Submitted:
20 March 2024
Posted:
22 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Details
2.1. Materials
2.2. Carbon Dots Synthesis: fs Laser Irradiation
2.3. Raman Spectroscopy
2.4. Transmission Electron Microscopy (TEM)
2.5. PL Properties of CDs and Other Luminophores
3. Results
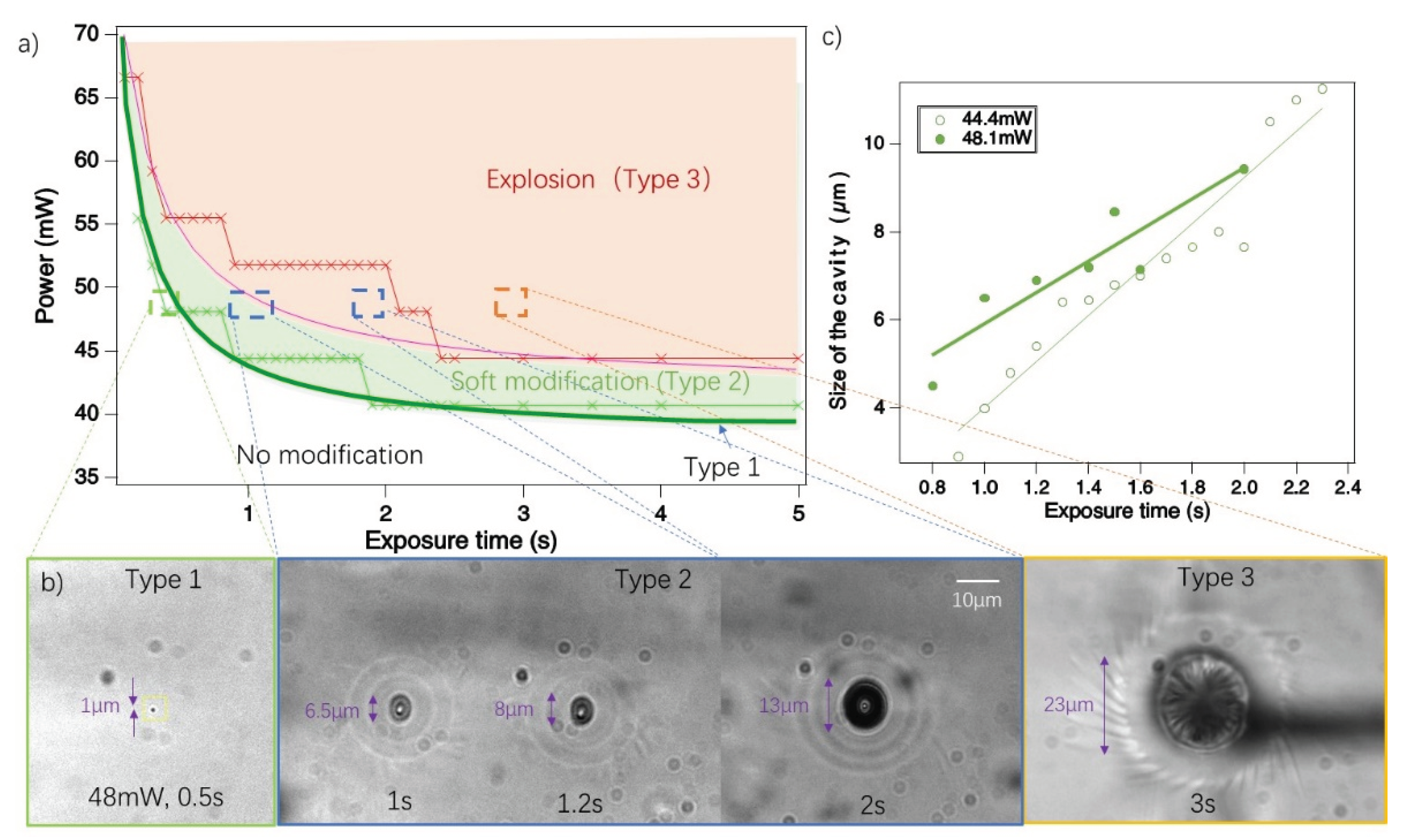
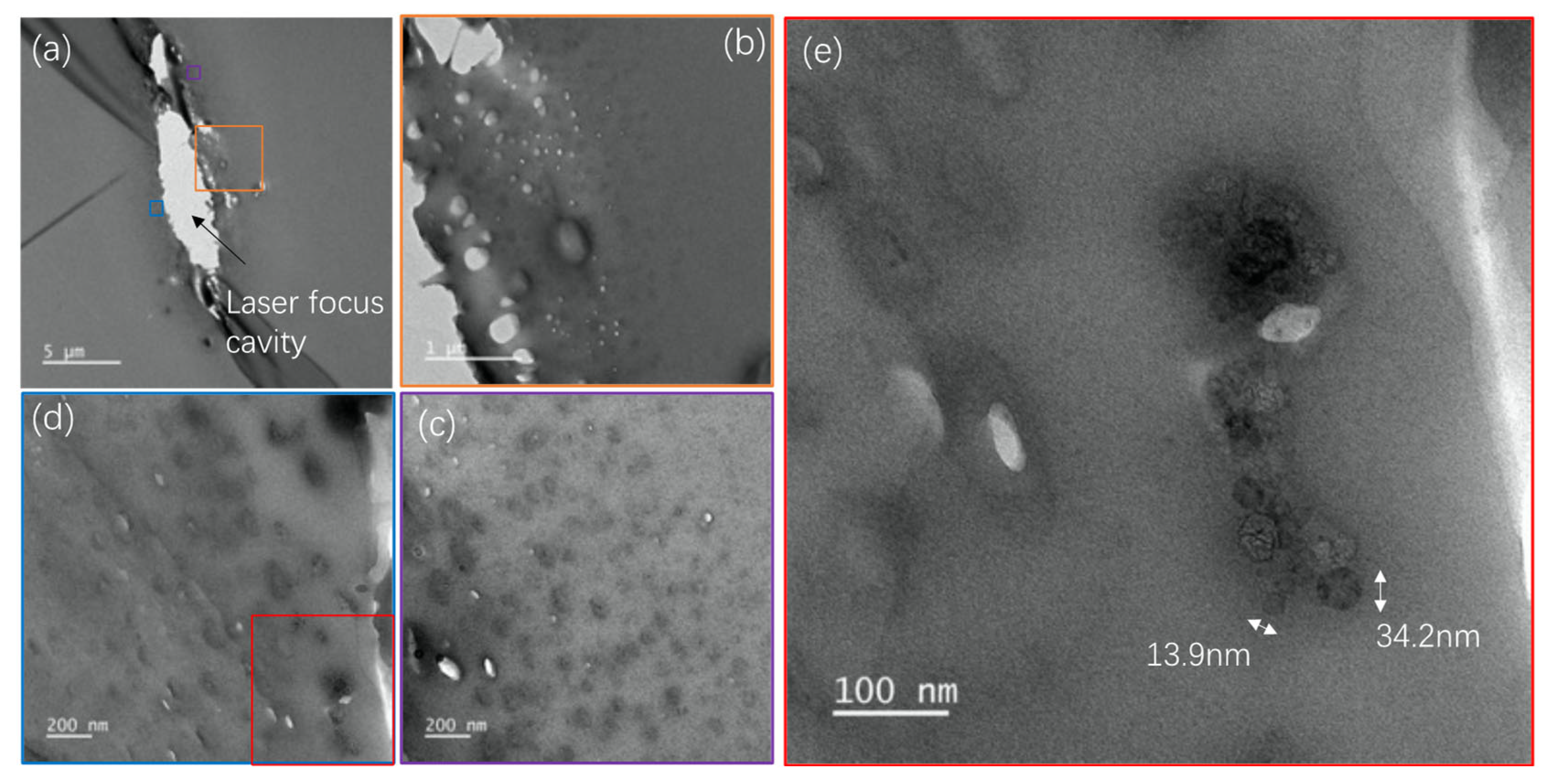
3.1. Fs laser Induced Modifications
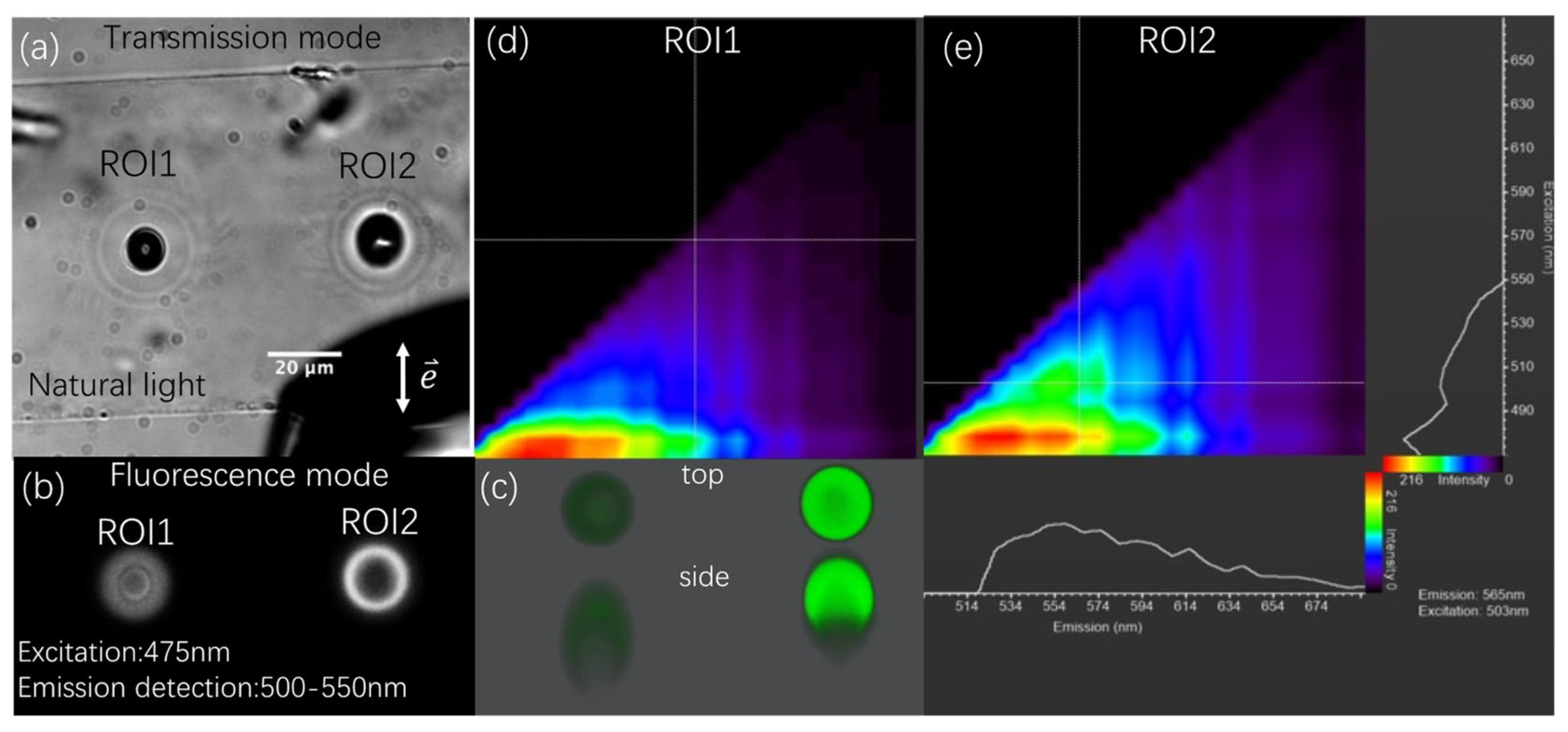
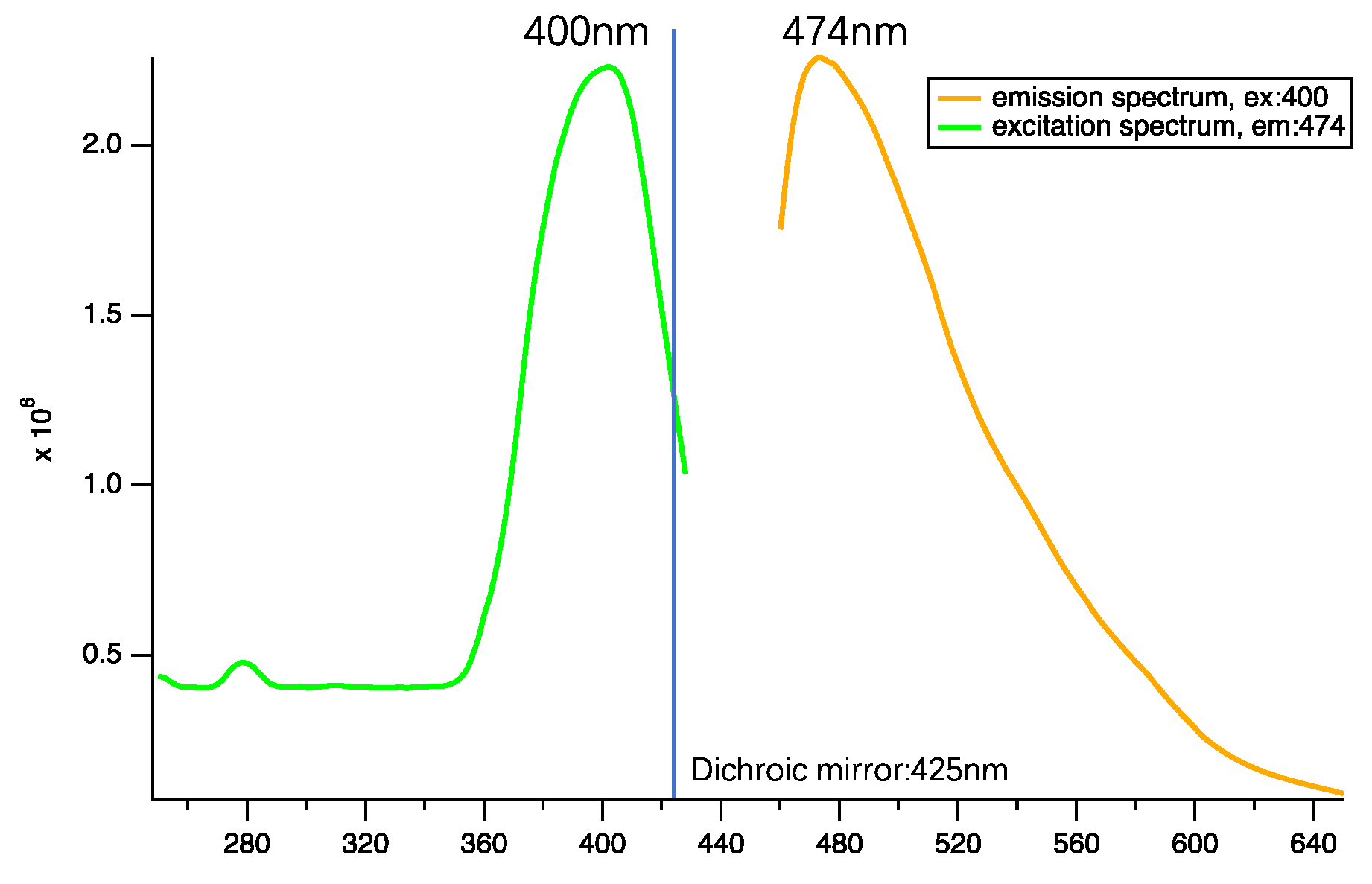
3.2. Photoluminescence (PL) Properties
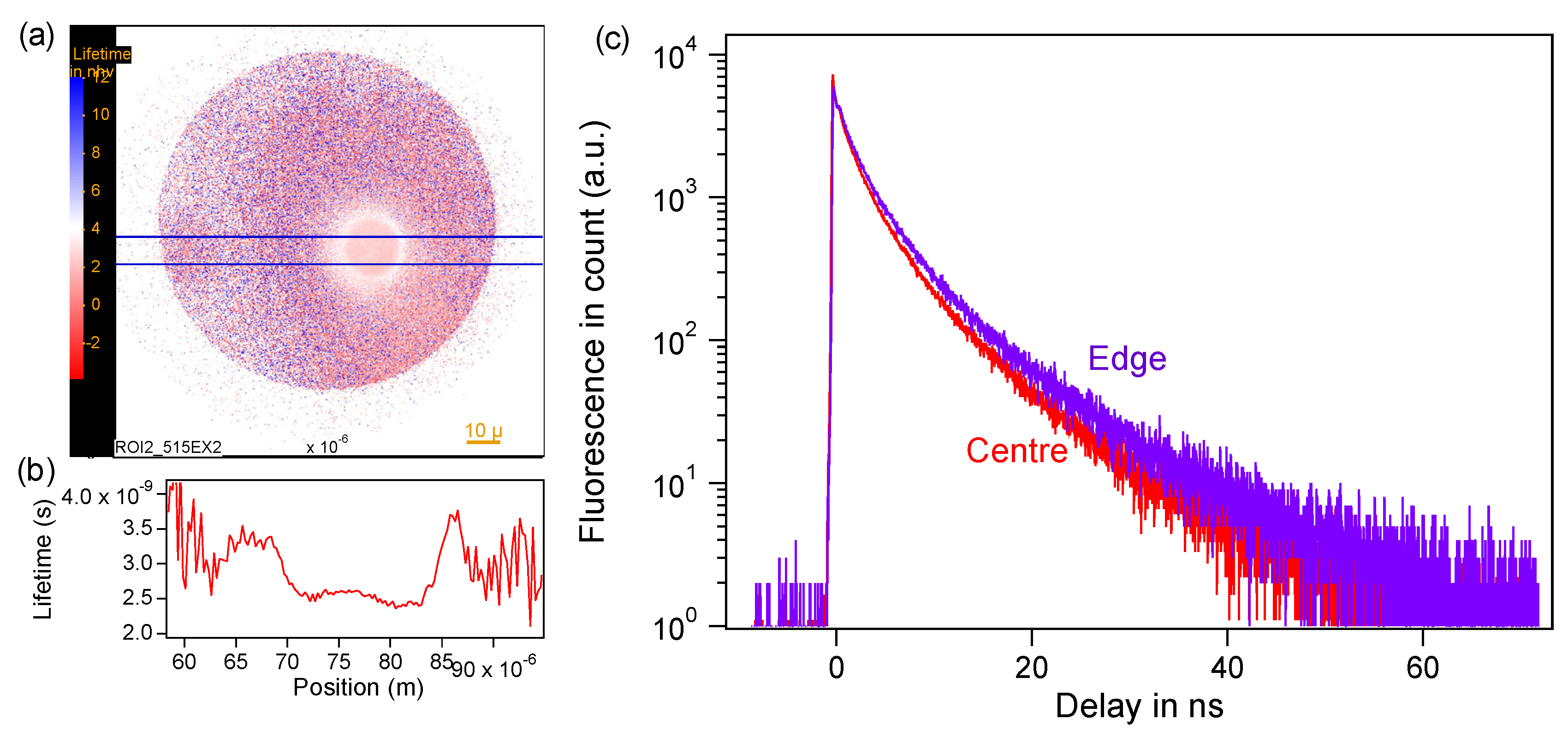
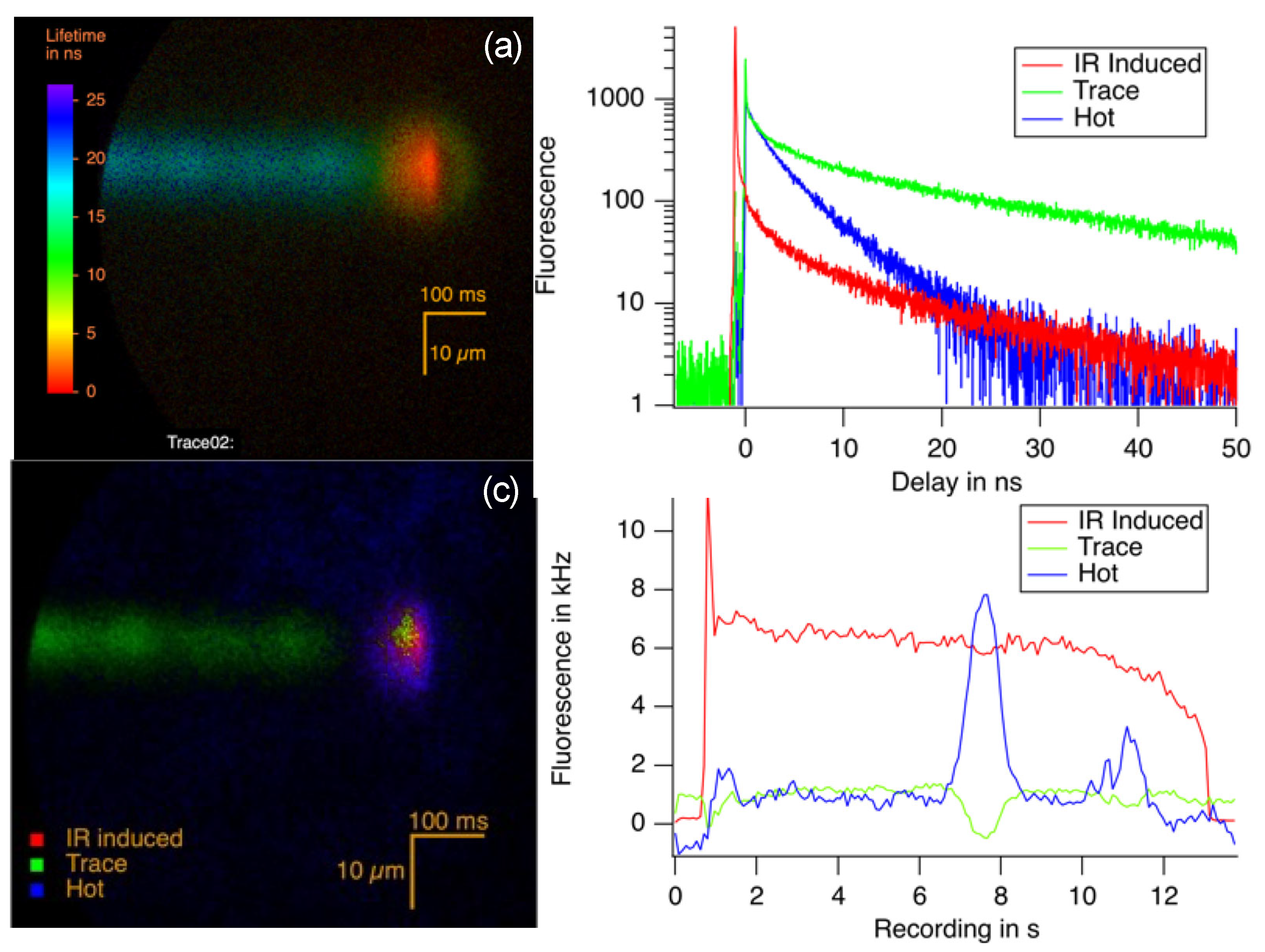
3.3. The Identification of Luminescent Region Containing Carbon Dots
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Appendix 1

Appendix 2
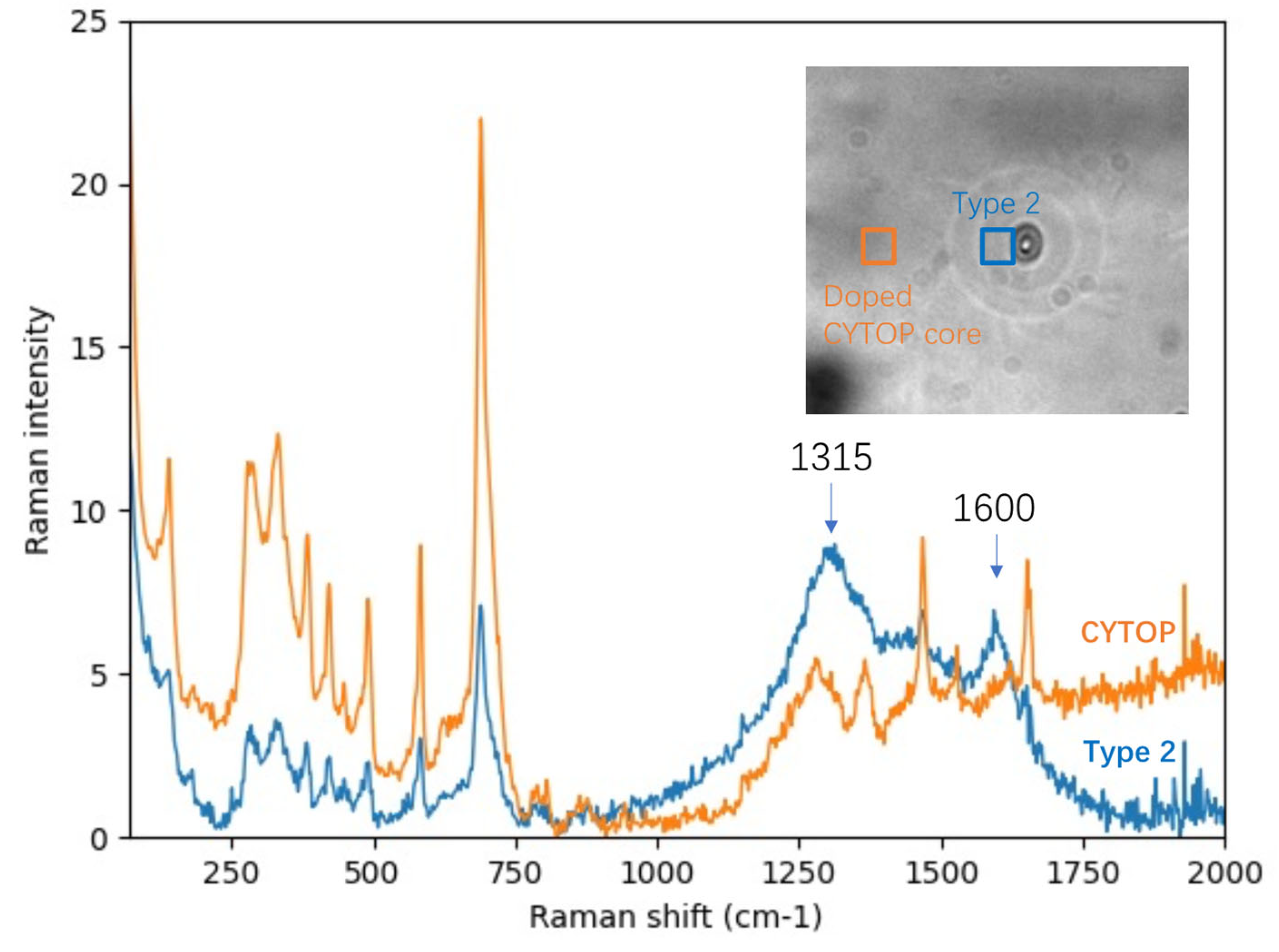
Composition Analysis of the CYTOP Fiber and Carbon Dots from the Raman Spectra

Appendix 3 (Information Extracted from [3])
References
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective. Nano Research 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. Journal of the American Chemical Society 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Riggs, J.E.; Guo, Z.; Carroll, D.L.; Sun, Y.-P. Strong luminescence of solubilized carbon nanotubes. Journal of the American Chemical Society 2000, 122, 5879–5880. [Google Scholar] [CrossRef]
- Kargozar, S.; Hoseini, S.J.; Milan, P.B.; Hooshmand, S.; Kim, H.W.; Mozafari, M. Quantum dots: a review from concept to clinic. Biotechnology Journal 2020, 15, 2000117. [Google Scholar] [CrossRef]
