Submitted:
20 March 2024
Posted:
20 March 2024
You are already at the latest version
Abstract
Keywords:
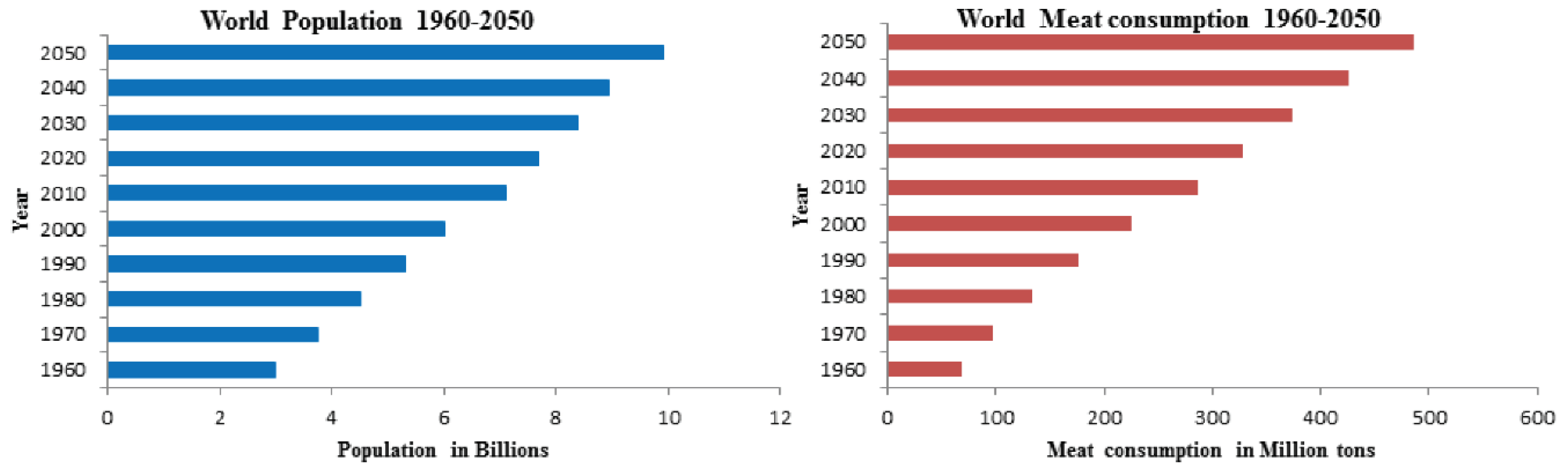
1. Introduction:
2. Conventional evaluation of meat quality:
2.1. Color
2.2. Tenderness
2.3. Water Holding Capacity
2.4. Beef Meat Grading Systems
3. Serum Biomarkers for Meat Quality:
3.1. Vitamin A
3.2. Blood Urea-Nitrogen (BUN)
3.3. Non-Esterified Fatty Acid (NEFA)
3.4. Total Cholesterol (TCH)
3.5. Paraoxonase 1 (PON1)
3.6. Insulin:
3.7. Leptin
3.8. Aspartic Acid Transaminase (AST) & Alanine Transaminase (ALT)
3.9. Total Protein (TP)
4. Cultured Meat and Limitations:
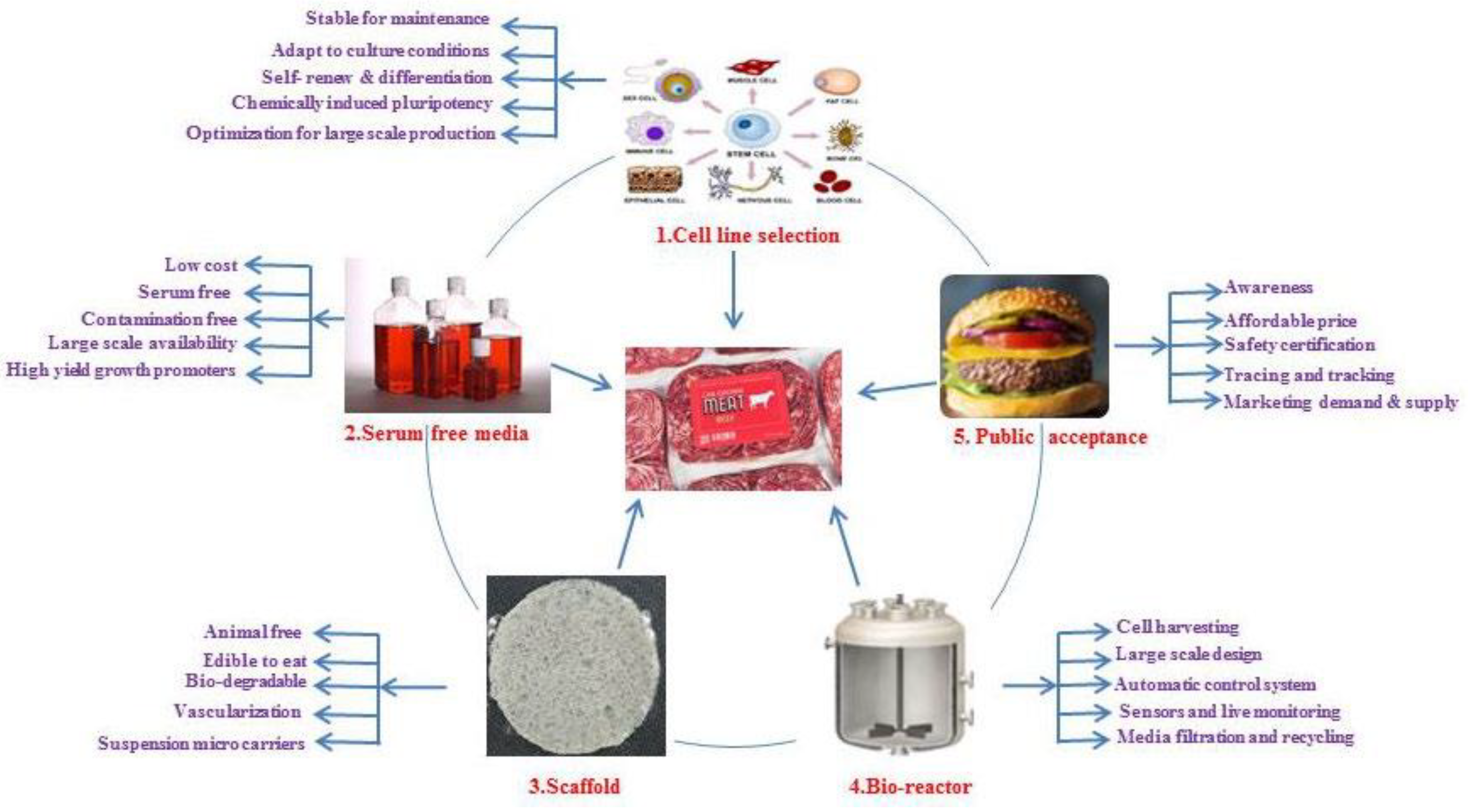
4.1. Cell Line Selection for Cultured Meat Production
4.2. Serum-Free Media Formulation
4.3. Scaffold
4.4. Limitations of Cultured Meat Production
4.5. Overcoming Solutions
5. Potential Application of Serum Markers for Culture Meat Production:
6. Conclusion:
Author Contributions
Funding
Conflicts of Interest
References
- Valin, H.; Sands, R.D.; van der Mensbrugghe, D.; Nelson, G.C.; Ahammad, H.; Blanc, E.; Bodirsky, B.; Fujimori, S.; Hasegawa, T.; Havlik, P.; et al. The Future of Food Demand: Understanding Differences in Global Economic Models. Agric. Econ. 2014, 45, 51–67. [Google Scholar] [CrossRef]
- Sans, P.; Combris, P. World Meat Consumption Patterns: An Overview of the Last Fifty Years (1961-2011). Meat Sci. 2015, 109, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.T.; Kim, G.D.; Hwang, Y.H.; Ryu, Y.C. Control of Fresh Meat Quality through Manipulation of Muscle Fiber Characteristics. Meat Sci. 2013, 95, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Geletu, U.S.; Usmael, M.A.; Mummed, Y.Y.; Ibrahim, A.M. Quality of Cattle Meat and Its Compositional Constituents. Vet. Med. Int. 2021, 2021. [Google Scholar] [CrossRef] [PubMed]
- Grunert, K.G.; Bredahl, L.; Brunsø, K. Consumer Perception of Meat Quality and Implications for Product Development in the Meat Sector-a Review. Meat Sci. 2004, 66, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Sharma, N.; Sharma, S.; Mehta, N.; Verma, A.K.; Chemmalar, S.; Sazili, A.Q. In-Vitro Meat: A Promising Solution forsustainability of Meat Sector. J. Anim. Sci. Technol. 2021, 63, 693. [Google Scholar] [CrossRef]
- Tuomisto, H.L.; Teixeira De Mattos, M.J. Environmental Impacts of Cultured Meat Production. Environ. Sci. Technol. 2011, 45, 6117–6123. [Google Scholar] [CrossRef]
- Post, M.J. Cultured Beef: Medical Technology to Produce Food. J. Sci. Food Agric. 2014, 94, 1039–1041. [Google Scholar] [CrossRef]
