Submitted:
20 March 2024
Posted:
20 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Quantification of Phenolic Contents from Australian Myrtles
2.2. Quantification of Antioxidant Activities from Australian Myrtles
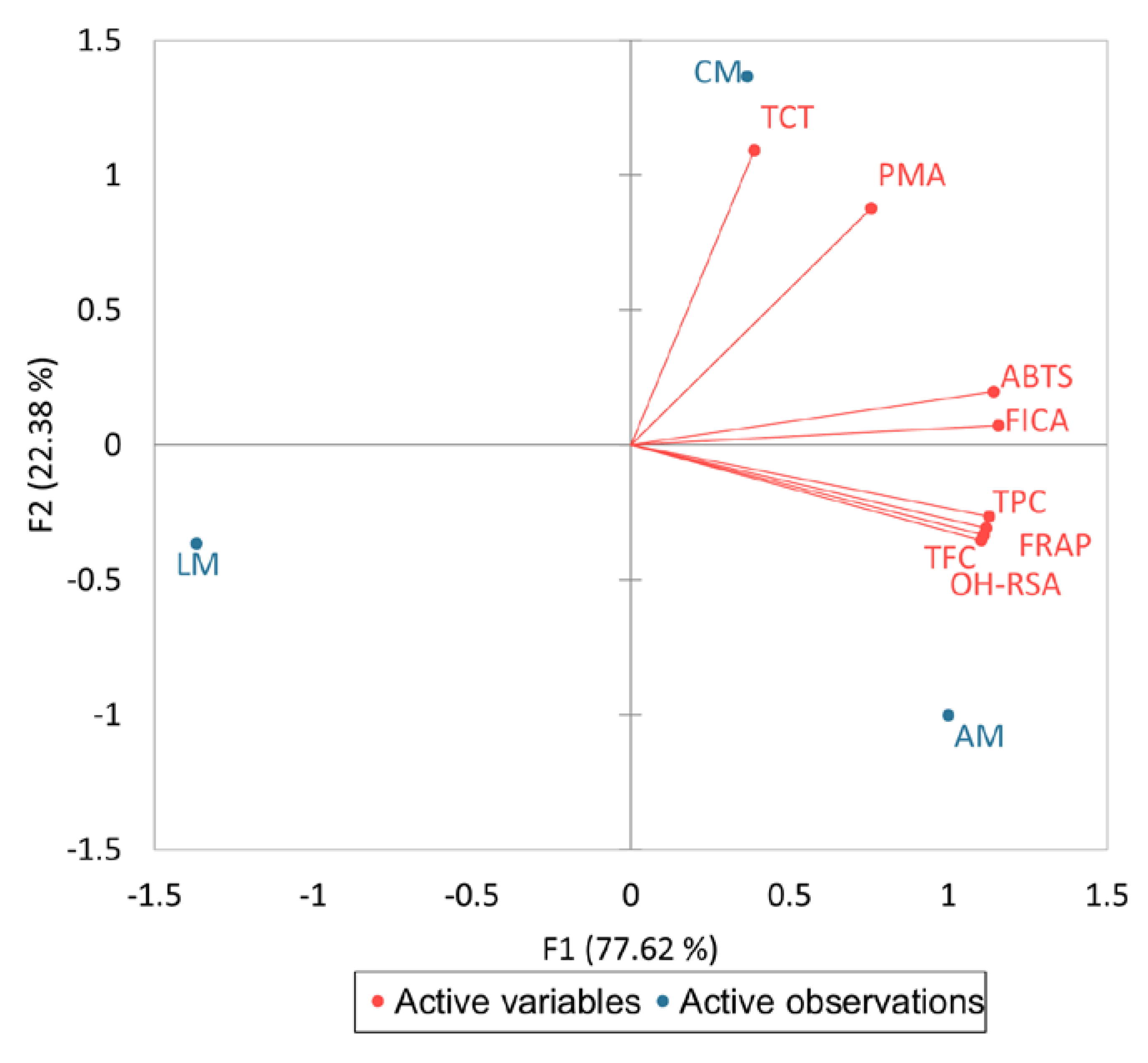
2.3. Pearson Correlation Analysis between Phenolic Contents and Their Antioxidant Activities
2.4. LC-MS Analysis
2.4.1. Phenolic Acids
2.4.2. Flavonoids
Flavonols
Flavanols
Flavanones
Flavones
Chalcones and Dihydrochalcones
2.4.3. Isoflavonoids
2.4.4. Tannins
2.4.5. Stilbenes
2.4.6. Lignans
2.4.7. Other Compounds
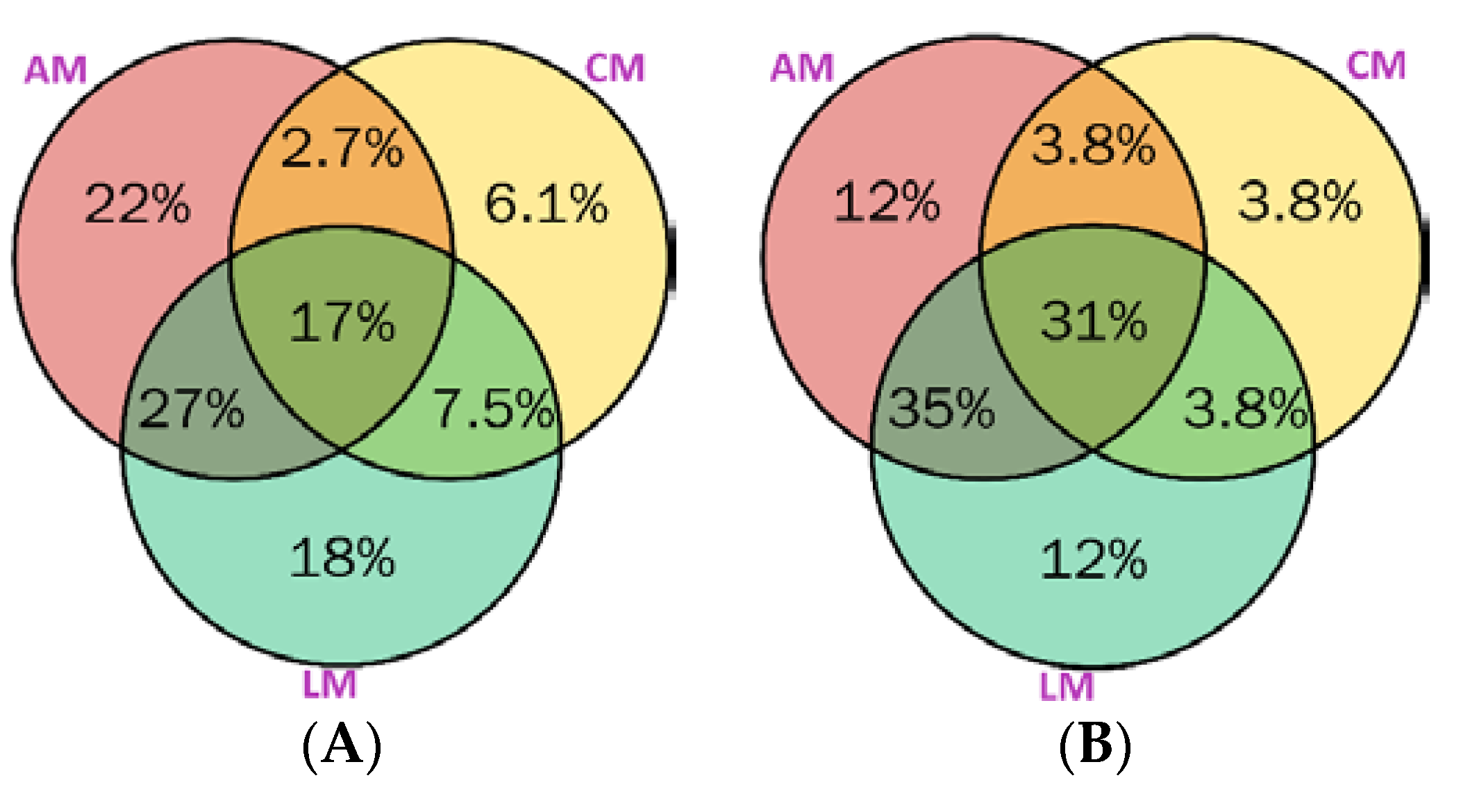
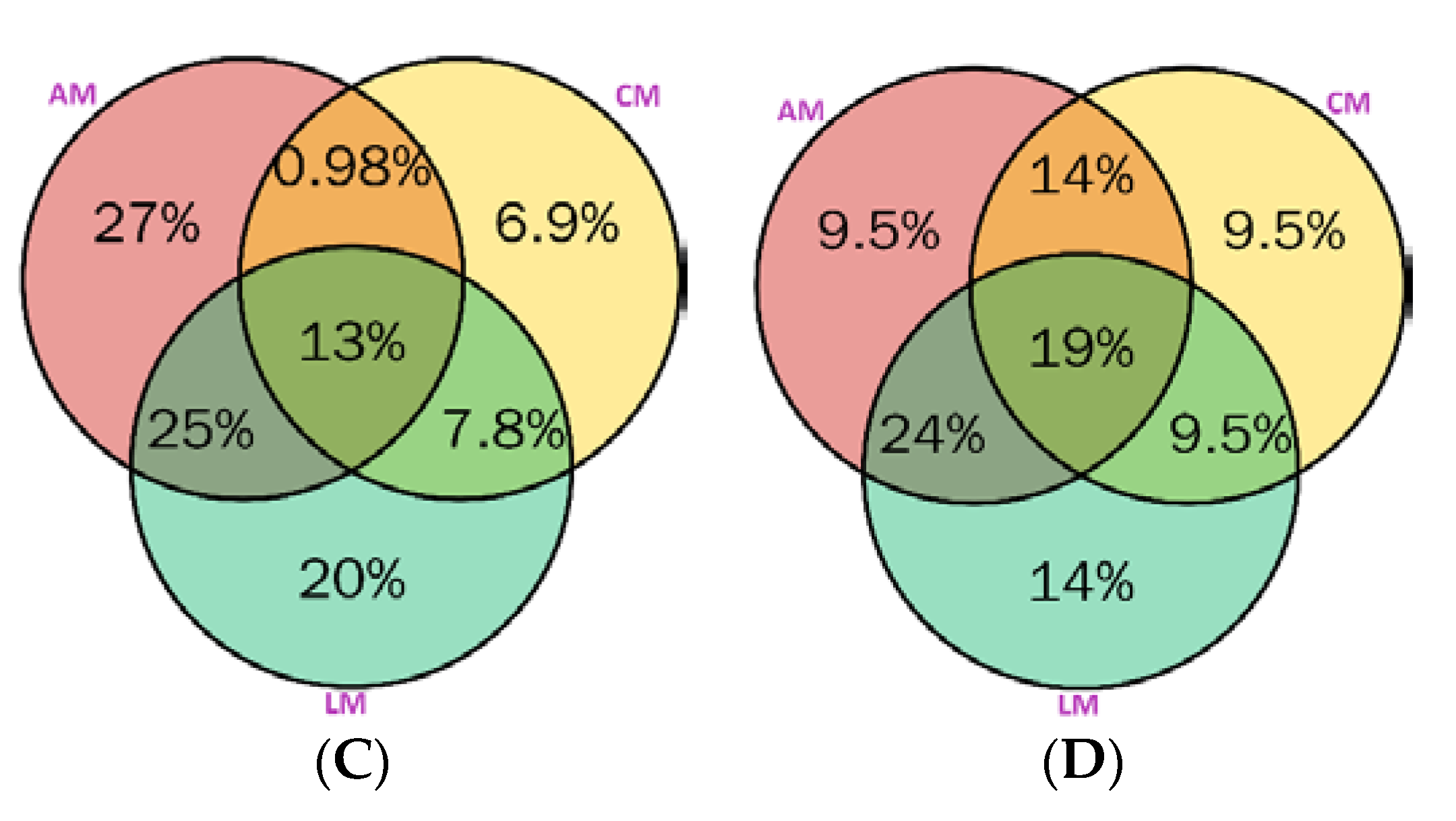
2.5. Distribution of Metabolites in Australian Myrtles
2.6. Quantification/Semi-Quantification of Individual Phenolic Compounds from Australian Myrtles
2.6.1. Phenolic Acids
2.6.2. Flavonoids and Non-Flavonoids
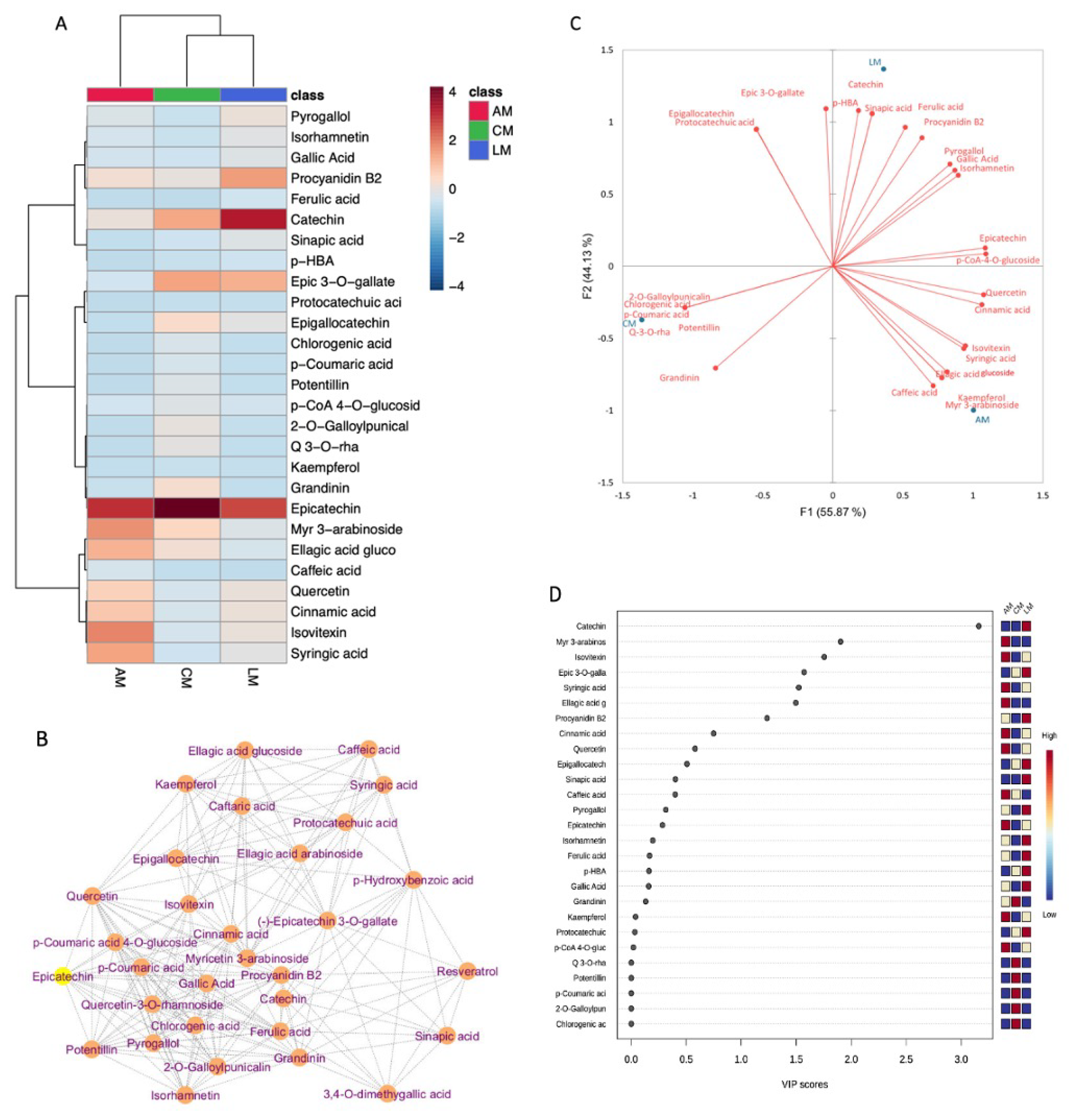
2.7. Heatmap Clustering, Debiased Sparse Partial Correlation Network and Chemometric Analysis
3. Materials and Methods
3.1. Sample Preparation and Extraction of Phenolic Compounds
3.2. Measurement of Phenolic Contents in Australian Myrtles
3.2.1. Total Phenolics
3.2.2. Total Flavonoids
3.2.3. Total Condensed Tannin
3.3. Quantification of Antioxidant Activities
3.3.1. ABTS Radical Scavenging Assay
3.3.2. FRAP Assay
3.3.3. OH-RSA
3.3.4. Fe2+ Chelating Activity
3.3.5. PMA Activity
3.4. HPLC-MS/MS Quantification of Phenolic Compounds
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, A.; Kiloni, S.M.; Cáceres-Vélez, P.R.; Jusuf, P.R.; Cottrell, J.J.; Dunshea, F.R. Phytochemicals, antioxidant activities, and toxicological screening of native australian fruits using zebrafish embryonic model. Foods 2022, 11, 4038. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Casado, A. The health potential of fruits and vegetables phytochemicals: Notable examples. Crit Rev Food Sci Nutr 2016, 56, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants (Basel, Switzerland) 2017, 6. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant physiology 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Ali, A. Characterization of phenolic compounds from the selected native australian flora, their bioaccessibility, bioactivities, safety evaluation, and their reciprocal interactions with the gut microbiota. University of Melbourne, 2023.
