Submitted:
19 March 2024
Posted:
21 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
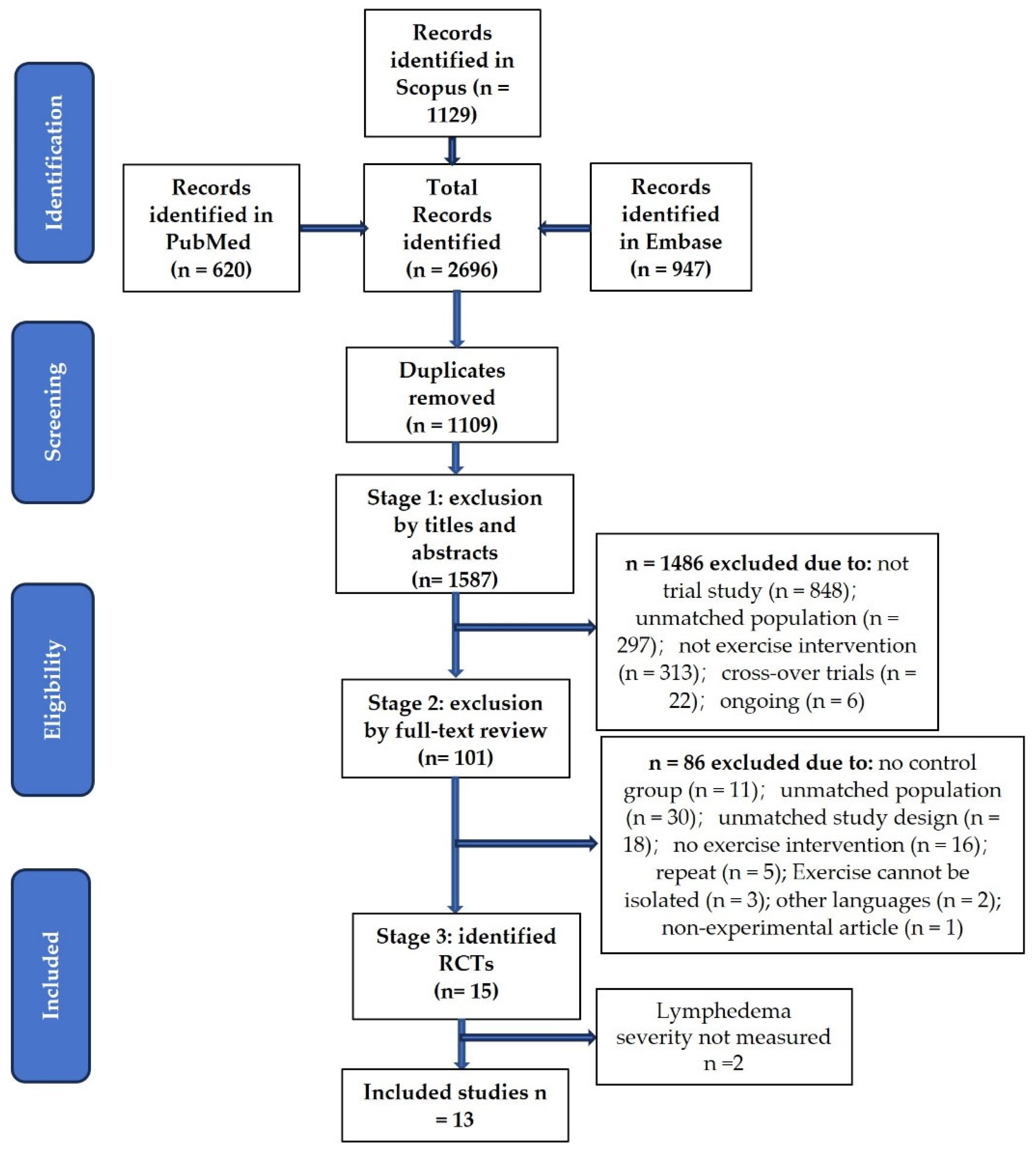
2. Methods
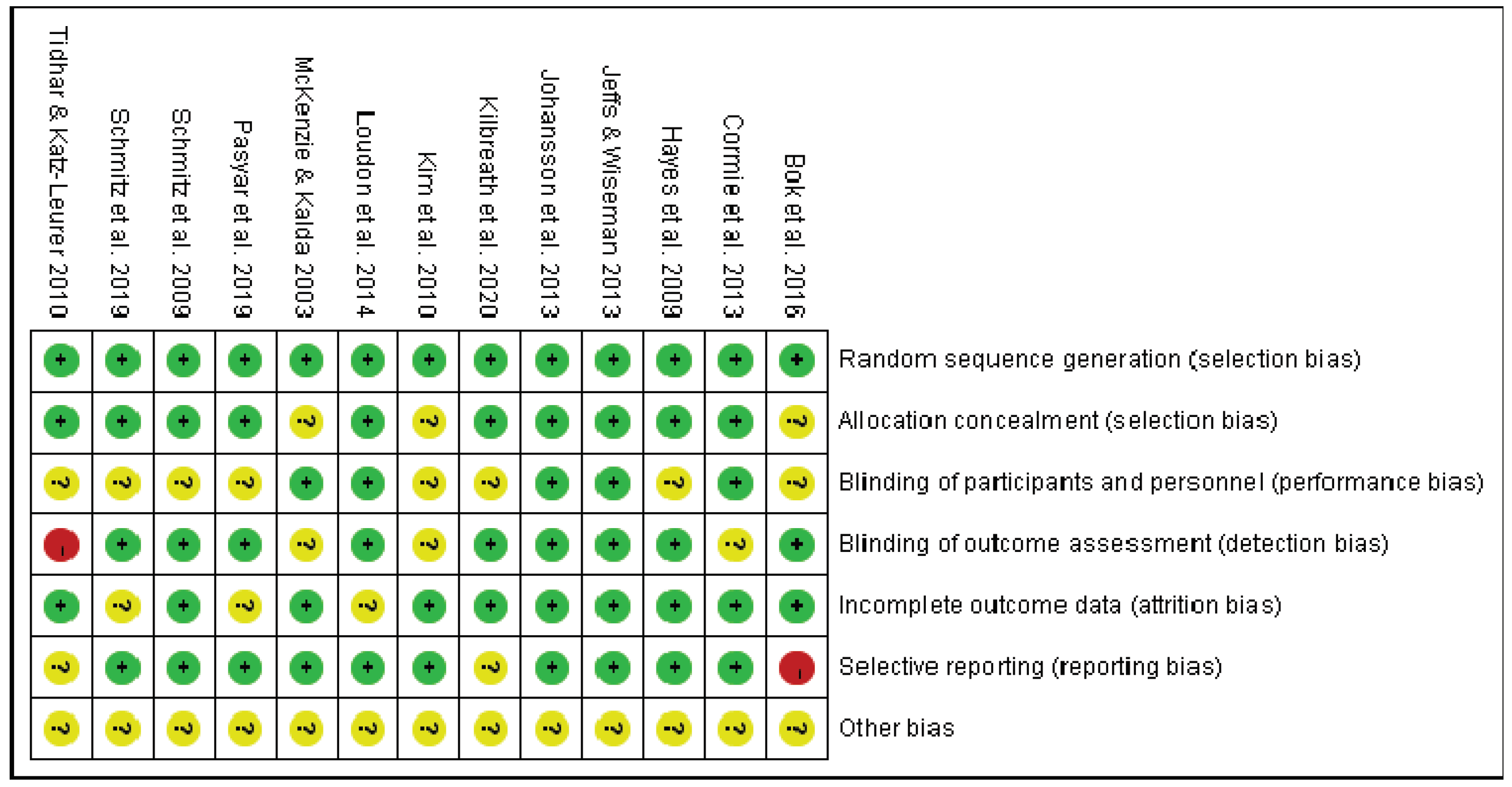
3. Results
3.1. Yoga
3.2. Resistance Exercise
3.3. Complex Exercise
3.4. Water-Based Exercise
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Cancer Research Fund International Breast Cancer Statistics. Available online: https://www.wcrf.org/cancer-trends/breast-cancer-statistics/ (accessed on 14 November 2023).
- World Health Organization Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 14 November 2023).
- American Cancer Society Survival Rates for Breast Cancer. Available online: https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html (accessed on 3 January 2023).
