1. Introduction
The historical definition of the terms “Advanced Maternal Age” (AMA) refers to a condition of maternal age over than 35 years[
1,
2]. The analysis of the data collected in the last three decades evidenced an increase in the average age of conception and delivery in women belonging to developed countries[
3]. These findings concern both women aged 35–39 years, in whom the increase in the birth rate was from 45.9 per 1000 women in 2010 to 52.7 per 1000 women in 2019, and women aged 40–44 years, who showed an increase in the birth age from 10.2 to 12 per 1000 women, in the same range of years[
4,
5,
6]. In addition to reasons related to work and career needs, the increase of AMA pregnant women could be explained also by the development in advanced reproductive technology, which has extended the reproductive window[
7,
8]. Thus, on the ground of these considerations, the above reported definition of AMA appears outdated, and it would be more appropriate to use it for pregnant women older than 40 years[
9,
10].
It should be kept in mind that AMA pregnancy could represent a risk factor for adverse maternal complications, such as pre-eclampsia, gestational diabetes mellitus, gestational hypertension, and Cesarean delivery, as well as, for fetal outcomes[
7,
8]. In addition to increase maternal mortality and morbidity, the above health complications also have repercussions in terms of increased costs to health systems worldwide[
11]: a great deal of effort should be made to try to counteract or even prevent them.
Actions aimed at preventing AMA pregnancy-related complications, could be focused on the modulation of its pathophysiological mechanisms. In this regard, an important role is played by the loss of placental functions[
12] and oxidative stress, which is the result of an imbalance between the production and the detoxification of reactive oxygen species (ROS)/reactive nitrogen species[
13].
In pregnancy, ROS and reactive nitrogen species are generated mainly by the placenta and vascular endothelium, as well, through the action of xanthine oxidase and nitric oxide synthase[
14]. An increased oxidants release not counteracted by antioxidants like superoxide dismutase, or glutathione (GSH) and glutathione peroxidase, may affect normal vasodilation and become an important factor in the pathogenesis of AMA pregnancy-related complications, like those affecting the cardiovascular system, kidney and liver, as well[
11,
13,
15].
Another possible target of the prevention/modulation of AMA pregnancy-related complications could be represented by the human umbilical cord mesenchymal stem cells (hUMSCs), which can protect the placental function by increasing its development and angiogenesis through paracrine actions, and the release of immunomodulatory factors[
16,
17,
18,
19]. Also, those cells can be involved in the modulation of oxidative stress/inflammation, have the ability to recover ovarian function in premature ovarian insufficiency or natural aging animal models, and exert cardioprotective effects through the release of microRNA[
20].
Despite existing knowledge about this issue, however, additional information regarding the plasma redox balance and the changes in hUMSCs function induced by circulating factors in AMA pregnant women at different time of pregnancy, would be mandatory to better understand the pathophysiological aspects.
Thus, our primary endpoint was to examine the plasma levels of lipid markers of peroxidation, GSH and NO at the first trimester (T0), at the second trimester during the morphologic ultrasound (T1), and at 48-72 hours after the delivery (T2), in pregnant women over 40 years compared with pregnant women younger than 40 years. Secondly, we wanted to analyze the effects of plasma taken from AMA pregnant women at different time-points on hUMSCs in order to highlight the role of circulating factors in the modulation of their viability and oxidants release.
2. Results
2.1. Patients
There were no statistically significant differences in the two groups’ anamnestic characteristics, except for maternal age and body mass index, which was higher in AMA patients (
Table 1).
In
Table 2, we describe the main delivery outcomes of patients and controls. The statistical analysis did not show a significant high percentage of complications in AMA patients vs controls (
P =0.12).
2.2. Plasma Thiobarbituric Acid Reactive Substances (TBARS), Glutathione (GSH) and Nitric Oxide (NO)
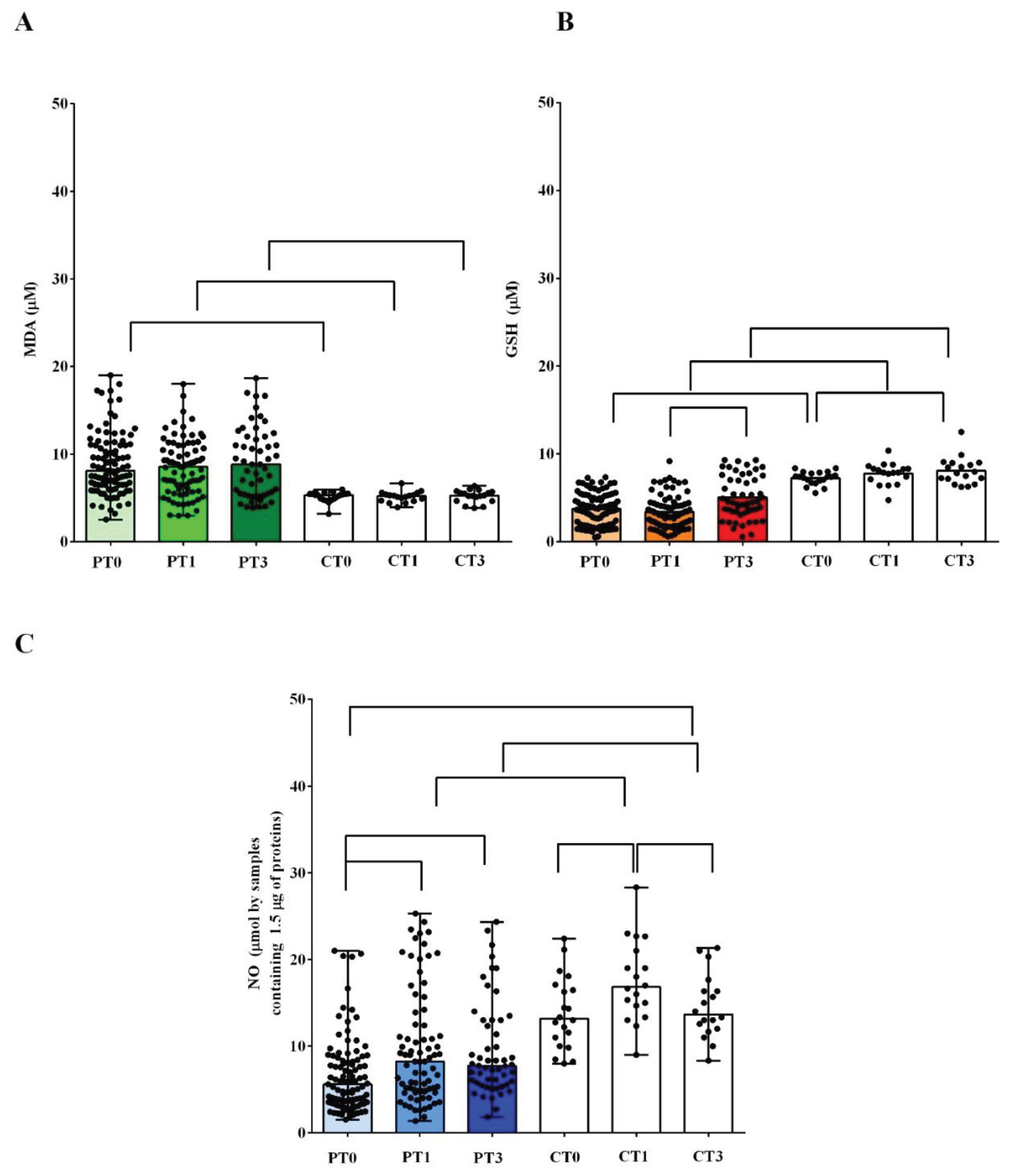
The results obtained from plasma measurements highlighted the presence of an alteration of the redox balance in AMA patients, already at T0. The TBARS levels amounted to 8.1 πM (6.5-11) in AMA patients vs 5.3 πM (4.8-5.4) of the controls (
P<0.05;
Figure 1A). At the same time, the GSH values in AMA patients amounted to 3.6 πM (1.9-5) vs 7.3 πM (6.7-7.8) of the controls (
P<0.05;
Figure 1B).
Significant differences were also found regarding the plasma NO levels. In AMA patients, the plasma NO at T0 amounted to 5.6 πM (3.5-8.4) vs 13.1 πM (8-17), which was found in the controls (
P <0.05;
Figure 1C).
In both groups of pregnant women, the plasma TBARS and GSH levels did not change significantly between T0 and T1 (
Figure 1A,B). On the contrary, we observed an increase in the plasma NO levels, between T0 and T1, in both the controls and AMA patients (
P <0.05;
Figure 1C).
At T3, in AMA patients, the levels of TBARS remained elevated and not significantly different from those observed at T0 or T1. Plasma GSH had increased compared to that measured at T1 (
P <0.05), but it did not reach the levels measured in the controls at the same timing (
Figure 1A,B). In controls, the plasma levels of TBARS and GSH did not change at T3 vs what was observed at T1 (
Figure 1A,B).
As regards NO, in AMA patients at T3 the levels remained similar to those measured at T1, while they returned to the same levels found at T0, in the controls (
Figure 1C).