- Vargas-Nadal, G.; Kober, M.; Nsamela, A.; Terenziani, F.; Sissa, C.; Pescina, S.; Sonvico, F.; Gazzali, A.M.; Wahab, H.A.; Grisanti, L.; Olivera, M.E.; Palena, M.C.; Guzman, M.L.; Luciani-Giacobbe, L.C.; Jimenez-Kairuz, A.; Ventosa, N.; Ratera, I.; Belfield, K.D.; Maoz, B.M. Fluorescent Multifunctional Organic Nanoparticles for Drug Delivery and Bioimaging: A Tutorial Review. Pharmaceutics 14( 2022. [CrossRef]
- Geys, J.; Nemmar, A.; Verbeken, E.; Smolders, E.; Ratoi, M.; Hoylaerts, M.F.; Nemery, B.; Hoet, P.H. Acute toxicity and prothrombotic effects of quantum dots: impact of surface charge. Environ Health Perspect 2008, 116, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of Carbon and Graphene Quantum Dots for Sensing. ACS Sensors 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
- Wang, B.; Cai, H.; Waterhouse, G.I.N.; Qu, X.; Yang, B.; Lu, S. Carbon Dots in Bioimaging, Biosensing and Therapeutics: A Comprehensive Review. Small Science 2022, 2. [Google Scholar] [CrossRef]
- Bayda, S.; Amadio, E.; Cailotto, S.; Frion-Herrera, Y.; Perosa, A.; Rizzolio, F. Carbon dots for cancer nanomedicine: a bright future. Nanoscale Adv 2021, 3, 5183–5221. [Google Scholar] [CrossRef]
- Han, M.; Zhu, S.; Lu, S.; Song, Y.; Feng, T.; Tao, S.; Liu, J.; Yang, B. Recent progress on the photocatalysis of carbon dots: Classification, mechanism and applications. Nano Today 2018, 19, 201–218. [Google Scholar] [CrossRef]
- Zeng, Q.; Shao, D.; He, X.; Ren, Z.; Ji, W.; Shan, C.; Qu, S.; Li, J.; Chen, L.; Li, Q. Carbon dots as a trackable drug delivery carrier for localized cancer therapy in vivo. J Mater Chem B 2016, 4, 5119–5126. [Google Scholar] [CrossRef]
