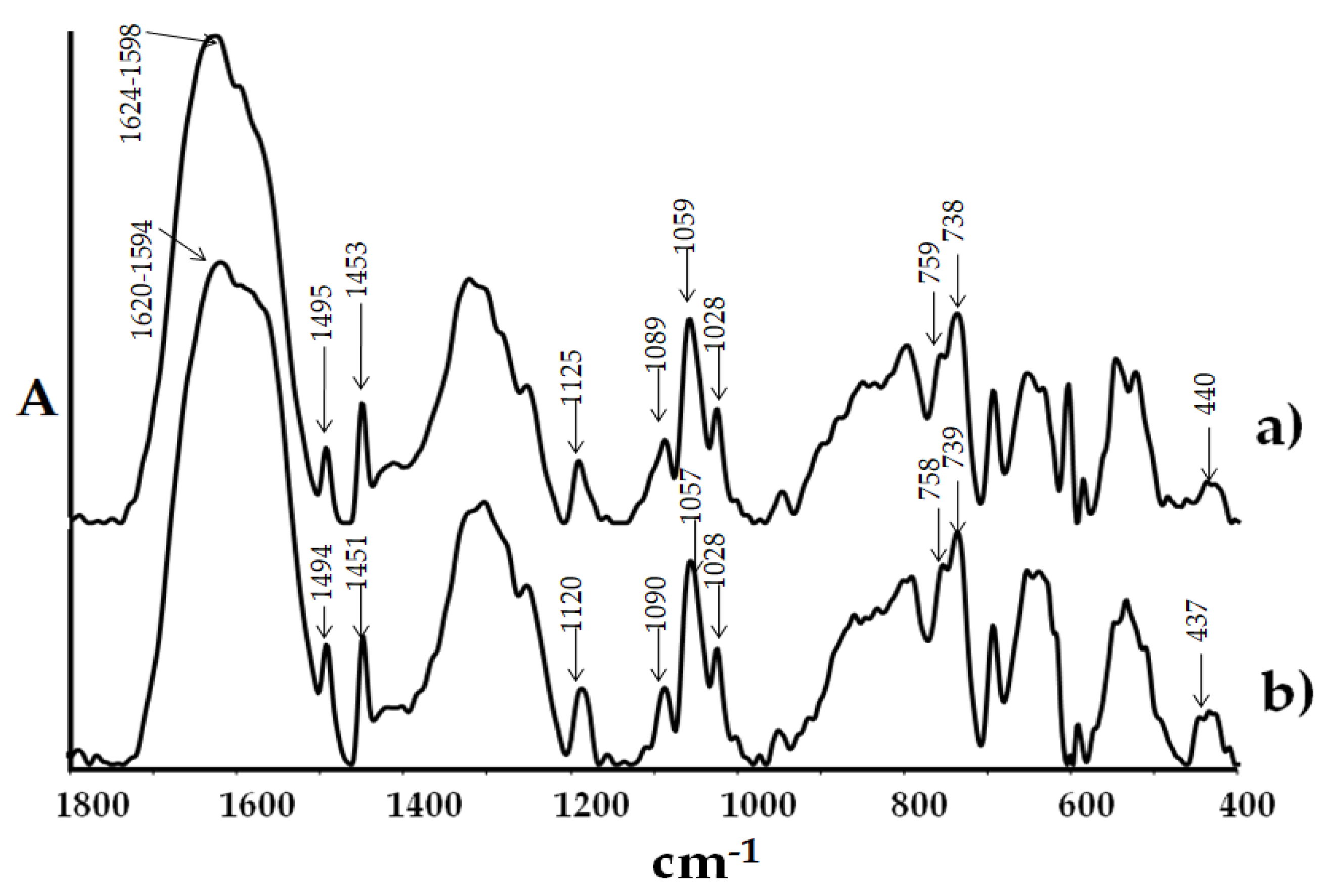
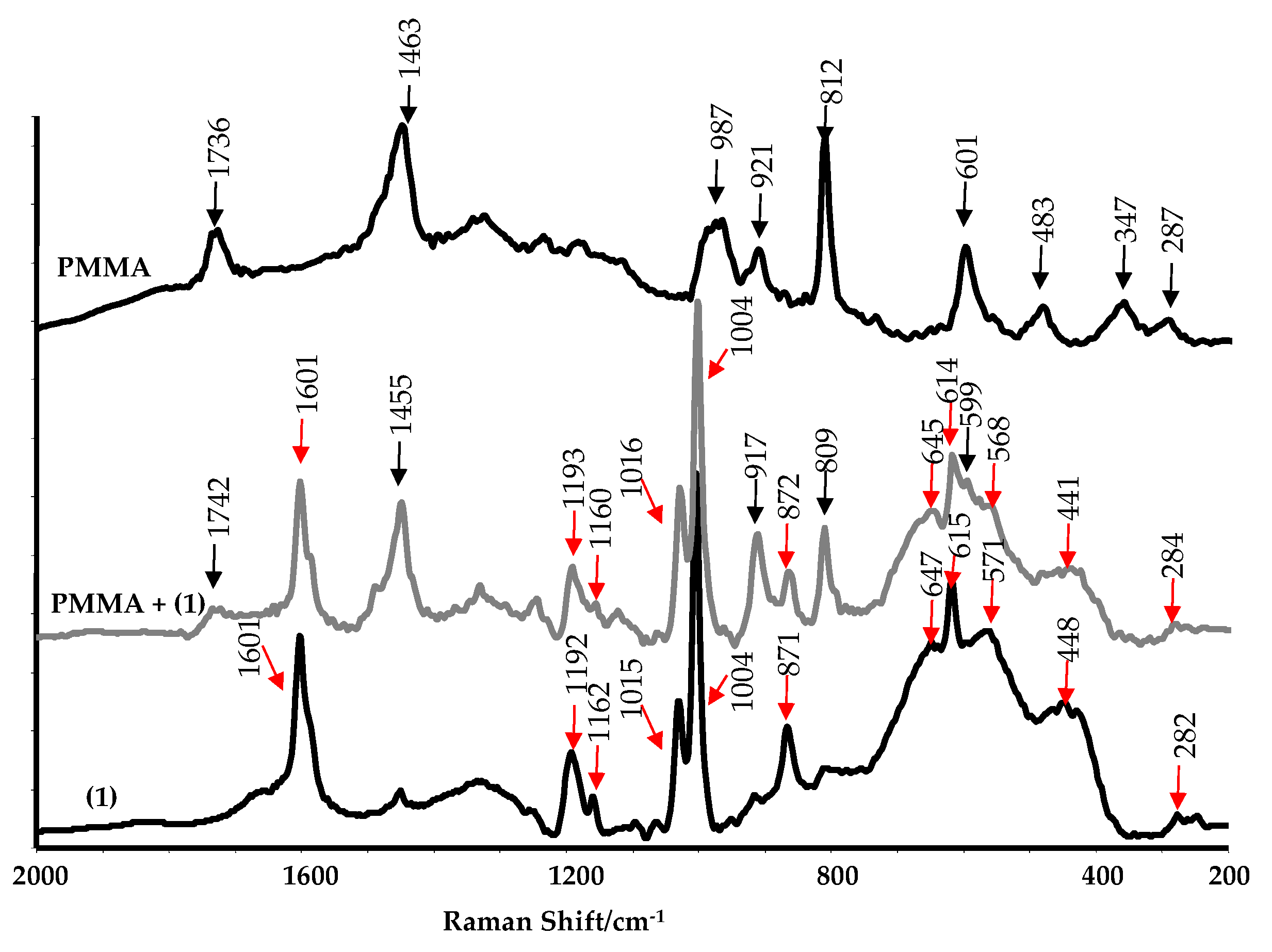
2.2. Spectral Cheracterization of (1) and Its Composite with Poly(Methyl Methacrylate)
2.3. Antimicrobial Activity of (1) and Its Composites
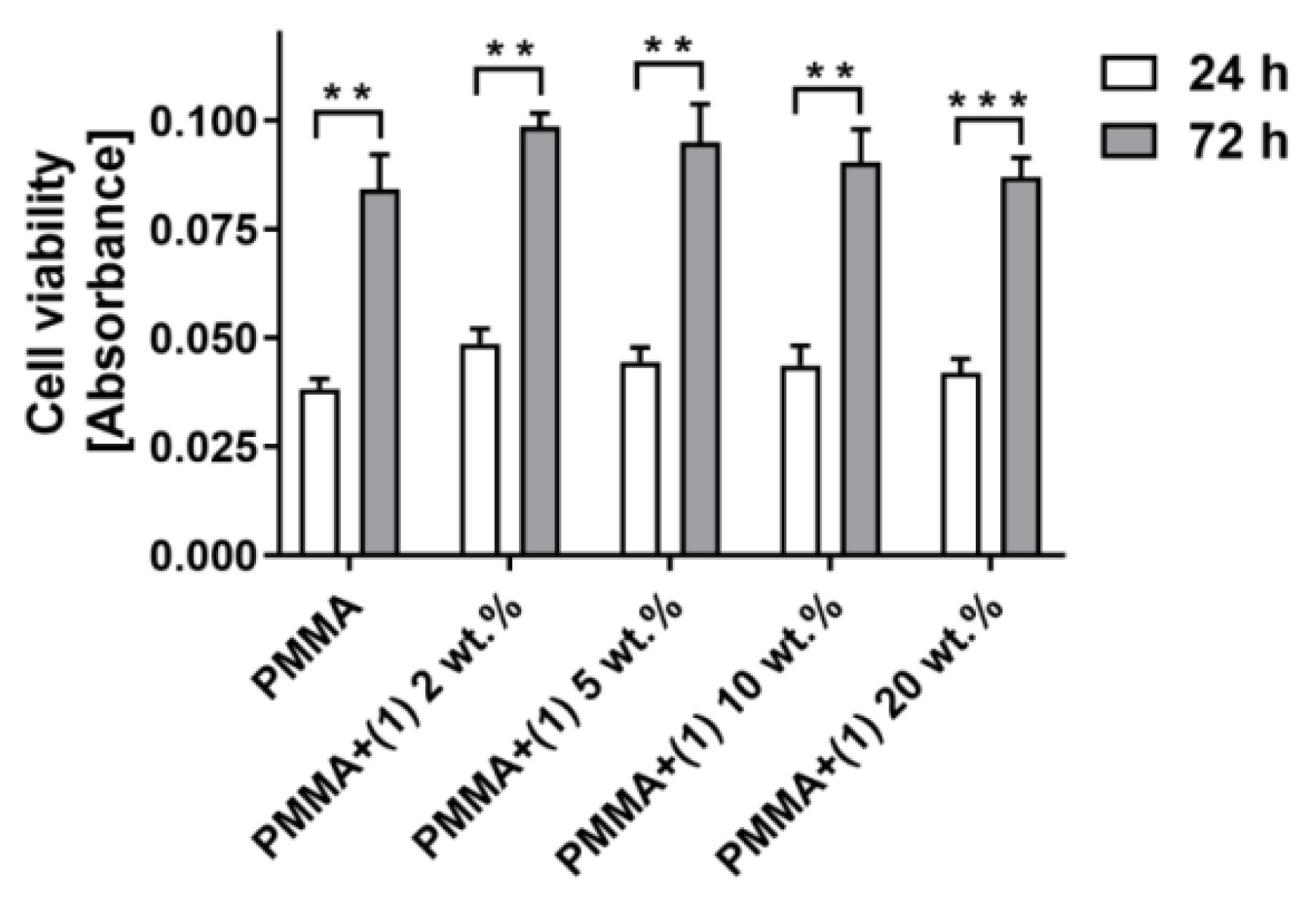
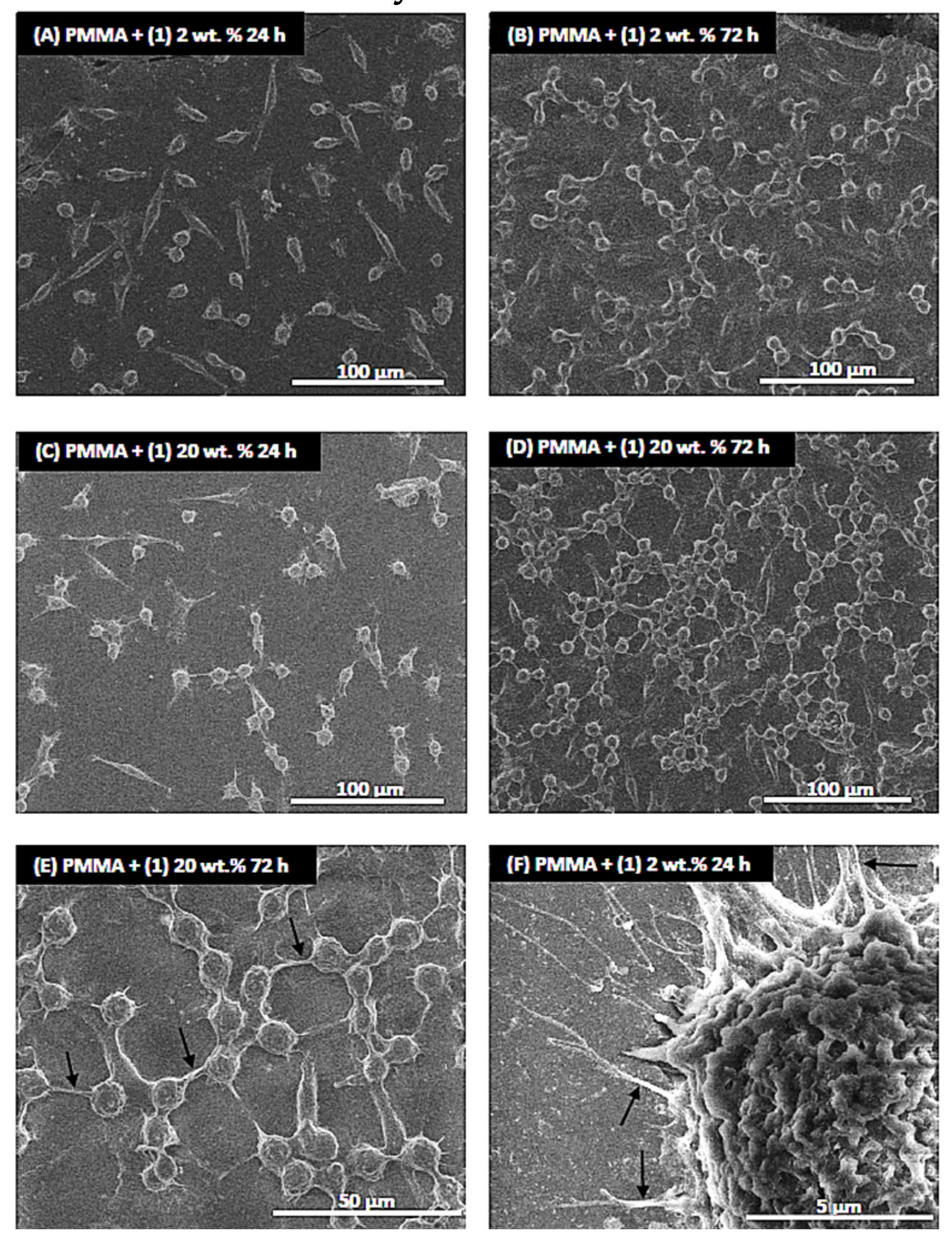
2.6. Cytotoxicity of PMMA + (1) Composites
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Ti(IV) Oxo-Complex (α-TOCs) and Preparation of PMMA/TOC COMPOSITES
4.2.1. The Synthesis of [Ti8O2(OiPr)20(man)4] (1)
4.2.2. PMMA/TOCs Composites Preparation
4.3. Analytical Methods
4.3.1. Structural and Spectroscopic Characterization of TOCs
4.3.2. Single Crystal X-ray Diffraction Measurement
4.3.3. X-Ray Diffraction of Powders
4.3.4. Characterization of PMMA +TOCs Composite Materials
4.3.5. The Electron Paramagnetic Resonance (EPR) Spectroscopy
4.4. Studies of the Biological Activity of Synthesized Materials
4.4.1. Antimicrobial Activity of PMMA + (1) Composites and Powder (1)
4.4.2. Assessment of Material Cytotoxicity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhuang, G.; Yan, J.; Wen, Y.; Zhuang, Z.; Yu, Y. Two-Dimensional Transition Metal Oxides and Chalcogenides for Advanced Photocatalysis: Progress, Challenges, and Opportunities. Sol. RRL 2021, 5, 2000403. [CrossRef]
- Wang, C.; Wang, S.-J.; Kong, F.-G. Calixarene-Protected Titanium-Oxo Clusters and Their Photocurrent Responses and Photocatalytic Performances. Inorg. Chem. 2021, 60, 5034–5041. [CrossRef]
- Ni, L.; Liang, D.; Cai, Y.; Diao, G.; Zhou, Z. A Novel Hexanuclear Titanium( iv )-Oxo-Iminodiacetate Cluster with a Ti 6 O 9 Core: Single-Crystal Structure and Photocatalytic Activities. Dalton Trans. 2016, 45, 7581–7588. [CrossRef]
- Lin, Y.; Zhu, Y.-F.; Chen, Z.-H.; Liu, F.-H.; Zhao, L.; Su, Z.-M. Synthesis, Structure, and Photocatalytic Hydrogen of Three Environmentally Friendly Titanium Oxo-Clusters. Inorganic Chemistry Communications 2014, 40, 22–25. [CrossRef]
- Kubiak, B.; Piszczek, P.; Radtke, A.; Muzioł, T.; Wrzeszcz, G.; Golińska, P. Photocatalytic and Antimicrobial Activity of Titanium(IV)-Oxo Clusters of Different Core Structure. Crystals 2023, 13, 998. [CrossRef]
- Janek, M.; Muzioł, T.M.; Piszczek, P. Trinuclear Oxo-Titanium Clusters: Synthesis, Structure, and Photocatalytic Activity. Materials 2019, 12, 3195. [CrossRef]
- Janek, M.; Radtke, A.; Muzioł, T.; Jerzykiewicz, M.; Piszczek, P. Tetranuclear Oxo-Titanium Clusters with Different Carboxylate Aromatic Ligands: Optical Properties, DFT Calculations, and Photoactivity. Materials 2018, 11, 1661. [CrossRef]