- Smith, G.C.; Belk, K.E.; Sofos, J.N.; Tatum, J.D.; Williams, S.N. Economic Implications of Improved Color Stability in Beef. Antioxidants muscle foods Nutr. Strateg. to Improv. Qual. 2000, 397–426. [Google Scholar]
- Gagaoua, M.; Hughes, J.; Terlouw, E.M.C.; Warner, R.D.; Purslow, P.P.; Lorenzo, J.M.; Picard, B. Proteomic Biomarkers of Beef Colour. Trends Food Sci. Technol. 2020, 101, 234–252. [Google Scholar] [CrossRef]
- O’Quinn, T.G.; Legako, J.F.; Brooks, J.C.; Miller, M.F. Evaluation of the Contribution of Tenderness, Juiciness, and Flavor to the Overall Consumer Beef Eating Experience. Transl. Anim. Sci. 2018, 2, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Dunshea, F.; Ha, M.; Purslow, P.; Miller, R.; Warner, R.; Vaskoska, R.S.; Wheeler, T.L.; Li, X. Meat Tenderness: Underlying Mechanisms, Instrumental Measurement, and Sensory Assessment. Meat Muscle Biol. 2021, 4. [Google Scholar] [CrossRef]
- Ouali, A.; Gagaoua, M.; Boudida, Y.; Becila, S.; Boudjellal, A.; Herrera-Mendez, C.H.; Sentandreu, M.A. Biomarkers of Meat Tenderness: Present Knowledge and Perspectives in Regards to Our Current Understanding of the Mechanisms Involved. Meat Sci. 2013, 95, 854–870. [Google Scholar] [CrossRef]
- Warner, R.D. The Eating Quality of Meat—IV Water-Holding Capacity and Juiciness. Lawrie’s Meat Sci. Eighth Ed. 2017, 419–459. [Google Scholar] [CrossRef]
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How Muscle Structure and Composition Influence Meat and Flesh Quality. ScientificWorldJournal. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Delele, M.A.; Kuffi, K.D.; Geeraerd, A.; De Smet, S.; Nicolai, B.M.; Verboven, P. Optimizing Precooling of Large Beef Carcasses Using a Comprehensive Computational Fluid Dynamics Model. J. Food Process Eng. 2019, 42. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of Water-Holding Capacity of Meat: The Role of Postmortem Biochemical and Structural Changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef]
- Font-i-Furnols, M.; Tous, N.; Esteve-Garcia, E.; Gispert, M. Do All the Consumers Accept Marbling in the Same Way? The Relationship between Eating and Visual Acceptability of Pork with Different Intramuscular Fat Content. Meat Sci. 2012, 91, 448–453. [Google Scholar] [CrossRef]
- What’s Your Beef – Prime, Choice or Select? | USDA. Available online: https://www.usda.gov/media/blog/2013/01/28/whats-your-beef-prime-choice-or-select?page=1 (accessed on 30 April 2023).