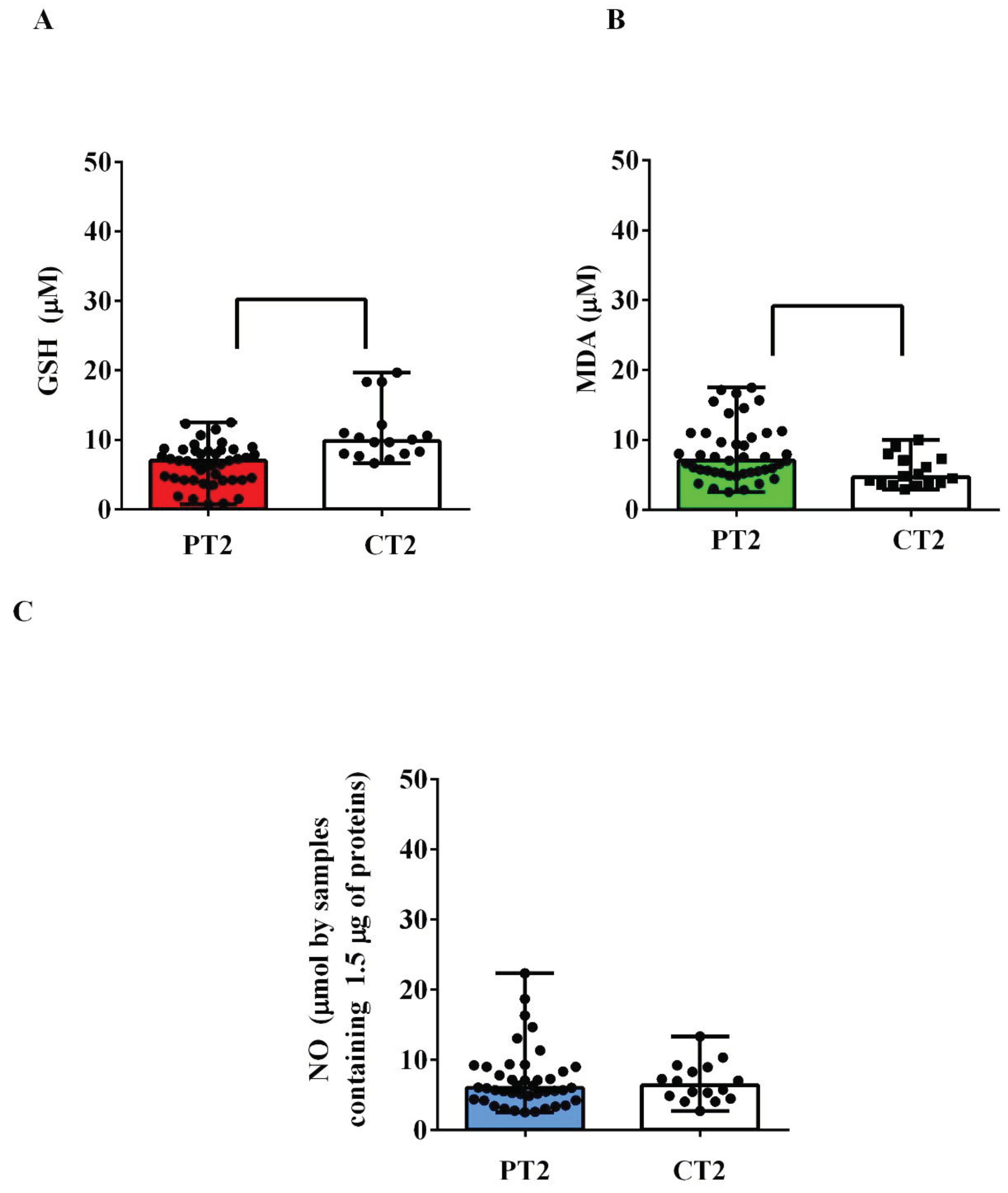
Also in the plasma of the umbilical cords, there was an alteration of the redox state in AMA patients. Indeed, GSH levels were lower than those observed in the controls (
P <0.05;
Figure 2A), while TBARS levels were higher (
P <0.05;
Figure 2B). Instead, we did not detect any significant differences regarding NO (
Figure 2C).
2.3. Effects of plasma on hUMSCs
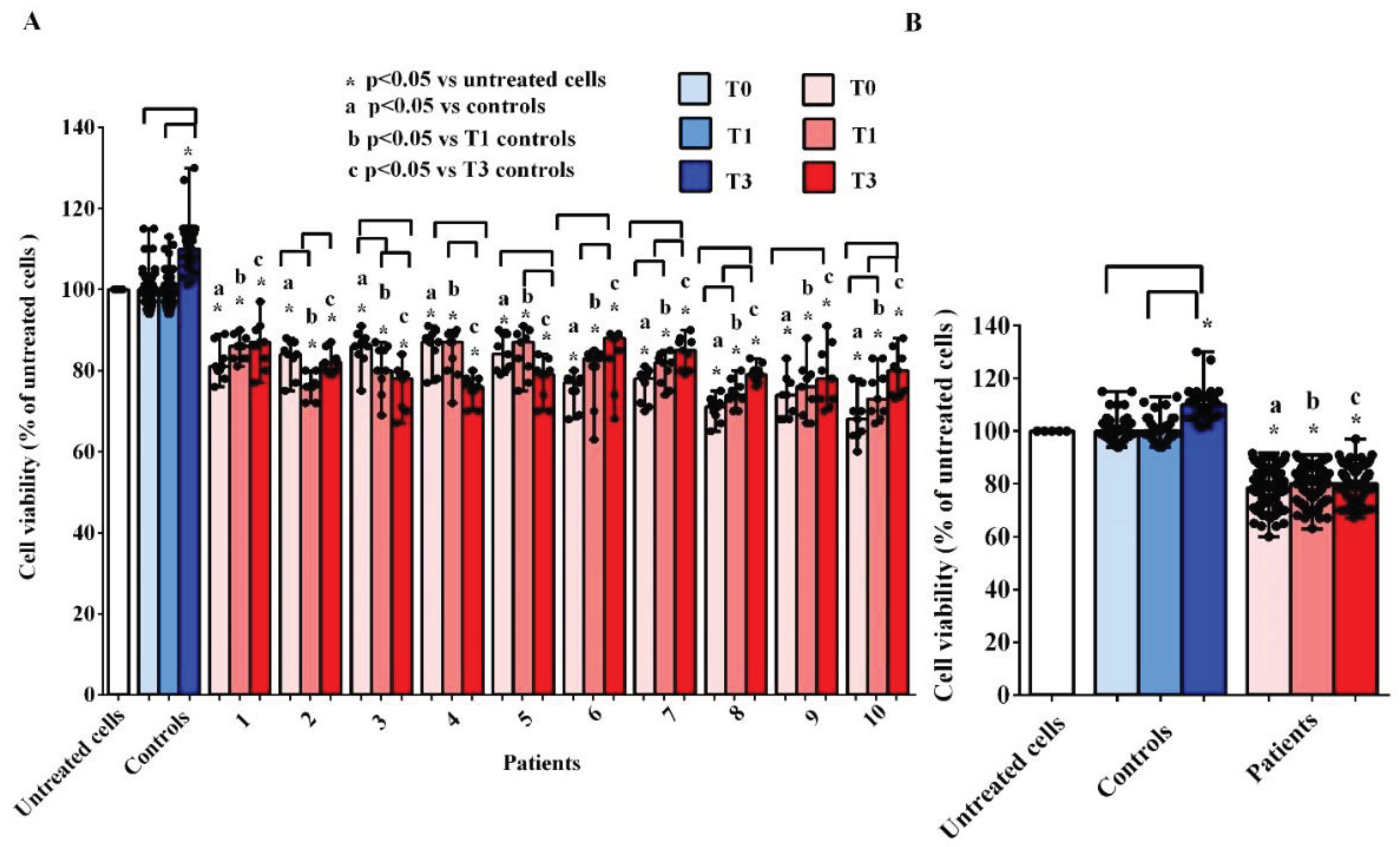
The
in vitro experiments showed that the treatment of hUMSCs with plasma taken from AMA patients at T0, T1 and T3, was able to cause harmful effects. As evidenced in
Figure 3A,B, the cell viability was lower than that measured both in hUMSCs treated with plasma of the controls and in the untreated hUMSCs.
If we analyze the results obtained at the three different time-points, it can be observed that there are no significant variations in the median values of cell viability in hUMSCs treated with plasma of AMA patients (
Figure 3B). In the case of the controls, cell viability was even increased at T3 in comparison with that found in the untreated hUMSCs (
P <0.05;
Figure 3B).