- Fenton, J.L.; Laaroussi, A.; Mobian, P.; Chaumont, C.; Khalil, G.; Huguenard, C.; Henry, M. Structural Investigation of Pyridinecarboxylato Titanium(IV) Complexes: An Uncommon Monomeric Octacoordinated Complex vs. a Hexaprismatic Architecture. Eur J Inorg Chem 2014, 2014, 357–363. [CrossRef]
- Seisenbaeva, G.A.; Ilina, E.; Håkansson, S.; Kessler, V.G. A New Concept for Titanium Oxo-Alkoxo-Carboxylates’ Encapsulated Biocompatible Time Temperature Food Indicators Based on Arising, Not Fading Color. J Sol-Gel Sci Technol 2010, 55, 1–8. [CrossRef]
- Benedict, J.B.; Freindorf, R.; Trzop, E.; Cogswell, J.; Coppens, P. Large Polyoxotitanate Clusters: Well-Defined Models for Pure-Phase TiO 2 Structures and Surfaces. J. Am. Chem. Soc. 2010, 132, 13669–13671. [CrossRef]
- Sokolow, J.D.; Trzop, E.; Chen, Y.; Tang, J.; Allen, L.J.; Crabtree, R.H.; Benedict, J.B.; Coppens, P. Binding Modes of Carboxylate- and Acetylacetonate-Linked Chromophores to Homodisperse Polyoxotitanate Nanoclusters. J. Am. Chem. Soc. 2012, 134, 11695–11700. [CrossRef]
- Zhang, L.; Fan, X.; Yi, X.; Lin, X.; Zhang, J. Coordination-Delayed-Hydrolysis Method for the Synthesis and Structural Modulation of Titanium-Oxo Clusters. Acc. Chem. Res. 2022, 55, 3150–3161. [CrossRef]
- Svensson, F.G.; Seisenbaeva, G.A.; Kessler, V.G. Mixed-Ligand Titanium “Oxo Clusters”: Structural Insights into the Formation and Binding of Organic Molecules and Transformation into Oxide Nanostructures on Hydrolysis and Thermolysis. Eur. J. Inorg. Chem. 2017, 2017, 4117–4122. [CrossRef]
- Wu, R.-H.; Guo, M.; Yu, M.-X.; Zhu, L.-G. Two Titanium( iv )-Oxo-Clusters: Synthesis, Structures, Characterization and Recycling Catalytic Activity in the Oxygenation of Sulfides. Dalton Trans. 2017, 46, 14348–14355. [CrossRef]
- Czakler, M.; Artner, C.; Schubert, U. Two New Hexanuclear Titanium Oxo Cluster Types and Their Structural Connection to Known Clusters. New J. Chem. 2018, 42, 12098–12103. [CrossRef]
- Yu, Y.-Z.; Zhang, Y.-R.; Geng, C.-H.; Sun, L.; Guo, Y.; Feng, Y.-R.; Wang, Y.-X.; Zhang, X.-M. Precise and Wide-Ranged Band-Gap Tuning of Ti 6 -Core-Based Titanium Oxo Clusters by the Type and Number of Chromophore Ligands. Inorg. Chem. 2019, 58, 16785–16791. [CrossRef]
- Guo, Y.-H.; Yu, Y.-Z.; Shen, Y.-H.; Yang, L.-G.; Liu, N.-N.; Zhou, Z.-Y.; Niu, Y.-S. “Three-in-One” Structural-Building-Mode-Based Ti 16 -Type Titanium Oxo Cluster Entirely Protected by the Ligands Benzoate and Salicylhydroxamate. Inorg. Chem. 2022, 61, 8685–8693. [CrossRef]
- Schubert, U. Titanium-Oxo Clusters with Bi- and Tridentate Organic Ligands: Gradual Evolution of the Structures from Small to Big. Chem. Eur. J. 2021, 27, 11239–11256. [CrossRef]
- Radtke, A.; Piszczek, P.; Muzioł, T.; Wojtczak, A. The Structural Conversion of Multinuclear Titanium(IV) μ-Oxo-Complexes. Inorg. Chem. 2014, 53, 10803–10810. [CrossRef]
