Introduction
Skin cancer is a common malignancy that affects millions of people worldwide. Traditional treatment methods, such as surgery or external beam radiation therapy, have been used for decades with varying success. [
1]
Surgical removal of the tumor is the preferred therapy option from a clinical point of view, but for several treatment areas not the patients choice of treatment, especially when very visible areas e.g. in the face are affected.
Additionally to surgical removal, radiation therapy options are available using linear accelerators, low- or higher energy gamma rays, or brachytherapy applicators using beta- or gamma emitting radio-isotopes. [
2]
Over 5 million cases of basal cell carcinoma and squamous cell carcinoma are diagnosed annually in the United States alone among 3.3 million people due to the fact that some patients are diagnosed with more than 1 cancer location. In Germany over 200.000 cases are registered.
Extrapolated that would indicate that there are likely 10 million cases annually and it has also been observed that the number of non-melanoma skin cancers has been growing for several years. [
3,
4]
Current treatment options of non-melanoma skin cancers include surgical removal (preferred option) and several different applications of radiation therapy.
There are reasons why the surgery option is not preferred or recommended, especially in very visible areas like the face including nose and ears.
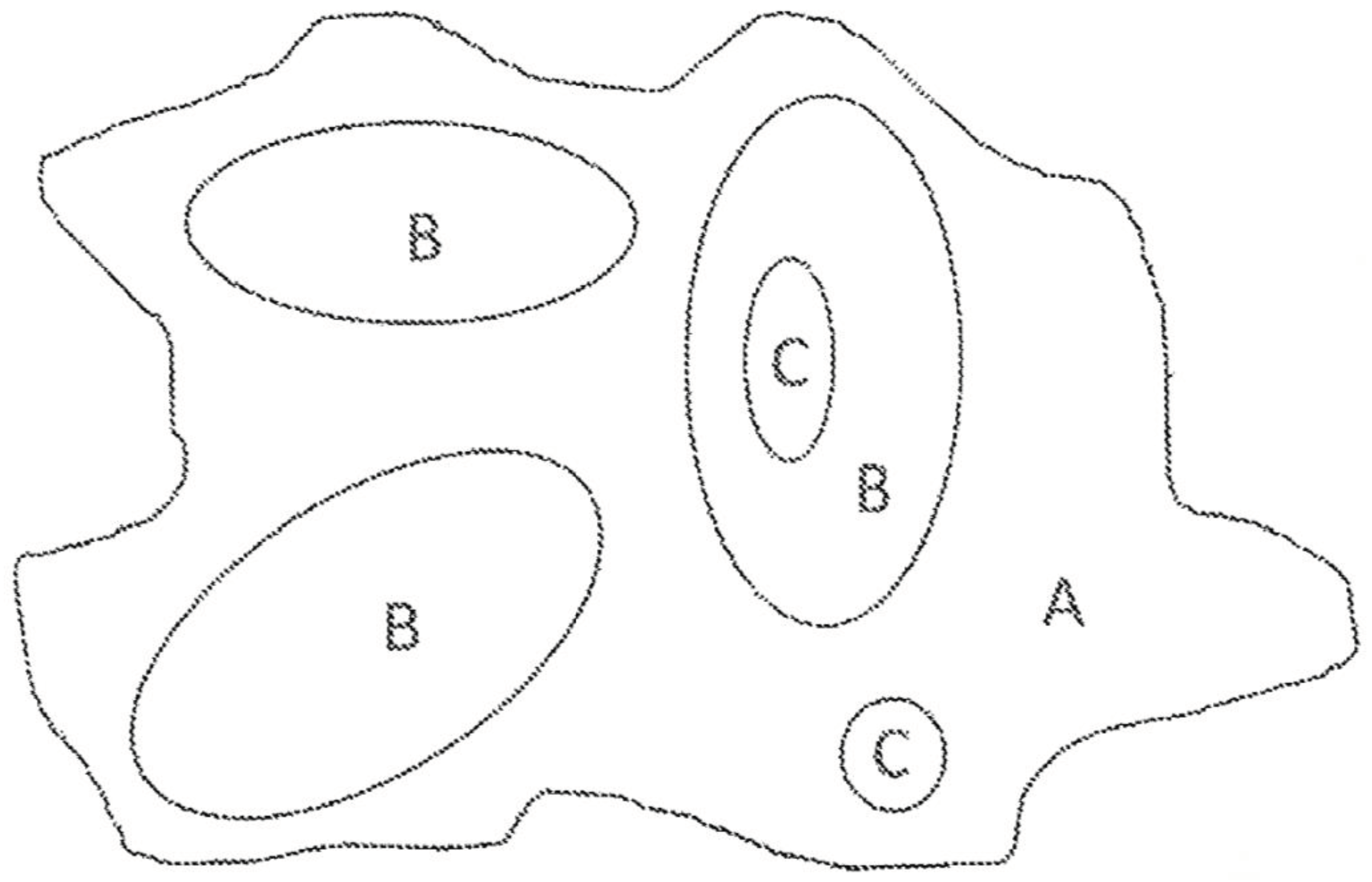