- What’s Your Beef – Prime, Choice or Select? | USDA. Available online: https://www.usda.gov/media/blog/2013/01/28/whats-your-beef-prime-choice-or-select?page=1 (accessed on 17 October 2022).
- Grading - Lone Mountain Cattle. Available online: https://www.lonemountaincattle.com/about-wagyu/grading/ (accessed on 30 April 2023).
- Australian Wagyu Grading – The Wagyu Shop. Available online: https://wagyushop.com/pages/australian-wagyu-grading (accessed on 30 April 2023).
- Wagyu Grading Comparison – Wagyu Prime. Available online: https://wagyuprime.com/pages/beef-grading-comparison (accessed on 30 April 2023).
- What the Beef? Canadian vs American Beef Grading -. Available online: https://smellaque.com/2020/08/12/what-the-beef-canadian-vs-american-beef-grading/ (accessed on 30 April 2023).
- Beef Quality Grading in Europe: Shifting to a Consumer Centred Perception of Beef Quality | Farming Connect. Available online: https://businesswales.gov.wales/farmingconnect/news-and-events/technical-articles/beef-quality-grading-europe-shifting-consumer-centred-perception-beef-quality (accessed on 30 April 2023).
- Liu, J.; Chriki, S.; Ellies-Oury, M.P.; Legrand, I.; Pogorzelski, G.; Wierzbicki, J.; Farmer, L.; Troy, D.; Polkinghorne, R.; Hocquette, J.F. European Conformation and Fat Scores of Bovine Carcasses Are Not Good Indicators of Marbling. Meat Sci. 2020, 170, 108233. [Google Scholar] [CrossRef]
- 축산물품질평가원 홈페이지. Available online: https://www.ekape.or.kr/index.do (accessed on 30 April 2023).
- Knutson, E.E.; Menezes, A.C.B.; Sun, X.; Fontoura, A.B.P.; Liu, J.H.; Bauer, M.L.; Maddock-Carlin, K.R.; Swanson, K.C.; Ward, A.K. Effect of Feeding a Low-Vitamin A Diet on Carcass and Production Characteristics of Steers with a High or Low Propensity for Marbling. Animal 2020, 14, 2308–2314. [Google Scholar] [CrossRef]
- Chen, W.; Chen, G. The Roles of Vitamin A in the Regulation of Carbohydrate, Lipid, and Protein Metabolism. J. Clin. Med. 2014, 3, 453. [Google Scholar] [CrossRef] [PubMed]
- Reichert, B.; Yasmeen, R.; Jeyakumar, S.M.; Yang, F.; Thomou, T.; Alder, H.; Duester, G.; Maiseyeu, A.; Mihai, G.; Harrison, E.H.; et al. Concerted Action of Aldehyde Dehydrogenases Influences Depot-Specific Fat Formation. Mol. Endocrinol. 2011, 25, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Gorocica-Buenfil, M.A.; Fluharty, F.L.; Bohn, T.; Schwartz, S.J.; Loerch, S.C. Effect of Low Vitamin A Diets with High-Moisture or Dry Corn on Marbling and Adipose Tissue Fatty Acid Composition of Beef Steers. J. Anim. Sci. 2007, 85, 3355–3366. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.C.; DeSantis, D.; Soltanian, H.; Croniger, C.M.; Noy, N. Retinoic Acid Upregulates Preadipocyte Genes to Block Adipogenesis and Suppress Diet-Induced Obesity. Diabetes 2012, 61, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Tshuma, T.; Fosgate, G.T.; Hamman, R.; Holm, D.E. Effect of Different Levels of Dietary Nitrogen Supplementation on the Relative Blood Urea Nitrogen Concentration of Beef Cows. Trop. Anim. Health Prod. 2019, 51, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.; Crispie, F.; Lewis, E.; Reid, M.; O’Toole, P.W.; Cotter, P.D. The Rumen Microbiome: A Crucial Consideration When Optimising Milk and Meat Production and Nitrogen Utilisation Efficiency. Gut Microbes 2019, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Xu, Z.; Shen, Z.; Tian, Y.; Shen, H. Dietary Energy Level Promotes Rumen Microbial Protein Synthesis by Improving the Energy Productivity of the Ruminal Microbiome. Front. Microbiol. 2019, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Spek, J.W.; Dijkstra, J.; Van Duinkerken, G.; Bannink, A. A Review of Factors Influencing Milk Urea Concentration and Its Relationship with Urinary Urea Excretion in Lactating Dairy Cattle. J. Agric. Sci. 2013, 151, 407–423. [Google Scholar] [CrossRef]
- Müller, C.B.M.; Görs, S.; Derno, M.; Tuchscherer, A.; Wimmers, K.; Zeyner, A.; Kuhla, B. Differences between Holstein Dairy Cows in Renal Clearance Rate of Urea Affect Milk Urea Concentration and the Relationship between Milk Urea and Urinary Nitrogen Excretion. Sci. Total Environ. 2021, 755. [Google Scholar] [CrossRef]
- Xia, C.; Rahman, M.A.U.; Yang, H.; Shao, T.; Qiu, Q.; Su, H.; Cao, B. Effect of Increased Dietary Crude Protein Levels on Production Performance, Nitrogen Utilisation, Blood Metabolites and Ruminal Fermentation of Holstein Bulls. Asian-Australasian J. Anim. Sci. 2018, 31, 1643–1653. [Google Scholar] [CrossRef]
- Schumacher, M.; Delcurto-Wyffels, H.; Thomson, J.; Boles, J. Fat Deposition and Fat Effects on Meat Quality-A Review. Anim. an open access J. from MDPI 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Kazala, E.C.; Lozeman, F.J.; Mir, P.S.; Laroche, A.; Bailey, D.R.C.; Weselake, R.J. Relationship of Fatty Acid Composition to Intramuscular Fat Content in Beef from Crossbred Wagyu Cattle. J. Anim. Sci. 1999, 77, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, M.M.; Schoonmaker, J.P.; Swanson, K.C.; Duckett, S.K.; Gionbelli, M.P.; Rodrigues, L.M.; Teixeira, P.D. Review: Nutrigenomics of Marbling and Fatty Acid Profile in Ruminant Meat. Animal 2018, 12, s282–s294. [Google Scholar] [CrossRef] [PubMed]