- Zheng, Y.-Z.; Zheng, Z.; Chen, X.-M. A Symbol Approach for Classification of Molecule-Based Magnetic Materials Exemplified by Coordination Polymers of Metal Carboxylates. Coordination Chemistry Reviews 2014, 258–259, 1–15. [CrossRef]
- Rozes, L.; Sanchez, C. Titanium Oxo-Clusters: Precursors for a Lego-like Construction of Nanostructured Hybrid Materials. Chem. Soc. Rev. 2011, 40, 1006. [CrossRef]
- Schubert, U. Chemical Modification of Titanium Alkoxides for Sol–Gel Processing. J. Mater. Chem. 2005, 15, 3701. [CrossRef]
- Wang, J.-F.; Fang, W.-H.; Li, D.-S.; Zhang, L.; Zhang, J. Cocrystal of {Ti 4 } and {Ti 6 } Clusters with Enhanced Photochemical Properties. Inorg. Chem. 2017, 56, 2367–2370. [CrossRef]
- Piszczek, P.; Kubiak, B.; Golińska, P.; Radtke, A. Oxo-Titanium(IV) Complex/Polymer Composites—Synthesis, Spectroscopic Characterization and Antimicrobial Activity Test. IJMS 2020, 21, 9663. [CrossRef]
- Kubiak, B.; Radtke, A.; Topolski, A.; Wrzeszcz, G.; Golińska, P.; Kaszkowiak, E.; Sobota, M.; Włodarczyk, J.; Stojko, M.; Piszczek, P. The Composites of PCL and Tetranuclear Titanium(IV)-Oxo Complexes as Materials Exhibiting the Photocatalytic and the Antimicrobial Activity. IJMS 2021, 22, 7021. [CrossRef]
- Cui, Y.; Zou, G.-D.; Li, H.-M.; Huang, Y.; Fan, Y. 4-Chlorosalicylate-Stabilized Titanium-Oxo Clusters with Structures Mediated by Tetrazole and Their Photophysical Properties. Polyhedron 2019, 157, 177–182. [CrossRef]
- Luo, W.; Shu, X.-P.; Liu, P.-Y.; Yu, S.-K.; Zhu, Q.-Y.; Dai, J. Lanthanide-Titanium Oxo-Clusters, New Precursors of Multifunctional Colloids for Effective Imaging and Photodynamic Therapy. Journal of Molecular Liquids 2020, 317, 113946. [CrossRef]
- Li, N.; Pranantyo, D.; Kang, E.-T.; Wright, D.S.; Luo, H.-K. A Simple Drop-and-Dry Approach to Grass-Like Multifunctional Nanocoating on Flexible Cotton Fabrics Using In Situ-Generated Coating Solution Comprising Titanium-Oxo Clusters and Silver Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 12093–12100. [CrossRef]
- Chen, S.; Fang, W.; Zhang, L.; Zhang, J. Atomically Precise Multimetallic Semiconductive Nanoclusters with Optical Limiting Effects. Angew Chem Int Ed 2018, 57, 11252–11256. [CrossRef]
- Luo, W.; Hu, B.; Zhang, H.-L.; Li, C.; Shi, Y.; Li, X.; Jin, L. Antibacterial, Photothermal and Stable Ag-Titanium-Oxo-Clusters Hydrogel Designed for Wound Healing. Materials & Design 2023, 226, 111674. [CrossRef]
- De Pasquale, I.; Lo Porto, C.; Dell’Edera, M.; Petronella, F.; Agostiano, A.; Curri, M.L.; Comparelli, R. Photocatalytic TiO2-Based Nanostructured Materials for Microbial Inactivation. Catalysts 2020, 10, 1382. [CrossRef]