- Ding, H.; Yu, S.B.; Wei, J.S.; Xiong, H.M. Full-Color Light-Emitting Carbon Dots with a Surface-State-Controlled Luminescence Mechanism. ACS Nano 2016, 10, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Tao, S.; Yue, D.; Zeng, Q.; Chen, W.; Yang, B. Recent Advances in Energy Conversion Applications of Carbon Dots: From Optoelectronic Devices to Electrocatalysis. Small 2020, 16, e2001295. [Google Scholar] [CrossRef]
- Shang, W.; Cai, T.; Zhang, Y.; Liu, D.; Liu, S. Facile one pot pyrolysis synthesis of carbon quantum dots and graphene oxide nanomaterials: All carbon hybrids as eco-environmental lubricants for low friction and remarkable wear-resistance. Tribology International 2018, 118, 373–380. [Google Scholar] [CrossRef]
- Chao-Mujica, F.J.; Garcia-Hernández, L.; Camacho-López, S.; Camacho-López, M.; Camacho-López, M.A.; Contreras, D.R.; Pérez-Rodríguez, A.; Peña-Caravaca, J.P.; Páez-Rodríguez, A.; Darias-Gonzalez, J.G.; Hernandez-Tabares, L.; de Fuentes, O.A.; Prokhorov, E.; Torres-Figueredo, N.; Reguera, E.; Desdin-García, L.F. Carbon quantum dots by submerged arc discharge in water: Synthesis, characterization, and mechanism of formation. Journal of Applied Physics 2021, 129. [Google Scholar] [CrossRef]
- Liu, X.; Pang, J.; Xu, F.; Zhang, X. Simple Approach to Synthesize Amino-Functionalized Carbon Dots by Carbonization of Chitosan. Sci Rep 2016, 6, 31100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chao, D.; Yu, C.; Zhu, Q.; Zhou, S.; Tian, L.; Zhou, L. One-Step Green Solvothermal Synthesis of Full-Color Carbon Quantum Dots Based on a Doping Strategy. The Journal of Physical Chemistry Letters 2021, 12, 8939–8946. [Google Scholar] [CrossRef]