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Characterization, antioxidant potential, and pharmacokinetics properties of phenolic compounds from native australian herbs and fruits. Plants 2023, 12, 993. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Lc-ms/ms characterization of phenolic metabolites and their antioxidant activities from australian native plants. Metabolites 2022, 12, 1016. [Google Scholar] [CrossRef]
- Balentine, D.A.; Albano, M.C.; Nair, M.G. Role of medicinal plants, herbs, and spices in protecting human health. Nutr Rev 1999, 57, S41–45. [Google Scholar] [CrossRef]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Antioxidant, alpha-glucosidase inhibition activities, in silico molecular docking and pharmacokinetics study of phenolic compounds from native australian fruits and spices. Antioxidants 2023, 12, 254. [Google Scholar] [CrossRef]
- Konczak, I.; Zabaras, D.; Dunstan, M.; Aguas, P. Antioxidant capacity and phenolic compounds in commercially grown native australian herbs and spices. Food Chemistry 2010, 122, 260–266. [Google Scholar] [CrossRef]
- de Falco, B.; Grauso, L.; Fiore, A.; Bonanomi, G.; Lanzotti, V. Metabolomics and chemometrics of seven aromatic plants: Carob, eucalyptus, laurel, mint, myrtle, rosemary and strawberry tree. Phytochemical analysis : PCA 2022, 33, 696–709. [Google Scholar] [CrossRef]
- Mathew, S.; Zhang, K.; Zhou, X.; Münch, G.; Bodkin, F.; Li, F.; Raju, R. Myrtinols a-f: New anti-inflammatory peltogynoid flavonoid derivatives from the leaves of australian indigenous plant backhousia myrtifolia. Molecules 2023, 28, 2160. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; Vuong, Q.V. Optimization of microwave-assisted extraction of polyphenols from lemon myrtle: Comparison of modern and conventional extraction techniques based on bioactivity and total polyphenols in dry extracts. Processes 2021, 9, 2212. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Vuong, Q. Effects of different drying methods on extractable phenolic compounds and antioxidant properties from lemon myrtle dried leaves. Heliyon 2019, 5, e03044. [Google Scholar] [CrossRef]
- Khan, J.; Tousif, M.I.; Saleem, M.; Nazir, M.; Touseef, S.; Saleem, K.; Asim, S.; Khan, A.; Asghar, M.A.; Zengin, G. , et al. Insight into the phytochemical composition, biological activities and docking studies of moringa oleifera lam. To authenticate its use in biopharmaceutical industries. Industrial Crops and Products 2021, 172, 114042. [Google Scholar] [CrossRef]
- Kang, E.J.; Lee, J.K.; Park, H.R.; Kim, H.; Kim, H.S.; Park, J. Antioxidant and anti-inflammatory activities of phenolic compounds extracted from lemon myrtle (backhousia citriodora) leaves at various extraction conditions. Food science and biotechnology 2020, 29, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Wu, H.; Ponnampalam, E.N.; Cottrell, J.J.; Dunshea, F.R.; Suleria, H.A.R. Comprehensive profiling of most widely used spices for their phenolic compounds through lc-esi-qtof-ms(2) and their antioxidant potential. Antioxidants (Basel) 2021, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof/ms characterization of australian herb and spices (garlic, ginger, and onion) and potential antioxidant activity. Journal of Food Processing and Preservation 2020, e14497. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The versatility of antioxidant assays in food science and safety-chemistry, applications, strengths, and limitations. Antioxidants (Basel, Switzerland) 2020, 9. [Google Scholar] [CrossRef]
- Medda, S.; Fadda, A.; Dessena, L.; Mulas, M. Quantification of total phenols, tannins, anthocyanins content in myrtus communis l. And antioxidant activity evaluation in function of plant development stages and altitude of origin site. 2021, 11, 1059. [Google Scholar]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Medda, S.; Fadda, A.; Dessena, L.; Mulas, M. Quantification of total phenols, tannins, anthocyanins content in myrtus communis l. And antioxidant activity evaluation in function of plant development stages and altitude of origin site. Agronomy 2021, 11, 1059. [Google Scholar] [CrossRef]
- Navarro, M.; Stanley, R.; Cusack, A.; Sultanbawa, Y. Combinations of plant-derived compounds against campylobacter in vitro. Journal of Applied Poultry Research 2015, 24, 352–363. [Google Scholar] [CrossRef]
- Ali, S.S.; Ahmad, W.; Budin, S.B.; Zainalabidin, S. Implication of dietary phenolic acids on inflammation in cardiovascular disease. Reviews in cardiovascular medicine 2020, 21, 225–240. [Google Scholar] [PubMed]
- Mani, J.S.; Johnson, J.B.; Hosking, H.; Ashwath, N.; Walsh, K.B.; Neilsen, P.M.; Broszczak, D.A.; Naiker, M. Antioxidative and therapeutic potential of selected australian plants: A review. J Ethnopharmacol 2021, 268, 113580. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K. An overview of antimicrobial properties of different classes of phytochemicals. In Dietary phytochemicals and microbes; Patra, A.K., Ed.; Springer: Netherlands: Dordrecht, 2012; pp. 1–32. [Google Scholar]
- Caruso, G.; Godos, J.; Privitera, A.; Lanza, G.; Castellano, S.; Chillemi, A.; Bruni, O.; Ferri, R.; Caraci, F.; Grosso, G. Phenolic acids and prevention of cognitive decline: Polyphenols with a neuroprotective role in cognitive disorders and alzheimer's disease. Nutrients 2022, 14, 819. [Google Scholar] [CrossRef] [PubMed]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H. , et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran J Basic Med Sci 2019, 22, 225–237. [Google Scholar] [PubMed]
- Dar, N.J.; Hamid, A.; Ahmad, M. Pharmacologic overview of withania somnifera, the indian ginseng. Cellular and molecular life sciences : CMLS 2015, 72, 4445–4460. [Google Scholar] [CrossRef]
- Mao, X.; Yang, Q.; Chen, D.; Yu, B.; He, J. Benzoic acid used as food and feed additives can regulate gut functions. Biomed Res Int 2019, 2019, 5721585. [Google Scholar] [CrossRef]