- Parcheta, M.; Świsłocka, R.; Świderski, G.; Matejczyk, M.; Lewandowski, W. Spectroscopic Characterization and Antioxidant Properties of Mandelic Acid and Its Derivatives in a Theoretical and Experimental Approach. Materials 2022, 15, 5413. [CrossRef]
- Egner, P.; Pavlačková, J.; Sedlaříková, J.; Pleva, P.; Mokrejš, P.; Janalíková, M. Non-Alcohol Hand Sanitiser Gels with Mandelic Acid and Essential Oils. IJMS 2023, 24, 3855. [CrossRef]
- Tang, S.-C.; Yang, J.-H. Dual Effects of Alpha-Hydroxy Acids on the Skin. Molecules 2018, 23, 863. [CrossRef]
- Hou, J.-L.; Luo, W.; Wu, Y.-Y.; Su, H.-C.; Zhang, G.-L.; Zhu, Q.-Y.; Dai, J. Two Ti 13 -Oxo-Clusters Showing Non-Compact Structures, Film Electrode Preparation and Photocurrent Properties. Dalton Trans. 2015, 44, 19829–19835. [CrossRef]
- Ding, Q.-R.; Liu, J.-X.; Narayanam, N.; Zhang, L.; Zhang, J. Construction of Molecular Rectangles with Titanium–Oxo Clusters and Rigid Aromatic Carboxylate Ligands. Dalton Trans. 2017, 46, 16000–16003. [CrossRef]
- Huang, Y.; Zou, G.-D.; Li, H.-M.; Cui, Y.; Fan, Y. A Photoactive {Ti 16 } Metal–Organic Capsule: Structural, Photoelectrochemical and Photocatalytic Properties. New J. Chem. 2018, 42, 14079–14082. [CrossRef]
- Kemmitt, T.; Al-Salim, N.I.; Gainsford, G.J.; Bubendorfer, A.; Waterland, M. Unprecedented Oxo-Titanium Citrate Complex Precipitated from Aqueous Citrate Solutions, Exhibiting a Novel Bilayered Ti 8 O 10 Structural Core. Inorg. Chem. 2004, 43, 6300–6306. [CrossRef]
- Salam, A.; Dadzie, O.E.; Galadari, H. Chemical Peeling in Ethnic Skin: An Update. Br J Dermatol 2013, 169 Suppl 3, 82–90. [CrossRef]
- Gentili, G.; Perugini, P.; Bugliaro, S.; D’Antonio, C. Efficacy and Safety of a New Peeling Formulated with a Pool of PHAs for the Treatment of All Skin Types, Even Sensitive. J Cosmet Dermatol 2023, 22, 517–528. [CrossRef]
- Dębowska, R.M.; Kaszuba, A.; Michalak, I.; Dzwigałowska, A.; Cieścińska, C.; Jakimiuk, E.; Zielińska, J.; Kaszuba, A. Evaluation of the Efficacy and Tolerability of Mandelic Acid-Containing Cosmetic Formulations for Acne Skin Care. pd 2015, 4, 316–321. [CrossRef]
- Greive, K.; Tran, D.; Townley, J.; Barnes, T. An Antiaging Skin Care System Containing Alpha Hydroxy Acids and Vitamins Improves the Biomechanical Parameters of Facial Skin. CCID 2014, 9. [CrossRef]
- Świsłocka, R.; Świderski, G.; Nasiłowska, J.; Sokołowska, B.; Wojtczak, A.; Lewandowski, W. Research on the Electron Structure and Antimicrobial Properties of Mandelic Acid and Its Alkali Metal Salts. IJMS 2023, 24, 3078. [CrossRef]
- Mamdouh S Masoud; Alaa E Ali; Almaza A Shokry; Sherif A. Kolkaila Chelation and Molecular Structure of Mandelic Acid Complexes. Journal of Chemical Research Advances 2021, 02, 1–9.