All currently available options based on cell-killing radiation are very complex and expensive. A viable alternative is skin brachytherapy, a form of radiation therapy, where a sealed radiation source is placed inside or next to the area requiring treatment, but they come with several logistics and treatment efficacy disadvantages. They are also expensive, complicated to use and handle from a clinical perspective, and are difficult to personalise, especially when there are different lesion areas with different tumor depth (see
Figure 1).
We are therefore proposing to create - additively manufactured - patches that could be individually printed using the surface area and the tumor depth obtained from diagnostic imaging modalities like Ultrasound, Magnetic Resonance Imaging, or other diagnostic imaging methods.
Materials and Methods
The penetration depth of beta-emitting isotopes is limited to around 10mm. A transverse dose profile radiation plan can be calculated to determine the amount of radiation needed at a specific surface location to ensure a cell-killing radiation boost delivery at the required max. depth location.
Patches to be applied to the skins surface containing several different emitters (Y-90, Re-188 or P-32 to just name a few) have been proposed in the past and several of those are also currently in clinical use. [
5,
6,
7,
8,
9]
Gamma and beta-emitting isotopes - liquid or semi-liquid as cream - can provide ionizing radiation that can penetrate the skin to varying depths. The patches are applied directly to the skin over the cancerous lesion, delivering a controlled dose of cell-killing radiation to the tumor, while minimizing the impact on surrounding healthy tissue and sensitive bone structures. These patches are surface matched to the tumor but often lead to under- or over-radiation as it is not possible to accurately create an individual patch that matches the tumor volume especially when there are several lesions in a close area / volume.