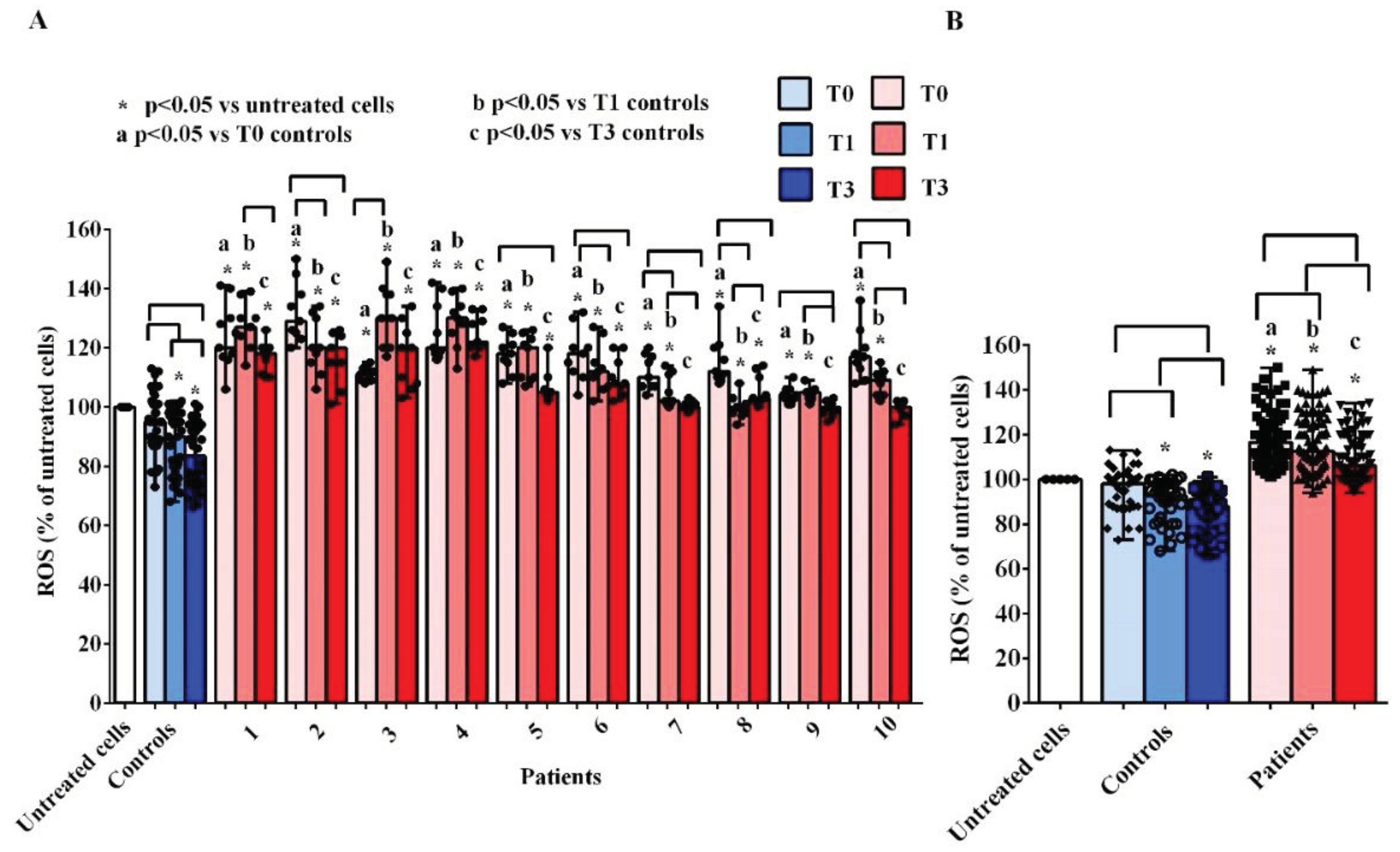
As concerning ROS, we observed an increased release by hUMSCs treated with plasma of AMA patients, at all time-points, both vs the untreated cells and vs hUMSCs treated with plasma of the controls (
Figure 4A,B). Considering the various time-points, the results obtained demonstrated a reduction in the release of ROS both by the hUMSCs treated with the plasma of AMA patients and of the controls (
Figure 4B). However, although the ROS release by hUMSCs treated with plasma of AMA patients at T3 was lower than that observed at T0, it remained higher than what found in the untreated cells and in hUMSCs treated with plasma of the controls.
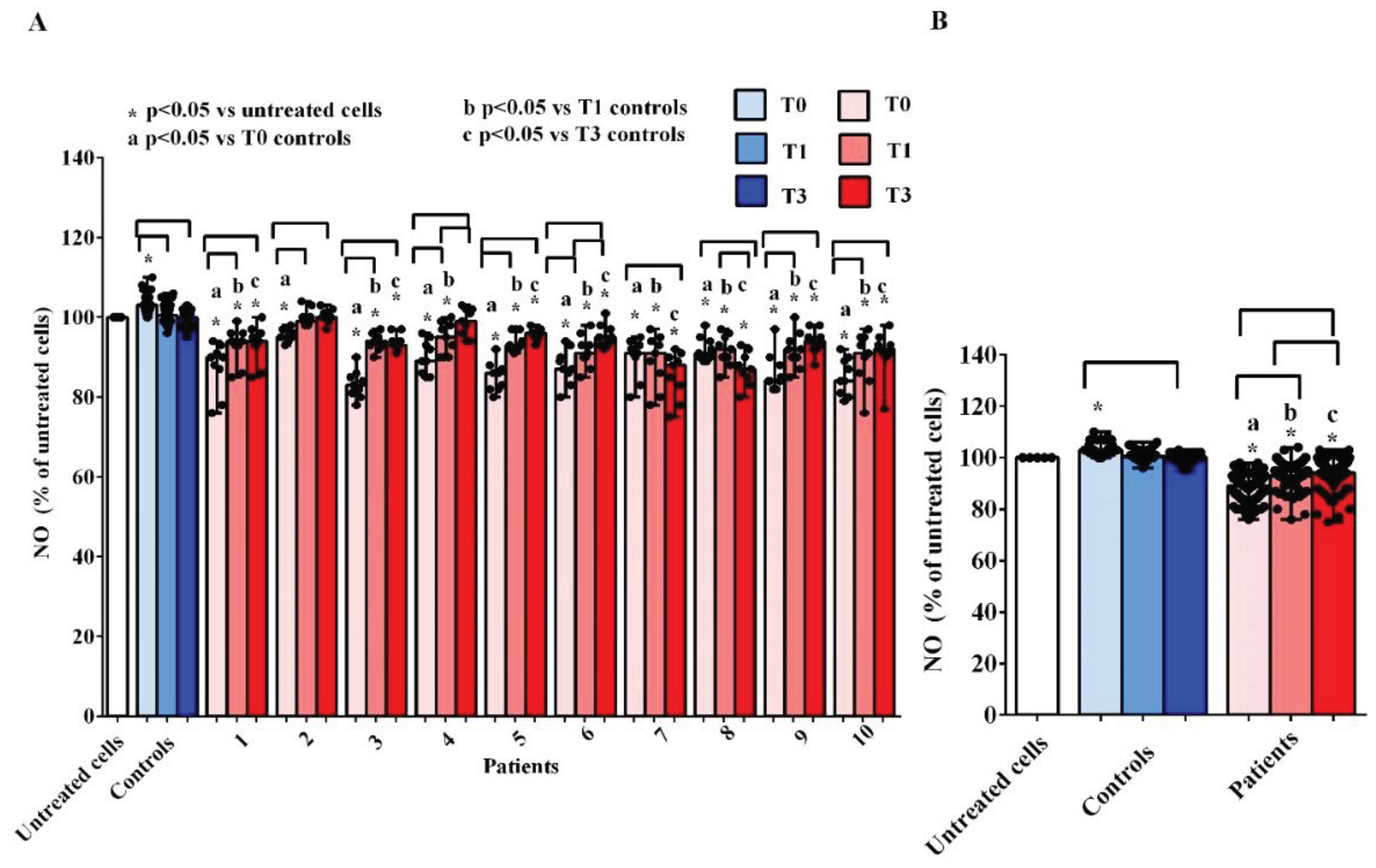
As observed for cell viability, treatment of hUMSCs with plasma of AMA patients was able to reduce the NO release at T0, T1 and T3 in comparison with what obtained in the untreated cells and in hUMSCs treated with plasma of the controls, where, instead, we found an increased NO release by hUMSCs, at T0 (
Figure 5A,B). At T1 and T3, the release of the NO by hUMSCs treated with plasma of AMA patients was augmented vs what found at T0, although the levels never reached what was found in the untreated cells or in hUMSCs treated with plasma of the controls.
2.4. Clinical Pattern
The analysis of the data regarding AMA patients suffering from pathologies during pregnancy vs controls, and even comparing patients with pregestational diseases vs uncomplicated ones, did not show any statistical significant difference. Thus, the altered redox balance we found in AMA patients can be linked to age rather than to any comorbidities.
3. Discussion
Our results highlighted an altered redox balance in the plasma of AMA patients, at diagnosis and throughout the duration of pregnancy up to 48 hours postpartum. Moreover, we showed that unknown factors capable of causing damages to hUMSCs circulate in the plasma of the aforementioned patients.
As previously reported, AMA pregnancy has been considered as an independent risk factor for several complications, varying across ages. In fact, these pregnancies are known to be linked to several comorbidities and adverse outcomes, such as pregnancy-related hypertensive disorders, blood transfusion, maternal and fetal mortality[
21,
37].