- Youzhu, Y.; Hui, W.; Leilei, L.; Yuhua, G.; Jing, F.; Yichao, L. Crystal Structure of Bis(μ 2 -2-Oxido-2-Phenylacetato-κ 3 O,O′:O′ )-Bis( N -Oxido-Benzamide-κ 2 O,O′ )-Bis(Propan-2-Olato-κ 1 O )Dititanium(IV), C 36 H 38 N 2 O 12 Ti 2. Zeitschrift für Kristallographie - New Crystal Structures 2022, 237, 957–959. [CrossRef]
- Youzhu, Y.; Yuhua, G.; Yongsheng, N.; Nana, L.; Hongfei, Z. Crystal Structure of Bis(μ 2 -2-Oxido-2-Phenylacetate-κ 3 O : O , O ′)-Bis(1-Isopropoxy-2-Oxo-2-Phenylethan-1-Olato-κ 2 O,O′)-Bis(Propan-2-Olato-κ 1 O)Dititanium(IV), C 44 H 52 O 14 Ti 2. Zeitschrift für Kristallographie - New Crystal Structures 2021, 236, 467–469. [CrossRef]
- Schetter, B.; Stosiek, C.; Ziemer, B.; Mahrwald, R. Multinuclear Enantiopure Titanium Self-Assembly Complexes—Synthesis, Characterization and Application to Organic Synthesis. Appl. Organometal. Chem. 2007, 21, 139–145. [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; Van Rijn, J.; Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of Copper( II ) Compounds Containing Nitrogen–Sulphur Donor Ligands; the Crystal and Molecular Structure of Aqua [1,7-Bis(N-Methylbenzimidazol-2′-Yl)-2,6-Dithiaheptane]Copper( II ) Perchlorate. J. Chem. Soc., Dalton Trans. 1984, 1349–1356. [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0 : From Visualization to Analysis, Design and Prediction. J Appl Crystallogr 2020, 53, 226–235. [CrossRef]
- Cicco, S.; Vona, D.; Gristina, R.; Sardella, E.; Ragni, R.; Lo Presti, M.; Farinola, G. Biosilica from Living Diatoms: Investigations on Biocompatibility of Bare and Chemically Modified Thalassiosira Weissflogii Silica Shells. Bioengineering 2016, 3, 35. [CrossRef]
- Xue, F.; Janzen, D.M.; Knecht, D.A. Contribution of Filopodia to Cell Migration: A Mechanical Link between Protrusion and Contraction. International Journal of Cell Biology 2010, 2010, 1–13. [CrossRef]
- Piszczek, P.; Richert, M.; Grodzicki, A.; Głowiak, T.; Wojtczak, A. Synthesis, Crystal Structures and Spectroscopic Characterization of [Ti8O8(OOCR)16] (Where R=But, CH2But, C(CH3)2Et). Polyhedron 2005, 24, 663–670. [CrossRef]
- Frot, T.; Cochet, S.; Laurent, G.; Sassoye, C.; Popall, M.; Sanchez, C.; Rozes, L. Ti 8 O 8 (OOCR) 16 , a New Family of Titanium–Oxo Clusters: Potential NBUs for Reticular Chemistry. Eur. J. Inorg. Chem. 2010, 2010, 5650–5659. [CrossRef]
- Fan, X.; Yuan, F.; Li, D.; Chen, S.; Cheng, Z.; Zhang, Z.; Xiang, S.; Zang, S.; Zhang, J.; Zhang, L. Threefold Collaborative Stabilization of Ag 14 -Nanorods by Hydrophobic Ti 16 -Oxo Clusters and Alkynes: Designable Assembly and Solid-State Optical-Limiting Application. Angew. Chem. Int. Ed. 2021, 60, 12949–12954. [CrossRef]