- Georgousaki, K.; Tsafantakis, N.; Gumeni, S.; Lambrinidis, G.; González-Menéndez, V.; Tormo, J.R.; Genilloud, O.; Trougakos, I.P.; Fokialakis, N. Biological evaluation and in silico study of benzoic acid derivatives from bjerkandera adusta targeting proteostasis network modules. Molecules 2020, 25, 666. [Google Scholar] [CrossRef]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic potential of plant phenolic acids in the treatment of cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Bourais, I.; Elmarrkechy, S.; Taha, D.; Mourabit, Y.; Bouyahya, A.; El Yadini, M.; Machich, O.; El Hajjaji, S.; El Boury, H.; Dakka, N. , et al. A review on medicinal uses, nutritional value, and antimicrobial, antioxidant, anti-inflammatory, antidiabetic, and anticancer potential related to bioactive compounds of j. Regia. Food Reviews International, 2022; 1–51. [Google Scholar]
- Thapliyal, S.; Singh, T.; Handu, S.; Bisht, M.; Kumari, P.; Arya, P.; Srivastava, P.; Gandham, R. A review on potential footprints of ferulic acid for treatment of neurological disorders. Neurochemical research 2021, 46, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front Oncol 2019, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Birková, A.; Hubková, B.; Bolerázska, B.; Mareková, M.; Čižmárová, B. Caffeic acid: A brief overview of its presence, metabolism, and bioactivity. Bioactive Compounds in Health and Disease 2020, 3, 74–81. [Google Scholar] [CrossRef]
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Gopi, M.; Karthik, K.; Malik, Y.S.; Dhama, K. Rosmarinic acid: Modes of action, medicinal values and health benefits. Anim Health Res Rev 2017, 18, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A comprehensive review of rosmarinic acid: From phytochemistry to pharmacology and its new insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Tan, W.; Li, Q.; Dong, F.; Guo, Z. Preparation of chitosan-rosmarinic acid derivatives with enhanced antioxidant and anti-inflammatory activities. Carbohydrate polymers 2022, 296, 119943. [Google Scholar] [CrossRef]
- Adisakwattana, S. Cinnamic acid and its derivatives: Mechanisms for prevention and management of diabetes and its complications. Nutrients 2017, 9, 163. [Google Scholar] [CrossRef]
- Rychlicka, M.; Rot, A.; Gliszczyńska, A. Biological properties, health benefits and enzymatic modifications of dietary methoxylated derivatives of cinnamic acid. Foods 2021, 10, 1417. [Google Scholar] [CrossRef]
- Sova, M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini Rev Med Chem 2012, 12, 749–767. [Google Scholar] [CrossRef]
- Wang, K.; Shi, J.; Zhou, Y.; He, Y.; Mi, J.; Yang, J.; Liu, S.; Tang, X.; Liu, W.; Tan, Z. , et al. Design, synthesis and evaluation of cinnamic acid hybrids as multi-target-directed agents for the treatment of alzheimer's disease. Bioorganic chemistry 2021, 112, 104879. [Google Scholar] [CrossRef]
- Pandi, A.; Kalappan, V.M. Pharmacological and therapeutic applications of sinapic acid-an updated review. Molecular biology reports 2021, 48, 3733–3745. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. Journal of nutritional science 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomedicine & Pharmacotherapy 2020, 128, 110301. [Google Scholar]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O. , et al. Therapeutic potential of quercetin: New insights and perspectives for human health. ACS omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxidative medicine and cellular longevity 2018, 2018, 6241017. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, M.; Farzaei, M.H.; Kiani, S.; Khodarahmi, R. Immunomodulatory; anti-inflammatory/antioxidant effects of polyphenols: A comparative review on the parental compounds and their metabolites. Food Reviews International 2021, 37, 759–811. [Google Scholar] [CrossRef]
- Hayder, N.; Bouhlel, I.; Skandrani, I.; Kadri, M.; Steiman, R.; Guiraud, P.; Mariotte, A.M.; Ghedira, K.; Dijoux-Franca, M.G.; Chekir-Ghedira, L. In vitro antioxidant and antigenotoxic potentials of myricetin-3-o-galactoside and myricetin-3-o-rhamnoside from myrtus communis: Modulation of expression of genes involved in cell defence system using cdna microarray. Toxicology in vitro : an international journal published in association with BIBRA 2008, 22, 567–581. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Szopa, A.; Ekiert, H. Citrus limon (lemon) phenomenon-a review of the chemistry, pharmacological properties, applications in the modern pharmaceutical, food, and cosmetics industries, and biotechnological studies. Plants (Basel, Switzerland) 2020, 9. [Google Scholar] [CrossRef]
- Shan, Z.; Nisar, M.F.; Li, M.; Zhang, C.; Wan, C.C. Theaflavin chemistry and its health benefits. Oxidative medicine and cellular longevity 2021, 2021, 6256618. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial properties of green tea catechins. International journal of molecular sciences 2020, 21. [Google Scholar] [CrossRef]
- Leyva-Soto, A.; Alejandra Chavez-Santoscoy, R.; Porras, O.; Hidalgo-Ledesma, M.; Serrano-Medina, A.; Alejandra Ramírez-Rodríguez, A.; Alejandra Castillo-Martinez, N. Epicatechin and quercetin exhibit in vitro antioxidant effect, improve biochemical parameters related to metabolic syndrome, and decrease cellular genotoxicity in humans. Food research international (Ottawa, Ont.) 2021, 142, 110101. [Google Scholar] [CrossRef]
- Barreca, D.; Gattuso, G.; Bellocco, E.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Laganà, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus phytochemical with health-promoting properties. BioFactors (Oxford, England) 2017, 43, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Islam, M.S.; Rahman, M.K.; Uddin, M.N.; Akanda, M.R. The pharmacological and biological roles of eriodictyol. Archives of pharmacal research 2020, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Lami, M.S.; Uddin, T.M.; Das, R.; Islam, F.; Anjum, J.; Hossain, M.J.; Emran, T.B. Prospective multifunctional roles and pharmacological potential of dietary flavonoid narirutin. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2022, 150, 112932. [Google Scholar]
