Submitted:
18 March 2024
Posted:
20 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. MTT Assay
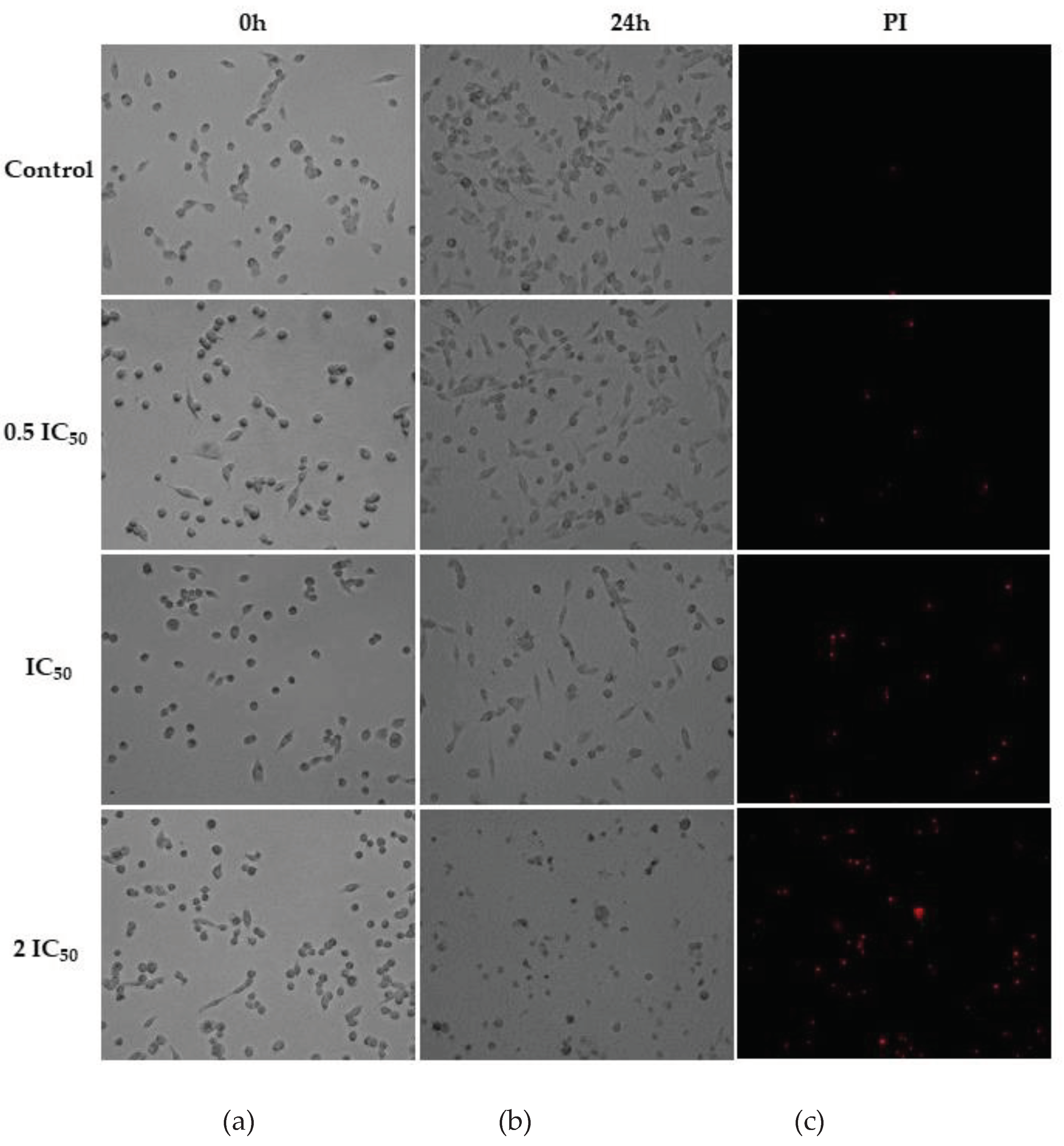
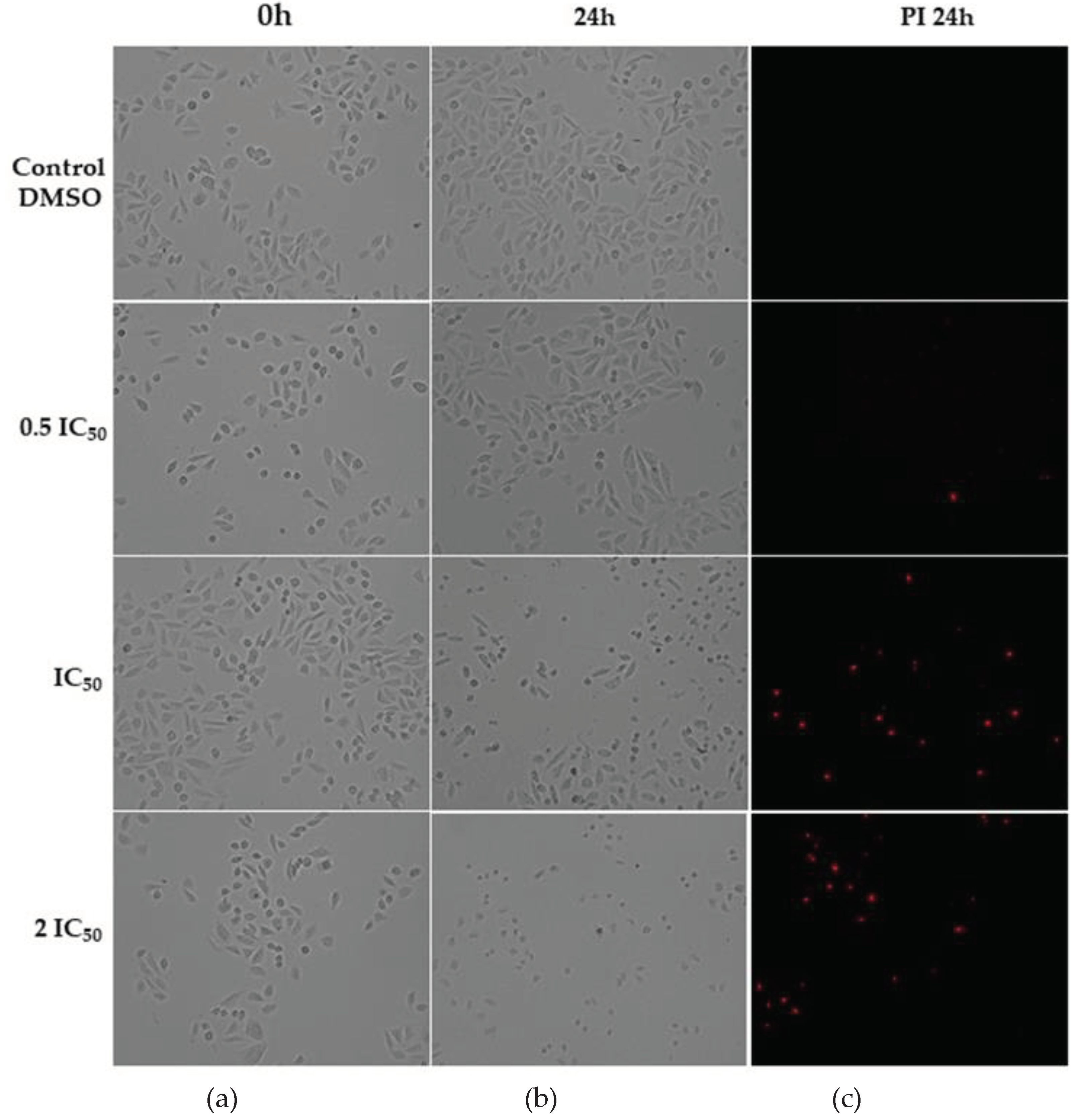
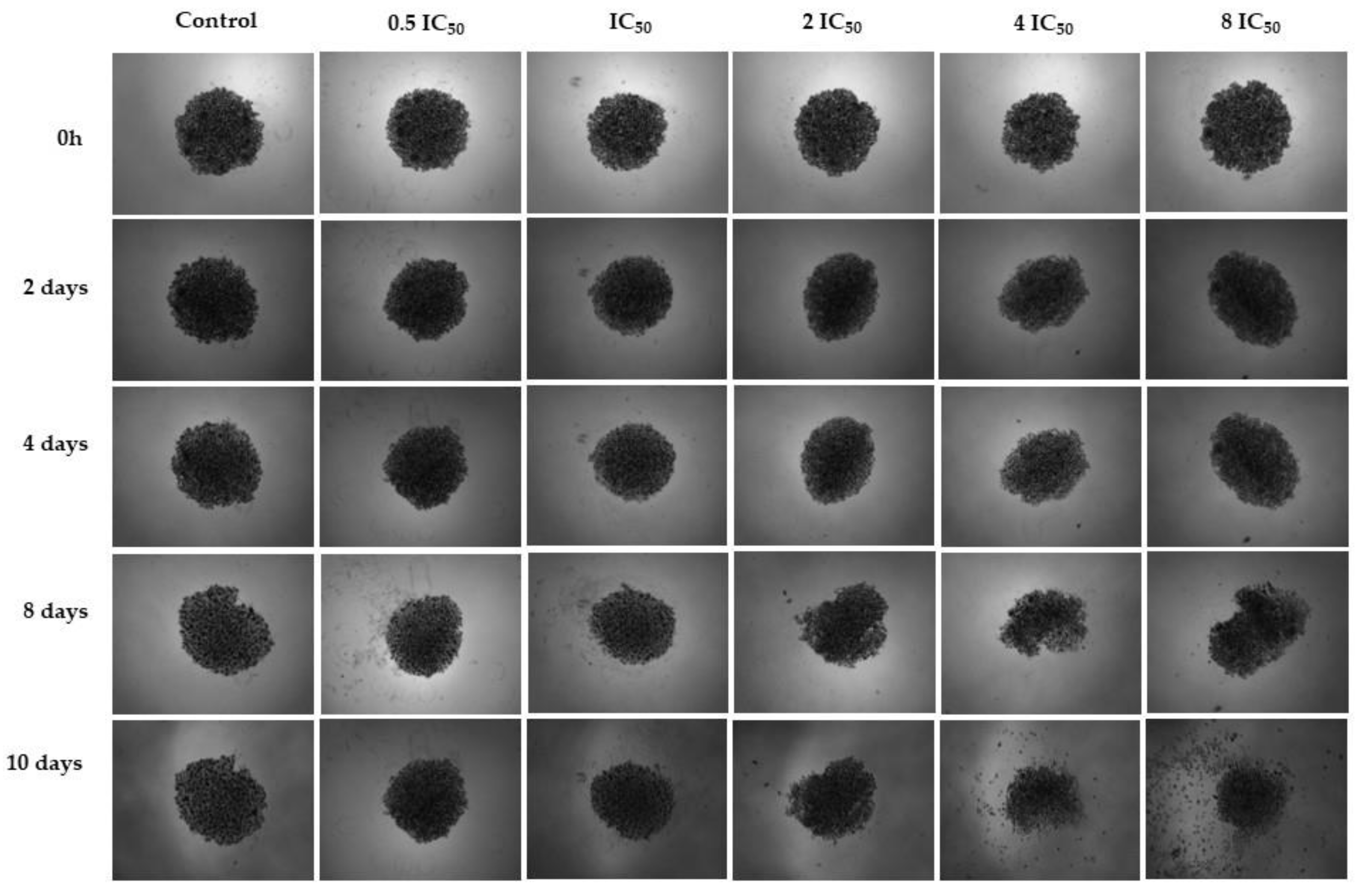
2.2. Cell Morphology Assay
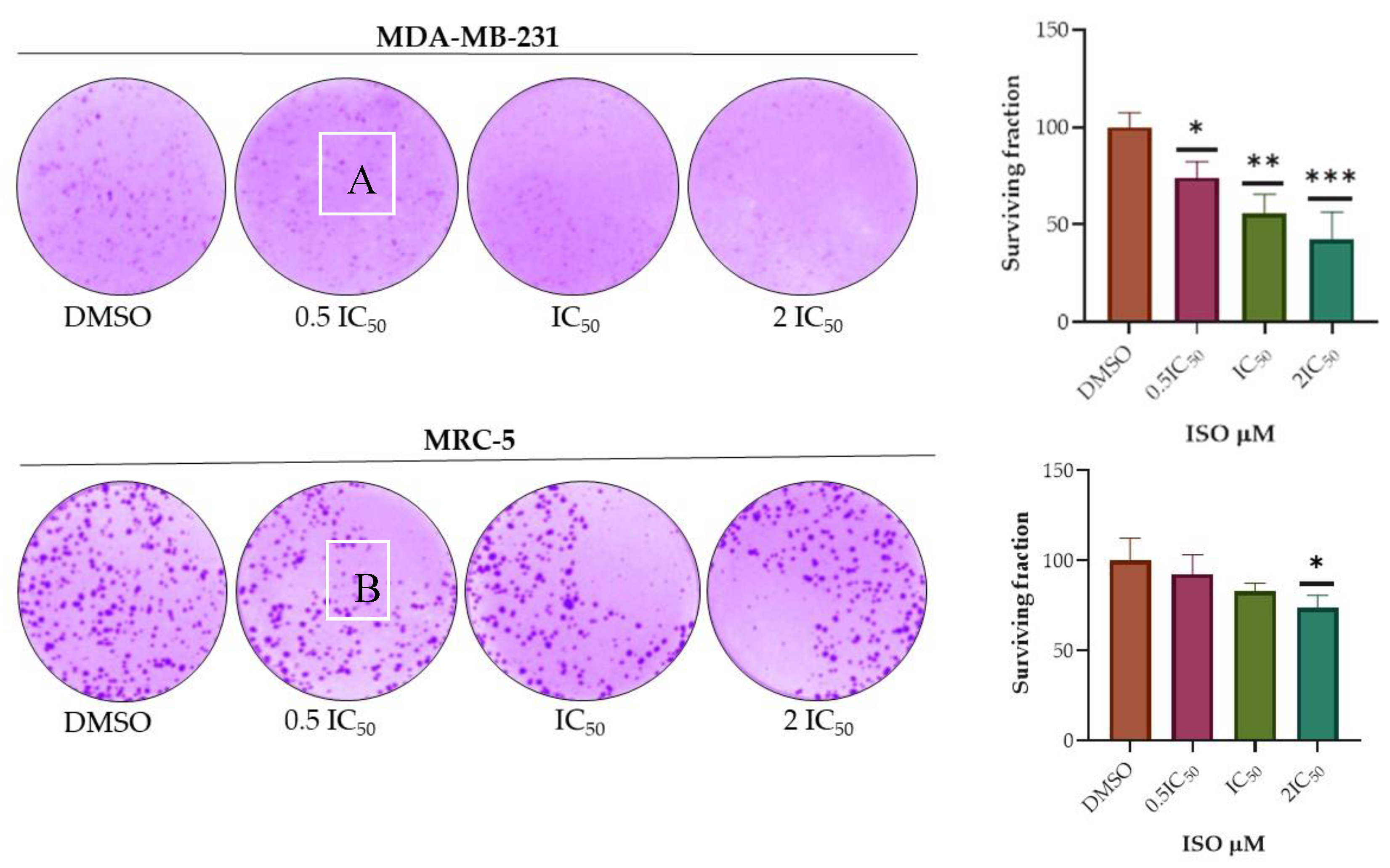
2.3. Clonogenicity Assay
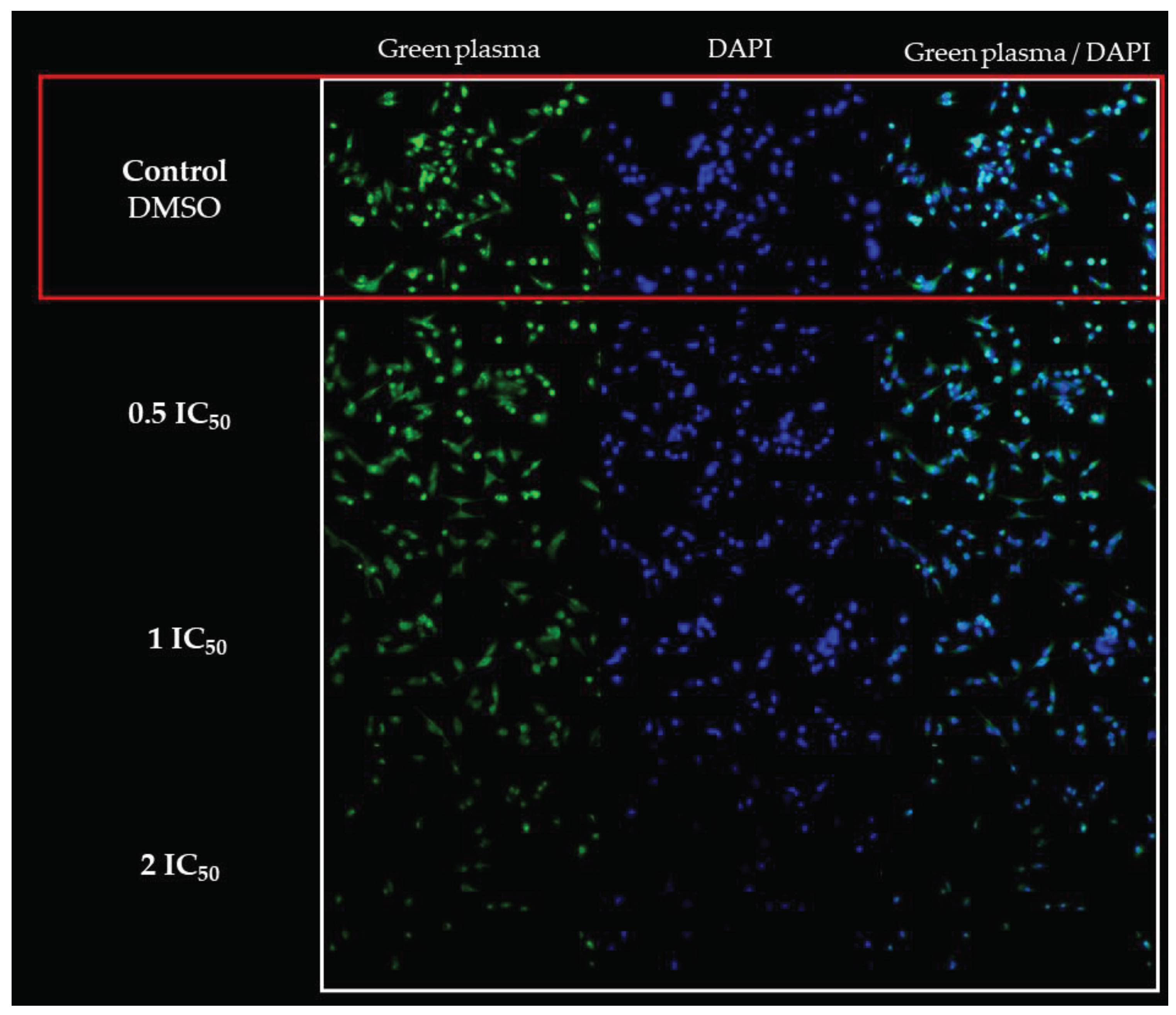
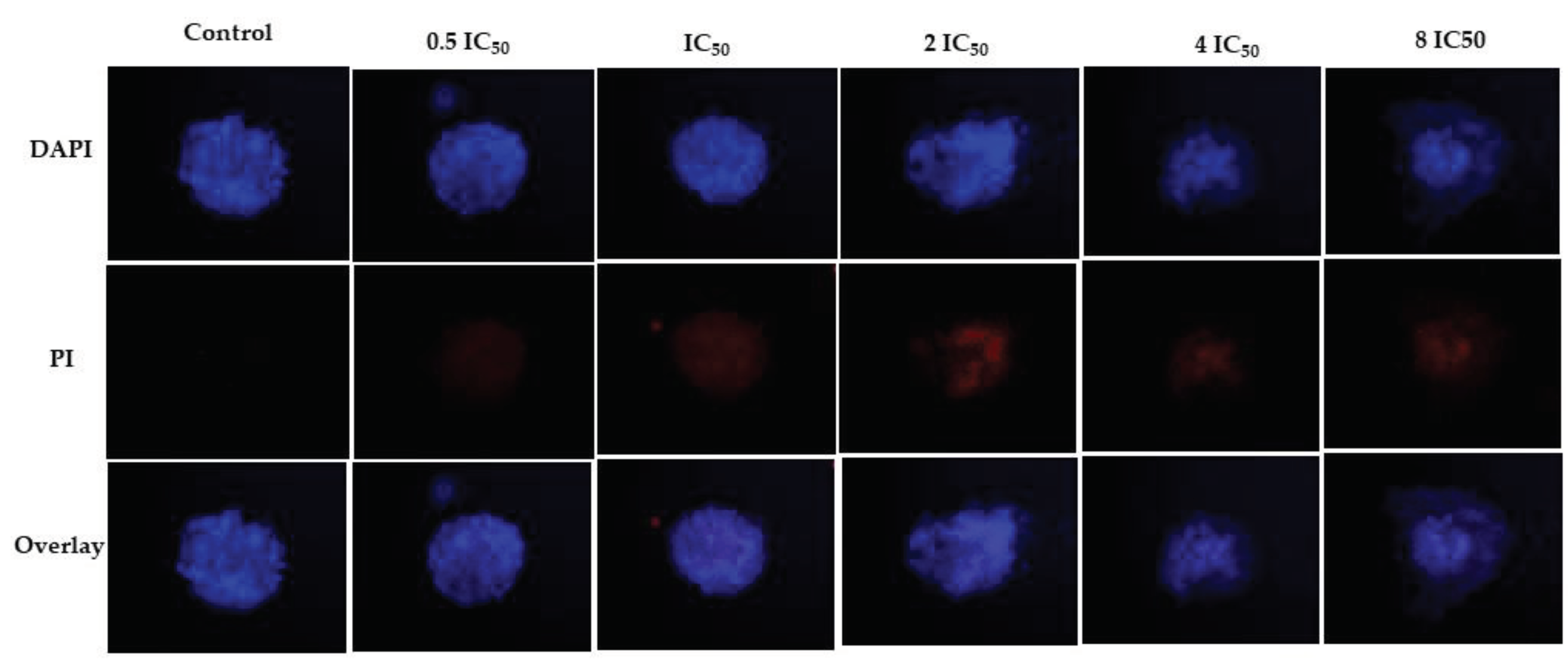
2.4.3. D Assay
3. Discussion
4. Materials and Methods
4.1. Obtaining and Identification of Isoespintanol
4.2. Cell Lines and Reagents
4.3. Cell Cultures
4.4. MTT Assay
4.5. Cell Morphology Assay
4.6. Clonogenicity Assay
- PE = № of colonies formed /№ of seeded cells × 100%
- SF = № of colonies formed after treatment /№ of seeded cells × PE
4.7. D Assay
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Budreviciute, A.; Damiati, S.; Sabir, D. K.; Onder, K.; Schuller-Goetzburg, P.; Plakys, G.; Katileviciute, A.; Khoja, S.; Kodzius, R. Management and prevention strategies for non-communicable diseases (NCDs) and their risk factors. Front. Public Health. 2020, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R. L.; Miller, K. D.; Jemal, A. Cancer statistics, 2017. CA. Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D. M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R. L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R. L.; Torre, L. A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. J.; Lei, K. F.; Han, F. Tumor microenvironment: Recent advances in various cancer treatments. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3855–3864. [Google Scholar] [PubMed]