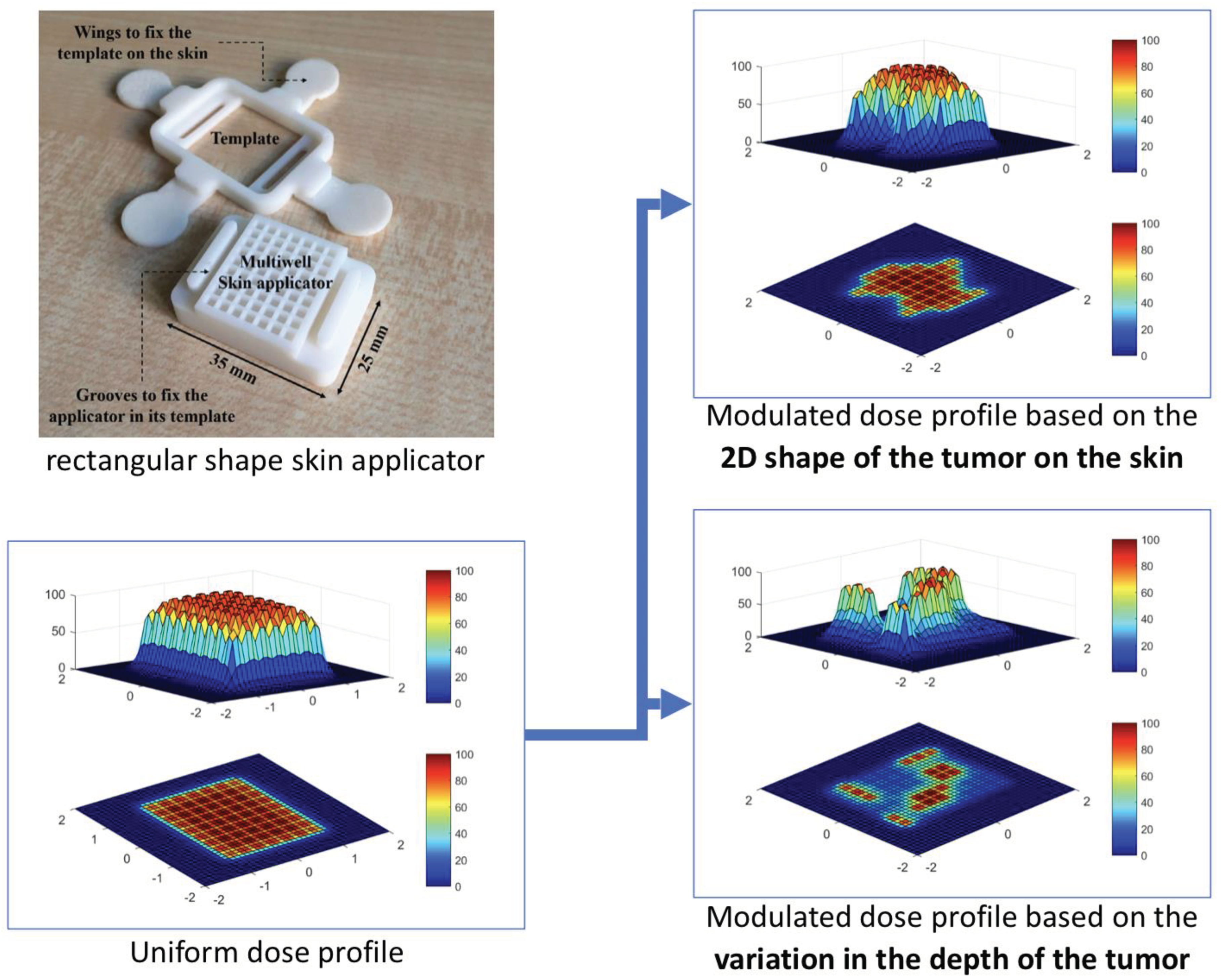
The tumour shape and depth determines the location, amount, activity level and configuration of the isotope to be applied to the surface. The required information for that are extracted from previously acquired diagnostic images and/or surface scans of the tumour site, which are then used to calculate a radiation plan based on the individual radiation energy and intensity of the used material (see
Figure 2 for details).