- DiSipio, T.; Rye, S.; Newman, B.; Hayes, S. Incidence of Unilateral Arm Lymphoedema after Breast Cancer: A Systematic Review and Meta-Analysis. Lancet Oncol 2013, 14, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Erickson, V.S.; Pearson, M.L.; Ganz, P.A.; Adams, J.; Kahn, K.L. Arm Edema in Breast Cancer Patients. JNCI: Journal of the National Cancer Institute 2001, 93, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Armer, J.M.; Stewart, B.R. POST-BREAST CANCER LYMPHEDEMA: INCIDENCE INCREASES FROM 12 TO 30 TO 60 MONTHS. Lymphology 2010, 43, 118. [Google Scholar] [PubMed]
- Manahan, M. Breast Cancer: Lymphedema After Treatment. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/breast-cancer/breast-cancer-lymphedema-after-treatment (accessed on 3 January 2023).
- Grada, A.A.; Phillips, T.J. Lymphedema: Pathophysiology and Clinical Manifestations. J Am Acad Dermatol 2017, 77, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Kayiran, O.; De La Cruz, C.; Tane, K.; Soran, A. Lymphedema: From Diagnosis to Treatment. Turk J Surg 2017, 33, 51–57. [Google Scholar] [CrossRef]
- Brix, B.; Sery, O.; Onorato, A.; Ure, C.; Roessler, A.; Goswami, N. Biology of Lymphedema. Biology (Basel) 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.M. Lymphedema in Women Treated for Breast Cancer. Semin Oncol Nurs 2000, 16, 226–237. [Google Scholar] [CrossRef]
- Hasenoehrl, T.; Palma, S.; Ramazanova, D.; Kölbl, H.; Dorner, T.E.; Keilani, M.; Crevenna, R. Resistance Exercise and Breast Cancer-Related Lymphedema-a Systematic Review Update and Meta-Analysis. Supportive Care in Cancer 2020, 28, 3593–3603. [Google Scholar] [CrossRef]
- Ciudad, P.; Sabbagh, M.D.; Agko, M.; Huang, T.C.T.; Manrique, O.J.; Carmen Román, L.; Reynaga, C.; Delgado, R.; Maruccia, M.; Chen, H.C. Surgical Management of Lower Extremity Lymphedema: A Comprehensive Review. Indian J Plast Surg 2019, 52, 81–92. [Google Scholar] [CrossRef]
- Ciudad, P.; Bolletta, A.; Kaciulyte, J.; Losco, L.; Manrique, O.J.; Cigna, E.; Mayer, H.F.; Escandón, J.M. The Breast Cancer-related Lymphedema Multidisciplinary Approach: Algorithm for Conservative and Multimodal Surgical Treatment. Microsurgery 2023, 43, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Shimbo, K.; Kawamoto, H.; Koshima, I. Comparative Study of Conservative Treatment and Lymphaticovenular Anastomosis with Compression Therapy for Early-Stage Breast Cancer-Related Lymphoedema. Journal of Plastic, Reconstructive & Aesthetic Surgery 2024, 88, 390–396. [Google Scholar] [CrossRef]
- Lasinski, B.B.; Thrift, K.M.K.; Squire, D.C.; Austin, M.K.; Smith, K.M.; Wanchai, A.; Green, J.M.; Stewart, B.R.; Cormier, J.N.; Armer, J.M. A Systematic Review of the Evidence for Complete Decongestive Therapy in the Treatment of Lymphedema From 2004 to 2011. PM&R 2012, 4, 580–601. [Google Scholar] [CrossRef]
- Michopoulos, E.; Papathanasiou, G.; Vasilopoulos, G.; Polikandrioti, M.; Dimakakos, E. Effectiveness and Safety of Complete Decongestive Therapy of Phase I: A Lymphedema Treatment Study in the Greek Population. Cureus 2020, 12. [Google Scholar] [CrossRef]
- S G Rockson Precipitating Factors in Lymphedema: Myths and Realities - PubMed. Cancer 1998, 83, 2814–2816. [CrossRef]
- McKenzie, DC. Abreast in a Boat—a Race against Breast Cancer. Cmaj 1998, 159, 376–378. [Google Scholar] [PubMed]
- Nelson, N.L. Breast Cancer-Related Lymphedema and Resistance Exercise: A Systematic Review. J Strength Cond Res 2016, 30, 2656–2665. [Google Scholar] [CrossRef] [PubMed]
- Wanchai, A.; Armer, J.M. The Effects of Yoga on Breast-Cancer-Related Lymphedema: A Systematic Review. J Health Res 2020, 34, 409–418. [Google Scholar] [CrossRef]