- Silva, B. I. M.; Nascimento, E. A.; Silva, C. J.; Silva, T. G.; Aguiar, J. S. Anticancer activity of monoterpenes: A systematic review. Mol. Biol. Rep. 2021, 48, 5775–5785. [Google Scholar] [CrossRef]
- Shrestha, S.; Song, Y. W.; Kim, H.; Lee, D. S.; Cho, S. K. Sageone, a diterpene from Rosmarinus officinalis, synergizes with cisplatin cytotoxicity in SNU-1 human gastric cancer cells. Phytomedicine 2016, 23, 1671–1679. [Google Scholar] [CrossRef]
- Wang, H.; Shu, L.; Su, Z.; Fuentes, F.; Lee, J.-H.; Kong, A.-N. T. Plants against cancer: A review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer. Agents Med. Chem. 2012, 12, 1281–1305. [Google Scholar] [CrossRef]
- Shamshoum, H.; Vlavcheski, F.; Tsiani, E. Anticancer effects of oleuropein. BioFactors 2017, 43, 517–528. [Google Scholar] [CrossRef]
- Hajdú, Z.; Hohmann, J.; Forgo, P.; Máthé, I.; Molnár, J.; Zupkó, I. Antiproliferative activity of Artemisia asiatica extract and its constituents on human tumor cell lines. Planta Med. 2014, 80, 1692–1697. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Li, M.; Mao, J.; Zhang, L.; Huang, R.; Jin, X.; Ye, L. Anti-tumor effect of α-pinene on human hepatoma cell lines through inducing G2/M cell cycle arrest. J. Pharmacol. Sci. 2015, 127, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Machado, T. Q.; Da Fonseca, A. C. C.; Duarte, A. B. S.; Robbs, B. K.; De Sousa, D. P. A Narrative review of the antitumor activity of monoterpenes from essential oils: An update. Biomed Res. Int. 2022, 2022. [Google Scholar] [CrossRef]