- Karrat, L.; Abajy, M.Y.; Nayal, R. Investigating the anti-inflammatory and analgesic properties of leaves ethanolic extracts of cedrus libani and pinus brutia. Heliyon 2022, 8, e09254. [Google Scholar] [CrossRef] [PubMed]
- Barrera, C.; Betoret, N.; Seguí, L. Phenolic profile of cane sugar derivatives exhibiting antioxidant and antibacterial properties. Sugar Tech 2020, 22, 798–811. [Google Scholar] [CrossRef]
- Refaat, J.; Desoukey, S.Y.; Ramadan, M.A.; Kamel, M.S. Rhoifolin: A review of sources and biological activities. Int. J. Pharmacogn 2015, 2, 102–109. [Google Scholar]
- Hassan, S.; Hamed, S.; Almuhayawi, M.; Hozzein, W.; Selim, S.; AbdElgawad, H. Bioactivity of ellagic acid and velutin: Two phenolic compounds isolated from marine algae. Egyptian Journal of Botany 2021, 61, 219–231. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Vinyagam, R.; Rajamanickam, V.; Sankaran, V.; Venkatesan, S.; David, E. Pharmacological aspects and potential use of phloretin: A systemic review. Mini reviews in medicinal chemistry 2019, 19, 1060–1067. [Google Scholar] [CrossRef]
- Vesaghhamedani, S.; Ebrahimzadeh, F.; Najafi, E.; Shabgah, O.G.; Askari, E.; Shabgah, A.G.; Mohammadi, H.; Jadidi-Niaragh, F.; Navashenaq, J.G. Xanthohumol: An underestimated, while potent and promising chemotherapeutic agent in cancer treatment. Progress in biophysics and molecular biology 2022, 172, 3–14. [Google Scholar] [CrossRef]
- Li, K.; Hu, W.; Yang, Y.; Wen, H.; Li, W.; Wang, B. Anti-inflammation of hydrogenated isoflavones in lps-stimulated raw264.7 cells via inhibition of nf-κb and mapk signaling pathways. Molecular immunology 2023, 153, 126–134. [Google Scholar] [CrossRef]
- Melo, L.F.M.; Aquino-Martins, V.G.Q.; Silva, A.P.D.; Oliveira Rocha, H.A.; Scortecci, K.C. Biological and pharmacological aspects of tannins and potential biotechnological applications. Food Chem 2023, 414, 135645. [Google Scholar] [CrossRef]
- Li, X.; Deng, Y.; Zheng, Z.; Huang, W.; Chen, L.; Tong, Q.; Ming, Y. Corilagin, a promising medicinal herbal agent. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2018, 99, 43–50. [Google Scholar]
- Saeed, M.; Naveed, M.; BiBi, J.; Kamboh, A.A.; Arain, M.A.; Shah, Q.A.; Alagawany, M.; El-Hack, M.E.A.; Abdel-Latif, M.A.; Yatoo, M.I. , et al. The promising pharmacological effects and therapeutic/medicinal applications of punica granatum l. (pomegranate) as a functional food in humans and animals. Recent patents on inflammation & allergy drug discovery 2018, 12, 24–38. [Google Scholar]
- N, I.K.; Olennikov, D.N. Phenolome of asian agrimony tea (agrimonia asiatica juz., rosaceae): Lc-ms profile, α-glucosidase inhibitory potential and stability. Foods (Basel, Switzerland) 2020, 9. [Google Scholar]
- Ríos, J.L.; Giner, R.M.; Marín, M.; Recio, M.C. A pharmacological update of ellagic acid. Planta medica 2018, 84, 1068–1093. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Y.; Chen, L.X.; Fang, L.; Zhang, Q. Cardioprotective effects of polydatin against myocardial injury in diabetic rats via inhibition of nadph oxidase and nf-κb activities. BMC complementary medicine and therapies 2020, 20, 378. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Baran, M.Y.; Arroo, R.; Kuruüzüm-Uz, A. Recent advances in chemistry, therapeutic properties and sources of polydatin. Phytochemistry Reviews 2018, 17, 973–1005. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, J.Y.; Jhin, C.; Shin, J.M.; Kim, M.; Ahn, H.R.; Yoo, G.; Son, Y.J.; Jung, S.H.; Nho, C.W. Reduction of hepatic lipogenesis by loliolide and pinoresinol from lysimachia vulgaris via degrading liver x receptors. Journal of agricultural and food chemistry 2019, 67, 12419–12427. [Google Scholar] [CrossRef]
- Dehmlow, C.; Erhard, J.; de Groot, H. Inhibition of kupffer cell functions as an explanation for the hepatoprotective properties of silibinin. Hepatology (Baltimore, Md.) 1996, 23, 749–754. [Google Scholar] [CrossRef]
- Cheung, C.W.; Gibbons, N.; Johnson, D.W.; Nicol, D.L. Silibinin--a promising new treatment for cancer. Anti-cancer agents in medicinal chemistry 2010, 10, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Antika, L.D.; Tasfiyati, A.N.; Hikmat, H.; Septama, A.W. Scopoletin: A review of its source, biosynthesis, methods of extraction, and pharmacological activities. Zeitschrift fur Naturforschung. C, Journal of biosciences 2022, 77, 303–316. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Babakir-Mina, M. Investigation of rosemary herbal extracts (rosmarinus officinalis) and their potential effects on immunity. Phytotherapy research : PTR 2020, 34, 1829–1837. [Google Scholar] [CrossRef]
- Benali, T.; Bakrim, S.; Ghchime, R.; Benkhaira, N.; El Omari, N.; Balahbib, A.; Taha, D.; Zengin, G.; Hasan, M.M.; Bibi, S. , et al. Pharmacological insights into the multifaceted biological properties of quinic acid. Biotechnology & genetic engineering reviews, 2022; 1–30. [Google Scholar]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of catechins and their applications. Biomedical Dermatology 2020, 4, 8. [Google Scholar] [CrossRef]
- Magnani, C.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic acid: A review of its potential use in medications and cosmetics. Analytical Methods 2014, 6, 3203–3210. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem 2022, 383, 132531. [Google Scholar] [CrossRef]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomás-Barberán, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum Nutr 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Abian, O.; Ortega-Alarcon, D.; Jimenez-Alesanco, A.; Ceballos-Laita, L.; Vega, S.; Reyburn, H.T.; Rizzuti, B.; Velazquez-Campoy, A. Structural stability of sars-cov-2 3clpro and identification of quercetin as an inhibitor by experimental screening. International Journal of Biological Macromolecules 2020, 164, 1693–1703. [Google Scholar] [CrossRef]