- Wei, C.W.; Wu, Y.C.; Chen, P.Y.; Chen, P.E.; Chi, C.C.; Tung, T.H. Effectiveness of Yoga Interventions in Breast Cancer-Related Lymphedema: A Systematic Review. Complement Ther Clin Pract 2019, 36, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Yeung, W.; Semciw, A.I. Aquatic Therapy for People with Lymphedema: A Systematic Review and Meta-Analysis. Lymphat Res Biol 2018, 16, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Loudon, A.; Barnett, T.; Piller, N.; Immink, M.A.; Williams, A.D. Yoga Management of Breast Cancer-Related Lymphoedema: A Randomised Controlled Pilot-Trial. BMC Complement Altern Med 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Pasyar, N.; Barshan Tashnizi, N.; Mansouri, P.; Tahmasebi, S. Effect of Yoga Exercise on the Quality of Life and Upper Extremity Volume among Women with Breast Cancer Related Lymphedema: A Pilot Study. European Journal of Oncology Nursing 2019, 42, 103–109. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Ahmed, R.L.; Troxel, A.; Cheville, A.; Smith, R.; Lewis-Grant, L.; Bryan, C.J.; Williams-Smith, C.T.; Greene, Q.P. Weight Lifting in Women with Breast-Cancer-Related Lymphedema. N Engl J Med 2009, 361, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Sim, Y.J.; Jeong, H.J.; Kim, G.C. Effect of Active Resistive Exercise on Breast Cancerrelated Lymphedema: A Randomized Controlled Trial. Arch Phys Med Rehabil 2010, 91, 1844–1848. [Google Scholar] [CrossRef] [PubMed]
- Jeffs, E.; Wiseman, T. Randomised Controlled Trial to Determine the Benefit of Daily Home-Based Exercise in Addition to Self-Care in the Management of Breast Cancer-Related Lymphoedema: A Feasibility Study. Support Care Cancer 2013, 21, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Bok, S.K.; Jeon, Y.; Hwang, P.S. Ultrasonographic Evaluation of the Effects of Progressive Resistive Exercise in Breast Cancer-Related Lymphedema. Lymphat Res Biol 2016, 14, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Cormie, P.; Pumpa, K.; Galvão, D.A.; Turner, E.; Spry, N.; Saunders, C.; Zissiadis, Y.; Newton, R.U. Is It Safe and Efficacious for Women with Lymphedema Secondary to Breast Cancer to Lift Heavy Weights during Exercise: A Randomised Controlled Trial. Journal of Cancer Survivorship 2013, 7, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.C.; Reul-Hirche, H.; Turner, J. Exercise and Secondary Lymphedema: Safety, Potential Benefits, and Research Issues. Med Sci Sports Exerc 2009, 41, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.H.; Troxel, A.B.; Dean, L.T.; Demichele, A.; Brown, J.C.; Sturgeon, K.; Zhang, Z.; Evangelisti, M.; Spinelli, B.; Kallan, M.J.; et al. Effect of Home-Based Exercise and Weight Loss Programs on Breast Cancer-Related Lymphedema Outcomes Among Overweight Breast Cancer Survivors: The WISER Survivor Randomized Clinical Trial. JAMA Oncol 2019, 5, 1605–1613. [Google Scholar] [CrossRef]
- Kilbreath, S.L.; Ward, L.C.; Davis, G.M.; Degnim, A.C.; Hackett, D.A.; Skinner, T.L.; Black, D. Reduction of Breast Lymphoedema Secondary to Breast Cancer: A Randomised Controlled Exercise Trial. Breast Cancer Res Treat 2020, 184, 459–467. [Google Scholar] [CrossRef]
- McKenzie, D.C.; Kalda, A.L. Effect of Upper Extremity Exercise on Secondary Lymphedema in Breast Cancer Patients: A Pilot Study. Journal of Clinical Oncology 2003, 21, 463–466. [Google Scholar] [CrossRef]
- Winkels, R.M.; Sturgeon, K.M.; Kallan, M.J.; Dean, L.T.; Zhang, Z.; Evangelisti, M.; Brown, J.C.; Sarwer, D.B.; Troxel, A.B.; Denlinger, C.; et al. The Women in Steady Exercise Research (WISER) Survivor Trial: The Innovative Transdisciplinary Design of a Randomized Controlled Trial of Exercise and Weight-Loss Interventions among Breast Cancer Survivors with Lymphedema. Contemp Clin Trials 2017, 61, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Tidhar, D.; Katz-Leurer, M. Aqua Lymphatic Therapy in Women Who Suffer from Breast Cancer Treatment-Related Lymphedema: A Randomized Controlled Study. Support Care Cancer 2010, 18, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Johansson, K.; Hayes, S.; Speck, R.M.; Schmitz, K.H. Water-Based Exercise for Patients with Chronic Arm Lymphedema: A Randomized Controlled Pilot Trial. Am J Phys Med Rehabil 2013, 92, 312–319. [Google Scholar] [CrossRef]