- Williams, P. Nutritional Composition of Red Meat. Nutr. Diet. 2007, 64, S113–S119. [Google Scholar] [CrossRef]
- Muchenje, V.; Hugo, A.; Dzama, K.; Chimonyo, M.; Strydom, P.E.; Raats, J.G. Cholesterol Levels and Fatty Acid Profiles of Beef from Three Cattle Breeds Raised on Natural Pasture. J. Food Compos. Anal. 2009, 22, 354–358. [Google Scholar] [CrossRef]
- Dinh, T.T.N.; Thompson, L.D.; Galyean, M.L.; Brooks, J.C.; Patterson, K.Y.; Boylan, L.M. Cholesterol Content and Methods for Cholesterol Determination in Meat and Poultry. Compr. Rev. Food Sci. Food Saf. 2011, 10, 269–289. [Google Scholar] [CrossRef]
- Kota, S.K.; Meher, L.K.; Kota, S.K.; Jammula, S.; Krishna, S.V.S.; Modi, K.D. Implications of Serum Paraoxonase Activity in Obesity, Diabetes Mellitus, and Dyslipidemia. Indian J. Endocrinol. Metab. 2013, 17, 402. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bacchetti, T.; Moroni, C.; Savino, S.; Liuzzi, A.; Balzola, F.; Bicchiega, V. Paraoxonase Activity in High-Density Lipoproteins: A Comparison between Healthy and Obese Females. J. Clin. Endocrinol. Metab. 2005, 90, 1728–1733. [Google Scholar] [CrossRef] [PubMed]
- Rincón, J.; Madeira, E.M.; Campos, F.T.; Mion, B.; Silva, J.F.; Absalón-Medina, V.A.; Butler, W.R.; Corrêa, M.N.; Pegoraro, L.; Schneider, A. Exogenous Paraoxonase-1 during Oocyte Maturation Improves Bovine Embryo Development in Vitro. Reprod. Domest. Anim. 2016, 51, 827–830. [Google Scholar] [CrossRef]
- Silveira, P.A.S.; Butler, W.R.; LaCount, S.E.; Overton, T.R.; Barros, C.C.; Schneider, A. Polymorphisms in the Anti-Oxidant Paraoxonase-1 (PON1) Gene Associated with Fertility of Postpartum Dairy Cows. Theriogenology 2019, 125, 302–309. [Google Scholar] [CrossRef]
- Rincón, J.; Madeira, E.M.; Campos, F.T.; Mion, B.; Silva, J.F.; Absalón-Medina, V.A.; Butler, W.R.; Corrêa, M.N.; Pegoraro, L.; Schneider, A. Exogenous Paraoxonase-1 during Oocyte Maturation Improves Bovine Embryo Development in Vitro. Reprod. Domest. Anim. 2016, 51, 827–830. [Google Scholar] [CrossRef]
- Silveira, P.A.S.; Butler, W.R.; LaCount, S.E.; Overton, T.R.; Barros, C.C.; Schneider, A. Polymorphisms in the Anti-Oxidant Paraoxonase-1 (PON1) Gene Associated with Fertility of Postpartum Dairy Cows. Theriogenology 2019, 125, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Ji, A.G.; Huai, Y.H.; Zhou, Z.K.; Li, J.Y.; Zhang, L.P.; Xu, S.Z.; Gao, X.; Ren, H.Y.; Chen, J.B. Association between PON1 Gene SNPs and Growth and Carcass Traits in Beef Cattle. Asian-Australasian J. Anim. Sci. 2008, 21, 1097–1102. [Google Scholar] [CrossRef]
- Stajkovic, S.; Vasilev, D.; Teodorovic, V.; Karabasil, N. Postmortem Glycolysis and Pork Quality. IOP Conf. Ser. Earth Environ. Sci. 2019, 333, 012032. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, F. Tissue-Specific Insulin Signaling in the Regulation of Metabolism and Aging. IUBMB Life 2014, 66, 485–495. [Google Scholar] [CrossRef]
- Newsholme, E.A.; Dimitriadis, G. Integration of Biochemical and Physiologic Effects of Insulin on Glucose Metabolism. Exp. Clin. Endocrinol. Diabetes, 2. [CrossRef]
- Goldberg, I.J.; Eckel, R.H.; Abumrad, N.A. Regulation of Fatty Acid Uptake into Tissues: Lipoprotein Lipase- and CD36-Mediated Pathways. J. Lipid Res. [CrossRef]
- Jindřichová, E.; Kratochvílová, S.; Kovář, J. Glucose Administration Downregulates Lipoprotein Lipase Activity in Vivo: A Study Using Repeated Intravenous Fat Tolerance Test. Physiol. Res. 2007, 56, 175–181. [Google Scholar] [CrossRef]
- Graham, T.E.; Yang, Q.; Blüher, M.; Hammarstedt, A.; Ciaraldi, T.P.; Henry, R.R.; Wason, C.J.; Oberbach, A.; Jansson, P.-A.; Smith, U.; et al. Retinol-Binding Protein 4 and Insulin Resistance in Lean, Obese, and Diabetic Subjects. N. Engl. J. Med. 2006, 354, 2552–2563. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.H.; Cho, W.K.; Lee, S.S. Investigation of Blood Biomarkers Related to Meat Quality and Quantity in Hanwoo Steers. Asian-Australasian J. Anim. Sci. 2018, 31, 1923. [Google Scholar] [CrossRef]
- Kuźnicka, E.; Gabryszuk, M.; Kunowska-Slósarz, M.; Gołębiewski, M.; Balcerak, M. Plasma Leptin as a Predictor for Carcass Composition in Growing Lambs. Can. J. Anim. Sci. 2017, 97, 193–198. [Google Scholar] [CrossRef]
- Geary, T.W.; McFadin, E.L.; MacNeil, M.D.; Grings, E.E.; Short, R.E.; Funston, R.N.; Keisler, D.H. Leptin as a Predictor of Carcass Composition in Beef Cattle. J. Anim. Sci. 2003, 81, 1–8. [Google Scholar] [CrossRef]