- Nigjeh, S. E.; Yeap, S. K.; Nordin, N.; Kamalideghan, B.; Ky, H.; Rosli, R. Citral induced apoptosis in MDA-MB-231 spheroid cells. BMC Complement. Altern. Med. 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Chen, M.; Fan, Y.; Li, M.; Wang, A.; Liu, G.; Xu, Y.; Ren, X.; Xiao, Y. Isolation of a new monoterpenoid glycoside from anhua dark tea based on an NMR-guided method and its cytotoxic activity against MDA-MB-231 and SH-SY5Y cell lines. Nat. Prod. Res. 2020, 36, 2015–2020. [Google Scholar] [CrossRef]
- Vandresen, F.; Falzirolli, H.; Almeida Batista, S. A.; Da Silva-Giardini, A. P. B.; De Oliveira, D. N.; Catharino, R. R.; Ruiz, A. L. T. G.; De Carvalho, J. E.; Foglio, M. A.; Da Silva, C. C. Novel R-(+)-limonene-based thiosemicarbazones and their antitumor activity against human tumor cell lines. Eur. J. Med. Chem. 2014, 79, 110–116. [Google Scholar] [CrossRef]
- Morales, I.; De La Fuente, J.; Sosa, V. Componentes de Eupatorium saltense. An Asoc Quim Argent 1991, 79, 141–144. [Google Scholar]
- Hocquemiller, R.; Cortes, D.; Arango, G. J.; Myint, S. H.; Cave, A. Isolement et synthese de L´espintanol, nouveau monoterpene antiparasitaire. J. Nat. Prod. 1991, 54, 445–452. [Google Scholar] [CrossRef]
- Rojano, B.; Sáez, J.; Schinella, G.; Quijano, J.; Vélez, E.; Gil, A.; Notario, R. Experimental and theoretical determination of the antioxidant properties of isoespintanol (2-Isopropyl-3,6-Dimethoxy-5-Methylphenol). J. Mol. Struct. 2008, 877, 1–6. [Google Scholar] [CrossRef]
- Rojano, B.; Pérez, E.; Figadère, B.; Martin, M. T.; Recio, M. C.; Giner, R.; Ríos, J. L.; Schinella, G.; Sáez, J. Constituents of Oxandra Cf. xylopioides with anti-inflammatory activity. J. Nat. Prod. 2007, 70, 835–838. [Google Scholar] [CrossRef]