One of the possible explanations of these phenomena could be the increased oxidative stress. About this issue, it should be pointed out that in physiological pregnancy the increase of oxidants is counterbalanced by the production of antioxidants and the occurrence of oxidative stress is limited[
22,
23]. Instead, in all conditions in which the systems for regulating the balance between oxidants and antioxidants are poorly effective, the development of oxidative stress is markedly greater. Certainly one of those conditions predisposing the onset of oxidative stress is aging[
24].
The primary endpoint of the study was the analysis of plasma redox balance at different time-points during pregnancy in AMA vs younger pregnant women. We found that patients and controls differed in terms of plasma TBARS and GSH levels, throughout the entire duration of pregnancy. Hence, lipid peroxidation markers were higher in the former while the antioxidant was higher in the latter. It is also to note that the same results were found in the plasma of umbilical cords. The differences we observed between the patients and the controls regarding the redox balance could be attributed to age, which was the only variable statistically different between the two groups in addition to body mass index, which, however, although being higher in AMA patients fell within the normal weight range[
25].
Also, although a role of the above reported pregnancy-related complications in the alteration of the balance between oxidants and antioxidants is conceivable[
26,
27], the statistical analysis showed that there were no significant differences as regarding the parameters of redox balance and oxidative stress between AMA patients with complications and without complications. For this reason we can attribute the increased values of plasma TBARS and reduced values of plasma GSH more to the advanced age of the patients than to their complications.
The data we obtained about the redox balance in AMA pregnant women up to 48 h after delivery and in umbilical blood, could have clinical implications in those subjects and newborn. In particular, our results could be helpful to increase knowledge and to better understand the most known AMA pregnancy-related comorbidities and stimulate studies on the efficacy of preventive or therapeutic treatments aimed at contrasting them. In addition, our results could have a prospective value related to the prevention of the development of cardiovascular disease, which has been reported for AMA pregnant women and their children[
28,
29].
About this issue, a key role as a pathogenic mechanism could be played by oxidative stress and endothelial dysfunction[
12,
30,
31,
32,
33]. The data we obtained about plasma levels of NO would confirm this hypothesis. Hence, in AMA pregnant women, the plasma NO levels were lower at T0 and, despite an increasing trend at T1 and T3, they did not reach the values observed in the younger pregnant women, at the same time-points. In the latter, the plasma NO levels increased from T0 to T1 and then decreased reaching values similar to those observed at T0, which were however, higher than those found in AMA patients at T0.
In order to better understand the pathophysiology of pregnancy in AMA both in relation to the effects on the mother and on the fetus, we performed some
in vitro experiments on hUMSCs, which could play a fundamental role for the proper functioning of the placenta and the continuation of a pregnancy without complications[
13]. The stimulation of those cells with plasma taken from AMA pregnant women at T0, T1 and T3 was able to reduce cell viability and increase ROS release. While for cell viability we did not observe any correlation between the observed harmful effects and the different time-points, as regards the release of ROS, the effect was greater at T0 and then it reduced at subsequent time-points, being, however, higher vs what found in untreated hUMSCs and in hUMSCs treated with plasma samples of controls. In case of pregnant women younger than 40 years, cell viability did not vary between T0 and T1, while it increased at T3. In parallel, the release of ROS progressively decreased at T1 and T3 starting from values similar to those of untreated cells observed at T0. As regards the release of NO, while in hUMSCs treated with plasma of pregnant women younger than 40 years, we observed an increase at T0 only, vs what was observed in the untreated cells, in the hUMSCs treated with plasma of AMA pregnant women, the NO release was reduced at all the three time-points and, especially, at T0.
About this issue, it is to note that hUMSCs can regulate trophoblast invasion, the angiogenesis and placental function through the modulation of inflammation and the release of mediators like vascular endothelial growth factor, the placental growth factor, miRNAs or NO[
16,
19,
34,
35,
36]. Alterations in the production of the aforementioned factors, with particular reference to NO, would, therefore, result in the loss of the role played by hUMSCs in the physiological regulation of pregnancy. For those reasons, our results, which highlight a possible mechanism underlying AMA pregnancy physiopathology, which could be related to unknown harmful circulating factors targetting hUMSCs, would add knowledge about this issue.
4. Materials and Methods
4.1. Patients
This observational case-control study was performed on 100 pregnant women older than 40 years (patients) and 20 pregnant women younger than 40 years (controls) enrolled at the Gynecology and Obstetrics Unit, Università del Piemonte Orientale, Azienda Ospedaliera Universitaria Maggiore della Carità, Novara, between June 30th 2021 and June 30th 2023, for a total of two years.
4.2. Clinical Evaluation
Patients and controls were subjected to anamnestic investigation. In each pregnant women, blood samples were collected for the analysis of oxidants/antioxidants markers, such as TBARS, GSH and NO, as below specified. The inclusion criteria were: singular or twin pregnancies; age ≥ 40 years for patients/< 40 years for the controls; written informed consent; acceptance to the follow-up and give birth at Gynecologic and Obstetrics Unit, Azienda Ospedaliera Universitaria Maggiore della Carità.
Women were excluded in case of: legal interdiction; childbirth in different centers; age < 40 years for patients and ≥40 years for the controls; positivity for Sars-Cov2, anti-HIV, anti-HCV.
Plasma samples were collected at T0, T1, T2 and T3. T0 was taken at 11-13 weeks of gestational age, during the first trimester screening, when the patients were counseled and recruited. T1 was taken at 20-22 weeks of gestational age, during the second trimester morphological ultrasound. T2 was at the time of delivery and umbilical cord blood was collected. Finally, T3 was taken before Cesarean section or within 48-72 hours after vaginal delivery.
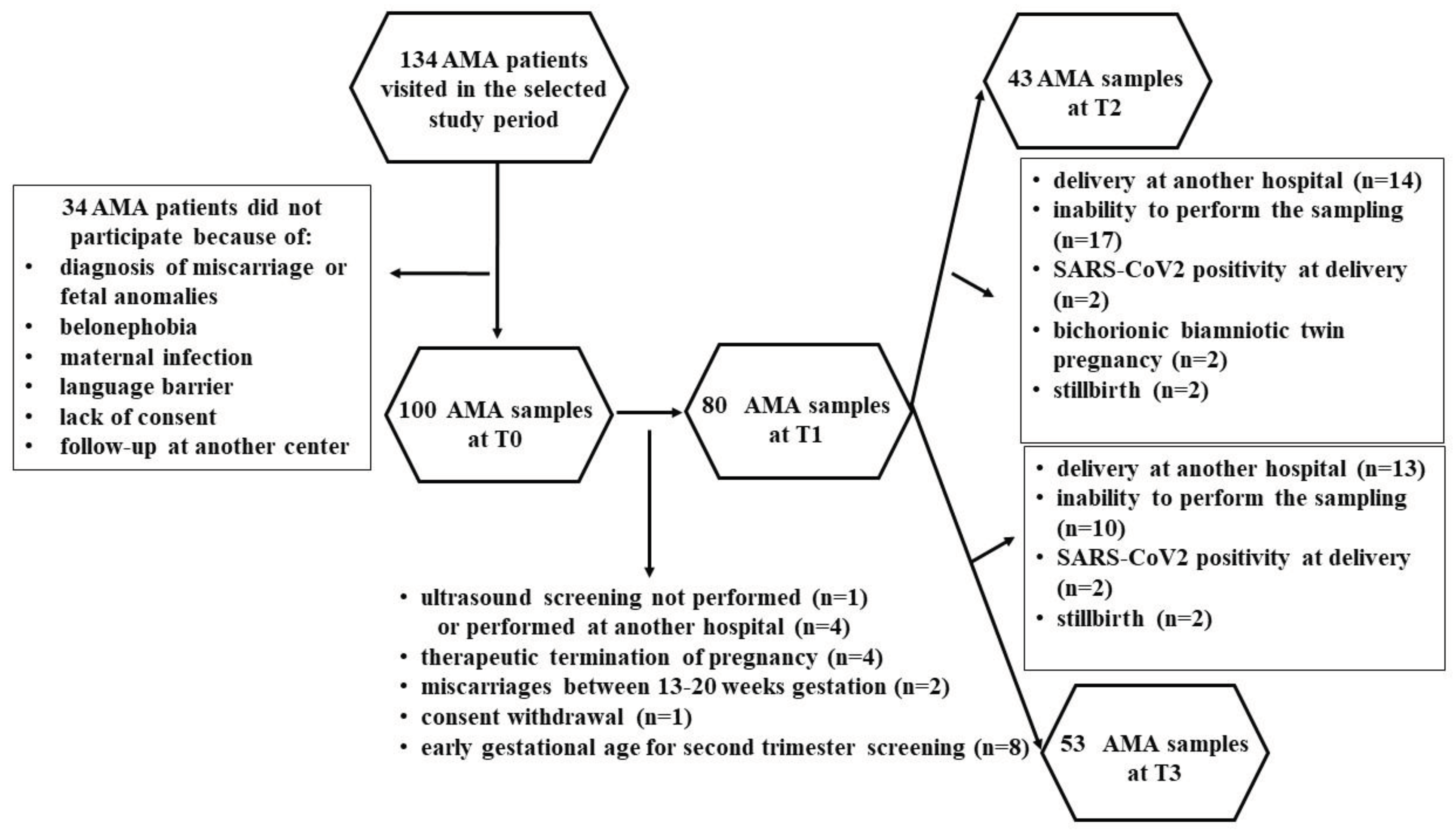
Of the 134 AMA patients visited in the selected study period, 34 women were excluded, as shown in
Figure 6.