- Kononoff, P.J.; Deobald, H.M.; Stewart, E.L.; Laycock, A.D.; Marquess, F.L.S. The Effect of a Leptin Single Nucleotide Polymorphism on Quality Grade, Yield Grade, and Carcass Weight of Beef Cattle. J. Anim. Sci. 2005, 83, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Asakuma, S.; Morishita, H.; Sugino, T.; Kurose, Y.; Kobayashi, S.; Terashima, Y. Circulating Leptin Response to Feeding and Exogenous Infusion of Insulin in Sheep Exposed to Thermoneutral and Cold Environments. Comp. Biochem. Physiol. - A Mol. Integr. Physiol. 2003, 134, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.; Faulconnier, Y.; Leroux, C.; Jurie, C.; Cassar-Malek, I.; Bauchart, D.; Boulesteix, P.; Pethick, D.; Hocquette, J.F.; Chilliard, Y. Glucose-6-Phosphate Dehydrogenase and Leptin Are Related to Marbling Differences among Limousin and Angus or Japanese Black x Angus Steers. J. Anim. Sci. 2007, 85, 2882–2894. [Google Scholar] [CrossRef]
- McGill, M.R. The Past and Present of Serum Aminotransferases and the Future of Liver Injury Biomarkers. EXCLI J. 2016, 15, 817. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R. The Past and Present of Serum Aminotransferases and the Future of Liver Injury Biomarkers. EXCLI J. 2016, 15, 817–828. [Google Scholar] [CrossRef]
- Lee, B.; Choi, Y.M. Association of Serum Glucose, Serotonin, Aspartate Aminotransferase, and Calcium Levels with Meat Quality and Palatability Characteristics of Broiler Pectoralis Major Muscle. Anim. an Open Access J. from MDPI 2022, 12. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Dai, C.; Li, J.; Huang, P.; Li, Y.; Ding, X.; Huang, J.; Hussain, T.; Yang, H. Effects of Dietary Energy on Growth Performance, Carcass Characteristics, Serum Biochemical Index, and Meat Quality of Female Hu Lambs. Anim. Nutr. 2020, 6, 499. [Google Scholar] [CrossRef] [PubMed]
- Dufour, D.R.; Lott, J.A.; Nolte, F.S.; Gretch, D.R.; Koff, R.S.; Seeff, L.B. Diagnosis and Monitoring of Hepatic Injury. I. Performance Characteristics of Laboratory Tests. Clin. Chem. 2000, 46, 2027–2049. [Google Scholar] [CrossRef] [PubMed]
- Salter, A.M. The Effects of Meat Consumption on Global Health. Rev. Sci. Tech. 2018, 37, 47–55. [Google Scholar] [CrossRef]
- Greger, M. The Human/Animal Interface: Emergence and Resurgence of Zoonotic Infectious Diseases. Crit. Rev. Microbiol. 2007, 33, 243–299. [Google Scholar] [CrossRef]
- Balasubramanian, B.; Liu, W.; Pushparaj, K.; Park, S. The Epic of In Vitro Meat Production—A Fiction into Reality. Foods 2021, 10. [Google Scholar] [CrossRef]
- Shaikh, S.; Lee, E.J.; Ahmad, K.; Ahmad, S.S.; Chun, H.J.; Lim, J.H.; Lee, Y.H.; Choi, I. Cell Types Used for Cultured Meat Production and the Importance of Myokines. Foods 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Stephens, N.; Di Silvio, L.; Dunsford, I.; Ellis, M.; Glencross, A.; Sexton, A. Bringing Cultured Meat to Market: Technical, Socio-Political, and Regulatory Challenges in Cellular Agriculture. Trends Food Sci. Technol. 2018, 78, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guttieres, D.; Koenigsberg, A.; Barone, P.W.; Sinskey, A.J.; Springs, S.L. Large-Scale Cultured Meat Production: Trends, Challenges and Promising Biomanufacturing Technologies. Biomaterials 2022, 280. [Google Scholar] [CrossRef]
- Karnieli, O.; Friedner, O.M.; Allickson, J.G.; Zhang, N.; Jung, S.; Fiorentini, D.; Abraham, E.; Eaker, S.S.; Yong, T.K.; Chan, A.; et al. A Consensus Introduction to Serum Replacements and Serum-Free Media for Cellular Therapies. Cytotherapy 2017, 19, 155–169. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.N.; Cosenza, Z.A.; Baar, K.; Block, D.E. Considerations for the Development of Cost-Effective Cell Culture Media for Cultivated Meat Production. Compr. Rev. food Sci. food Saf. 2021, 20, 686–709. [Google Scholar] [CrossRef]
- Fujita, H.; Endo, A.; Shimizu, K.; Nagamori, E. Evaluation of Serum-Free Differentiation Conditions for C2C12 Myoblast Cells Assessed as to Active Tension Generation Capability. Biotechnol. Bioeng. 2010, 107, 894–901. [Google Scholar] [CrossRef]
- Kuo, H.H.; Gao, X.; DeKeyser, J.M.; Fetterman, K.A.; Pinheiro, E.A.; Weddle, C.J.; Fonoudi, H.; Orman, M. V.; Romero-Tejeda, M.; Jouni, M.; et al. Negligible-Cost and Weekend-Free Chemically Defined Human IPSC Culture. Stem cell reports 2020, 14, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Khan, S.; Murid, M.; Asif, Z.; Oboturova, N.P.; Nagdalian, A.A.; Blinov, A.V.; Ibrahim, S.A.; Jafari, S.M. Marketing Strategies for Cultured Meat: A Review. Appl. Sci. 2022, 12, 8795. [Google Scholar] [CrossRef]
- Benjaminson, M.A.; Gilchriest, J.A.; Lorenz, M. In Vitro Edible Muscle Protein Production System (MPPS): Stage 1, Fish. Acta Astronaut. 2002, 51, 879–889. [Google Scholar] [CrossRef]
- Ben-Arye, T.; Shandalov, Y.; Ben-Shaul, S.; Landau, S.; Zagury, Y.; Ianovici, I.; Lavon, N.; Levenberg, S. Textured Soy Protein Scaffolds Enable the Generation of Three-Dimensional Bovine Skeletal Muscle Tissue for Cell-Based Meat. Nat. Food 2020, 1, 210–220. [Google Scholar] [CrossRef]