- Gavilánez Buñay, T. C.; Colareda, G. A.; Ragone, M. I.; Bonilla, M.; Rojano, B. A.; Schinella, G. R.; Consolini, A. E. Intestinal, urinary and uterine antispasmodic effects of isoespintanol, metabolite from Oxandra xylopioides leaves. Phytomedicine 2018, 51, 20–28. [Google Scholar] [CrossRef]
- Rinaldi, G. J.; Rojano, B.; Schinella, G.; Mosca, S. M. Participation of NO in the vasodilatory action of isoespintanol. Vitae 2019, 26, 78–83. [Google Scholar] [CrossRef]
- Usuga, A.; Tejera, I.; Gómez, J.; Restrepo, O.; Rojano, B.; Restrepo, G. Cryoprotective effects of ergothioneine and isoespintanol on canine semen. Animals 2021, 11, 2757. [Google Scholar] [CrossRef]
- Rojano, B. A.; Montoya, S.; Yépez, F.; Sáez, J. Evaluación de isoespintanol aislado de Oxandra Cf. xylopioides (Annonaceae) sobre Spodoptera frugiperda J.E. Smith (Lepidoptera: Noctuidae). 2007, 60, 3691–3702. [Google Scholar]
- Arango, N.; Vanegas, N.; Sáez, J.; García, C.; Rojano, B. Actividad antifungica del isoespintanol sobre hongos del género Colletotricum. Sci. Tech. 2007, 33, 279–280. [Google Scholar]
- Contreras Martínez, O. I.; Angulo Ortíz, A.; Santafé Patiño, G. Antibacterial screening of isoespintanol, an aromatic monoterpene isolated from Oxandra xylopioides Diels. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Contreras Martínez, O. I.; Angulo Ortíz, A.; Santafé Patiño, G. Antifungal potential of isoespintanol extracted from Oxandra xylopioides Diels (Annonaceae) against intrahospital isolations of Candida spp. Heliyon 