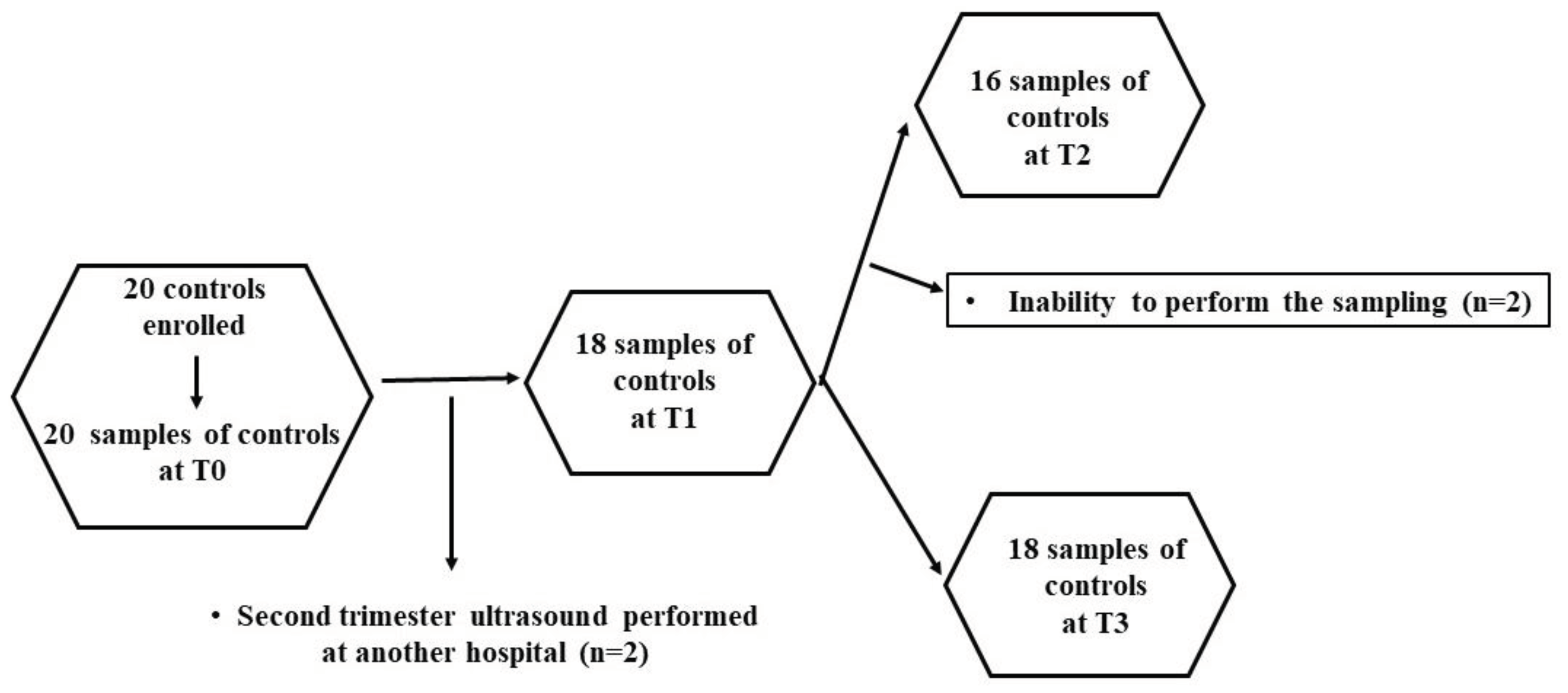
Therefore, 100 patients and 20 controls were enrolled. At various time-points, some patients were lost, as shown in
Figure 6 and
Figure 7. Thus, the evaluations regarding the plasma redox balance and NO were executed on: 100 patients at T0, 80 patients at T1, 43 patients at T2, 53 patients at T3; 20 controls at T0, 18 controls at T1, 16 controls at T2, and 18 controls at T3.
4.3. Biological Sample Analysis
In each pregnant woman, 15 ml blood samples were taken at 9 am in fasting conditions by using BD Vacutainer tubes (sodium heparin or ethylenediaminetetraacetic acid as anticoagulant). Each sample was centrifuged for 15 min through a centrifuge (Eppendorf, mod. 5702 with rotor A-4-38), at 1600 rpm, 4°C. The plasma was aliquoted into 5 tubes which were stored at -80°C at the Physiology laboratory, Università del Piemonte Orientale, until they were used for the quantification of TBARS, GSH e NO and to perform the in vitro experiments on hUMSCs. Plasma samples were handled in pseudonymized conditions and measurements were performed at least in triplicate
4.4. Quantification of Plasma TBARS
Plasma TBARS were measured by using the TBARS assay Kit (Cayman Chemical, Ann Arbor, MI, USA), which evaluates the malonyldialdeide release[
37,
38]. Briefly, 100 µl of each plasma sample was added to sodium dodecyl sulfate solution (100 µl) and Color Reagent (2 ml). Samples were boiled for 1 h and then put on ice for 10 min to stop the reaction. After centrifugation for 10 min at 1600 g at 4°C, 150 μl of each sample was transferred to 96-well plates for malonyldialdeide detection through a spectrophotometer (VICTOR™ X Multilabel Plate Reader; PerkinElmer; Waltham, Massachusetts, USA), at 530–540 nm excitation/emission. A standard curve with the TBARS Standard was performed as a reference for TBARS in each sample (expressed as malonyldialdeide in µM).
4.5. Quantification of Plasma GSH
Plasma GSH levels were quantified through the Glutathione Assay Kit (Cayman Chemical) [
37,
38]. Briefly, each plasma sample was deproteinated through addition of meta- phosphoric acid solution. After centrifugation (2000 g for 2 min), the supernatant of each sample was collected, and 50 μl/ml of TEAM reagent was added. Thereafter, 50 μl of each sample was moved to 96-well plates where the measurement of GSH was executed through a spectrophotometer (VICTOR™ X Multilabel Plate Reader), at 405-414 nm excitation/emission. To perform an accurate GSH quantification (as µM), a standard curve was prepared, as well, by using the GSH Standard.
4.6. Quantification of Plasma NO
Plasma NO was quantified by the Griess assay (Promega Italia Srl, Milan, Italy)[
37,
39]. To do this, 5 ml plasma sample was deproteinated by adding 10 ml sulfosalicylic acid. The samples have been, then, vortexed every 5 min and let to react for 30 min at room temperature. After centrifugation (10000 g for 15 min), 50 μL of the supernatant was added to saline (1:2 dilution) for the subsequent analysis. The remaining microliters were used without dilution. In order to reduce nitrate to nitrite, the samples were passed through a copper–cadmium column of an autoanalyzer (Autoanalyzer; Technicon Instruments Corp., Tarrytown, NY, USA) and then they were mixed with an equal volume of Griess reagents. After 10 min, the absorbance was measured by a spectrometer (VICTOR™ X Multilabel Plate Reader) at 570 nm. NO release was examined in comparison with a standard curve and expressed as nitrites (μM).
4.8. In Vitro Experiments
4.8.1. Effects of Plasma on hUMSCs
4.8.1.1. Cell Culture
The hUMSCs (StemBioSys, San Antonio, Texas USA) were cultured in α-Minimum Essential Medium Eagle (VWR International srl, Milan, Italy) supplemented with 2.5% heparin free human pooled platelet lysate, 1% Antibiotic-Antimycotic (VWR International srl), and 1% GlutaMax (Euroclone, Pero, Italy).
Experiments were carried out using the plasma of 10 AMA patients at the time-points, T0, T1 and T3, and of 5 patients belonging to the controls at the same time-points. For the in vitro experiments we selected patients/controls characterized by a physiological pregnancy, without the onset of any complications, and for whom samples were available and adequate at the three chosen time-points.