- Hasan, M.R.; Saha, N.; Quaid, T.; Reza, M.T. Formation of Carbon Quantum Dots via Hydrothermal Carbonization: Investigate the Effect of Precursors. Energies 2021, 14. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, W. Hydrothermal synthesis of highly fluorescent nitrogen-doped carbon quantum dots with good biocompatibility and the application for sensing ellagic acid. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2020, 240, 118580. [Google Scholar] [CrossRef]
- Xie, Y.; Zheng, J.; Wang, Y.; Wang, J.; Yang, Y.; Liu, X.; Chen, Y. One-step hydrothermal synthesis of fluorescence carbon quantum dots with high product yield and quantum yield. Nanotechnology 2019, 30, 085406. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, X.; Jing, N.; Zhang, Y. Hydrothermal synthesis of carbon quantum dots as fluorescent probes for the sensitive and rapid detection of picric acid. Analytical Methods 2018, 10, 2775–2784. [Google Scholar] [CrossRef]
- Liu, Y.; Li, W.; Wu, P.; Ma, C.; Wu, X.; Xu, M.; Luo, S.; Xu, Z.; Liu, S. Hydrothermal synthesis of nitrogen and boron co-doped carbon quantum dots for application in acetone and dopamine sensors and multicolor cellular imaging. Sensors and Actuators B: Chemical 2019, 281, 34–43. [Google Scholar] [CrossRef]
- de Yro, P.A.N.; Quaichon, G.M.O.; Cruz, R.A.T.; Emolaga, C.S.; Que, M.C.O.; Magdaluyo, E.R., Jr.; Basilia, B.A. Hydrothermal synthesis of carbon quantum dots from biowaste for bio-imaging. AIP Conference Proceedings 2019, 2083. [Google Scholar]