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Contreras Martínez, O. I.; Angulo Ortíz, A.; Santafé Patiño, G.; Peñata-Taborda, A.; Berrio Soto, R. Isoespintanol antifungal activity involves mitochondrial dysfunction, inhibition of biofilm formation, and damage to cell wall integrity in Candida tropicalis. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef]
- Contreras Martínez, O. I.; Angulo Ortíz, A.; Santafé Patiño, G. Mechanism of antifungal action of monoterpene isoespintanol against clinical isolates of Candida tropicalis. Molecules 2022, 27, 5808. [Google Scholar] [CrossRef]
- Marquez-Fernandez, M.; Munoz-Lasso, D.; Bautista Lopez, J.; Zapata, K.; Puertas Mejia, M.; Lopez-Alarcon, C.; Rojano, B. A. Effect of isoespintanol isolated from Oxandra Cf. xylopioides against DNA damage of human lymphocytes. Pak. J. Pharm. Sci 2018, 31, 1777–1782. [Google Scholar]
- Zapata, K.; Arias, J.; Cortés, F.; Alarcon, C.; Durango, D.; Rojano, B. Oxidative stabilization of palm olein with isoespintanol (2-Isopropyl-3,6-Dimethoxy-5-Methylphenol) isolated from Oxandra Cf xylopioides. J. Med. Plants Res. 2017, 11, 218–225. [Google Scholar] [CrossRef]
- Jaganathan, H.; Gage, J.; Leonard, F.; Srinivasan, S.; Souza, G. R.; Dave, B.; Godin, B. Three-dimensional in vitro co-culture model of breast tumor using magnetic levitation. Sci. Rep. 2014, 4, 1–9. [Google Scholar] [CrossRef]
- Brajša, K.; Trzun, M.; Zlatar, I.; Jelić, D. Three-dimensional cell cultures as a new tool in drug discovery. Period. Biol. 2016, 118, 59–65. [Google Scholar] [CrossRef]
