Submitted:
19 March 2024
Posted:
20 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
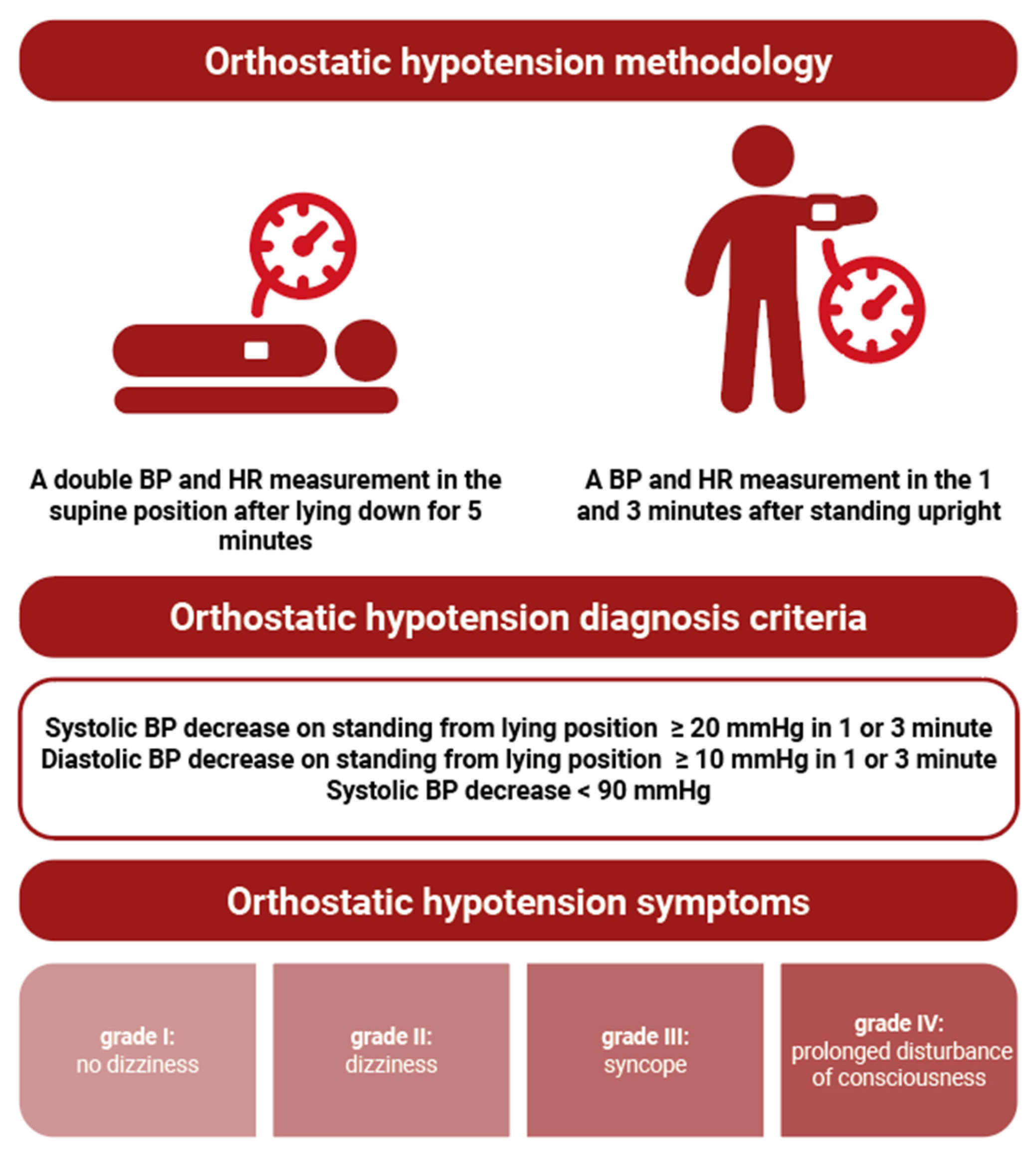
2. Materials and Methods
Statistical Analysis
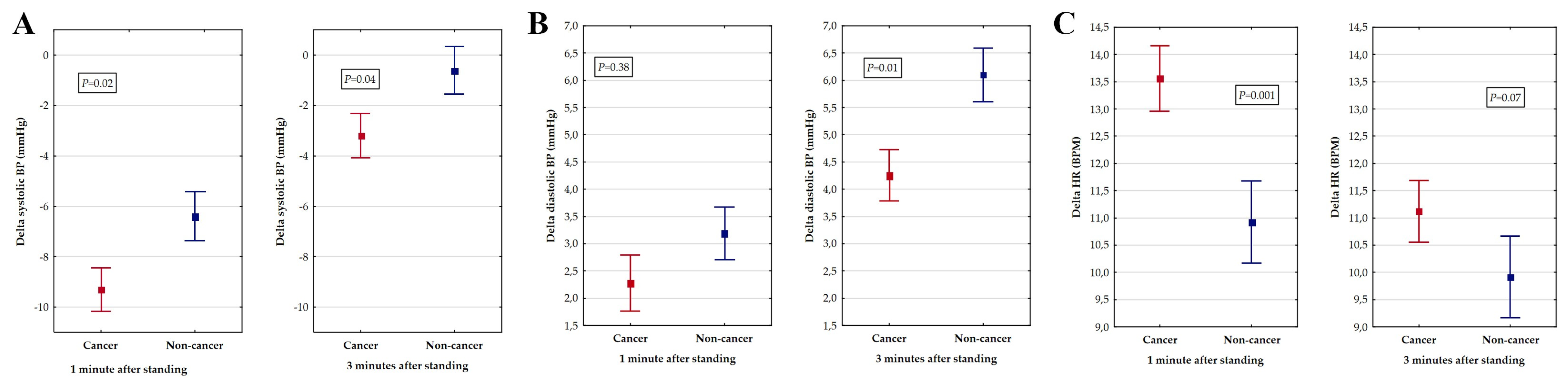
3. Results
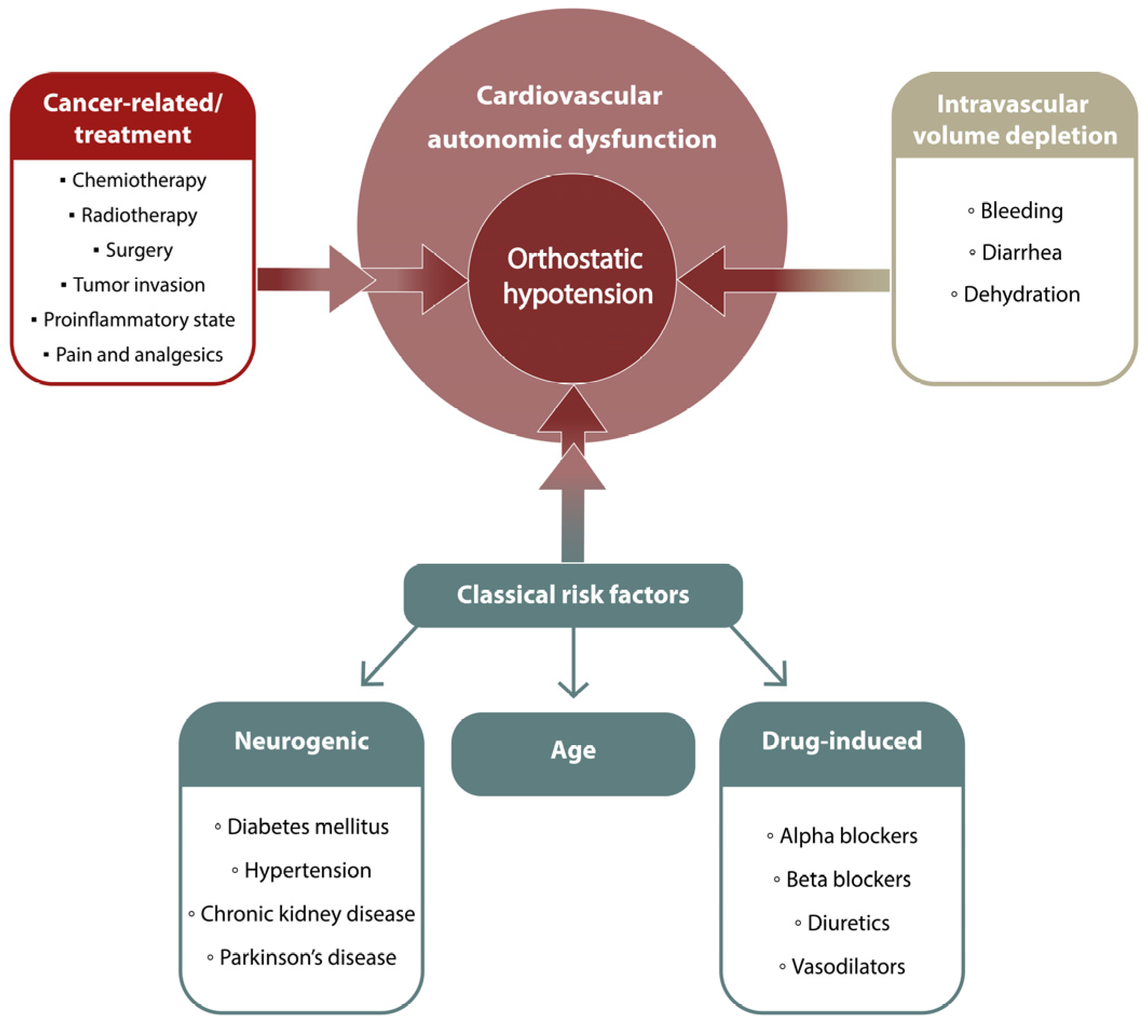
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Magkas, N.; Tsioufis, C.; Thomopoulos, C.; Dilaveris, P.; Georgiopoulos, G.; Sanidas, E.; Papademetriou, V.; Tousoulis, D. Orthostatic hypotension: From pathophysiology to clinical applications and therapeutic considerations. J. Clin. Hypertens. 2019, 21, 546–554. [Google Scholar] [CrossRef]
- Freeman, R.; Abuzinadah, A.R.; Gibbons, C.; Jones, P.; Miglis, M.G.; Sinn, D.I. Orthostatic Hypotension: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 1294–1309. [Google Scholar] [CrossRef]
- Walsh, D.; Nelson, K.A. Autonomic nervous system dysfunction in advanced cancer. Support. Care Cancer 2002, 10, 523–528. [Google Scholar] [CrossRef]
- Noor, B.; Akhavan, S.; Leuchter, M.; Yang, E.H.; Ajijola, O.A. Quantitative assessment of cardiovascular autonomic impairment in cancer survivors: a single center case series. Cardiooncology 2020, 6, 11. [Google Scholar] [CrossRef]
- Teng, A.E.; Noor, B.; Ajijola, O.A.; Yang, E.H. Chemotherapy and Radiation-Associated Cardiac Autonomic Dysfunction. Curr Oncol Rep. 2021, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; Cutter, D.J.; de Azambuja, E.; de Boer, R.A.; Dent, S.F.; Farmakis, D.; Gevaert, S.A.; Gorog, D.A.; Herrmann, J.; Lenihan, D.; Moslehi, J.; Moura, B.; Salinger, S.S.; Stephens, R.; Suter, T.M.; Szmit, S.; Tamargo, J.; Thavendiranathan, P.; Tocchetti, C.G.; van der Meer, P.; van der Pal, H.J.H.; ESC Scientific Document Group. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Mosnaim, A.D.; Abiola, R.; Wolf, M.E.; Perlmuter, L.C. Etiology and risk factors for developing orthostatic hypotension. Am. J. Ther. 2010, 17, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Streeten, D.H., Jr. Anderson, G.H. Mechanisms of orthostatic hypotension and tachycardia in patients with pheochromocytoma. Am. J. Hypertens. 1996, 9, 760–769. [Google Scholar] [CrossRef]
- Gosney, M.A.; Gosney, J.R.; Lye, M. Orthostatic hypotension and vasodilatory peptides in bronchial carcinoma. J. Clin. Pathol. 1995, 48, 1102–1105. [Google Scholar] [CrossRef]
- Tykarski, A.; Filipiak, K.J.; Januszewicz, A.; Litwin, M.; Narkiewicz, K.; Prejbisz, A.; Ostalska-Nowicka, D.; Widecka, K.; Kostka-Jeziorny, K. Zasady postępowania w nadciśnieniu tętniczym – 2019 rok. Wytyczne Polskiego Towarzystwa Nadciśnienia Tętniczego. Nadciśnienie Tętnicze w Praktyce. 2019, 5, 1–86. [Google Scholar]
- Lavi, S.; Aharon-Peretz, J.; Haim, N.; Vlodavsky, E.; Jacob, G. Unusual cause of partially reversible severe cardiovascular autonomic failure. Am. J. Med. Sci. 2003, 326, 159–163. [Google Scholar] [CrossRef]
- Shah, R.V.; Patel, K.P.; Manion, C.; Runkana, A.; Hama Amin, A.; Jain, A. Third-degree atrioventricular block followed by syncope, labile hypertension, and orthostatic hypotension in a patient with nasopharyngeal cancer: baroreflex failure. Am. J. Cardiovasc. Dis. 2018, 8, 39–42. [Google Scholar] [PubMed]
- Ashraf, W.; Farrow, L.J. Severe orthostatic hypotension in carcinoma of the pancreas. Br. J. Clin. Pract. 1992, 46, 278–279. [Google Scholar] [CrossRef] [PubMed]
- Bortnik, M.; Occhetta, E.; Marino, P. Orthostatic hypotension as an unusual clinical manifestation of pheochromocytoma: a case report. J. Cardiovasc. Med. 2008, 9, 839–841. [Google Scholar] [CrossRef]