- Atchudan, R.; Edison, T.N.J.I.; Shanmugam, M.; Perumal, S.; Somanathan, T.; Lee, Y.R. Sustainable synthesis of carbon quantum dots from banana peel waste using hydrothermal process for in vivo bioimaging. Physica E: Low-dimensional Systems and Nanostructures 2021, 126, 114417. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, I.; Gathania, A.K. Synthesis, characterization and potential sensing application of carbon dots synthesized via the hydrothermal treatment of cow milk. Scientific Reports 2022, 12, 22495. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhu, Z.; Chen, M.; Chen, W.; Zhou, X. Microwave-assisted synthesis of xylan-derived carbon quantum dots for tetracycline sensing. Optical Materials 2018, 85, 329–336. [Google Scholar] [CrossRef]
- Das, S.K.; Gawas, R.; Chakrabarty, S.; Harini, G.; Patidar, R.; Jasuja, K. An Unexpected Transformation of Organic Solvents into 2D Fluorescent Quantum Dots during Ultrasonication-Assisted Liquid-Phase Exfoliation. The Journal of Physical Chemistry C 2019, 123, 25412–25421. [Google Scholar] [CrossRef]
- Jiang, G.; Jiang, T.; Zhou, H.; Yao, J.; Kong, X. Preparation of N-doped carbon quantum dots for highly sensitive detection of dopamine by an electrochemical method. RSC Advances 2015, 5, 9064–9068. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, Y. A simple and green synthesis of carbon quantum dots from coke for white light-emitting devices. RSC Advances 2019, 9, 33789–33793. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Zhang, R.; Zhang, G.; Liu, X.; Qu, F.; Lu, L. Embedding carbon dots and gold nanoclusters in metal-organic frameworks for ratiometric fluorescence detection of Cu(2). Anal Bioanal Chem 2020, 412, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, H.M.; Duarte, A.J.; Davis, F.; Higson, S.P.; da Silva, J.C.E. Layer-by-layer immobilization of carbon dots fluorescent nanomaterials on single optical fiber. Analytica chimica acta 2012, 735, 90–95. [Google Scholar] [CrossRef]