- Wang, S.; Reinsch, H.; Heymans, N.; Wahiduzzaman, M.; Martineau-Corcos, C.; De Weireld, G.; Maurin, G.; Serre, C. Toward a Rational Design of Titanium Metal-Organic Frameworks. Matter 2020, 2, 440–450. [CrossRef]
- Kim, B.; Keum, Y.; Chen, Y.-P.; Oh, H.S.; Lee, J.Y.; Park, J. Stimuli-Responsive Ti-Organic Gels and Aerogels Derived from Ti-Oxo Clusters: Hierarchical Porosity and Photocatalytic Activity. Inorg. Chem. 2019, 58, 15936–15941. [CrossRef]
- Czakler, M.; Artner, C.; Schubert, U. Influence of the Phosphonate Ligand on the Structure of Phosphonate-Substituted Titanium Oxo Clusters. Eur. J. Inorg. Chem. 2013, 2013, 5790–5796. [CrossRef]
- Fan, X.; Fu, H.; Gao, M.-Y.; Zhang, L.; Zhang, J. One-Pot and Postsynthetic Phenol-Thermal Synthesis toward Highly Stable Titanium-Oxo Clusters. Inorg. Chem. 2019, 58, 13353–13359. [CrossRef]
- Liu, J.-X.; Gao, M.-Y.; Fang, W.-H.; Zhang, L.; Zhang, J. Bandgap Engineering of Titanium-Oxo Clusters: Labile Surface Sites Used for Ligand Substitution and Metal Incorporation. Angew. Chem. Int. Ed. 2016, 55, 5160–5165. [CrossRef]
- Wu, Y.-Y.; Luo, W.; Wang, Y.-H.; Pu, Y.-Y.; Zhang, X.; You, L.-S.; Zhu, Q.-Y.; Dai, J. Titanium–Oxo–Clusters with Dicarboxylates: Single-Crystal Structure and Photochromic Effect. Inorg. Chem. 2012, 51, 8982–8988. [CrossRef]
- He, W.; Liu, Y.; Wamer, W.G.; Yin, J.-J. Electron Spin Resonance Spectroscopy for the Study of Nanomaterial-Mediated Generation of Reactive Oxygen Species. Journal of Food and Drug Analysis 2014, 22, 49–63. [CrossRef]
- Xiong, L.-B.; Li, J.-L.; Yang, B.; Yu, Y. Ti 3 + in the Surface of Titanium Dioxide: Generation, Properties and Photocatalytic Application. Journal of Nanomaterials 2012, 2012, 1–13. [CrossRef]
- Suriye, K.; Lobo-Lapidus, R.J.; Yeagle, G.J.; Praserthdam, P.; Britt, R.D.; Gates, B.C. Probing Defect Sites on TiO2 with [Re3(CO)12H3]: Spectroscopic Characterization of the Surface Species. Chem. Eur. J. 2008, 14, 1402–1414. [CrossRef]
- Dan-Hardi, M.; Serre, C.; Frot, T.; Rozes, L.; Maurin, G.; Sanchez, C.; Férey, G. A New Photoactive Crystalline Highly Porous Titanium(IV) Dicarboxylate. J. Am. Chem. Soc. 2009, 131, 10857–10859. [CrossRef]
- Richards, E.; Murphy, D.M.; Che, M. An EPR Characterisation of Stable and Transient Reactive Oxygen Species Formed under Radiative and Non-Radiative Conditions. Res Chem Intermed 2019, 45, 5763–5779. [CrossRef]
- Śmigiel, J.; Piszczek, P.; Wrzeszcz, G.; Jędrzejewski, T.; Golińska, P.; Radtke, A. The Composites of PCL and Tetranuclear Titanium(IV)–Oxo Complex with Acetylsalicylate Ligands—Assessment of Their Biocompatibility and Antimicrobial Activity with the Correlation to EPR Spectroscopy. Materials 2022, 16, 297. [CrossRef]