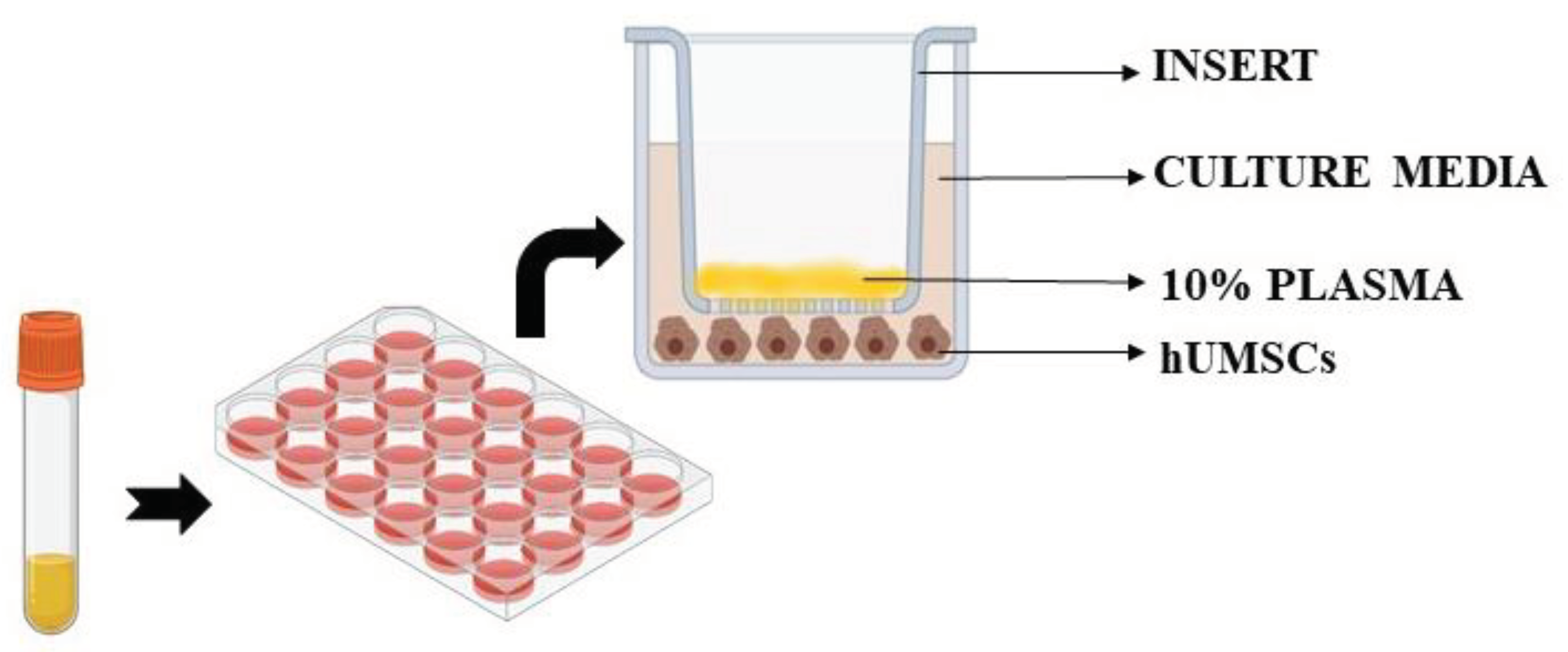
Untreated cells were also included in the analysis. In particular, we used specific Transwell inserts in order to analyze cell viability, ROS and NO release in hUMSCs treated with plasma samples, as previously performed. As depicted in
Figure 8, 10% plasma samples calculated in relation to total volume of each insert, were positioned in the apical surface of the insert itself for 3 h, while hUMSCs were plated in the basal one. After 3 h stimulation with plasma, the inserts were removed and various assays were performed, as below described. Experiments were executed in triplicate and repeated three times on different pools of hUMSCs.
4.8.1.2. Cell Viability
Cell viability of hUMSCs was investigated by using the 1% 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT assay; Cayman Chemical, Ann Arbor, MI, USA), as previously performed [
39,
40]. Briefly, after the 10% plasma stimulation of hUMSCs (50000 cells/well in 24-well plates), the media was removed and 200 µL of the MTT solution diluted in Dulbecco’s Modified Eagle Medium high glucose w/o phenol red, supplemented with 2 mM L-glutamine, and 1% penicillin-streptomycin was added to each well. After the addition of 100 µL of dimethyl sulfoxide (Sigma, Milan, Italy) to dissolve the formazan crystals, cell viability was determined by measuring the absorbance through a spectrophotometer (VICTOR™ X Multilabel Plate Reader; PerkinElmer; Waltham, MA, USA), with a wavelength of 570 nm. Cell viability was calculated by setting control cells (untreated cells) as 100%.
4.8.1.3. ROS Release
ROS release by hUMSCs was analyzed through the DCFDA assay which evaluated the oxidation of 2,7-dichlorodihydrofluorescein diacetate into 2,7-dichlorodihydrofluorescein (Abcam, Cambridge, UK).[
39,
40]. To do this, 50000 hUMSCs/well were plated in 24-Transwells plates in complete medium, as executed for MTT assay. After stimulation with 10% plasma for 3 h, the medium was removed and washing was performed with phosphate buffer saline, which was followed by staining with 10 µM 2,7-dichlorodihydrofluorescein diacetate for 20 min at 37°C. The fluorescence intensity of 2,7-dichlorodihydrofluorescein was measured at 485 nm/530 nm excitation/emission by using a spectrophotometer (VICTOR™ X Multilabel Plate Reader; PerkinElmer). Results were expressed as 2,7-dichlorodihydrofluorescein fluorescence intensity, which was proportional to the amount of intracellular ROS.
4.8.1.4. NO Release
NO release by hUMSCs was quantified through the Griess assay (Promega)[
39,
40]. To do this, 50000 hUMSCs were plated in 24-Transwells plates in complete medium, and then the same experimental protocol followed for MTT and ROS assays was used. At the end of the stimulations, NO was examined in the sample's supernatants by adding Griess reagent following the manufacturer's instruction. Thereafter, the absorbance of each sample was read at 570 nm through a spectrometer (VICTOR™ X Multilabel Plate Reader). A standard curve was prepared to quantify the NO production, which was expressed as nitrites (μM).
4.8.1.5. Statistical Analyses
All data were collected using the Research Electronic Data Capture software (RED-Cap, Vanderbilt University, Nashville, TN, USA). The mean of the multiple measurements taken on each patient/control was considered for the analysis. For plasma quantification and the in vitro experiments, the results werepresented as median and interquartile (IQR) range. The differences of quantitative variables between the two groups were assessed using the Mann Whitney test. Spearman’s correlation coefficient was used to calculate the correlation between quantitative variables. The association between categorical variables was assessed using the chi-square test. A P-value <0.05 was considered statistically significant. Statistical analysis was performed by using STATA version 17 (College Station, TX: StataCorp LLC) and Graph PAD (GraphPad Software, San Diego, California USA).
5. Conclusions
Our results have highlighted an altered redox balance and a reduction of NO in plasma of AMA pregnant women, and evidenced the existence of unknown circulating factors capable of inducing damages in hUMSCs. Those alterations were shown early and persisted up to 48 hours after delivery. Beyond the possible obstetric complications, the increased plasma levels of TBARS, the reduced plasma levels of GSH and the harmful circulating factors could represent risk factors for the development of cardiovascular or other chronic disease, which could arise in women after advanced maternal age pregnancy and in children[
13]. In future, these data could have interesting clinical repercussions in terms of diagnosis, treatment and follow up of several widespread diseases related to AMA pregnancy.
Author Contributions
Conceptualization: EG, CIA, RV, VR, DS. Investigation and data curation: CIA, SV, LT, ET, FF. Formal analysis: EG, SV, LT, ET, FF, DF, RV, VR, DS. Methodology: EG, SV, LT, ET, FF, DF, RV, VR, DS. Funding acquisition and resources: EG, RV, VR, DS. Project administration: EG, RV, VR, DS. Writing the original draft: EG, CIA, SV, DF, RV, VR, DS. Writing, review and editing: EG, CIA, SV, LT, ET, FF, DF, RV, VR, DS. Supervision: EG, RV, VR, DS. Validation: EG, CIA, SV, LT, ET, FF, DF, RV, VR, DS. Visualization: EG, CIA, SV, LT, ET, FF, DF, RV, VR, DS.
Funding
This research was supported by the Italian Ministry of Education, University and Research (MIUR) program “Departments of Excellence 2018–2022,” Aging Project—Department of Translational Medicine, Università del Piemonte Orientale.
Institutional Review Board Statement
The study was approved by the Intercompany Ethics Committee n. CE 119/21 and complies with the Declaration of Helsinki and principles of Good Clinical Practice.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability
The data that support the findings of the present study are available from the corresponding author upon reasonable request.
Acknowledgments
We are thankful to all the patients for giving the consent to provide blood samples and to the staff from the Gynecology and Obstetrics, Department of Translational Medicine, Università del Piemonte Orientale, Azienda Ospedaliera Universitaria (AOU) Maggiore della Carità, Novara, Italy, who were involved in patients’ management and providing blood samples.
Conflicts of Interest
The authors declare that no competing interests exist. All authors have thoroughly reviewed and complied with the journal's authorship agreement.