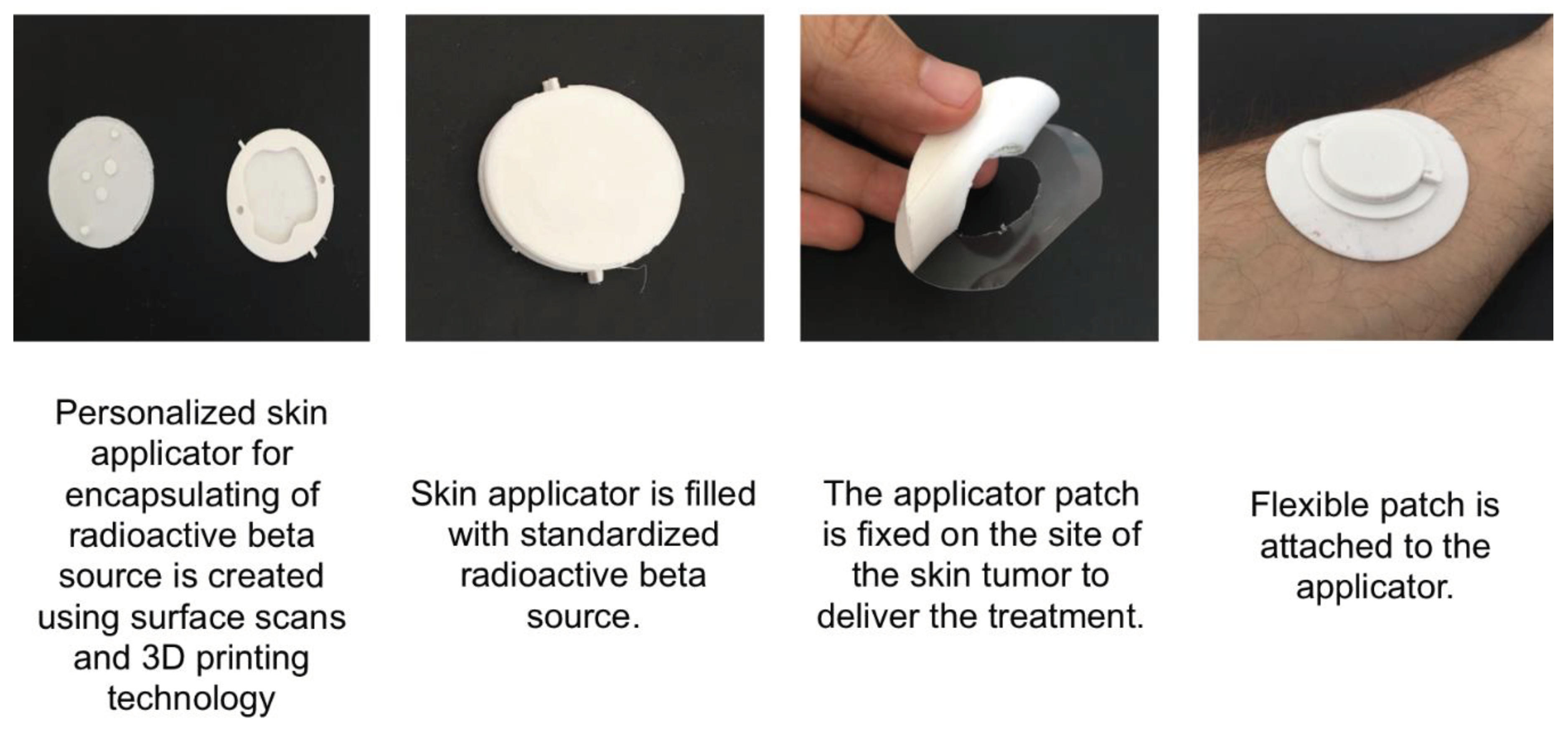
In a next step this radiation plan is translated into a 3D printing plan for a skin applicator that can be filled with the radioactive source material.
Figure 3 shows an individualised round applicator with the negative fill volume representing the tumour shape and volume printed based on a radiation plan and a subsequent conversion for the 3D printer.
If you provide these isotopes controlled you can achieve desired surface - depth profiles with the needed radiation exposure to guarantee cell killing in one application.