- Yamada, K. M.; Cukierman, E. Modeling tissue morphogenesis and cancer in 3D. Cell 2007, 130, 601–610. [Google Scholar] [CrossRef]
- Timm, D. M.; Chen, J.; Sing, D.; Gage, J. A.; Haisler, W. L.; Neeley, S. K.; Raphael, R. M.; Dehghani, M.; Rosenblatt, K. P.; Killian, T. C.; Tseng, H.; Souza, G. R. A High-throughput three-dimensional cell migration assay for toxicity screening with mobile device-based macroscopic image analysis. Sci. Rep. 2013, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yu, P.; Tang, J. Characterization of triple-negative breast cancer MDA-MB-231 cell spheroid model. Onco. Targets. Ther. 2020, 13, 5395–5405. [Google Scholar] [CrossRef]
- Liao, Z.; Tan, Z. W.; Zhu, P.; Tan, N. S. Cancer-associated fibroblasts in tumor microenvironment – accomplices in tumor malignancy. Cell. Immunol. 2018, 343, 0–1. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. Addressing cancer’s grand challenges. Nat. Rev. Drug Discov. 2020, 19, 825–826. [Google Scholar] [CrossRef]
- Aylate, A.; Agize, M.; Ekero, D.; Kiros, A.; Ayledo, G.; Gendiche, K. In-vitro and in-vivo antibacterial activities of Croton macrostachyus methanol extract against E. coli and S. aureus. Adv. Anim. Vet. Sci. 2017, 5, 107–114. [Google Scholar] [CrossRef]
- Chaouki, W.; Leger, D. Y.; Liagre, B.; Beneytout, J. L.; Hmamouchi, M. Citral inhibits cell proliferation and induces apoptosis and cell cycle arrest in MCF-7 cells. Fundam. Clin. Pharmacol. 2009, 23, 549–556. [Google Scholar] [CrossRef]
- Avato, P. Editorial to the special issue –“Natural products and drug discovery". Molecules 2020, 25, 1128. [Google Scholar] [CrossRef] [PubMed]