- MacQueen, L.A.; Alver, C.G.; Chantre, C.O.; Ahn, S.; Cera, L.; Gonzalez, G.M.; O’Connor, B.B.; Drennan, D.J.; Peters, M.M.; Motta, S.E.; et al. Muscle Tissue Engineering in Fibrous Gelatin: Implications for Meat Analogs. npj Sci. Food 2019, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Orellana, N.; Sánchez, E.; Benavente, D.; Prieto, P.; Enrione, J.; Acevedo, C.A. A New Edible Film to Produce In Vitro Meat. Foods 2020, 9. [Google Scholar] [CrossRef]
- Wollschlaeger, J.O.; Maatz, R.; Albrecht, F.B.; Klatt, A.; Heine, S.; Blaeser, A.; Kluger, P.J. Scaffolds for Cultured Meat on the Basis of Polysaccharide Hydrogels Enriched with Plant-Based Proteins. Gels 2022, 8, 94. [Google Scholar] [CrossRef]
- Xiang, N.; Yuen, J.S.K.; Stout, A.J.; Rubio, N.R.; Chen, Y.; Kaplan, D.L. 3D Porous Scaffolds from Wheat Glutenin for Cultured Meat Applications. Biomaterials 2022, 285. [Google Scholar] [CrossRef]
- Bomkamp, C.; Skaalure, S.C.; Fernando, G.F.; Ben-Arye, T.; Swartz, E.W.; Specht, E.A. Scaffolding Biomaterials for 3D Cultivated Meat: Prospects and Challenges. Adv. Sci. 2022, 9, 2102908. [Google Scholar] [CrossRef] [PubMed]
- Prasopdee, T.; Sinthuvanich, C.; Chollakup, R.; Uttayarat, P.; Smitthipong, W. The Albumin/Starch Scaffold and Its Biocompatibility with Living Cells. Mater. Today Commun. 2021, 27, 102164. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Kumar, S.; Fayaz, H. In Vitro Meat Production: Challenges and Benefits over Conventional Meat Production. J. Integr. Agric. 2015, 14, 241–248. [Google Scholar] [CrossRef]
- Allan, S.J.; De Bank, P.A.; Ellis, M.J. Bioprocess Design Considerations for Cultured Meat Production With a Focus on the Expansion Bioreactor. Front. Sustain. Food Syst. 2019, 3, 44. [Google Scholar] [CrossRef]
- Faustman, C.; Hamernik, D.; Looper, M.; Zinn, S.A. Cell-Based Meat: The Need to Assess Holistically. J. Anim. Sci. 2020, 98, 1–7. [Google Scholar] [CrossRef]
- Zidarič, T.; Milojević, M.; Vajda, J.; Vihar, B.; Maver, U. Cultured Meat: Meat Industry Hand in Hand with Biomedical Production Methods. Food Eng. Rev. 2020, 12, 498–519. [Google Scholar] [CrossRef]
- Moritz, M.S.M.; Verbruggen, S.E.L.; Post, M.J. Alternatives for Large-Scale Production of Cultured Beef: A Review. J. Integr. Agric. 2015, 14, 208–216. [Google Scholar] [CrossRef]
- Pajčin, I.; Knežić, T.; Azoulay, I.S.; Vlajkov, V.; Djisalov, M.; Janjušević, L.; Grahovac, J.; Gadjanski, I. Bioengineering Outlook on Cultivated Meat Production. Micromachines 2022, 13. [Google Scholar] [CrossRef]
- Escobar, M.I.R.; Cadena, E.; Nhu, T.T.; Cooreman-Algoed, M.; De Smet, S.; Dewulf, J. Analysis of the Cultured Meat Production System in Function of Its Environmental Footprint: Current Status, Gaps and Recommendations. Foods 2021, 10, 2941. [Google Scholar] [CrossRef] [PubMed]
- Reiss, J.; Robertson, S.; Suzuki, M. Cell Sources for Cultivated Meat: Applications and Considerations throughout the Production Workflow. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef]
- Tomiyama, A.J.; Kawecki, N.S.; Rosenfeld, D.L.; Jay, J.A.; Rajagopal, D.; Rowat, A.C. Bridging the Gap between the Science of Cultured Meat and Public Perceptions. Trends Food Sci. Technol. 2020, 104, 144–152. [Google Scholar] [CrossRef]
- Teixeira, A.; Rodrigues, S. Consumer Perceptions towards Healthier Meat Products. Curr. Opin. Food Sci. 2021, 38, 147–154. [Google Scholar] [CrossRef]
- Chriki, S.; Hocquette, J.F. The Myth of Cultured Meat: A Review. Front. Nutr. 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Post, M.J.; Hocquette, J.F. New Sources of Animal Proteins: Cultured Meat. New Asp. Meat Qual. From Genes to Ethics 2017, 425–441. [Google Scholar] [CrossRef]
- Simsa, R.; Yuen, J.; Stout, A.; Rubio, N.; Fogelstrand, P.; Kaplan, D.L. Extracellular Heme Proteins Influence Bovine Myosatellite Cell Proliferation and the Color of Cell-Based Meat. Foods 2019, 8. [Google Scholar] [CrossRef]
- Fraeye, I.; Kratka, M.; Vandenburgh, H.; Thorrez, L. Sensorial and Nutritional Aspects of Cultured Meat in Comparison to Traditional Meat: Much to Be Inferred. Front. Nutr. 2020, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.T.; Choi, J.S.; Hur, S.J.; Kim, G.D.; Kim, C.J.; Lee, E.Y.; Bakhsh, A.; Hwang, Y.H. A Comparative Study on the Taste Characteristics of Satellite Cell Cultured Meat Derived from Chicken and Cattle Muscles. Food Sci. Anim. Resour. 2022, 42, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Cui, J.; Liu, X.; Zhang, Y.; Qin, N.; Luo, Y. Application of Artificial Neural Network to Predict the Change of Inosine Monophosphate for Lightly Salted Silver Carp (Hypophthalmichthys Molitrix) during Thermal Treatment and Storage. J. Food Process. Preserv. 2017, 41. [Google Scholar] [CrossRef]