References
- Frederiksen, L.E.; Ernst, A.; Brix, N.; Braskhøj Lauridsen, L.L.; Roos, L.; Ramlau-Hansen, C.H.; Ekelund, C.K. Risk of Adverse Pregnancy Outcomes at Advanced Maternal Age. Obstet Gynecol 2018, 131, 457–463. [Google Scholar] [CrossRef]
- Pinheiro, R.L.; Areia, A.L.; Mota Pinto, A.; Donato, H. Advanced Maternal Age: Adverse Outcomes of Pregnancy, A Meta-Analysis. Acta Med Port 2019, 32, 219–226. [Google Scholar] [CrossRef]
- Glick, I.; Kadish, E.; Rottenstreich, M. Management of Pregnancy in Women of Advanced Maternal Age: Improving Outcomes for Mother and Baby. Int J Womens Health 2021, 13, 751–759. [Google Scholar] [CrossRef]
- Martin, J.A.; Hamilton, B.E.; Ventura, S.J.; Osterman, M.J.; Wilson, E.C.; Mathews, T.J. Births: Final data for 2010. Natl Vital Stat Rep 2012, 61, 1–72. [Google Scholar]
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.K.; Driscoll, A.K. Births: Final Data for 2019. Natl Vital Stat Rep 2021, 70, 1–51. [Google Scholar]
- Matthews, T.J.; Hamilton, B.E. First births to older women continue to rise. NCHS data brief 2014, 152, 1–8. [Google Scholar]
- Sauer, M.V. Reproduction at an advanced maternal age and maternal health. Fertil Steril 2015, 103, 1136–1143. [Google Scholar] [CrossRef]
- Kawwass, J.F.; Badell, M.L. Maternal and Fetal Risk Associated With Assisted Reproductive Technology. Obstet Gynecol 2018, 132, 763–772. [Google Scholar] [CrossRef]
- Cleary-Goldman, J.; Malone, F.D.; Vidaver, J.; Ball, R.H.; Nyberg, D.A.; Comstock, C.H.; Saade, G.R.; Eddleman, K.A.; Klugman, S.; Dugoff, L.; et al. Impact of maternal age on obstetric outcome. Obstet Gynecol 2005, 105, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Jacobsson, B.; Ladfors, L.; Milsom, I. Advanced maternal age and adverse perinatal outcome. Obstet Gynecol 2014, 104, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Di Fabrizio, C.; Giorgione, V.; Khalil, A.; Murdoch, C.E. Antioxidants in Pregnancy: Do We Really Need More Trials? Antioxidants (Basel) 2022, 11, 812. [Google Scholar] [CrossRef] [PubMed]
- Cooke, C.M.; Davidge, S.T. Advanced maternal age and the impact on maternal and offspring cardiovascular health. Am J Physiol Heart Circ Physiol 2019, 317, H387–H394. [Google Scholar] [CrossRef] [PubMed]
- Miriyala, S.; Spasojevic, I.; Tovmasyan, A.; Salvemini, D.; Vujaskovic, Z.; St Clair, D.; Batinic-Haberle, I. Manganese superoxide dismutase, MnSOD and its mimics. Biochim Biophys Acta 2012, 1822, 794–814. [Google Scholar] [CrossRef] [PubMed]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis 2018, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediators Inflamm. 2021, 9962860. [Google Scholar] [CrossRef] [PubMed]
- Surico, D.; Bordino, V.; Cantaluppi, V.; Mary, D.; Gentilli, S.; Oldani, A.; Farruggio, S.; Melluzza, C.; Raina, G.; Grossini, E. Preeclampsia and intrauterine growth restriction: Role of human umbilical cord mesenchymal stem cells-trophoblast cross-talk. PLoS ONE 2019, 14, e0218437. [Google Scholar] [CrossRef] [PubMed]
- Kamath, U.; Rao, G.; Kamath, S.U.; Rai, L. Maternal and fetal indicators of oxidative stress during intrauterine growth retardation (IUGR). Indian J Clin Biochem 2006, 21, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, G.; Fan, H.; Zhao, X.; Li, P.; Wang, Z.; Hu, Y.; Hou, Y. Mesenchymal stem cells ameliorate Th1-induced pre-eclampsia-like symptoms in mice via the suppression of TNF-α expression. PLoS ONE 2014, 9, e88036. [Google Scholar] [CrossRef]
- Choi, J.H.; Jung, J.; Na, K.H.; Cho, K.J.; Yoon, T.K.; Kim, G.J. Effect of mesenchymal stem cells and extracts derived from the placenta on trophoblast invasion and immune responses. Stem Cells Dev 2014, 23, 132–145. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, J.; Xu, B.; He, Y.; Liu, W.; Li, J.; Zhang, S.; Lin, X.; Su, D.; Wu, T.; et al. HucMSC-Derived Exosomes Mitigate the Age-Related Retardation of Fertility in Female Mice. Mol Ther 2020, 28, 1200–1213. [Google Scholar] [CrossRef]
- Machado-Gédéon, A.; Badeghiesh, A.; Baghlaf, H.; Dahan, M.H. Adverse pregnancy, delivery and neonatal outcomes across different advanced maternal ages: A population-based retrospective cohort study. Eur J Obstet Gynecol Reprod Biol X 2023, 17, 100180. [Google Scholar] [CrossRef]
- Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Vázquez, C.M.; Mate, A.; Sobrevia, L.; Marín, R. Oxidative stress: Normal pregnancy versus preeclampsia. Biochim Biophys Acta Mol Basis Dis 2020, 1866, 165354. [Google Scholar] [CrossRef]
- de Lucca, L.; Jantsch, L.B.; Vendrame, S.A.; de Paula, H.L.; Dos Santos Stein, C.; Gallarreta, F.M.P.; Moresco, R.N.; de Lima Gonçalves, T. Variation of the Oxidative Profile in Pregnant Women With and Without Gestational Complications. Matern Child Health J 2022, 26, 2155–2168. [Google Scholar] [CrossRef]
- Husain, S.; Hillmann, K.; Hengst, K.; Englert, H. Effects of a lifestyle intervention on the biomarkers of oxidative stress in non-communicable diseases: A systematic review. Front Aging 2023, 4, 1085511. [Google Scholar] [CrossRef] [PubMed]
- Malti, N.; Merzouk, H.; Merzouk, S.A.; Loukidi, B.; Karaouzene, N.; Malti, A.; Narce, M. Oxidative stress and maternal obesity: Feto-placental unit interaction. Placenta 2014, 35, 411–416. [Google Scholar] [CrossRef]
- Velazquez, M.A.; Smith, C.G.; Smyth, N.R.; Osmond, C.; Fleming, T.P. Advanced maternal age causes adverse programming of mouse blastocysts leading to altered growth and impaired cardiometabolic health in post-natal life. Hum Reprod 2016, 31, 1970–1980. [Google Scholar] [CrossRef]
- Davidge, S.T.; Morton, J.S.; Rueda-Clausen, C.F. Oxygen and perinatal origins of adulthood diseases: Is oxidative stress the unifying element? Hypertension 2008, 52, 808–810. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Clausen, C.F.; Morton, J.S.; Davidge, S.T. The early origins of cardiovascular health and disease: Who, when, and how. Semin Reprod Med 2011, 29, 197–210. [Google Scholar] [CrossRef]
- Care, A.S.; Bourque, S.L.; Morton, J.S.; Hjartarson, E.P.; Davidge, S.T. Effect of advanced maternal age on pregnancy outcomes and vascular function in the rat. Hypertension 2015, 65, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 2008, 359, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Leiva, A.; Pardo, F.; Ramírez, M.A.; Farías, M.; Casanello, P.; Sobrevia, L. Fetoplacental vascular endothelial dysfunction as an early phenomenon in the programming of human adult diseases in subjects born from gestational diabetes mellitus or obesity in pregnancy. Exp Diabetes Res 2011, 349286. [Google Scholar] [CrossRef]
- Choi, J.W.; Im, M.W.; Pai, S.H. Nitric oxide production increases during normal pregnancy and decreases in preeclampsia. Ann Clin Lab Sci 2002, 32, 257–263. [Google Scholar]
- de Lucca, L.; Jantsch, L.B.; Vendrame, S.A.; de Paula, H.L.; Dos Santos Stein, C.; Gallarreta, F.M.P.; Moresco, R.N.; de Lima Gonçalves, T. Variation of the Oxidative Profile in Pregnant Women With and Without Gestational Complications. Matern Child Health J 2022, 26, 2155–2168. [Google Scholar] [CrossRef]
- Castrechini, N.M.; Murthi, P.; Gude, N.M.; Erwich, J.J.; Gronthos, S.; Zannettino, A.; Brennecke, S.P.; Kalionis, B. Mesenchymal stem cells in human placental chorionic villi reside in a vascular Niche. Placenta 2010, 31, 203–212. [Google Scholar] [CrossRef]
- Demir, R.; Kaufmann, P.; Castellucci, M.; Erbengi, T.; Kotowski, A. Fetal vasculogenesis and angiogenesis in human placental villi. Acta Anat (Basel) 1989, 136, 190–203. [Google Scholar] [CrossRef]
- Awoyemi, T.; Cerdeira, A.S.; Zhang, W.; Jiang, S.; Rahbar, M.; Logenthiran, P.; Redman, C.; Vatish, M. Preeclampsia and syncytiotrophoblast membrane extracellular vesicles (STB-EVs). Clin Sci (Lond) 2022, 136, 1793–1807. [Google Scholar] [CrossRef] [PubMed]
- Grossini, E.; Garhwal, D.; Venkatesan, S.; Ferrante, D.; Mele, A.; Saraceno, M.; Scognamiglio, A.; Mandrioli, J.; Amedei, A.; De Marchi, F.; et al. The Potential Role of Peripheral Oxidative Stress on the Neurovascular Unit in Amyotrophic Lateral Sclerosis Pathogenesis: A Preliminary Report from Human and In Vitro Evaluations. Biomedicines 2022, 10, 691. [Google Scholar] [CrossRef]
- Grossini, E.; Concina, D.; Rinaldi, C.; Russotto, S.; Garhwal, D.; Zeppegno, P.; Gramaglia, C.; Kul, S.; Panella, M. Association Between Plasma Redox State/Mitochondria Function and a Flu-Like Syndrome/COVID-19 in the Elderly Admitted to a Long-Term Care Unit. Front Physiol 2021, 12, 707587. [Google Scholar] [CrossRef] [PubMed]
- Grossini, E.; Smirne, C.; Venkatesan, S.; Tonello, S.; D'Onghia, D.; Minisini, R.; Cantaluppi, V.; Sainaghi, P.P.; Comi, C.; Tanzi, A.; et al. Plasma Pattern of Extracellular Vesicles Isolated from Hepatitis C Virus Patients and Their Effects on Human Vascular Endothelial Cells. Int J Mol Sci 2023, 24, 10197. [Google Scholar] [CrossRef] [PubMed]
- Grossini, E.; Esposito, T.; Viretto, M.; Venkatesan, S.; Licari, I.; Surico, D.; Della Corte, F.; Castello, L.; Bruno, S.; Quaglia, M.; et al. Circulating Extracellular Vesicles in Subarachnoid Hemorrhage Patients: Characterization and Cellular Effects. Int J Mol Sci 2023, 24, 14913. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Plasma levels of malonyldialdeide (MDA, A), glutathione (GSH, B) and nitric oxide (NO, C) in pregnant women older and younger than 40 years. P: pregnant women older than 40 years. C: pregnant women younger than 40 years. T0: at the 11-13 weeks of gestational age; T1:at the 20-22 weeks of gestational age; T3: before Cesarean section or at 48-72 hours after the delivery. The results are expressed as median and range of different measurements. Square brackets indicate significance between groups (P <0.05).