- Lee, S.; Chary, M.; Petersen, B.; Mascarenhas, J.O. Paraneoplastic orthostatic hypotension associated with acute myeloid leukemia. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2934–2937. [Google Scholar] [PubMed]
- Sharabi, Y.; Dendi, R.; Holmes, C.; Goldstein, D.S. Baroreflex failure as a late sequela of neck irradiation. Hypertension. 2003, 42, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, H.; Norcliffe-Kaufmann, L.; Palma, J.A. Baroreflex Dysfunction. N. Engl. J. Med. 2020, 382, 163–178. [Google Scholar] [CrossRef]
- Dermitzakis, E.V.; Kimiskidis, V.K.; Lazaridis, G.; Alexopoulou, Z.; Timotheadou, E.; Papanikolaou, A.; Romanidou, O.; Georgiadis, G.; Kalogeras, K.T.; Tsiptsios, I.; Tarlatzis, B.; Fountzilas, G. The impact of paclitaxel and carboplatin chemotherapy on the autonomous nervous system of patients with ovarian cancer. BMC Neurol. 2016, 16, 190. [Google Scholar] [CrossRef]
- Vassilomanolakis, M.; Koumakis, G.; Demiri, M.; Missitzis, J.; Barbounis, V.; Efremidis, A.P. Vinorelbine and cisplatin for metastatic breast cancer: a salvage regimen in patients progressing after docetaxel and anthracycline treatment. Cancer Invest. 2003, 21, 497–504. [Google Scholar] [CrossRef]
- Ho, M.; Moscvin, M.; Low, S.K.; Evans, B.; Close, S.; Schlossman, R.; Laubach, J.; Prada, C.P.; Glotzbecker, B.; Richardson, P.G.; Bianchi, G. Risk factors for the development of orthostatic hypotension during autologous stem cell transplant in patients with multiple myeloma. Leuk. Lymphoma. 2022, 63, 2403–2412. [Google Scholar] [CrossRef]
- Vecchié, A.; Thomas, G.; Bressi, E.; Bonaventura, A.; Canada, J.M.; Chuquin, D.; Kadariya, D.; Piracha, U.; Endicott, D.; Markley, R.; Toor, A.; Hess, M.; Abbate, A. Orthostatic intolerance syndromes after hematopoietic cell transplantation: clinical characteristics and therapeutic interventions in a single-center experience. Cardiooncology. 