- Vaishampayan, A.; Grohmann, E. Antimicrobials Functioning through ROS-Mediated Mechanisms: Current Insights. Microorganisms 2021, 10, 61. [CrossRef]
- Mazur, P.; Skiba-Kurek, I.; Mrowiec, P.; Karczewska, E.; Drożdż, R. Synergistic ROS-Associated Antimicrobial Activity of Silver Nanoparticles and Gentamicin Against Staphylococcus Epidermidis. IJN 2020, Volume 15, 3551–3562. [CrossRef]
- Lam, P.-L.; Wong, R.S.-M.; Lam, K.-H.; Hung, L.-K.; Wong, M.-M.; Yung, L.-H.; Ho, Y.-W.; Wong, W.-Y.; Hau, D.K.-P.; Gambari, R.; et al. The Role of Reactive Oxygen Species in the Biological Activity of Antimicrobial Agents: An Updated Mini Review. Chemico-Biological Interactions 2020, 320, 109023. [CrossRef]
- Joe, A.; Park, S.-H.; Kim, D.-J.; Lee, Y.-J.; Jhee, K.-H.; Sohn, Y.; Jang, E.-S. Antimicrobial Activity of ZnO Nanoplates and Its Ag Nanocomposites: Insight into an ROS-Mediated Antibacterial Mechanism under UV Light. Journal of Solid State Chemistry 2018, 267, 124–133. [CrossRef]
- Beyene, B.B.; Mihirteu, A.M.; Ayana, M.T.; Yibeltal, A.W. Synthesis, Characterization and Antibacterial Activity of Metalloporphyrins: Role of Central Metal Ion. Results in Chemistry 2020, 2, 100073. [CrossRef]
- Chen, S.; Wu, G.; Zeng, H. Preparation of High Antimicrobial Activity Thiourea Chitosan–Ag+ Complex. Carbohydrate Polymers 2005, 60, 33–38. [CrossRef]
- Burns, J.; McCoy, C.P.; Irwin, N.J. Synergistic Activity of Weak Organic Acids against Uropathogens. Journal of Hospital Infection 2021, 111, 78–88. [CrossRef]
- Tshuva, E.Y.; Peri, D. Modern Cytotoxic Titanium(IV) Complexes; Insights on the Enigmatic Involvement of Hydrolysis. Coordination Chemistry Reviews 2009, 253, 2098–2115. [CrossRef]
- Immel, T.A.; Grützke, M.; Batroff, E.; Groth, U.; Huhn, T. Cytotoxic Dinuclear Titanium-Salan Complexes: Structural and Biological Characterization. Journal of Inorganic Biochemistry 2012, 106, 68–75. [CrossRef]
- E. CrysAlis Red and CrysAlis CCD. Oxford Diffraction Ltd. Abingdon, Oxfordshire, 2000.
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr C Struct Chem 2015, 71, 3–8. [CrossRef]
- Brandenburg, K. Berndt, M. Diamond, Release 2.1e, Crystal Impact GbR, Bonn, Germany, 2001.
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Crystal Growth & Design 2014, 14, 3576–3586. [CrossRef]
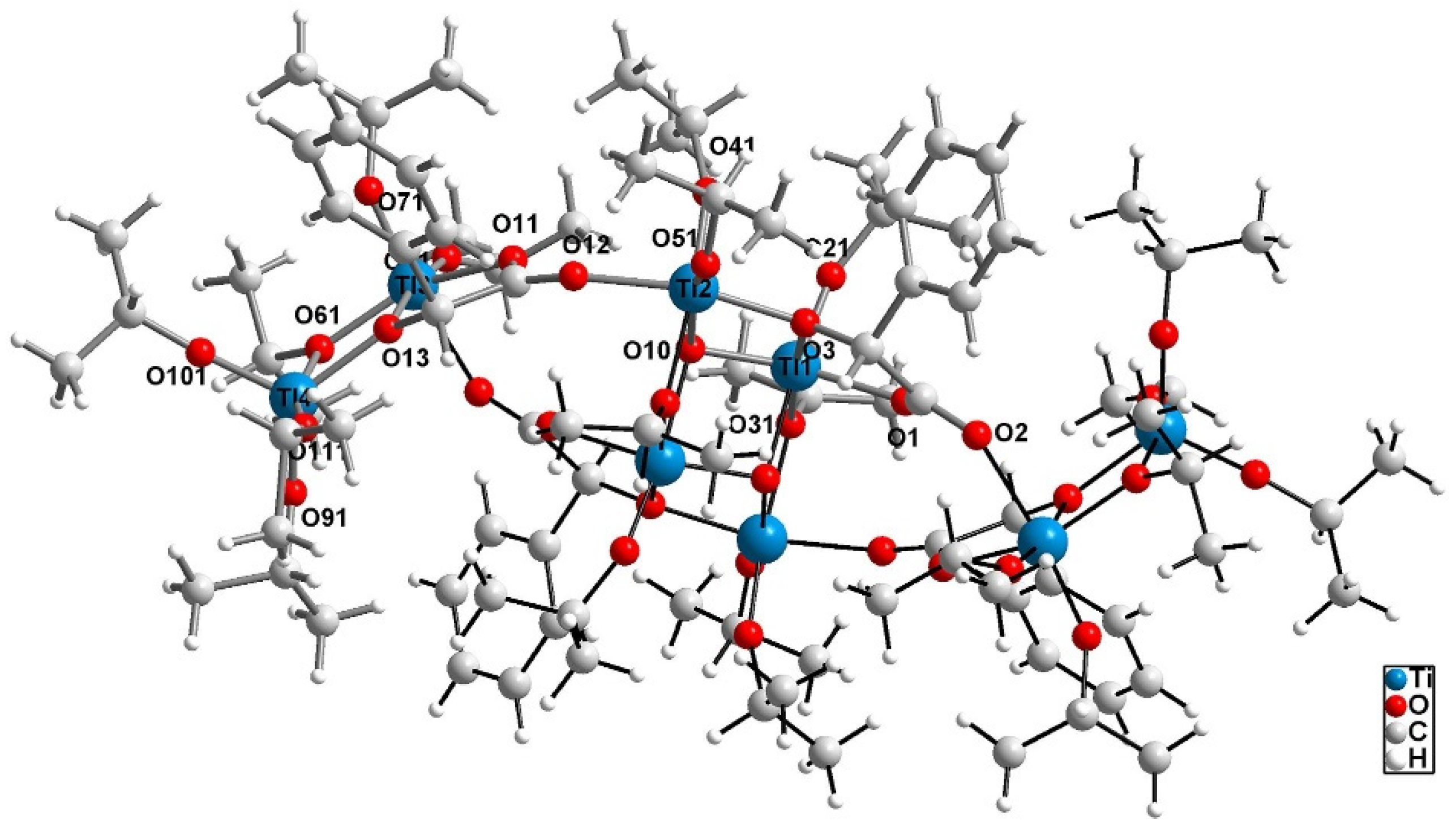
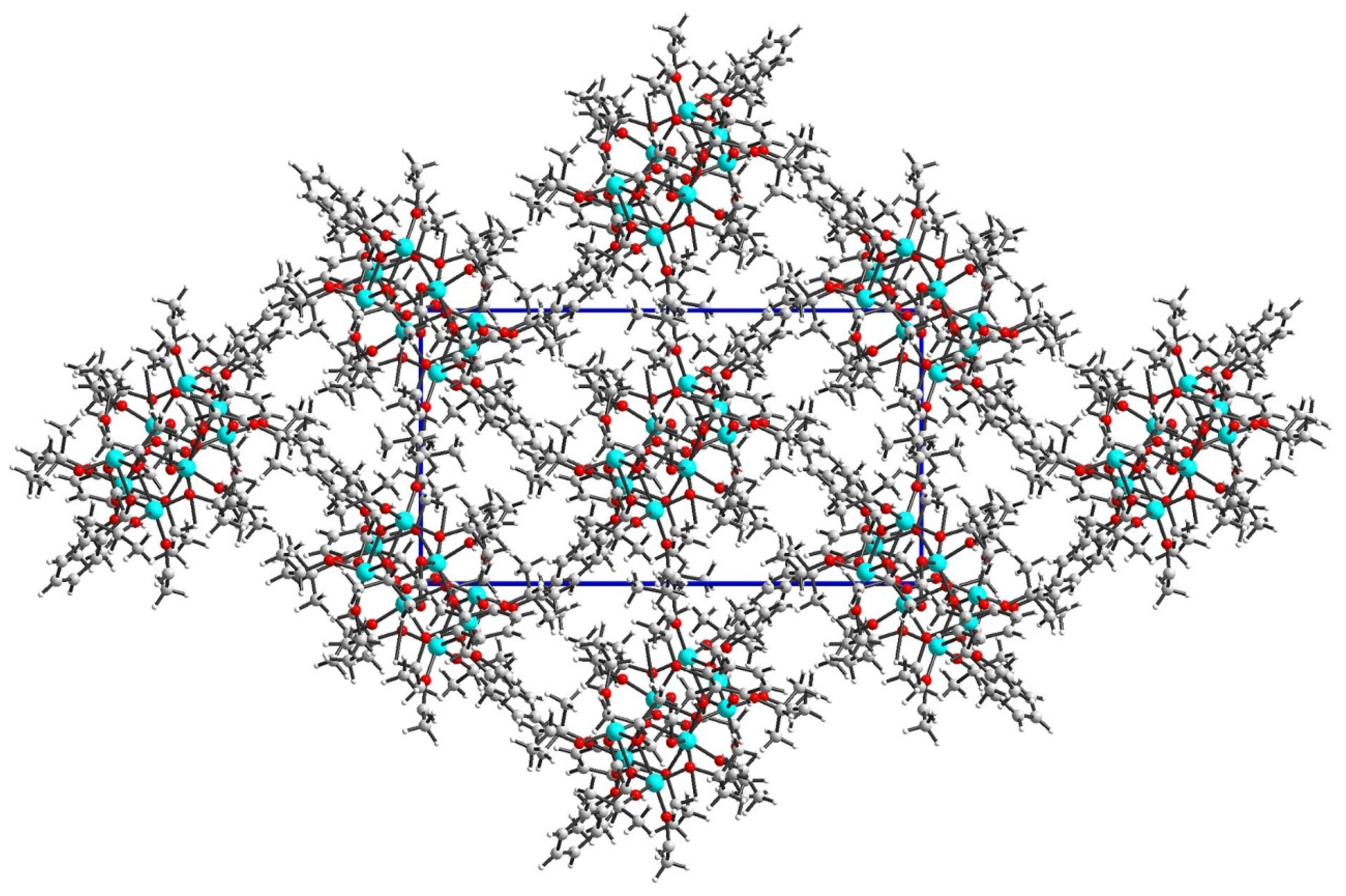
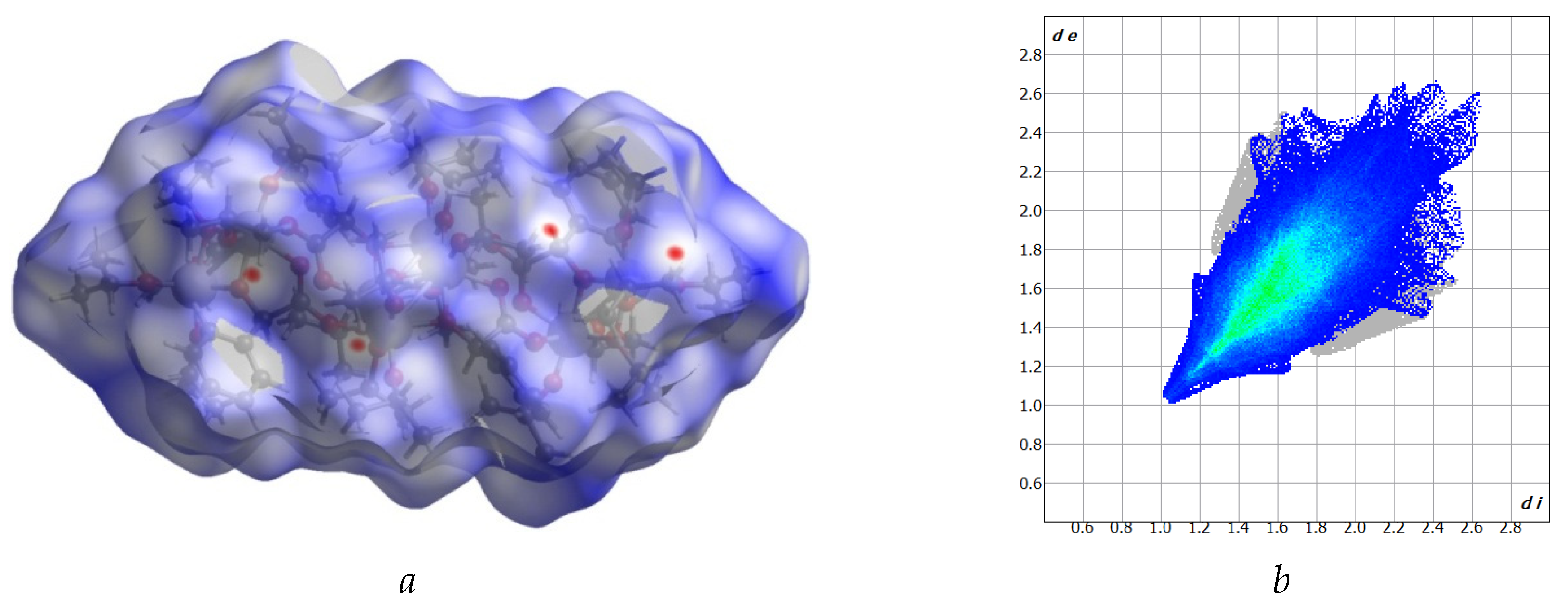
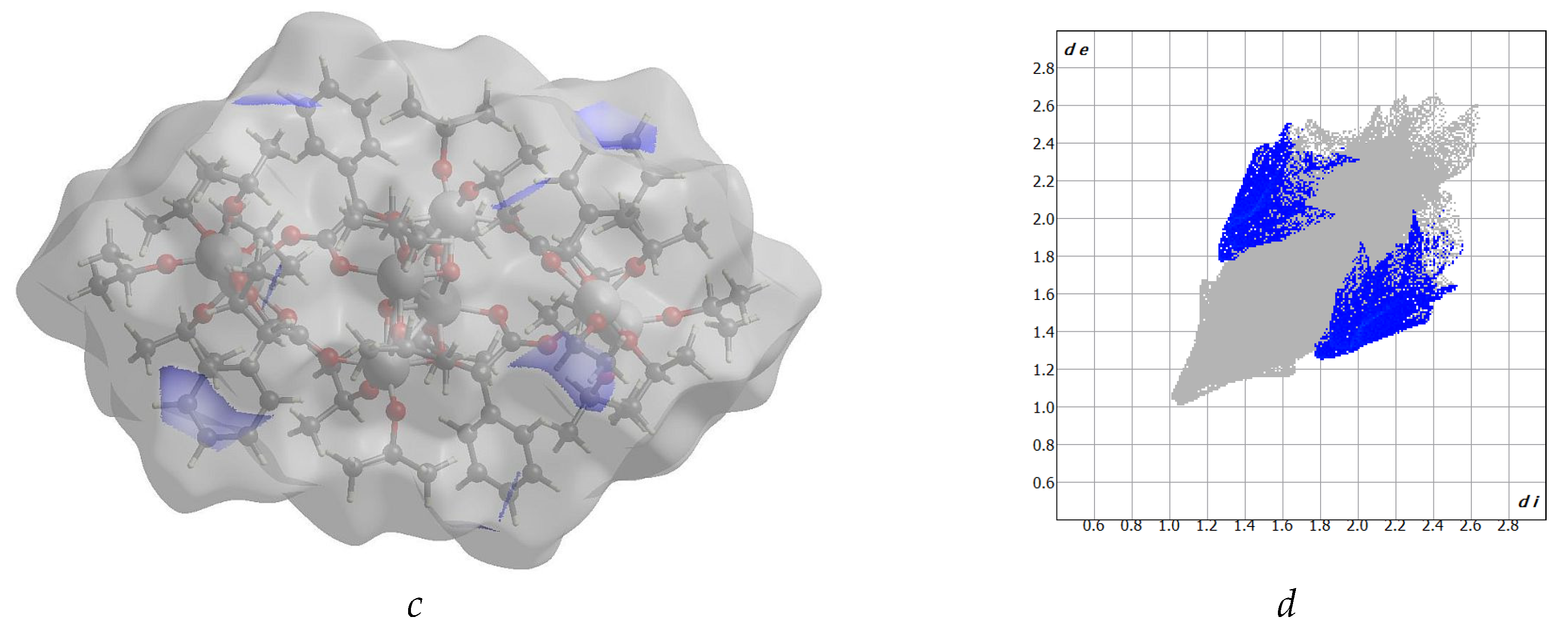
| Composite | C | O | Al | Ti |
|---|---|---|---|---|
| PMMA | 26.10 | 72.23 | 1.67 | - |
| PMMA + (1) 2wt.% | 28.27 | 71.00 | 0.45 | 0.27 |
| PMMA + (1) 5wt.% | 24.75 | 66.45 | 0.59 | 8.21 |
| PMMA + (1) 10wt.% | 19.02 | 67.24 | 0.52 | 13.22 |