- Crowell, P. L. Prevention and therapy of cancer by dietary monoterpenes. J. Nutr. 1999, 129, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Filho, H. G. de; dos Santos, J. F.; Carvalho, M. T. B.; Picot, L.; Fruitier-Arnaudin, I.; Groult, H.; Quintans-Júnior, L. J.; Quintans, J. S. S. Anticancer activity of limonene: A systematic review of target signaling pathways. Phyther. Res. 2021, 35, 4957–4970. [Google Scholar] [CrossRef] [PubMed]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M. K. D-limonene: A multifunctional compound with potent therapeutic effects. J. Food Biochem. 2021, 45, 1–10. [Google Scholar] [CrossRef]
- Mączka, W.; Wińska, K.; Grabarczyk, M. One hundred faces of geraniol. Molecules. 2020, 25, 3303. [Google Scholar] [CrossRef]
- Silva, G. dos S. E.; Marques, J. N. de J.; Linhares, E. P. M.; Bonora, C. M.; Costa, É. T.; Saraiva, M. F. Review of anticancer activity of monoterpenoids: Geraniol, nerol, geranial and neral. Chem. Biol. Interact. 2022, 362. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Khan, I. A.; Shahbaz, M.; Qaisrani, T. B.; Fatmawati, S.; Abu-Izneid, T.; Imran, A.; Rahman, K. U.; Gondal, T. A. Thymoquinone: A novel strategy to combat cancer: A review. Biomed. Pharmacother. 2018, 106, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Kim, C. W.; Choi, K. C. Potential roles of iridoid glycosides and their underlying mechanisms against diverse cancer growth and metastasis: Do they have an inhibitory effect on cancer progression? Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide—natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Park, K. R.; Nam, D.; Yun, H. M.; Lee, S. G.; Jang, H. J.; Sethi, G.; Cho, S. K.; Ahn, K. S. β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/MTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011, 312, 178–188. [Google Scholar] [CrossRef]