2021, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Fradley, M.G.; Beckie, T.M.; Brown, S.A.; Cheng, R.K.; Dent, S.F.; Nohria, A.; Patton, K.K.; Singh, J.P.; Olshansky, B. Recognition, Prevention, and Management of Arrhythmias and Autonomic Disorders in Cardio-Oncology: A Scientific Statement From the American Heart Association. Circulation. 2021, 144, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, J.R.; Munk-Madsen, P.; Kehlet, H.; Gögenur, I. Orthostatic intolerance in enhanced recovery laparoscopic colorectal resection. Acta Anaesthesiol. Scand. 2019, 63, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Unaldi, H.E. Effects of mobilization within the first 4 h following anatomical lung resection with thoracotomy. Updates Surg. 2023, 75, 2027–2031. [Google Scholar] [CrossRef]
- Nakada, T.; Shirai, S.; Oya, Y.; Takahashi, Y.; Sakakura, N.; Ohtsuka, T.; Kuroda, H. Four Hours Postoperative Mobilization is Feasible After Thoracoscopic Anatomical Pulmonary Resection. World J. Surg. 2021, 45, 631–637. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Wang, Y.; Feng, F.; Wang, H.; Zhang, Y.; Lin, X.; Xu, B. Lambert-Eaton Myasthenic Syndrome in Lung Cancer. Contrast Media Mol. Imaging. 2022. [Google Scholar] [CrossRef]
- Worrall, A.P.; Doyle, C.P.; Ní Dhomhnall, R.; Lorton, C.; Barrett, M.; Uí Dhuibhir, P.; O’Higgins, C.; Brady, B.; Walsh, D. Blood Pressure, Orthostatic Hypotension and Falls in Patients with Advanced Cancer. Ir. Med. J. 2022, 115, 596. [Google Scholar]
- Ricci, F.; De Caterina, R.; Fedorowski, A. Orthostatic Hypotension: Epidemiology, Prognosis, and Treatment. J. Am. Coll. Cardiol. 2015, 66, 848–860. [Google Scholar] [CrossRef]
- Fedorowski, A.; Ricci, F.; Sutton, R. Orthostatic hypotension and cardiovascular risk. Kardiol. Pol. 2019, 77, 1020–1027. [Google Scholar] [CrossRef]