- Shahbazi, M.; Cundiff, P.; Zhou, W.; Lee, P.; Patel, A.; D’Souza, S.L.; Abbasi, F.; Quertermous, T.; Knowles, J.W. The Role of Insulin as a Key Regulator of Seeding, Proliferation, and MRNA Transcription of Human Pluripotent Stem Cells. Stem Cell Res. Ther. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Khillan, J.S. Vitamin A/Retinol and Maintenance of Pluripotency of Stem Cells. Nutrients 2014, 6, 1209–1222. [Google Scholar] [CrossRef]
- Rajala, K.; Vaajasaari, H.; Suuronen, R.; Hovatta, O.; Skottman, H. Effects of the Physiochemical Culture Environment on the Stemness and Pluripotency of Human Embryonic Stem Cells. Stem Cell Stud. 2011, 1, e3. [Google Scholar] [CrossRef]
- Kawaguchi, R.; Yu, J.; Honda, J.; Hu, J.; Whitelegge, J.; Ping, P.; Wiita, P.; Bok, D.; Sun, H. A Membrane Receptor for Retinol Binding Protein Mediates Cellular Uptake of Vitamin A. Science 2007, 315, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Bernecker, C.; Köfeler, H.; Pabst, G.; Trötzmüller, M.; Kolb, D.; Strohmayer, K.; Trajanoski, S.; Holzapfel, G.A.; Schlenke, P.; Dorn, I. Cholesterol Deficiency Causes Impaired Osmotic Stability of Cultured Red Blood Cells. Front. Physiol. 2019, 10, 1529. [Google Scholar] [CrossRef]
- Fernández, C.; Lobo, M.D.V.T.; Gómez-Coronado, D.; Lasunción, M.A. Cholesterol Is Essential for Mitosis Progression and Its Deficiency Induces Polyploid Cell Formation. Exp. Cell Res. 2004, 300, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Kulka, M.; Kołodziejska-Lesisz, J.; Kluciński, W. Serum Paraoxonase 1 (PON1) Activity and Lipid Metabolism Parameters Changes in Different Production Cycle Periods of Holstein-Friesian, Polish Red and Norwegian Breeds. Pol. J. Vet. Sci. 2016, 19, 165–173. [Google Scholar] [CrossRef]
- Kulka, M.; Bełtowski, J.; Kluciński, W.; Orłowska, M.; Kołodziejska, J.; Kleczkowski, M. Serum Paraoxonase-1 Activity of Dairy Holstein-Fresian Cows in Different Lactation Stages--Preliminary Study. Pol. J. Vet. Sci. 2014, 17, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Zhang, M.; Yang, L.; Jin, Y. Correlations between Circulating Leptin Concentrations and Growth Performance, Carcass Traits, and Meat Quality Indexes in Finishing Simmental × Luxi Bulls Fed High-Concentrate Diets. Anim. Sci. J. 2020, 91. [Google Scholar] [CrossRef] [PubMed]
- Berg, E.P.; McFadin, E.L.; Maddock, K.R.; Goodwin, R.N.; Baas, T.J.; Keisler, D.H. Serum Concentrations of Leptin in Six Genetic Lines of Swine and Relationship with Growth and Carcass Characteristics. J. Anim. Sci. 2003, 81, 167–171. [Google Scholar] [CrossRef]
- Luo, G.; Wang, L.; Hu, S.; Du, K.; Wang, J.; Lai, S. Association of Leptin MRNA Expression with Meat Quality Trait in Tianfu Black Rabbits. Anim. Biotechnol. 2022, 33, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Fish, K.D.; Rubio, N.R.; Stout, A.J.; Yuen, J.S.K.; Kaplan, D.L. Prospects and Challenges for Cell-Cultured Fat as a Novel Food Ingredient. Trends food Sci. Technol. 2020, 98, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Tor, M.; Vilaró, F.; Ros-Freixedes, R.; Álvarez-Rodríguez, J.; Bosch, L.; Gol, S.; Pena, R.N.; Reixach, J.; Estany, J. Circulating Non-Esterified Fatty Acids as Biomarkers for Fat Content and Composition in Pigs. Anim. an Open Access J. from MDPI 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Yeo, G.C.; Weiss, A.S. Soluble Matrix Protein Is a Potent Modulator of Mesenchymal Stem Cell Performance. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 2042–2051. [Google Scholar] [CrossRef]
- Wang, C.; Chen, H.; Ying, W. Cytosolic Aspartate Aminotransferase Mediates the Mitochondrial Membrane Potential and Cell Survival by Maintaining the Calcium Homeostasis of BV2 Microglia. Neuroreport 2018, 29, 99–105. [Google Scholar] [CrossRef]
| Country | Board | Type of Grade | Classification | Quality (Intra muscular fat/ Beef marbling grade) | References |
|---|---|---|---|---|---|
| USA | USDA (United States Department of Agriculture) |
Prime Choice Select Standard Commercial Utility Cutter Canner |
USDA classified the beef grades based on marbling and maturity of the steak Marbling (Abundant to Practically Devoid) Maturity- (Grade A to E) Maturity divide into 5 groups based on carcass age A-9- 30 Months, B- 30–42 Months, C- 42–72 Months, D- 72-96 Months, E- > 96 Months Colour and tenderness of the steak based on maturity Grade-A- Red, porous and soft Grade-B- Slightly red and slightly soft Grade-C- Tinged with red, slightly hard Grade-D- Rather white, moderately hard Grade-E-White, nonporous, extremely hard |
Prime-Abundant &above (Grade A&B) Choice – Moderate(Grade A&B) Select - Slight (Grade A) Standard-Slight traces (Grade A&B) Commercial – Small (Grade C, D&E) Utility – Traces ( Grade C, D&E) Cutter - Practically Devoid (Grade E) Canner- Practically Devoid (Grade E) |
[19,20] |
| Japan | JMGA (Japanese Meat Grading Association) | A (A5, A4, A3, A2, A1) B C |