- Whatley, J.; Street, R.; Kay, S. Experiences of Breast Cancer Related Lymphoedema and the Use of Reflexology for Managing Swelling: A Qualitative Study. Complement Ther Clin Pract 2018, 32, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, C.S.; Watroba, N.L.; Rezaishiraz, H.; Giese, W.; Hurd, T.; Fassl, K.A.; Edge, S.B. Lymphedema Secondary to Postmastectomy Radiation: Incidence and Risk Factors. Ann Surg Oncol 2004, 11, 573–580. [Google Scholar] [CrossRef]
- Hasenoehrl, T.; Keilani, M.; Palma, S.; Crevenna, R. Resistance Exercise and Breast Cancer Related Lymphedema – a Systematic Review Update. https://doi-org.nuigalway.idm.oclc.org/10.1080/09638288.2018.1514663 2019, 42, 26–35. [Google Scholar] [CrossRef]
- Saraswathi, V.; Latha, S.; Niraimathi, K.; Vidhubala, E. Managing Lymphedema, Increasing Range of Motion, and Quality of Life through Yoga Therapy among Breast Cancer Survivors: A Systematic Review. Int J Yoga 2021, 14, 3. [Google Scholar] [CrossRef]
- Wang, L.; Shi, Y.X.; Wang, T.T.; Chen, K.X.; Shang, S.M. Breast Cancer-Related Lymphoedema and Resistance Exercise: An Evidence-Based Review of Guidelines, Consensus Statements and Systematic Reviews. J Clin Nurs 2023, 32, 2208–2227. [Google Scholar] [CrossRef]
| Study | No.of Participants and Characteristics | Intervention Details (Frequency, Time, Duration, and Supervision) | Measure and Outcomes |
|---|---|---|---|
| Loudon et al. (2014) [24] | Twenty-eight women (age from 34 to 80) completed breast cancer treatment before ≥ 6 months, with stage one unilateral BCRL | 90-min teacher-led Yoga weekly, 45-min DVD-led Yoga daily for 8 weeks | No significant mean change in arm volume (assessed by a Jobst non-stretch tape), and extracellular fluid (measured by BIS) between the two groups were detected after 8 weeks. The mean arm volume reduced to 72.03 ± 80.77 from 101.45 ± 75.08 in the intervention group after 8 weeks. After another 4 weeks of follow-up, the mean arm volume in the intervention group was increased |
| Pasyar et al. (2019) [25] | Forty women (> 18 years old) with BCRL and an average age of 51.7 years old | Three sessions weekly (2 sessions under supervision and 1 session by using DVD at home) for 8 weeks | The mean upper extremity volume was not reduced in the intervention group after 8 weeks. No statistical difference in upper extremity volume was assessed by water displacement between the two groups. |
| Study | No. of Participants and Characteristics | Intervention Details (Frequency, Intensity, Time, Duration, and Supervision) | Measure and Outcomes |
|---|---|---|---|
| Schmitz et al. (2009) [26] | One hundred and forty-one patients (average age of 56 ± 9 in the study group and 58 ± 10 in the control group) with stable BCRL after unilateral non-metastatic breast cancer 1 to 15 years |
Frequency and duration: Twice weekly for 1 year Intensity, time, progression, and supervision: in the first 13 weeks, 90-minute professional-led sessions in the group, starting from little-to-no resistance and 1-3 new exercises were taught each session (of those weeks, in the first 5 weeks, increasing the number of sets from 2 to 3, after 2 sessions of 3 sets of 10 reps, the resistance were increased gradually), and then unsupervised exercise for the rest 39 weeks |
Limb swelling was measured by water displacement. The percentage of women who had a change in interlimb volume difference of 5% or more and the percentage of mean interlimb volume discrepancy between baseline and 12 months were not statistically different between the two groups. |
| Kim et al. (2010) [27] | Forty women (age from 27 to 76) with unilateral BCRL |
Frequency and duration: 5 days per week for 8 weeks Intensity, time, progression: 6 exercises (seated row, bench press, latissimus dorsi pull-down, 1-arm bent-over row, triceps extension, and biceps curl) with 0.5-kg dumbbells for 15 minutes; after the first 2 weeks, 1-kg dumbbells were used unless participants felt they were too heavy. Supervision: for the first 2 weeks |