- Shimotsuma, Y.; Kazansky, P.G.; Qiu, J.; Hirao, K. Self-Organized Nanogratings in Glass Irradiated by Ultrashort Light Pulses. Physical Review Letters 2003, 91, 247405. [Google Scholar] [CrossRef]
- Xie, Q.; Cavillon, M.; Pugliese, D.; Janner, D.; Poumellec, B.; Lancry, M. On the Formation of Nanogratings in Commercial Oxide Glasses by Femtosecond Laser Direct Writing. Nanomaterials 2022, 12, 2986. [Google Scholar] [CrossRef]
- Cao, J.; Lancry, M.; Brisset, F.; Mazerolles, L.; Saint-Martin, R.; Poumellec, B. Femtosecond laser-induced crystallization in glasses: growth dynamics for orientable nanostructure and nanocrystallization. Crystal Growth & Design 2019, 19, 2189–2205. [Google Scholar]
- Deepak, K.L.N.; Kuladeep, R.; Venugopal Rao, S.; Narayana Rao, D. "Luminescent microstructures in bulk and thin films of PMMA, PDMS, PVA, and PS fabricated using femtosecond direct writing technique. Chemical Physics Letters 2011, 503, 57–60. [Google Scholar]
- Que, R.; Houel-Renault, L.; Temagoult, M.; Herrero, C.; Lancry, M.; Poumellec, B. Space-selective creation of photonics functions in a new organic material: Femtosecond laser direct writing in Zeonex glass of refractive index change and photoluminescence. Optical Materials 2022, 133, 112651. [Google Scholar]
- Que, R.; Houel-Renault, L.; Lancry, M.; Fontaine-Aupart, M.; Nait, T.; Poumellec, B. Space-selective Luminescence Creation in Organic Crystal by Femtosecond Laser Irradiation. in Integrated Photonics Research, Silicon and Nanophotonics, (Optica Publishing Group, 2020), ITu4A. 9.