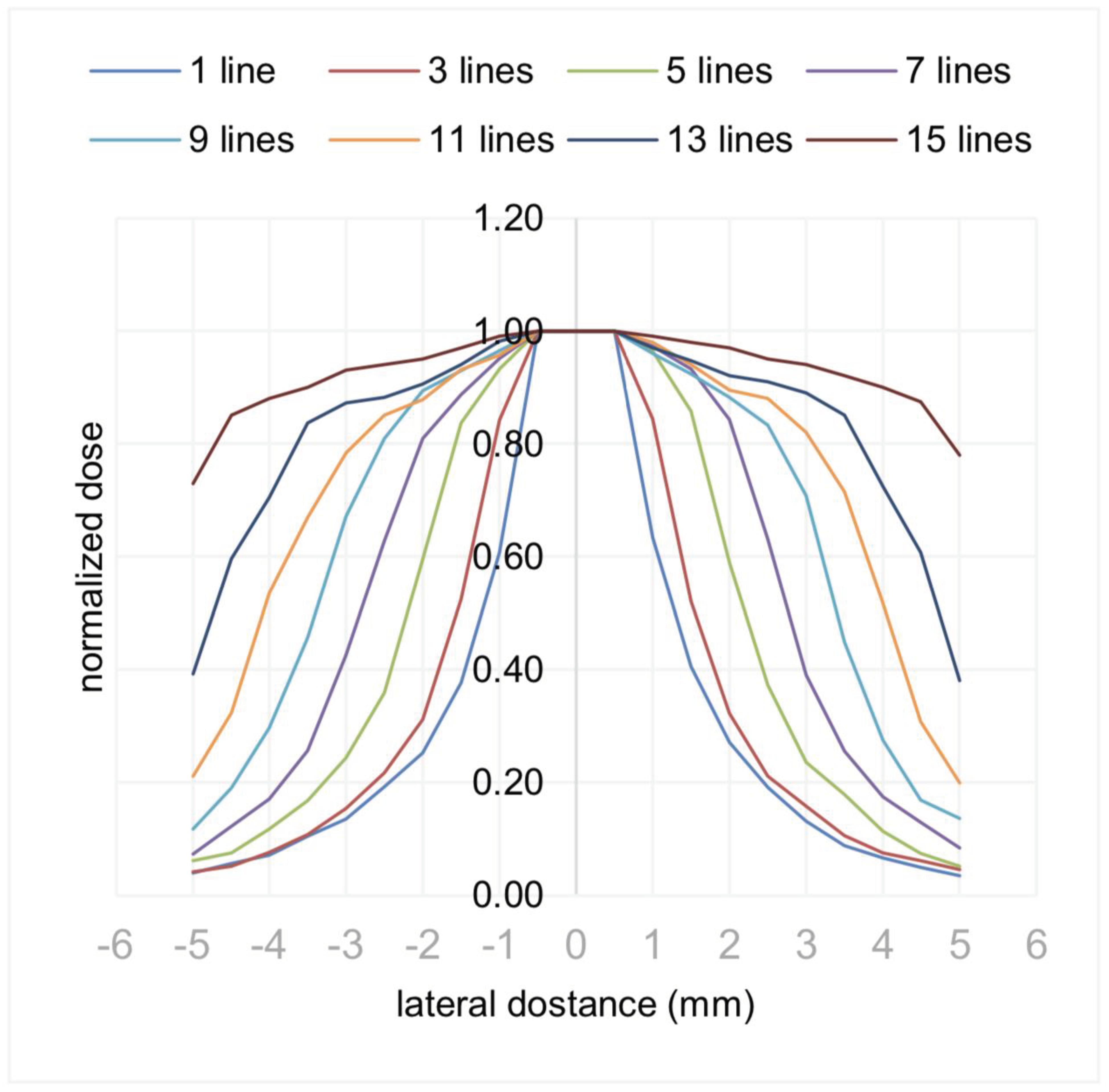
Figure 4. shows a Monte Carlo simulation result of using 0.7mm lines of 20mm length placed
next to each other on the surface and the resulting increase with more lines in the obtained normalised
dose. The simulation used a beta-emitting isotope Yttrium-90 (Y-90), with a half-life of about 64 hours
and a maximum beta particle emission energy of 2.28 MeV and an average energy of 0.93 MeV that
can penetrate up to approximately 11 mm in tissue.