- Low, P.A. Prevalence of ortostatic hypotension. Clin. Auton. Res. 2008, 18, 8–13. [Google Scholar] [CrossRef]
- Fedorowski, A.; Ricci, F.; Hamrefors, V.; Sandau, K.E.; Hwan Chung, T.; Muldowney, J.A.S.; Gopinathannair, R.; Olshansky, B. Orthostatic Hypotension: Management of a Complex, But Common, Medical Problem. Circ. Arrhythm. Electrophysiol. 2022, 15, e010573. [Google Scholar] [CrossRef]
- Ricci, F.; Radico, F.; Romanello, M. Morbidity and mortality related to orthostatic hypotension: results of a meta-analysis of non-randomized observational studies. Eur. Heart J. 2013, 34, 4462. [Google Scholar] [CrossRef]
- Ricci, F.; Fedorowski, A.; Radico, F.; Romanello, M.; Tatasciore, A.; Di Nicola, M.; Zimarino, M.; De Caterina, R. Cardiovascular morbidity and mortality related to orthostatic hypotension: a meta-analysis of prospective observational studies. Eur. Heart J. 2015, 36, 1609–1617. [Google Scholar] [CrossRef]
- Ali, A.; Ali, N.S.; Waqas, N.; Bhan, C.; Iftikhar, W.; Sapna, F.; Jitidhar, F.; Cheema, A.M.; Ahmad, M.Q.; Nasir, U.; Sami, S.A.; Zulfiqar, A.; Ahmed, A. Management of Orthostatic Hypotension: A Literature Review. Cureus. 2018, 10, 3166. [Google Scholar] [CrossRef]
- Rivasi, G.; Rafanelli, M.; Mossello, E.; Brignole, M.; Ungar, A. Drug-Related Orthostatic Hypotension: Beyond Anti-Hypertensive Medications. Drugs Aging. 2020, 37, 725–738. [Google Scholar] [CrossRef]
- Sever, P. Severe orthostatic hypotension and weight loss associated with cancer therapy. Br. J. Cardiol. 2021, 28, 40. [Google Scholar] [CrossRef] [PubMed]
- Grześkowiak, A.; Rojek, A.; Szyndler, A.; Śmiałek, K.; Szczęch, R.; Chrostowska, M.; Narkiewicz, K. Prevalence of postural hypotension in patients with treated hypertension. Arterial Hypertension. 2005, 9, 452–457, https://journals.viamedica.pl/arterial_hypertension/article/view/12599. [Google Scholar]