- Astafiev, A.A.; Shakhov, A.M.; Osychenko, A.A.; Syrchina, M.S.; Karmenyan, A.V.; Tochilo, U.A.; Nadtochenko, V.A. Probing intracellular dynamics using fluorescent carbon dots produced by femtosecond laser in situ. ACS omega 2020, 5, 12527–12538. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Suzuki, K.; Sugie, A.; Yoshida, H.; Yoshida, M.; Suzuki, Y. Effect of end group of amorphous perfluoro-polymer electrets on electron trapping. Science and Technology of advanced MaTerialS 2018, 19, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Yeniay, A.; Gao, R.; Takayama, K.; Gao, R.; Garito, A.F. Ultra-low-loss polymer waveguides. Journal of lightwave technology 2004, 22, 154–158. [Google Scholar] [CrossRef]
- Naritomi, M.; Murofushi, H.; Nakashima, N. Dopants for a Perfluorinated Graded Index Polymer Optical Fiber. Bulletin of the Chemical Society of Japan 2004, 77, 2121–2127. [Google Scholar] [CrossRef]
- Available online: https://www.agc-chemicals.com/file.jsp?id=jp/en/fluorine/products/cytop/download/pdf/CYTOP_EN_Brochure.pdf.
- van den Boom, H.P.A.; Li, W.; van Bennekom, P.K.; Monroy, I.T.; Giok-Djan, K. High-capacity transmission over polymer optical fiber. IEEE Journal of Selected Topics in Quantum Electronics 2001, 7, 461–470. [Google Scholar] [CrossRef]
- Association, G.P. , Physical properties of glycerine and its solutions (Glycerine Producers' Association, 1963).
- Spitz, J.A.; Yasukuni, R.; Sandeau, N.; Takano, M.; Vachon, J.J.; Méallet-Renault, R.; Pansu, R.B. Scanning-less wide-field single-photon counting device for fluorescence intensity, lifetime and time-resolved anisotropy imaging microscopy. Journal of microscopy 2008, 229, 104–114. [Google Scholar] [CrossRef]
- Kallepalli, D.L.N.; Godfrey, A.T.K.; Walia, J.; Variola, F.; Staudte, A.; Zhang, C.; Jakubek, Z.J.; Corkum, P.B. Multiphoton laser-induced confined chemical changes in polymer films. Opt Express 2020, 28, 11267–11279. [Google Scholar] [CrossRef]
- Hayashi, S.; Tsunemitsu, K.; Terakawa, M. Laser Direct Writing of Graphene Quantum Dots inside a Transparent Polymer. Nano Lett 2022, 22, 775–782. [Google Scholar] [CrossRef]
- Miller, F.A.; Harney, B.M. The infrared and Raman spectra of perfluorocyclohexane. Spectrochimica Acta Part A: Molecular Spectroscopy 1972, 28, 1059–1066. [Google Scholar] [CrossRef]
- Childres, I.; Jauregui, L.A.; Park, W.; Cao, H.; Chen, Y.P. Raman spectroscopy of graphene and related materials. New developments in photon and materials research 2013, 1, 1–20. [Google Scholar]
- Sfyris, D.; Sfyris, G.; Galiotis, C. Stress intrepretation of graphene E-2g and A-1g vibrational modes: theoretical analysis. arXiv preprint arXiv:1706.04465, 2017.
- Childres, I.; Jauregui, L.A.; Tian, J.; Chen, Y.P. Effect of oxygen plasma etching on graphene studied using Raman spectroscopy and electronic transport measurements. New Journal of Physics 2011, 13. [Google Scholar] [CrossRef]
- Nair, R.R.; Ren, W.; Jalil, R.; Riaz, I.; Kravets, V.G.; Britnell, L.; Blake, P.; Schedin, F.; Mayorov, A.S.; Yuan, S. Fluorographene: a two-dimensional counterpart of Teflon. small 2010, 6, 2877–2884. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, C.B.; Brodeur, A.; Mazur, E. Laser-induced breakdown and damage in bulk transparent materials induced by tightly focused femtosecond laser pulses. Measurement Science and Technology 2001, 12, 1784. [Google Scholar] [CrossRef]
- Beresna, M.; Gecevičius, M.; Bulgakova, N.M.; Kazansky, P.G. Twisting light with micro-spheres produced by ultrashort light pulses. Opt. Express 2011, 19, 18989–18996. [Google Scholar] [CrossRef]
- Cvecek, K.; Miyamoto, I.; Schmidt, M. Gas bubble formation in fused silica generated by ultra-short laser pulses. Opt. Express 2014, 22, 15877–15893. [Google Scholar] [CrossRef] [PubMed]
- Glezer, E.N.; Mazur, E. Ultrafast-laser driven micro-explosions in transparent materials. Applied Physics Letters 1997, 71, 882–884. [Google Scholar] [CrossRef]
- Bellouard, Y.; Hongler, M.-O. Femtosecond-laser generation of self-organized bubble patterns in fused silica. Opt. Express 2011, 19, 6807–6821. [Google Scholar] [CrossRef] [PubMed]
- Juodkazis, S.; Nishimura, K.; Tanaka, S.; Misawa, H.; Gamaly, E.G.; Luther-Davies, B.; Hallo, L.; Nicolai, P.; Tikhonchuk, V.T. Laser-induced microexplosion confined in the bulk of a sapphire crystal: evidence of multimegabar pressures. Physical review letters 2006, 96, 166101. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.crystran.co.uk/optical-materials/silica-glass-sio2.