| PMMA + (1) 20wt.% | 18.77 | 58.68 | 0.48 | 22.07 |
| Sample | g-factor | Species |
|---|---|---|
| (1) | 2.025, 2.011,2.003 | O2- |
| 1.992 | Ti(III) | |
| PMMA | - | - |
| PMMA + (1) 5 wt.% | 2.010, 2.002 | O2- |
| 2.005, 2.000 | O- | |
| 1.992 | Ti(III) | |
| PMMA + (1) 10 wt.% | 2.025, 2.010, 2.000 | O2- |
| 2.016 | O- | |
| 1.992 | Ti(III) | |
| PMMA + (1) 20 wt.% | 2.025, 2.010, 2.002 | O2- |
| 2.016, 2.005, 2.000 | O- | |
| 1.992, 1.972 | Ti(III) |
|
No. |
Microorganisms | |||||
| Samples |
E. coli ATCC 8739 |
E. coli ATCC 25922 |
S. aureus ATCC 6538 |
S. aureus ATCC 25923 |
C. albicans ATCC 10231 |
|
| 1 | (1) 2 wt.% | 6.0 (>99.99%) | 6.0 (>99.99%) | 4.2 (>99.99%) | 5.4 (>99.99%) | 0 (0%) |
| 2 | (1) 5 wt.% | 6.0 (>99.99%) | 5.7 (>99.99%) | 5.9 (>99.99%) | 6.0 (>99.99%) | 0.3 (53.20%) |
| 3 | (1) 10 wt.% | 6.0 (>99.99%) | 6.0 (>99.99%) | 6.2 (>99.99%) | 6.0 (>99.99%) | 0.9 (87.45%) |
| 4 | (1) 20 wt.% | 6.0 (>99.99%) | 6.0 (>99.99%) | 6.5 (>99.99%) | 5.7 (>99.99%) | 6.7 (>99.99%) |
| 5 | PMMA | none (0%) | none (0%) | none (0%) | none (0%) | none (0%) |
| 6 | PMMA + (1) 2 wt.% | 2.0 (99.00%) | 3.1 (>99.90%) | 4.7 (>99.99%) | 5.1 (>99.99%) | +0.9 (+87.41%) |
| 7 | PMMA + (1) 5 wt.% | 3.1 (>99.90%) | 4.9 (>99.99%) | 4.7 (>99.99%) | 5.1 (>99.99%) | +0.82 (+84.86%) |
| 8 | PMMA + (1) 10 wt.% | 4.9 (>99.99%) | 3.9 (>99.90%) | 4.7 (>99.99%) | 5.1 (>99.99%) | +0.8 (+84.20%) |
| 9 | PMMA + (1) 20 wt.% | 4.9 (>99.99%) | 4.7 (>99.99%) | 4.7 (>99.99%) | 5.1 (>99.99%) | +0.7 (+80.05%) |
| Empirical formula | C92 H164 O34 Ti8 (1) |
| Formula weight | 2197.42 |
| Temperature | 100(2) K |
| Wavelength [Å] | 1.54184 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| Unit cell dimensions [Å] and [°] | a = 19.9172(6) |
| b = 12.3935(3) | |
| c = 24.4651(8) | |
| α = 90 | |
| β = 111.483(4) | |
| γ = 90 | |
| Volume [Å3] | 5619.5(3) |
| Z, calculated density [Mg/m3] | 2, 1.299 |
| Absorption coefficient [mm-1] | 5.193 |
| F(000) | 2328 |
| Crystalsize [mm3] | 0.220 x 0.180 x 0.080 |
| Theta range for data collection [°] | 2.384 to 74.492 |
| Index ranges | -24<=h<=24 |
| -15<=k<=14 | |
| -30<=l<=21 | |
| Reflections collected/unique | 44138/ 11218 [R(int) = 0.0943 |
| Completeness to theta | 67.684° 99.9 % |
| Absorption correction | Gaussian |
| Max. and min. transmission | 1.000 and 0.414 |
| Refinementmethod | Full-matrix least-squares on F2 |
| Data / restraints / parameters | 11218 / 35 / 654 |
| Goodness-of-fit on F2 | 1.054 |
| Final R indices [I>2sigma(I)] | R1a = 0.0865, wR2b = 0.2451 |
| R indices (all data) | R1a = 0.1092, wR2b = 0.2674 |
| Largest diff. peak and hole | 0.766 and -0.915 e·Å-3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