- Borella, R.; Forti, L.; Gibellini, L.; De Gaetano, A.; De Biasi, S.; Nasi, M.; Cossarizza, A.; Pinti, M. Synthesis and anticancer activity of CDDO and CDDO-Me, two derivatives of natural triterpenoids. Molecules 2019, 24, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Motlhatlego, K.; Ali, M.; Leonard, C.; Eloff, J.; McGaw, L. Inhibitory effect of newtonia extracts and myricetin-3-O-rhamnoside (myricitrin) on bacterial biofilm formation. BMC Complement. Med. Ther. 2020, 20, 358. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, C.; Bagga, M.; Kaur, A.; Westermarck, J.; Abankwa, D. Colony Area: An imageJ plugin to automatically quantify colony formation in clonogenic assays. PLoS One 2014, 9, 14–17. [Google Scholar] [CrossRef]
- Franken, N. A. P.; Rodermond, H. M.; Stap, J.; Haveman, J.; Van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Buch, K.; Peters, T.; Nawroth, T.; Sänger, M.; Schmidberger, H.; Langguth, P. Determination of cell survival after irradiation via clonogenic assay versus multiple MTT assay - a comparative study. Radiat. Oncol. 2012, 7, 1–6. [Google Scholar] [CrossRef]
| Cell lines | IC50 ISO (µM) |
|---|---|
| MDA-MB-231 | 52.39 ± 3.20 |
| A549 | 59.70 ± 2.74 |
| DU145 | 47.84 ± 3.52 |
| A2780 Cis | 60.35 ± 8.4 |
| A2780 | 42.15 ± 1.39 |
| MRC5 | 39.95 ± 3.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