Results
A planar source of Y- 90, consisting of 15 extruded lines (0.7 mm by 20 mm), printed side by side, can deliver a therapeutic dose of 40 Gy to a cuboid (10 mm by 20 mm by 3 mm) in about 6 mCi-h. Considering the specific activity of the Y-90 (5.38 × 10E9 mCi/gram), that would require about 1.1 nanograms of recently-produced Y-90 to treat this tumor in one hour. This simulation result shows that this allows to print a patch that would be able to match a tumor volume or several tumor volumes quite well.
The technology would allow to create very individual patches based on the almost every body surface and through the use of different filling materials could also achieve a very good match of cell-killing dose volume to tumor volume and with that prevent damage of sensitive adjacent structures.
Alternatively a dose profile can be obtained by printing with different activity levels of radioisotopes embedded in collagen / starch or other suitable materials.
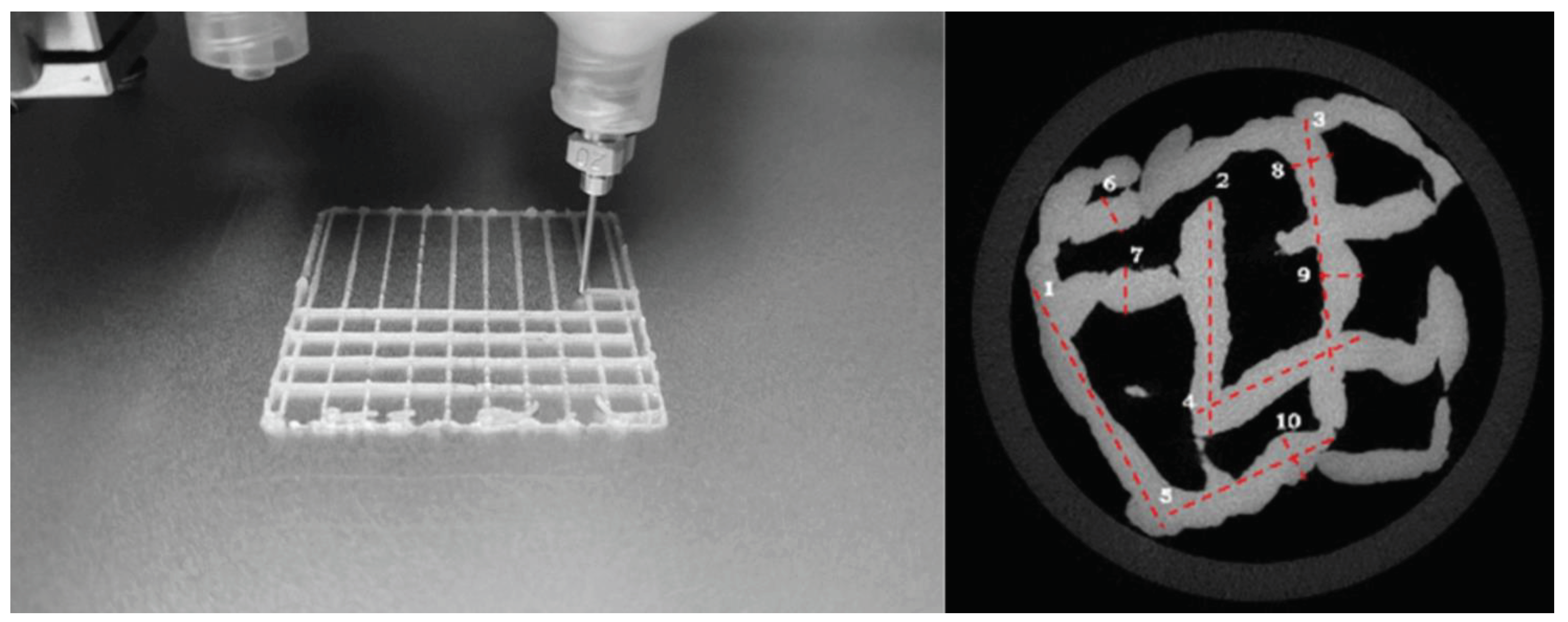
Figure 5 shows a scaffold structure and a micro-Computed Tomography (CT) image of a scaffold structure that contained magnesium (for creating radio-opaqueness) instead of Y-90 for example.