- Argilés, J.M.; López-Soriano, F.J.; Stemmler, B.; Busquets, S. Cancer-associated cachexia - understanding the tumour macroenvironment and microenvironment to improve management. Nat. Rev. Clin. Oncol. 2023, 20, 250–264. [Google Scholar] [CrossRef]
- van Hateren, K.J.; Kleefstra, N.; Blanker, M.H.; Ubink-Veltmaat, L.J.; Groenier, K.H.; Houweling, S.T.; Kamper, A.M. , van der Meer, K.; Bilo, H.J. Orthostatic hypotension, diabetes, and falling in older patients: a cross-sectional study. Br. J. Gen. Pract. 2012, 62, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Beretta, M.V.; Milan, V.B.; Hoffmeister, M.C.; Rodrigues, T.C. Orthostatic hypotension, falls and in-hospital mortality among elderly patients with and without type 2 diabetes. J. Hypertens. 2023, 41, 388–392. [Google Scholar] [CrossRef]
| Hospital Department | Number of Cases | % |
|---|---|---|
| Clinical Oncology | 106 | 25.8 |
| Oncological Gynecology | 58 | 14.1 |
| Radiotherapy | 56 | 13.6 |
| Neurology | 108 | 26.3 |
| Internal diseases | 49 | 11.9 |
| Dermatology | 20 | 4.9 |
| Rehabilitation | 10 | 2.4 |
| Urology | 4 | 1.0 |
| Total | 411 | 100 |
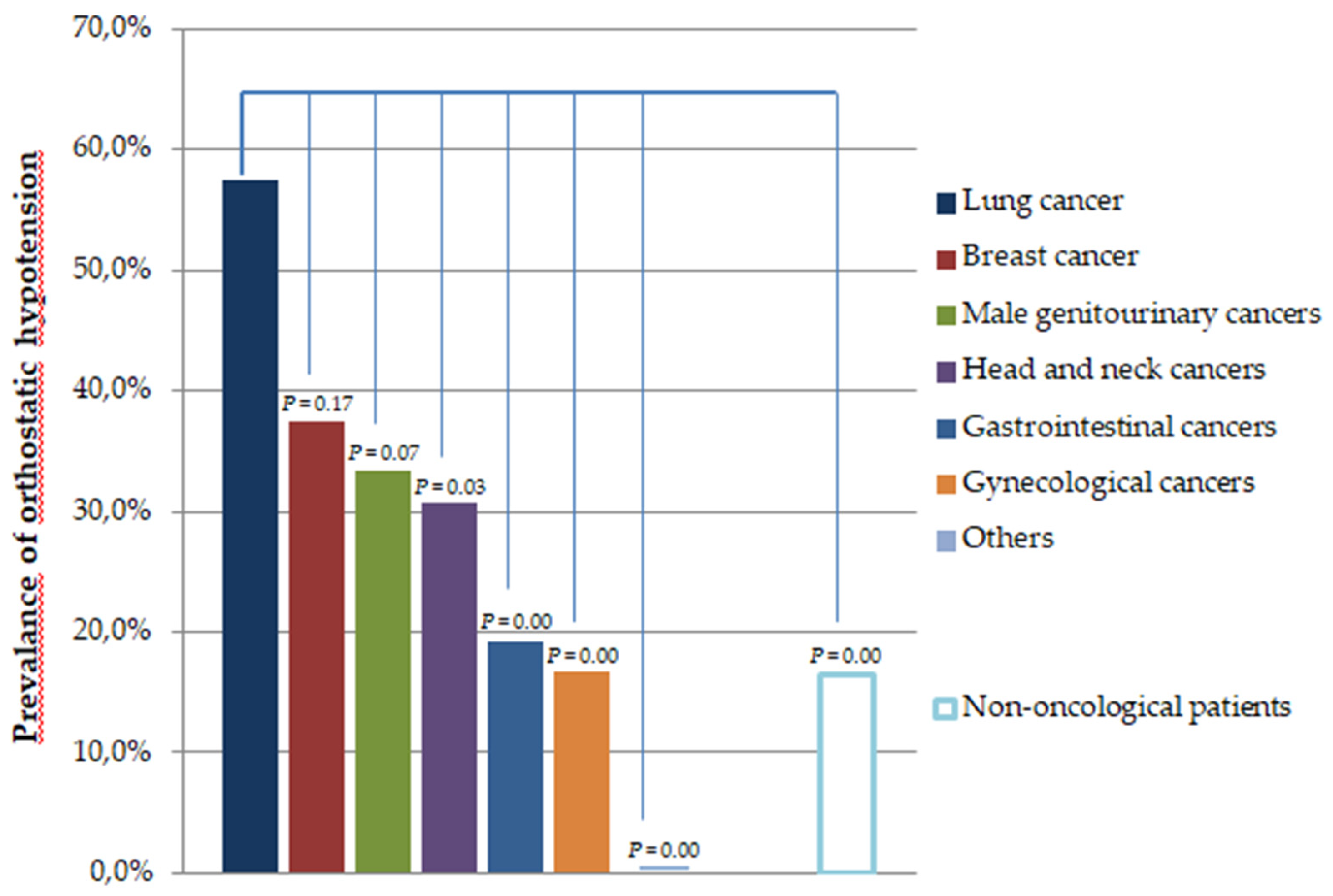
| Type of cancer | Number of Cases | % |
| Lung cancer | 40 | 17.9 |
| Breast cancer | 16 | 7.2 |
| Male genitourinary cancers | 21 | 9.4 |
| Head and neck cancers | 26 | 11.7 |
| Gastrointestinal cancers | 52 | 23.3 |
| Gynecological cancers | 60 | 26.9 |
| Others | 8 | 3.6 |
| Total | 223 | 100 |
| Oncological (n = 223) | Non-oncological (n = 188) | P-value | |
|---|---|---|---|
| Age in years, mean (SD) | 65.0 (8.9) | 61.5 (12.2) | 0.01 |
| Female sex, n (%) | 116 (52.0) | 113 (60.1) | 0.09 |
| BMI (kg/m2), mean (SD) | 26.2 (5.7) | 28.1 (5.0) | 0.01 |