The volume (measured circumference by tape) of the proximal arm statistically reduced within both groups after 8 weeks. The reduced volume of the proximal arm was significantly greater in the intervention group than in the control group. |
| Cormie et al. (2013) [30] | Sixty-two women (average age of 56.1±8.1 in the high-load group, 57.0±10.0 in the low-load group, and 58.6±6.7 in the control group) diagnosed with breast cancer at least 1 year before |
Frequency and duration: twice per week (in a group of 8-10 persons) for 12 weeks Intensity and supervision: fully supervised moderate to high-intensity exercise (low-load group: 55-65% of 1RM using 20-15 RM; high-load group 75-85% of 1RM using 10-6 RM) Time: 1-4 sets per exercise (chest press, seated row/lat pulldown, shoulder press/lateral raise, bicep curl, triceps extension, wrist curl, leg press/leg extension, squat/lunge) for a total of 60 minutes per session Progression: increased in 5–10 % increments if participants were able to perform more repetitions than the prescribed RM during a set without worsening arm symptoms. |
There was no mean change difference in the extent of swelling (measured by three methods: (1) BIS, (2) dual energy X-ray absorptiometry, and (3) arm circumference measurements) between groups either at the baseline or at 12 weeks. |
| Jeffs & Wiseman (2013) [28] | Twenty-three women (age from 51 to 73.5) with stable unilateral BCRL ≥ 3 months |
Frequency, time, and duration: 10-15 mins of breathing and gravity-resistive isotonic arm exercises daily for 26 weeks Supervision: no |
%ELV (calculated from limb volume measured by Perometer) decreased significantly in the intervention group but not in the control group, with no statistically significant difference between the two groups. |
| Bok et al. (2016) [29] | Thirty-two women (average age of 45.4 ± 8.8 in the PRE group and 53.3 ± 9.54 in the non-PRE group) with unilateral BCRL |
Frequency: Twice daily for 8 weeks Intensity and progression: 5 repetitions of 6 exercises (dumbbell fly, triceps extension, one-arm bent-over row, biceps curl, dumbbell side raise, and lifting the arms forward) with 0.5-kg dumbbells, adding 5 additional repetitions every week Supervision: no |
Both distal and proximal upper limb circumferences measured by tape reduced significantly in the intervention group after 8 weeks. |
| Study | No. of participants and characteristics | Intervention details (Frequency, Intensity, time, duration, and supervision) | Measure and outcomes |
|---|---|---|---|
| McKenzie & Kalda (2003) [34] | Fourteen women (an average age of 56.6 ± 9.0 years old) completed stage I or II breast cancer treatment for at least 6 months with unilateral lymphedema. |
Frequency and duration: 3 times per week for 8 weeks Intensity, time, progression, and supervision: Resistance exercise started from 2 sets of 10 repetitions of 6 exercises (seated row, bench press, latissimus dorsi pull down, one arm bent-over rowing, triceps extension, and bicep curl) with light weights; after the first week, it increased to 3 sets of 10 repetitions, and weights were gradually processed for each participant. The aerobic exercise started from 5-7 minutes of warm-up aerobic exercise; after 2 weeks, an arm cycle ergometer was used (under supervision), 5 bouts of 1-min cycling at a resistance of 8.3 W and then processed to 20 minutes of continuous cycling with a resistance of up to 25 W |
No statistical change in arm circumference and arm volume (calculated from arm circumference and measured by water displacement) in either group was observed. |
| Hayes et al. (2009) [31] | Thirty-two women (average age of 59 ± 7 in the intervention group and 60 ± 11 in the control group) with unilateral BCRL at least 6 months |