These kind of applicators can then also be used for intra-operative applications or for tumor-bed radiation after surgery and when combined with a radio-opaque material in a biocompatible structure even left in the patient for a longer period of time.
Discussion
We have utilised a radiation delivery simulation that was subsequently used with 3D printing technology and a non-radiating substitute to create patches for a directed, effective and controlled dose delivery to the skin tumors, thus mitigating exposure to sensitive structures.
This approach is relatively easy in its setup and use, inexpensive, has advantages with handling and delivery, and could provide a more personalised and effective therapy for several tumor types.
Different materials or a combination of the same material with different activity levels for creating the patch would open even more options.
Additionally, further research and clinical trials are needed to fully understand the efficacy and safety of this approach for superficial treatment of skin cancers.
Conclusions
This personalised (size, shape, radiation dose) and targeted approach can result in more effective treatment with fewer side effects compared to traditional radiation therapy methods, which may require larger fields of radiation and may be less precise in targeting the tumor.
This approach promises to reduce the total radiation exposure for the treatment of superficial tumors, while at the same time providing a more efficient therapy option.
The hardware and planning setup, that are the base of the invention, would provide high patient comfort combined with a very affordable solution.
The invention would allow to additively manufacture a patch to be filled with radio-isotopes that would provide a cell-killing dose volume/profile to closely match the tumour volume with greatly reduced harm of healthy tissue and underlying bone structures.
Alternatively individual lines or volumes of radio-isotope material could be printed for a skin surface application containing different activity levels and with that provide different dose depth profiles.
Even after surgery the proposed personalised patch could also potentially provide an easy solution for tumor bed radiation maybe even using biocompatible patches that are left in the human body for a longer period of time.
Supplementary Materials
There are no supplementary materials provided with this article.
Authorship contribution statement
Michael Friebe: Conceptualization, Methodology, Writing - original draft. Axel Boese: Methodology, Writing - review & editing Nathan Castro: Investigation, Writing - review & editing Dietmar Hutmacher: Conceptualisation, Writing - review & editing Ali Pashazadeh: Validation, Investigation, Writing - review & editing.
Ethics statement
The invention was used with humans and therefore no ethics approval required.
Acknowledgement
The initial research was supported by a grant of the Federal Ministry of Education and Research (BMBF) in the context of the 'INKA' project. Grant Number: 03IPT7100X . Prototyping and initial validation and investigation was conducted at the INKA Laboratories, Medical Faculty, Otto-von-Guericke-University, Magdeburg.
Declaration of Competing Interest
The authors are also the inventors and owners of the presented invention. All inventors have an existing relation to a research institution and are interested to continue additional research in this field. The presented invention is potentially licensable. The authors declare that they are not developing competing technology and that they have no current and known competing financial interest that could have appeared to influence the work reported in this paper.