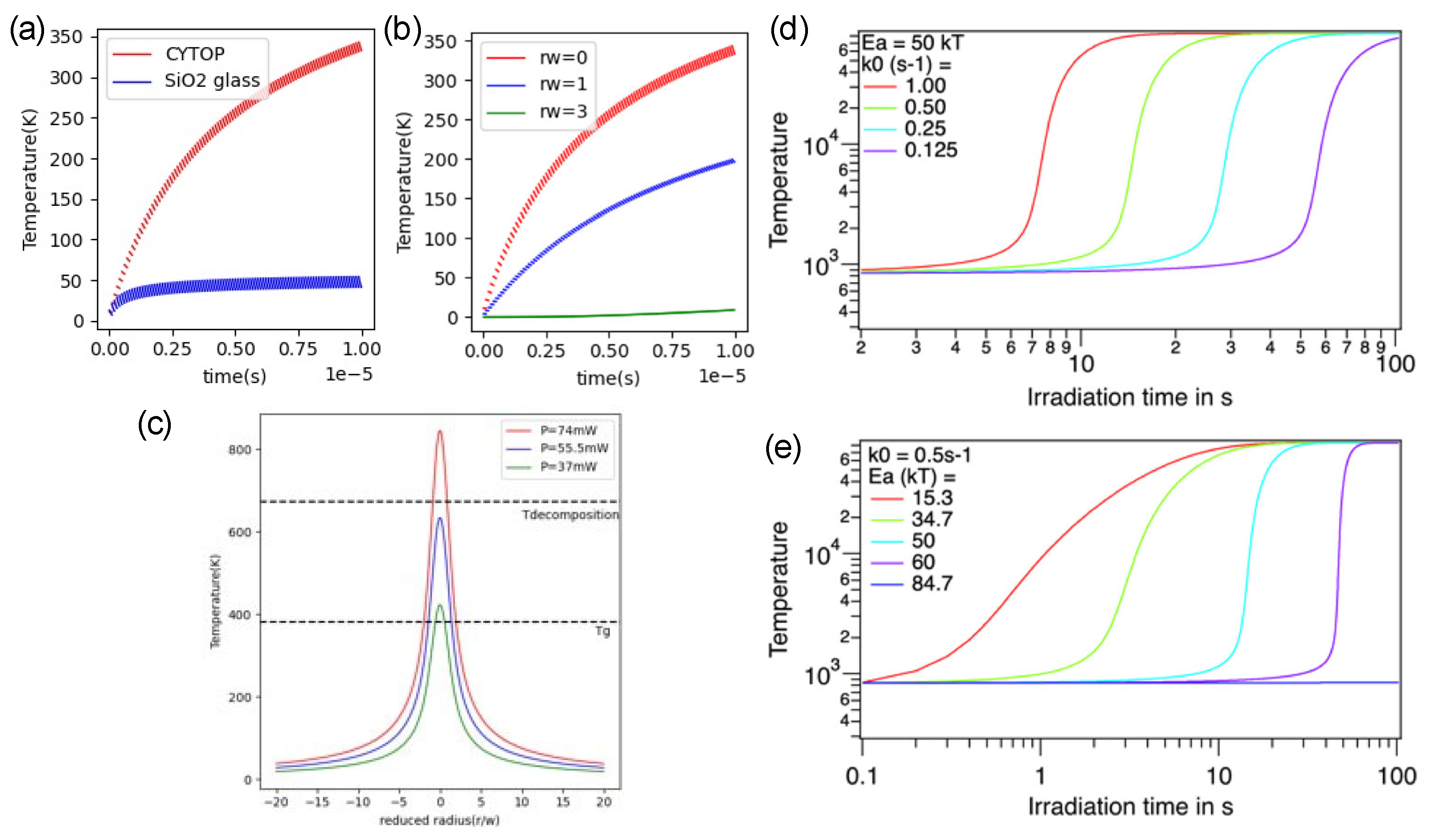
- Que, R.; Lancry, M.; Poumellec, B. Usable Analytical Expressions for Temperature Distribution Induced by Ultrafast Laser Pulses in Dielectric Solids. Micromachines 2024, 15. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Gandla, D.; Venkatesh, Y.; Bangal, P.R.; Ghosh, S.; Yang, Y.; Misra, S. Graphene quantum dots from graphite by liquid exfoliation showing excitation-independent emission, fluorescence upconversion and delayed fluorescence. Phys Chem Chem Phys 2016, 18, 21278–21287. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lian, Z.; Si, C.; Li, B. Revealing the role of nitrogen dopants in tuning the electronic and optical properties of graphene quantum dots via a TD-DFT study. Phys Chem Chem Phys 2020, 22, 28230–28237. [Google Scholar] [CrossRef] [PubMed]
- Müllen, K. Evolution of Graphene Molecules: Structural and Functional Complexity as Driving Forces behind Nanoscience. ACS Nano 2014, 8, 6531–6541. [Google Scholar] [CrossRef]
- Patterson, J.W. The ultraviolet absorption spectra of coronene. Journal of the American Chemical Society 1942, 64, 1485–1486. [Google Scholar] [CrossRef]
- Clar, E.; Schmidt, W. Correlations between photoelectron and ultraviolet absorption spectra of polycyclic hydrocarbons: the terrylene and peropyrene series. Tetrahedron 1978, 34, 3219–3224. [Google Scholar]
- Ma, J.; Bian, L.; Zhao, L.; Feng, X.; Zhao, L.; Wang, Z.; Pu, Q. Dialysed caramel as an effective fluorophore for the simultaneous detection of three nitrophenols. Talanta 2019, 197, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.A.; Harney, B.M. The infrared and Raman spectra of perfluorocyclohexane. Spectrochimica Acta Part A: Molecular Spectroscopy 1972, 28, 1059–1066. [Google Scholar] [CrossRef]
- Sharts, C.M.; Gorelik, V.S.; Agoltsov, A.; Zlobina, L.I.; Sharts, O.N. Detection of carbon-fluorine bonds in organofluorine compounds by Raman spectroscopy using a copper-vapor laser. in Electro-Optic, Integrated Optic, and Electronic Technologies for Online Chemical Process Monitoring, (SPIE, 1999), 317-326.
- Saito, R.; Hofmann, M.; Dresselhaus, G.; Jorio, A.; Dresselhaus, M.S. Raman spectroscopy of graphene and carbon nanotubes. Advances in Physics 2011, 60, 413–550. [Google Scholar] [CrossRef]
- Que, R.; Lancry, M.; Poumellec, B. Usable Analytical Expressions for Temperature Distribution Induced by Ultrafast Laser Pulses in Dielectric Solids. Micromachines 2024, 15. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