Frequency and supervision: 3 times (2 supervised) per week at week 1-4, 4 times (2 supervised) per week at week 5-8, and at least 4 times (1 supervised) per week at week 9-12 Intensity, time, and progression: Low-to-moderate-intensity (RPE 3-5) aerobic exercise only for 20-30 minutes in the first 2 weeks; Low-to-moderate-intensity (RPE 3-5) aerobic exercise and low-intensity (20 reps per exercise) resistance exercise for 20-30 minutes at week 3 and 4; moderate-intensity aerobic (RPE 4-6) and moderate-intensity (finally completed 15 reps per exercise) resistance exercises for 30-45 minutes at week 5-8;moderate-to-high-intensity (RPE 4-7) aerobic and moderate- to-high (finally completed 10 reps per exercise) resistance exercises for 45+ minutes at week 9-12 |
The changes in lymphedema status (measured by BIS and Perometry) were not statistically different between the two groups. No significant change within the groups was observed. |
| Schmitz et al. (2019) [32] | One hundred and seventy-seven (the number in the control and exercise groups) women (body mass index of 25 to 50) diagnosed with BCRL and completed treatment for at least 6 months, not engaging in resistance training or 3 or more weekly aerobic sessions in the past 1 year, no current weight management surgery or medication taken, no weight loss greater than 4.5 kg in the past 3 months |
Frequency and duration: Twice weekly for 52 weeks Intensity, time, and progression: Two sets of 10 repetitions of 9 resistance exercises with 0.45 to 9.45 kg dumbbells and one 90-minute weekly class in the first 6 weeks; from week 7, it increased to 3 sets per session with increased weights by 0.45 to 0.9 kg every two weeks with an upper limit of 9.45 kg and monthly classes provided; aerobic exercise started from 90 minutes per week, gradually increased walking time to 180 minutes per week |
There was no significant difference between the two groups in the percentage of interlimb volume differences (measured by Perometry) either at the baseline or after 12 months |
| Kilbreath et al. (2020) [33] | Eighty-nine women (≥ 18 years old, average age of 59.5±8.0 in the control group and 53.7±10.4 in the exercise group) with BCRL at least 3 months |
Frequency and duration: Three sessions per week for 12 weeks Intensity and time: One-hour exercise session consisted of 10-min warm-up, 30-min resistance exercise, and 20-min aerobic exercise. Resistance exercise: 10–12 repetitions moderate-intensity (5-7 on the OMNI-RES) for weeks 1-6 and high-intensity resistance exercise (7-9 on the OMNI-RES) for weeks 7–12. Aerobic exercise: 60%-85% of HRR, perceived exertion of 11-17. Progression: For resistance exercise, the intensity was increased if the weight could be lifted more than 12 times, but if participants could not lift the new weight, the number of repetitions was increased from 12 to 14 and then increased weight. For aerobic exercise, new types of exercise (stationary bike, treadmill, rower, etc.) were added every 4 weeks. |
The change in BIS ratio was significant for breasts but not significant for arms and legs between the two group |
| Study | No. of Participants and Characteristics | Intervention Details (Frequency, Intensity, Time, Duration, and Supervision) | Measure and Outcomes |
|---|---|---|---|
| Johansson et al. (2013) [37] | Twenty-nine women (age 56-74 years old) with unilateral BCRL for at least 6 months but without active treatment for the last 3 months |
Frequency, duration, supervision: three 30-minute sessions per week for 8 weeks, supervision only for the first session Intensity: moderate intensity (11-13 RPE on the Borg scale) |
No change in lymphedema status (measured by Perometry and BIS) was identified. |
| Tidhar & Katz-Leurer (2010) [36] | Forty-eight women with (average age of 56.2 ±10.7 years old in the study group and 56.5±8.8 years old in the control group) unilateral BCRL and completed intensive CDT for the last 2 weeks |
Frequency, intensity, time, and duration: one 45-minute session of gentle exercises (in a group setting) for 12 weeks Supervision: no |
No long-term effect of water-based exercise on limb volume (assessed by water displacement) was observed although it reduced limb volume immediately after sessions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