Patent Information
| Subject Code |
2708 Dermatology, 2730 Oncology |
| Specific subject area |
Additive manufactured radiation patch for application to the skin |
| Industry Code |
A61N 5/10 radiation therapy, A61K 51/12 Preparations containing radioactive substances for use in therapy or testing in vivo |
| Dates of Invention |
early 2018 first ideas and experiments, german patent application filed 14. August 2018 (DE 10 2018 119745) |
| Patent Details |
US 11,116,994 granted Sept. 14, 2021 Patent Owners: see inventorsPatent attorney: Schneiders & Behrendt Partner, Rechts- und Patentanwälte, 44787 Bochum, DELink to patent: https://patentimages.storage.googleapis.com/31/0c/86/f80ffe619187b2/US11116994.pdf |
| Intended Use |
The „Personalized 3D printed patches“ invention is available for licensing and for commercialisation as well as for cooperative research activities |
| Funding / Sponsor Acknowledgement |
The initial research was supported by a grant of the Federal Ministry of Education and Research (BMBF) in the context of the 'INKA' project. Grant Number: 03IPT7100X - INKA Innovation laboratory, Otto-von-Guericke-University, Magdeburg, Germany |
| Related Research Articles |
A. Pashazedeh, et al., Feasibility of 3D printing for customized radiotherapeutic models to be used in superficial skin cancer therapy, Additive Manufacturing Meets Medicine 2019, Conference Paper, https://doi.org/10.18416/AMMM.2019.1909S03P20A. Pashazadeh, M. Friebe, Transverse dose profile simulation of extruded lines for a 3D printed models for superficial skin cancer therapy, Current Directions in Biomedical Engineering 2020;6(3): 20203143, https://doi.org/10.1515/cdbme-2020-3143 A. Pashazadeh, M. Robatjazi, N. Castro, M. Friebe, A multiwell applicator for conformal brachytherapy of superficial skin tumors: a simulation study, Skin Res. Technol., 2020; 26:537-541, https://doi.org/10.1111/srt.12826 |
References
- Pashazadeh, A.; Boese, A.; Friebe, M. Radiation therapy techniques in the treatment of skin cancer: an overview of the current status and outlook. J Dermatolog Treat 2019, 1–41. [Google Scholar] [CrossRef]
- US Patent 11,116,994 granted Sept. 14, 2021, available at (viewed 15.04.2023) https://patentimages.storage.googleapis.com/31/0c/86/f80ffe619187b2/US11116994.pdf.
- Skin Cancer Statistics (non-melanoma), cancer.net, viewed 15.04.2023, https://www.cancer.net/cancer-types/skin-cancer-non-melanoma/statistics#:~:text=It is estimated that 5.4,been growing for several years.
- Robert-Koch-Institut, Zentrum für Krebsregisterfragen, viewed 15. 23, https://www.krebsdaten.de/Krebs/EN/Content/Cancer_sites/Non-melanoma_skin_cancer/non-melanoma_skin_cancer_node.html. 20 April.
- Chung, Y.L.; Lee, J.D.; Bang, D.; Lee, J.B.; Park, K.B.; Lee, M.G. Treatment of Bowen's disease with a specially designed radioactive skin patch. Eur J Nucl Med 2000, 27, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Pandey, U.; Sarma, H.D.; Pillai, M.R.; Venkatesh, M. Preparation and evaluation of 90 Y skin patches for therapy of superficial tumours in mice. Nucl Med Commun 2002, 23, 243–247. [Google Scholar] [CrossRef]
- Koneru, B.; et al. Radiotherapeutic bandage for the treatment of squamous cell carcinoma of the skin. Nucl Med Biol 2016, 43, 333–338. [Google Scholar] [CrossRef] [PubMed]
- BShukla, J.; Mittal, B.R. 188 Re Tailor Made Skin Patch for the Treatment of Skin Cancers and Keloid: Overview and Technical Considerations. International Journal of Nuclear Medicine Research 2017, 107–113. [Google Scholar]
- Salgueiro, M.J.; et al. Design and bioevaluation of a 32P- patch for brachytherapy of skin diseases. Appl Radiat Isot 2008, 66, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Pashazadeh, A.; Friebe, M. Transverse dose profile simulation of extruded lines for a 3D printed models for superficial skin cancer therapy. Current Directions in Biomedical Engineering 2020, 6, 20203143. [Google Scholar] [CrossRef]
- Pashazadeh, A.; Landes, R.; Boese, A.; Kreissl, M.C.; Klopfleisch, M.; Friebe, M. Superficial skin cancer therapy with Y- 90 microspheres: A feasibility study on patch preparation. Skin Res Technol 2019. [Google Scholar] [CrossRef] [PubMed]
- Pashazedeh, A.; et al. Feasibility of 3D printing for customized radiotherapeutic models to be used in superficial skin cancer therapy, Additive Manufacturing Meets Medicine 2019, Conference Paper. 2019. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).