| SBP (SD) | 127.7 (16.6) | 129.2 (18.4) | 0.35 |
| DBP (SD) | 80.0 (10.4) | 79.3 (9.7) | 0.42 |
| HR (SD) | 69.4 (12.4) | 78.4 (14.0) | 0.001 |
| Orthostatic hypotension, n (%) | 64 (28.7) | 31 (16.5) | 0.003 |
| Orthostatic hypotension symptoms: grade I, n (%) grade II, n (%) grade III, n (%) grade IV, n (%) |
198 (88.8) 25 (11.2) 0 0 |
187 (99.5) 1 (0.5) 0 0 |
0.00001 |
| Hypertension, n (%) | 114 (51.1) | 98 (52.1) | 0.83 |
| CAD, n (%) | 22 (9.9) | 21 (11.2) | 0.66 |
| Stroke, n (%) | 5 (2.2) | 9 (4.8) | 0.15 |
| Dyslipidemia, n (%) | 25 (11.2) | 43 (22.9) | 0.001 |
| Diabetes, n (%) | 38 (17.0) | 48 (25.5) | 0.03 |
| CKD, n (%) | 5 (2.2) | 7 (3.7) | 0.37 |
| Thyroid diseases, n (%) | 19 (8.5) | 20 (10.6) | 0.46 |
| VTE, n (%) | 15 (6.7) | 7 (3.7) | 0.17 |
| PD, n (%) | 1 (0.5) | 3 (1.6) | 0.23 |
| Diuretics, n (%) | 42 (18.8) | 27 (14.4) | 0.22 |
| ACEi, n (%) | 59 (26.5) | 56 (29.8) | 0.45 |
| ARBs, n (%) | 20 (9.0) | 22 (11.7) | 0.36 |
| Beta- blockers, n (%) | 82 (36.8) | 60 (31.9) | 0.30 |
| Nitrates, n (%) | 10 (4.5) | 6 (3.2) | 0.49 |
| Alpha-blockers, n (%) | 5 (2.2) | 6 (3.2) | 0.55 |
| CCB, n (%) | 34 (15.3) | 38 (20.2) | 0.18 |
| Antiparkinsonian agents, n (%) | 1 (0.5) | 3 (1.6) | 0.23 |
| Variable | Single-variable analysis | Multivariable analysis | ||||
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Cancer | 2.04 | 1.26-3.31 | 0.003 | 2.06 | 1.24-3.43 | 0.005 |
| Age ≥ 65 | 2.45 | 1.44-4.18 | 0.0002 | - | - | - |
| Male sex | 1.31 | 0.83-2.08 | 0.24 | - | - | - |
| BMI ≥ 30 kg/m2 | 0.47 | 0.26-0.82 | 0.007 | 0.40 | 0.22-0.72 | 0.002 |
| Hypertension | 1.47 | 0.92-2.35 | 0.10 | - | - | - |
| Diabetes | 1.61 | 0.94-2.74 | 0.07 | 1.90 | 1.06-3.40 | 0.03 |
| Stroke | 2.60 | 0.87-7.70 | 0.08 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