Figure 1.
Plasma levels of malonyldialdeide (MDA, A), glutathione (GSH, B) and nitric oxide (NO, C) in pregnant women older and younger than 40 years. P: pregnant women older than 40 years. C: pregnant women younger than 40 years. T0: at the 11-13 weeks of gestational age; T1:at the 20-22 weeks of gestational age; T3: before Cesarean section or at 48-72 hours after the delivery. The results are expressed as median and range of different measurements. Square brackets indicate significance between groups (P <0.05).
Figure 2.
Plasma levels of glutathione (GSH, A), malonyldialdeide (MDA, B) and nitric oxide (NO, C) in umbilical cords taken from pregnant women older and younger than 40 years. P: pregnant women older than 40 years. C: pregnant women younger than 40 years. T2: at the delivery. The results are expressed as median and range of different measurements. Square brackets indicate significance between groups (P <0.05).
Figure 2.
Plasma levels of glutathione (GSH, A), malonyldialdeide (MDA, B) and nitric oxide (NO, C) in umbilical cords taken from pregnant women older and younger than 40 years. P: pregnant women older than 40 years. C: pregnant women younger than 40 years. T2: at the delivery. The results are expressed as median and range of different measurements. Square brackets indicate significance between groups (P <0.05).
Figure 3.
Effects of plasma taken from pregnant women older (patients) and younger than 40 years (controls) on cell viability of hUMSCs. In A, effects of plasma of each patient and controls, at various time-points, are shown. In B, effects of plasma of all 10 patients and all 5 controls, at various time-points, are shown. T0: at the 11-13 weeks of gestational age; T1:at the 20-22 weeks of gestational age; T3: before Cesarean section or at 48-72 hours after the delivery. The results are the median and range of repeated experiments. Untreated cells: non-treated hUMSCs. Square brackets indicate significance between the groups (P <0.05).
Figure 3.
Effects of plasma taken from pregnant women older (patients) and younger than 40 years (controls) on cell viability of hUMSCs. In A, effects of plasma of each patient and controls, at various time-points, are shown. In B, effects of plasma of all 10 patients and all 5 controls, at various time-points, are shown. T0: at the 11-13 weeks of gestational age; T1:at the 20-22 weeks of gestational age; T3: before Cesarean section or at 48-72 hours after the delivery. The results are the median and range of repeated experiments. Untreated cells: non-treated hUMSCs. Square brackets indicate significance between the groups (P <0.05).
Figure 4.
Effects of plasma taken from pregnant women older (patients) and younger than 40 years (controls) on reactive oxygen species (ROS) release by hUMSCs. In A, effects of plasma of each patient and controls, at various time-points, are shown. In B, effects of plasma of all 10 patients and all 5 controls, at various time-points, are shown. T0: at the 11-13 weeks of gestational age; T1:at the 20-22 weeks of gestational age; T3: before Cesarean section or at 48-72 hours after the delivery. The results are the median and range of repeated experiments. Untreated cells: non-treated hUMSCs. Square brackets indicate significance between the groups (P <0.05).
Figure 4.
Effects of plasma taken from pregnant women older (patients) and younger than 40 years (controls) on reactive oxygen species (ROS) release by hUMSCs. In A, effects of plasma of each patient and controls, at various time-points, are shown. In B, effects of plasma of all 10 patients and all 5 controls, at various time-points, are shown. T0: at the 11-13 weeks of gestational age; T1:at the 20-22 weeks of gestational age; T3: before Cesarean section or at 48-72 hours after the delivery. The results are the median and range of repeated experiments. Untreated cells: non-treated hUMSCs. Square brackets indicate significance between the groups (P <0.05).
Figure 5.
Effects of plasma taken from pregnant women older (patients) and younger than 40 years (controls) on nitric oxide (NO) release by hUMSCs. In A, effects of plasma of each patient and controls, at various time-points, are shown. In B, effects of plasma of all 10 patients and all 5 controls, at various time-points, are shown. T0: at the 11-13 weeks of gestational age; T1:at the 20-22 weeks of gestational age; T3: before Cesarean section or at 48-72 hours after the delivery. The results are the median and range of repeated experiments. Untreated cells: non-treated hUMSCs. Square brackets indicate significance between the groups (P <0.05).
Figure 5.
Effects of plasma taken from pregnant women older (patients) and younger than 40 years (controls) on nitric oxide (NO) release by hUMSCs. In A, effects of plasma of each patient and controls, at various time-points, are shown. In B, effects of plasma of all 10 patients and all 5 controls, at various time-points, are shown. T0: at the 11-13 weeks of gestational age; T1:at the 20-22 weeks of gestational age; T3: before Cesarean section or at 48-72 hours after the delivery. The results are the median and range of repeated experiments. Untreated cells: non-treated hUMSCs. Square brackets indicate significance between the groups (P <0.05).
Figure 6.
Flowchart describing advanced maternal age (AMA) pregnant women undergone the analysis of plasma redox balance and NO at various time-points.
Figure 6.
Flowchart describing advanced maternal age (AMA) pregnant women undergone the analysis of plasma redox balance and NO at various time-points.
Figure 7.
Flowchart describing the controls undergone the analysis of plasma redox balance and NO at various time-points. .
Figure 7.
Flowchart describing the controls undergone the analysis of plasma redox balance and NO at various time-points. .
Figure 8.
Experiments on hUMSCs. hUMSCs: human umbilical cord mesenchymal stem cells .
Figure 8.
Experiments on hUMSCs. hUMSCs: human umbilical cord mesenchymal stem cells .
Table 1.
Characteristics of the total population at enrollment.
Table 1.
Characteristics of the total population at enrollment.
| |
AMA patients
(n=100) |
Controls
(n=20) |
P-value |
| |
|
|
|
| Age median (IQR) |
41 (40-42) |
30 (27-32) |
<0.0001 |
BMI before pregnancy
(kg/m2) median (IQR)
|
23.7 (20.9-27.4) |
21.5 (19.8-23.3) |
0.04 |
Ethnicity n (%)
Caucasic
Other
|
80 (80.0)
20 (20.0) |
18 (0.90)
2 (0.10) |
0.47 |
| Nulliparous n (%) |
36 (36.0) |
7 (35.0) |
0.93 |
Miscarriages n (%)
0
1
>=2
|
45 (45.0)
37 (37.0)
18 (18.0) |
12 (60.0)
7 (35.0)
1 (5.0) |
0.29 |
| Before-pregnancy pathologies n (%) |
18 (18.0) |
1 (5.0) |
0.25 |
| Positive combined prenatal screening test and positive at second level test |
26 (26.0) |
1 (5.0) |
0.06 |
Table 2.
Delivery outcomes in patients and controls who gave birth by the end of the study.
Table 2.
Delivery outcomes in patients and controls who gave birth by the end of the study.
| |
AMA patients
(n=53) |
Controls
(n=18) |
P-value |
Type of delivery n (%)
Spontaneous vaginal
Induced
Cesarean section
Dystocic
|
23 (43.4)
5 (9.4)
22 (41.5)
3 (5.7) |
11 (61.1)
3 (16.7)
3 (16.7)
1 (5.6) |
0.23 |
| Gestational age at delivery median (IQR) |
39 weeks (38-40) |
39 weeks (38-40) |
0.50 |
| Birth weight at delivery (g) mean (SD) |
3073 (575.2) |
3288 (445.9) |
0.15 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).