JMGA classified the meat grades based on yield, and quality Yield is determined by rib eye area, rib thickness, subcutaneous fat thickness, and chilled carcass weight. Yield grade divide into A,B,C (A: 72 and greater B: 69 -72 C: less than 69) Quality grade is classified by yield percentages estimated by an equation. Quality grades divide into five groups (5-1) based on marbling, meat color, texture, fat color and quality. Based on marbling, meat color, quality scores divide into 1-12 classes A5 - No.8~No.12 A4 - No.5~No.7 A3 - No.3~No.4 A2 - No.2 A1 - No.1 A- Represents yield grade. 5- 1- Represents quality grade. No 1-12 - Represents quality score. |
A5- Very abundant (No.8~No.12) A4- Abundant (No.5~No.7) A3- Standard (No.3~No.4) A2- Scarce (No.2) A1-Very scarce (No.1) |
[21] |
|
Australia |
Meat and Livestock Australia (MLA) |
AUS-MEAT - M9 to M0 MSA - 100-1100+ |
MSA classified the meat grades based on meat colour, marbling, fat depth, carcass weight, maturity and ultimate pH Based on intramuscular fat, meat grades divide into M9-M0 (AUS-MEAT) MSA grades on a scale of 100 (no intramuscular fat) to 1190 (extreme amounts of intramuscular fat) |
M9- Extreme (1100+) M8- Excellent (1000-1100) M7- Very Good (900-1000) M6- Good (800-900) M5- Good (700-800) M4- Average (600-700) M3- Fair (500-600) M2- Poor (400-500) M1- Poor (300-400) M0- Poor (100-200) |
[22,23] |
| Canada | CBGA (Canadian Beef Grading Agency) |
Prime A (AAA, AA, A) B (B1-B4) D (D1-D4) E |
CBGA classified the beef, based on maturity, sex, conformation (muscling), fat and meat (color, texture and marbling). Youthful cattle may receive a quality grade of Canada Prime, AAA, AA, A or B1-B4, Carcasses from mature cattle may receive a grade of D1-D4 or E. Yield grades 1, 2, 3 and 4 estimates the percentage of the carcass that is saleable at retail. |
Prime-Superior (11.5%) AAA- Best (4.62%) AA -Better (3.11%) A -Good (2.92%) B1 - Better B2 - Good B3 - Good B4 - Fair |
[24] |
| Europe | EUROP grid | E. U. R. O. P | EUROP grid is used to classify a carcass according to its conformation (shape) and fat level Conformation is assessed on an E to P basis, with E being a convex and shapely carcass, R being an average shape or straight profile, and P being a plainer carcass with a concave profile. Fat is assessed on a 1 to 5 basis, with 1 being very lean and 5 being very fat. U, O and P are subdivided to give a high (+) or low (–) classification, and fat classes 4 and 5 are subdivided to low (L) and high (H). |
E- excellent U- Very Good R- Good O- Fair P- Poor |
[25,26] |
|
Korea |
KAPE (Korea Institute for Animal Products Quality Evaluation) |
Grade 1++ Grade 1+ Grade 1 Grade 2 Grade 3 |
South Korea has its own meat grading system. Beef meat is classified by 5 grades that include 1++, 1+, 1, 2, and 3, depending on the degree of marbling, meat color, fat color, firmness of rib eye and maturity. |
Grade 1++ - Superior (15.6-17 %) Grade 1+ - Excellent (12.3-15.5%) Grade1 - Good (9-12.2%) Grade 2 - Fair (5-9%) Grade 3 - Poor (0-5 %) |
[27] |
| Serum markers | Serum levels | Correlation | References |
|---|---|---|---|
| Retinol | 1.10 ± 0.012 IU/ mL |
High retinol content (> 1.10) poorly/negatively associated with meat marbling score. Retinol concentration positively correlates with meat color. |
[28] |
| Blood Urea Nitrogen (BUN) | 6.02 ± 0.6 mg/10 mL |
An indication of the cattle's protein energy status may be found in the blood urea nitrogen concentration (BUN) level. |
[33] |
|
Non-esterified fatty acid (NEFA) |
2.237 ± 0.12 μEq/10mL |
Palatability and intramuscular fat positively correlate with non-esterified fatty acid content. |
[40] |
| Total cholesterol | 128.03 ± 6.00 mg/mL |
The amount of total blood cholesterol was strongly/positively connected with carcass body weight & negatively correlated with marbling score. |
[43] |
| Paraoxonase 1(PON1) | 72.3 ± 35.6 mU/mL |
PON1 positively correlated with Marbling score, it is higher in female and castrated male than in male, indicating a gender-dependent difference. |
[51] |
| Insulin | 35.35 ± 2.37 ng/mL |
Meat marbling score and meat color are both favorably and adversely linked with insulin. |
[59] |
| Leptin | 30.34 ± 1.37 ng/mL |
Leptin have a negatively correlation with meat marbling score |
[59] |
| Aspartic acid transaminase (AST) | 0.54 ± 0.0324 U/10mL |
AST is positively connected with meat color and adversely correlated with the yield index and meat marbling score. |
[69] |
| Alanine transaminase (ALT) | 0.51 ± 0.043 U/10mL |
ALT has a positive correlation with the yield index and the meat marbling score and a negative correlation with the color of the meat. |
[68] |
| Total Protein (TP) | 1.1 ± 0.04 g/10 mL |
TP has a positive correlation with the yield index and the meat marbling score |
[44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