- He, M.; Min, J.-W.; Kong, W.-L.; He, X.-H.; Li, J.-X.; Peng, B.-W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 2016, 115, 74–85. [Google Scholar] [CrossRef]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Identification and characterization of anthocyanins and non-anthocyanin phenolics from australian native fruits and their antioxidant, antidiabetic, and anti-alzheimer potential. Food Research International 2022, 162, 111951. [Google Scholar] [CrossRef] [PubMed]
- Severo, J.; Tiecher, A.; Chaves, F.C.; Silva, J.A.; Rombaldi, C.V. Gene transcript accumulation associated with physiological and chemical changes during developmental stages of strawberry cv. Camarosa. Food Chemistry 2011, 126, 995–1000. [Google Scholar] [CrossRef]
- Kiani, H.S.; Ahmad, W.; Nawaz, S.; Farah, M.A.; Ali, A. Optimized extraction of polyphenols from unconventional edible plants: Lc-ms/ms profiling of polyphenols, biological functions, molecular docking, and pharmacokinetics study. Molecules 2023, 28, 6703. [Google Scholar] [CrossRef] [PubMed]
| Variables | AM | CM | LM |
| TPC (mg GAE/g) | 52.49 ± 3.55 a | 41.31 ± 3.23 b | 28.77±1.03 c |
| TFC (mg QE/g) | 23.73 ± 2.32 a | 19.41 ± 1.57 b | 15.73 ± 1.34 c |
| TCT (mg CE/g) | 1.52 ± 0.12 a | 1.83 ± 0.19 a | 1.49 ± 0.09 a |
| Variables | AM | CM | LM |
| FRAP (mg AAE/g) | 14.30 ± 1.92 a | 9.21 ± 1.03 b | 4.60 ± 0.23 c |
| ABTS (mg AAE/g) | 148.16 ± 3.74 a | 142.66 ± 3.87 ab | 92.36 ± 0.75 c |
| PMA (mg AAE/g) | 13.41 ± 0.28 b | 17.09 ± 0.38 a | 10.57 ± 0.18 c |
| FICA (μg EDTA/g) | 1.80 ± 0.10 a | 1.63 ± 0.05 a | 0.98 ± 0.03 ab |
| •OH-RSA (mg AAE/g) | 23.62 ± 0.47 a | 21.62 ± 0.21 a | 19.66 ± 0.31 ab |
| Variables | TPC | TFC | TCT | FRAP | ABTS | PMA | FICA |
| TFC | 0.99 | ||||||
| TCT | 0.11 | 0.03 | |||||
| FRAP | 0.99 | 1.00 | 0.05 | ||||
| ABTS | 0.92 | 0.89 | 0.49 | 0.90 | |||
| PMA | 0.46 | 0.39 | 0.93 | 0.40 | 0.77 | ||
| FICA | 0.96 | 0.93 | 0.40 | 0.94 | 0.99 | 0.70 | |
| OH-RSA | 0.99 | 0.99 | 0.07 | 1.00 | 0.91 | 0.43 | 0.95 |
| No. | Proposed compounds | Molecular Formula | RT (min) | Mode of ionization | Theoretical (m/z) | Observed (m/z) | Mass Error (ppm) | MS/MS | Samples |
| Phenolic acids | |||||||||
| Hydroxybenzoic acids and derivatives | |||||||||
| 1 | Gallic acid 4-O-glucoside | C13H16O10 | 5.258 | [M−H]− | 331.0666 | 331.0675 | 2.7 | 169, 125 | AM, LM |
| 2 | Gallic acid | C7H6O5 | 6.084 | * [M−H]− | 169.0137 | 169.0145 | 4.7 | 125 | LM, AM, CM |
| 3 | Protocatechuic acid 4-O-glucoside | C13H16O9 | 7.915 | [M−H]− | 315.0716 | 315.0737 | 6.7 | 153 | LM, AM |
| 4 | Protocatechuic acid | C7H6O4 | 9.906 | * [M-H]- | 153.0193 | 153.0195 | 1.3 | 109 | AM, LM, CM |
| 5 | Benzoic acid | C7H6O2 | 15.442 | [M+H]+ | 123.0446 | 123.0449 | 2.4 | 105, 77 | CM, AM, LM |
| 6 | p-Hydroxybenzoic acid | C7H6O3 | 15.442 | * [M−H]− | 139.0395 | 137.0230 | 0.7 | 93 | CM, AM, LM |
| 7 | Vanillic acid | C8H8O4 | 15.583 | [M−H]− | 167.0345 | 167.0353 | 4.8 | 152, 123, 108 | LM, CM |
| 8 | Ellagic acid glucoside | C20H16O13 | 18.379 | * [M−H]− | 463.0513 | 463.0531 | 3.9 | 301, 284 | AM |
| 9 | Syringic acid | C9H10O5 | 18.431 | * [M−H]− | 197.0530 | 197.0521 | −3.7 | 169, 151, 125 | AM, CM |
| 10 | Paeoniflorin | C23H28O11 | 53.231 | [M+H]+ | 481.1710 | 481.1734 | 5.0 | 463, 359, 319, 301, 197 | LM |
| Hydroxycinnamic acids and derivatives | |||||||||
| 11 | 3-p-Coumaroylquinic acid | C16H18O8 | 6.088 | [M−H]− | 337.0924 | 337.0957 | 9.8 | 191, 163, 119 | CM |
| 12 | Dihydroferulic acid | C10H12O4 | 14.255 | * [M−H]− | 195.0658 | 195.0669 | 5.6 | 151 | LM, AM |
| 13 | p-Coumaric acid | C9H8O3 | 14.295 | ** [M−H]− | 163.0395 | 163.0389 | −3.7 | 119, 65 | LM, AM, CM |
| 14 | p-Coumaric acid 4-O-glucoside | C15H18O8 | 15.492 | [M−H]− | 325.0924 | 325.0927 | 0.9 | 163, 119 | AM, LM |
| 15 | 2-S-Glutathionyl caftaric acid | C23H27N3O15S | 16.529 | * [M−H]− | 616.1085 | 616.1096 | 1.8 | 598, 594 | LM |
| 16 | Cinnamic acid | C9H8O2 | 17.329 | [M−H]− | 149.0602 | 149.0606 | 2.7 | 10 | LM, AM |
| 17 | Sinapic acid | C11H12O5 | 23.312 | [M−H]− | 223.0607 | 223.0610 | 1.2 | 205, 193, 179, 149 | AM, LM |
| 18 | 3-Caffeoylquinic acid (chlorogenic acid) | C16H18O9 | 24.472 | * [M-H]- | 353.0878 | 353.0897 | 5.4 | 191, 179, 161 | LC, AM, CM |
| 19 | Ferulic acid | C10H10O4 | 24.003 | [M-H]- | 193.0506 | 193.0509 | 1.6 | 178, 163, 149, 135 | AM, CM, LM |
| 20 | 3,4-Dimetoxycinnamic acid | C11H12O4 | 28.319 | [M+H]+ | 209.0814 | 209.0811 | -1.4 | 149 | AM |
| 21 | Caffeic acid | C9H8O4 | 28.573 | [M−H]− | 179.0345 | 179.0348 | 1.7 | 135 | CM, LM, AM |
| 22 | p-Coumaroyl malic acid | C13H12O7 | 36.005 | [M+H]+ | 281.0661 | 281.0655 | −2.1 | 119 | AM |
| 23 | 1,5-Dicaffeoylquinic acid | C25H24O12 | 36.918 | [M−H]− | 515.1190 | 515.1193 | 0.6 | 353, 191, 179, 161 | AM, LM |
| 24 | 1-Caffeoyl-5-feruloylquinic acid | C26H26O12 | 43.148 | [M−H]− | 529.1346 | 529.1365 | 3.6 | 373, 191, 161 | LM, AM |
| 25 | Rosmarinic acid | C18H16O8 | 55.937 | [M−H]− | 359.0767 | 359.0762 | −1.4 | 197, 179, 161 | LM, AM |
| Flavonoids | |||||||||
| Flavonols | |||||||||
| 26 | Kaempferol 3-O-xylosyl-glucoside | C26H28O15 | 16.150 | [M−H]− | 579.1350 | 579.1370 | 3.5 | 285 | LM |
| 27 | 3-Methoxynobiletin | C22H24O9 | 17.715 | [M−H]− | 431.1342 | 431.1326 | −3.7 | 401, 387 | LM, AM |
| 28 | Limocitrin | C17H14O8 | 17.721 | [M−H]− | 345.0611 | 345.0638 | 7.8 | 315, 301, 181 | LM, CM |
| 29 | Myricitrin 3-rhamnoside (Myricitrin) | C21H20O12 | 18.920 | * [M−H]− | 463.0877 | 463.0888 | 3.4 | 317 | AM, LM |
| 30 | Quercetin 4'-O-glucuronide | C21H18O13 | 19.871 | [M−H]− | 477.0669 | 477.0657 | −2.5 | 301 | AM |
| 31 | Isomyricitrin | C21H20O13 | 20.592 | * [M−H]− | 479.0826 | 479.0815 | −1.5 | 317 | AM |
| 32 | Myricetin 3-O-arabinoside | C20H18O12 | 21.955 | [M+H]+ | 449.0721 | 449.0857 | 8.4 | 317, 271 | AM |
| 33 | Quercetin 3-O-glucoside | C21H20O12 | 25.190 | * [M−H]− | 463.0877 | 463.0808 | −9.4 | 301, 271 | AM, LM |
| 34 | Myricetin 3-O-rutinoside | C27H30O17 | 23.769 | [M+H]+ | 627.1561 | 627.1541 | −3.2 | 319 | AM |
| 35 | 3'-O-Methylmyricetin (Laricitrin) | C16H12O8 | 24.849 | [M−H]− | 331.0454 | 331.0447 | −2.6 | 316, 287, 271 | LM |
| 36 | Quercetin 3-O-glucosyl-xyloside | C26H28O16 | 25.305 | [M+H]+ | 597.1455 | 597.1460 | 0.8 | 303 | AM |
| 37 | Dihydromyricetin 3-O-rhamnoside | C21H22O12 | 25.702 | [M−H]− | 465.1033 | 465.1056 | 4.9 | 319 | LM |
| 38 | Rutin | C27H30O16 | 25.972 | [M+H]+ | 611.1612 | 611.1600 | −2.0 | 303 | AM, LM, CM |
| 39 | Europetin 3-galactoside | C22H22O13 | 26.228 | [M−H]− | 493.0982 | 493.0971 | −2.2 | 331 | LM |
| 40 | Quercetin 3-O-xyloside | C20H18O11 | 28.548 | * [M−H]− | 435.0927 | 435.0926 | −0.2 | 300, 301, 271 | AM |
| 41 | Quercetin 3-O-rhamnoside (quercitrin) | C21H20O11 | 29.541 | [M−H]− | 447.0928 | 447.0948 | 4.6 | 301, 271, 151 | AM |
| 42 | Taxifolin 4',7-diglucoside | C27H32O17 | 29.932 | [M−H]− | 627.1562 | 627.1579 | 2.7 | 303 | AM |
| 43 | Quercetin | C15H10O7 | 30.394 | [M−H]− | 301.0348 | 301.0352 | 1.3 | 271, 179, 151 | LM, AM, CM |
| 44 | Kaempferol 3,7,4'-O-triglucoside | C33H40O21 | 33.048 | [M+H]+ | 773.2140 | 773.2140 | 0.0 | 287 | AM |
| 45 | Isorhamnetin | C16H12O7 | 35.937 | [M−H]− | 315.0505 | 315.0530 | 7.9 | 300, 151, 107 | LM, AM, CM |
| 46 | Myricetin | C15H10O8 | 39.695 | * [M−H]− | 317.0298 | 317.0302 | 1.3 | 299, 179, 151 | LM, AM |
| 47 | 3-Methoxysinensetin | C21H22O8 | 40.377 | [M+H]+ | 403.1393 | 403.1396 | 0.7 | 373, 359, 211 | LM, CM |
| 48 | 3,7-Dimethylquercetin | C17H14O7 | 41.104 | * [M+H]+ | 331.0818 | 331.0833 | 4.5 | 313, 150, 139, 121 | LM |
| Flavanols | |||||||||
| 49 | 4'-O-Methylepigallocatechin | C16H16O7 | 3.888 | [M−H]− | 319.0818 | 319.0816 | −0.6 | 289, 245 | AM |
| 50 | Prodelphinidin trimer GC-GC-C | C45H38O20 | 5.218 | [M−H]− | 897.1878 | 897.1892 | 1.6 | 879, 305, 289, 125 | AM, LM |
| 51 | (-)-Epigallocatechin | C15H14O7 | 9.624 | * [M−H]− | 305.0662 | 305.0681 | 6.2 | 179, 169, 139, 125 | LM, AM, CM |
| 52 | Cinnamtannin A2 | C60H50O24 | 15.174 | [M+H]+ | 1155.2770 | 1155.2775 | 0.4 | 1137, 985, 865, 579 | CM, LM |
| 53 | Epicatechin | C15H14O6 | 15.945 | [M−H]− | 289.0712 | 289.0729 | 5.9 | 245, 205 | AM, LM, CM |
| 54 | Catechin | C15H14O6 | 16.453 | [M−H]− | 289.0712 | 289.0729 | 5.9 | 245, 179, 139, 123 | AM, LM, CM |
| 55 | Catechin 3'-glucoside | C21H24O11 | 20.675 | [M−H]− | 451.1241 | 451.1239 | −0.4 | 289 | AM, LM |
| 56 | 3'-O-Methylcatechin | C16H16O6 | 22.910 | [M−H]− | 303.0869 | 303.0863 | −2.0 | 289, 245 | LM, AM |
| 57 | (-)-Epigallocatechin 7-O-glucuronide | C21H22O13 | 22.910 | * [M−H]− | 481.0982 | 481.0988 | 1.2 | 289, 271, 151 | LM |
| 58 | (-)-Epicatechin 3-O-gallate | C22H18O10 | 26.538 | [M−H]− | 441.0822 | 441.0845 | 5.2 | 289, 169, 125 | AM, LM |
| 59 | Theaflavin 3,3'-O-digallate | C43H32O20 | 28.942 | [M−H]− | 867.1409 | 867.1367 | −4.8 | 715, 563, 169, 125 | AM |
| Flavanones | |||||||||
| 60 | Neoeriocitrin | C27H32O15 | 14.825 | [M−H]− | 595.1663 | 595.1684 | 3.5 | 459, 287, 151 | AM, LM |
| 61 | 6''-Acetylliquiritin | C23H24O10 | 15.278 | [M−H]− | 459.1292 | 459.1301 | 2.0 | 441, 255 | CM, LM |
| 62 | Naringenin 7-O-glucoside | C21H22O10 | 24.514 | * [M−H]− | 433.1135 | 433.1134 | −0.2 | 271 | AM, LM, CM |
| 63 | Narirutin | C27H32O14 | 25.305 | [M+H]+ | 581.1870 | 581.1873 | 0.5 | 563, 273, 255 | AM |
| 64 | Hesperetin 3'-O-glucuronide | C22H22O12 | 26.645 | [M−H]− | 477.1033 | 477.1023 | −2.1 | 301 | AM |
| 65 | Eriodictyol | C15H12O6 | 26.960 | [M−H]− | 287.0556 | 287.0563 | 2.4 | 269, 151, 135 | AM, LM |
| 66 | Pinocembrin 7-O-benzoate | C22H16O5 | 27.263 | [M+H]+ | 361.1076 | 361.1073 | −0.8 | 256 | CM |
| 67 | 6-Geranylnaringenin | C25H28O5 | 27.808 | * [M−H]− | 407.1859 | 407.1883 | 5.9 | 287, 243, 159, 119 | AM, CM |
| 68 | Kaempferol 7-(6''-galloylglucoside) | C28H24O15 | 28.564 | [M−H]− | 599.1037 | 599.1043 | 1.0 | 285 | AM, LM |
| 69 | Hesperetin 5-glucoside | C22H24O11 | 34.336 | [M+H]+ | 465.1397 | 465.1398 | 0.2 | 303 | AM |
| Flavones | |||||||||
| 70 | Diosmin (Diosmetin 7-O-rutinoside) | C28H32O15 | 4.350 | [M−H]− | 607.1663 | 607.1682 | 3.1 | 301, 300 | AM |
| 71 | Artocarpetin B | C22H22O6 | 4.688 | [M+H]+ | 383.1494 | 383.1498 | 1.0 | 365, 339, 327, 259 | LM, CM, AM |
| 72 | Hibiscetin 3-glucoside | C21H20O14 | 4.708 | [M+H]+ | 497.0931 | 497.0926 | −1.0 | 335 | AM |
| 73 | Kanzonol E | C25H24O4 | 15.195 | [M−H]− | 387.1597 | 387.1600 | 0.8 | 387 | LM |
| 74 | Chrysoeriol 7-O-glucoside | C22H22O11 | 15.678 | * [M−H]− | 461.1084 | 461.1076 | −1.7 | 299 | LM, AM |
| 75 | Quercetin 3-(2-galloylglucoside) | C28H24O16 | 16.150 | [M−H]− | 615.0986 | 615.1026 | 6.5 | 301 | LM |
| 76 | Myricetin 3-glucuronide | C21H18O14 | 18.088 | [M+H]+ | 495.0775 | 495.0814 | 7.9 | 319 | CM |
| 77 | Apigenin 6,8-di-C-glucoside | C27H30O15 | 19.490 | [M+H]+ | 595.1663 | 595.1673 | 1.7 | 271 | AM |
| 78 | Syringetin-3-O-glucoside | C23H24O13 | 22.686 | [M+H]+ | 509.1295 | 509.1261 | −6.7 | 347 | LM |
| 79 | 6-Hydroxyluteolin 7-O-rhamnoside | C21H20O11 | 23.048 | [M+H]+ | 449.1084 | 449.1084 | 0.0 | 303, 285 | AM |
| 80 | Multijugin | C24H22O7 | 24.308 | [M−H]− | 421.1288 | 421.1288 | 0.0 | 421 | LM, AM, CM |
| 81 | Rhoifolin | C27H30O14 | 24.308 | * [M−H]− | 577.1558 | 577.1523 | −6.1 | 431, 269 | LM, CM |
| 82 | Apigenin 6-C-glucoside | C21H20O10 | 25.484 | * [M−H]− | 433.1134 | 433.1134 | 0.0 | 269 | AM, LM |
| 83 | Quercetin 3-(2''-galloylrhamnoside) | C28H24O15 | 26.331 | [M−H]− | 599.1037 | 599.1037 | 0.0 | 301, 169, 125 | LM |
| 84 | Velutin | C17H14O6 | 27.263 | [M+H]+ | 315.0868 | 315.0878 | 3.2 | 300, 272, 257 | CM, LM |
| 85 | 6-Hydroxyluteolin 7-O-rhamnoside | C21H20O11 | 30.394 | [M−H]− | 447.0928 | 447.0945 | 3.8 | 301, 171, 151 | LM |
| 86 | Luteolin 8-C-glucoside (orientin) | C21H20O11 | 20.394 | [M−H]− | 447.0928 | 447.0945 | 3.8 | 357, 327 | AM, LM |
| 87 | Isorhamnetin 3-galactoside | C22H22O12 | 29.712 | [M−H]− | 477.1033 | 477.1047 | 4.7 | 315 | LM, AM |
| 88 | 3,4',7-Tetrahydroxyflavone | C15H10O6 | 30.619 | [M−H]− | 285.0455 | 285.0423 | −4.7 | 256, 241, 229, 165 | AM, LM |
| 89 | Kaempferol-3-O-rhamnoside | C21H20O10 | 35.354 | [M−H]− | 431.0978 | 431.1041 | 5.6 | 285, 255, 227 | AM |
| 90 | Kaempferol | C15H10O6 | 35.809 | [M−H]− | 285.0399 | 285.0414 | 5.3 | 267, 255, 227, 151 | LM |
| 91 | Apigenin 7-O-glucuronide | C21H18O11 | 35.927 | [M−H]− | 445.0771 | 445.0771 | 0.0 | 269 | LM |
| 92 | 3,5-Dimethylquercetin glucoside | C23H24O12 | 37.310 | [M−H]− | 491.1190 | 491.1211 | 4.3 | 329, 301 | LM, AM |
| 93 | Diosmetin 7-glucuronide | C22H20O12 | 40.896 | [M−H]− | 475.0877 | 475.0873 | −0.8 | 299 | LM |
| 94 | 7-Methoxyflavone | C16H12O3 | 41.780 | * [M+H]+ | 253.0864 | 253.0863 | −0.4 | 238, 210 | LM, AM |
| 95 | Tricin | C17H14O7 | 42.419 | [M−H]− | 329.0662 | 329.0684 | 6.7 | 313, 285, 257, 151 | LM |
| 96 | 3,5-Diacetyltambulin | C22H20O9 | 52.419 | [M−H]− | 427.1029 | 427.1029 | 0.0 | 427 | LM |
| Chalcones and dihydrochalcones | |||||||||
| 97 | Dihydropedicin | C18H20O6 | 4.572 | * [M+H]+ | 333.1338 | 333.1341 | 0.9 | 333 | AM, LM, CM |
| 98 | Phloretin 2'-O-glucuronide | C21H22O11 | 20.839 | [M−H]− | 449.1084 | 449.1084 | 0.0 | 373 | AM |
| 99 | Phloretin | C15H14O5 | 32.886 | [M−H]− | 273.0763 | 273.0780 | 4.5 | 179, 167, 151, 123 | LM, AM, AM |
| 100 | Xanthohumol | C21H22O5 | 29.765 | [M+H]+ | 355.1545 | 355.1552 | 2.0 | 337, 229, 179 | AM |
| 101 | 2'-Hydroxy-4',6'-dimethoxychalcone | C17H16O4 | 37.640 | [M+H]+ | 285.1127 | 285.1130 | 1.1 | 267, 253, 181, 131 | LM |
| 102 | Phloretin 2'-O-xylosyl-glucoside | C26H32O14 | 39.772 | [M−H]− | 567.1714 | 567.1706 | −1.4 | 273 | LM |
| Isoflavonoids | |||||||||
| 103 | 6''-O-Acetylglycitin | C24H24O11 | 15.202 | * [M−H]− | 487.1241 | 487.1245 | 0.8 | 283, 267, 59 | AM, LM |
| 104 | 3',4',7-Trihydroxyisoflavan | C15H14O4 | 15.756 | [M+H]+ | 259.0970 | 259.0974 | 1.5 | 241, 231, 149, 123 | CM |
| 105 | 3',4',7-Trihydroxyisoflavanone | C15H12O5 | 17.278 | [M+H]+ | 273.0763 | 273.0752 | −4.0 | 255, 161, 137, 121 | AM, LM |
| 106 | 3'-O-Methylequol | C16H16O4 | 18.765 | [M+H]+ | 273.1127 | 273.1129 | 0.7 | 255, 149, 121 | CM, AM |
| 107 | Daidzein 7-O-glucuronide | C21H18O10 | 22.546 | [M+H]+ | 431.0978 | 431.0978 | 0.0 | 255 | AM, LM |
| 108 | Dolineone | C19H12O6 | 25.484 | [M+H]+ | 337.0712 | 337.0717 | 1.5 | 319, 307, 161 | AM |
| 109 | 6''-O-Acetylgenistin | C23H22O11 | 32.916 | [M−H]− | 473.1084 | 473.1090 | 1.3 | 455, 269, 227 | LM |
| 110 | Violanone | C17H16O6 | 37.992 | [M+H]+ | 317.1025 | 317.1020 | −1.6 | 299, 191, 179, 137 | CM |
| 111 | 3'-Hydroxymelanettin | C16H12O6 | 41.555 | [M+H]+ | 301.0712 | 301.0712 | 0.0 | 301 | LM, AM, CM |
| 112 | Dihydroformononetin | C16H14O4 | 41.780 | [M+H]+ | 271.0970 | 271.0961 | −3.3 | 253, 137 | LM, CM |
| 113 | Dihydrobiochanin A | C16H14O5 | 52.724 | [M+H]+ | 287.0919 | 287.0913 | −2.1 | 269, 259, 179 | CM |
| Tannins | |||||||||
| 114 | Grandinin | C46H34O30 | 5.317 | * [M−H]− | 1065.1057 | 1065.1090 | 3.1 | 1047, 933, 783, 169, 125 | AM, CM |
| 115 | Prodelphinidin B3 | C30H26O14 | 5.357 | [M−H]− | 609.1245 | 609.1265 | 3.3 | 591, 539 | LM, AM |
| 116 | 2-O-Galloylpunicalin | C41H26O26 | 5.386 | * [M−H]− | 933.0634 | 933.0666 | 3.4 | 783, 169, 125 | AM, CM |
| 117 | Glucosyringic acid | C1520HO10 | 7.324 | [M−H]− | 359.0978 | 359.0978 | 0.0 | 315, 197, 153, 125 | AM, LM |
| 118 | Kurigalin | C27H24O18 | 13.912 | [M−H]− | 635.0885 | 635.0905 | 3.1 | 466, 313, 211, 169, 125 | AM |
| 119 | Corilagin | C27H22O18 | 14.062 | [M−H]− | 633.0728 | 633.0742 | 2.2 | 301, 169, 125 | LM, AM |
| 120 | Potentillin | C41H28O26 | 14.081 | [M−H]− | 935.0791 | 935.0866 | 8.2 | 784, 634, 169 | AM |
| 121 | Procyanidin dimer B2 | C30H26O12 | 14.255 | [M−H]− | 577.1346 | 577.1373 | 4.7 | 451, 425, 289, 245 | LM, AM |
| 122 | Punicafolin | C41H30O26 | 14.390 | [M+H]+ | 939.1103 | 939.1111 | 0.9 | 169, 125 | CM |
| 123 | Procyanidin trimer C1 | C45H38O18 | 14.412 | * [M−H]− | 865.1980 | 865.1981 | 0.1 | 696, 577, 425, 289, 125 | LM, AM, CM |
| 124 | Procyanidin B2 3-gallate | C37H30O16 | 19.170 | [M−H]− | 729.1456 | 729.1460 | 1.3 | 408, 289, 245, 169, 125 | LM |
| 125 | Ellagic acid | C14H6O8 | 26.341 | * [M−H]− | 300.9985 | 300.9985 | 0.0 | 284, 257 | LM, AM, CM |
| Stilbenes | |||||||||
| 126 | 3'-Hydroxy-3,4,5,4'-tetramethoxystilbene | C17H18O5 | 27.546 | [M+H]+ | 303.1232 | 303.1236 | 1.3 | 285, 257, 165 | AM, CM |
| 127 | Dihydroresveratrol | C14H14O3 | 28.279 | [M+H]+ | 231.1021 | 231.1024 | 1.3 | 217, 137, 107 | CM |
| 128 | Polydatin | C20H22O8 | 49.650 | [M+H]+ | 391.1393 | 391.1393 | 0.0 | 229 | LM |
| Lignans | |||||||||
| 129 | Deoxyschisandrin | C24H32O6 | 21.934 | [M−H]− | 415.2121 | 415.2121 | 0.0 | 402, 347, 361, 301 | CM, LM |
| 130 | Lariciresinol-sesquilignan | C30H36O10 | 23.749 | [M−H]− | 555.2230 | 555.2228 | −0.4 | 537, 509, 359, 343 | LM |
| 131 | Silibinin | C25H22O10 | 25.415 | [M−H]− | 481.1135 | 481.1131 | −0.8 | 301, 179, 165, 151 | LM, AM |
| 132 | Lariciresinol | C20H24O6 | 32.572 | * [M−H]− | 359.1495 | 359.1492 | −0.8 | 329, 192, 178, 175, 160 | CM, LM |
| 133 | Pinoresinol | C20H22O6 | 33.476 | * [M+H]+ | 359.1494 | 359.1496 | 0.6 | 359 | AM, CM |
| Other compounds | |||||||||
| 134 | Psoralen | C11H6O3 | 2.321 | [M−H]− | 185.0239 | 185.0287 | 141, 125, 80 | AM, LM | |
| 135 | Quinic acid | C7H12O6 | 3.995 | [M−H]− | 191.0561 | 191.0564 | 1.3 | 173, 127, 111, 93, 85 | LM, AM, CM |
| 136 | Caffeine | C8H10N4O2 | 5.086 | [M+H]+ | 195.0877 | 195.0885 | 4.4 | 138, 110 | AM, LM |
| 137 | Pyrogallol | C6H6O3 | 5.329 | [M−H]− | 125.0239 | 125.0238 | −0.8 | 107, 97 | LM, AM, CM |
| 138 | Catechol | C6H6O2 | 5.377 | [M+H]+ | 111.0446 | 111.0448 | 1.8 | 93, 81, 65 | LM |
| 139 | Rosmanol | C20H26O5 | 21.759 | * [M−H]− | 345.1702 | 345.1700 | −0.6 | 301 | LM, CM, AM |
| 140 | Urolithin C | C13H8O5 | 29.016 | [M−H]− | 243.0294 | 243.0293 | −0.3 | 199, 175 | AM |
| 141 | Urolithin A | C13H8O4 | 35.750 | [M−H]− | 227.0345 | 227.0377 | 4.2 | 183, 167 | AM |
| 142 | Carnosol | C20H26O4 | 36.214 | [M+H]+ | 331.1909 | 331.1905 | −1.2 | 287 | CM |
| 143 | Scopoletin | C10H8O4 | 32.315 | [M−H]− | 191.0345 | 191.0350 | 1.3 | 147 | LM, AM |
| 144 | Carvacrol | C10H14O | 58.692 | * [M−H]− | 149.0967 | 149.0970 | 2.0 | 105 | LM, AM, CM |
| 145 | Vanillin | C8H8O3 | 59.456 | [M+H]+ | 153.0551 | 153.0552 | 0.7 | 137, 125, 93, 65 | LM, AM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





