Submitted:
22 March 2024
Posted:
25 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Antibodies
2.2. Human Tissue and Cells
2.2.1. Bronchoscopy Sampling and Subject Selection
2.2.2. BAL Processing for Primary Alveolar Macrophages
2.2.3. THP-1 Macrophages
2.2.4. Monocyte-Derived Macrophages (MDM)
2.2.5. Human lung Tissue
2.3. Mouse Tissue and Alveolar Macrophages
2.4. Cell Models of Cigarette Smoke Exposure and Macrolide Treatment
2.5. Immunofluorescence Staining and Multi-Fluorescence Quantitative Confocal Microscopy (MQCM)
2.6. Colocalization Analysis
2.7. Flow-Cytometry
2.8. Western Blot Analysis
2.9. Histochemistry
2.10. Statistical Analysis
3. Results
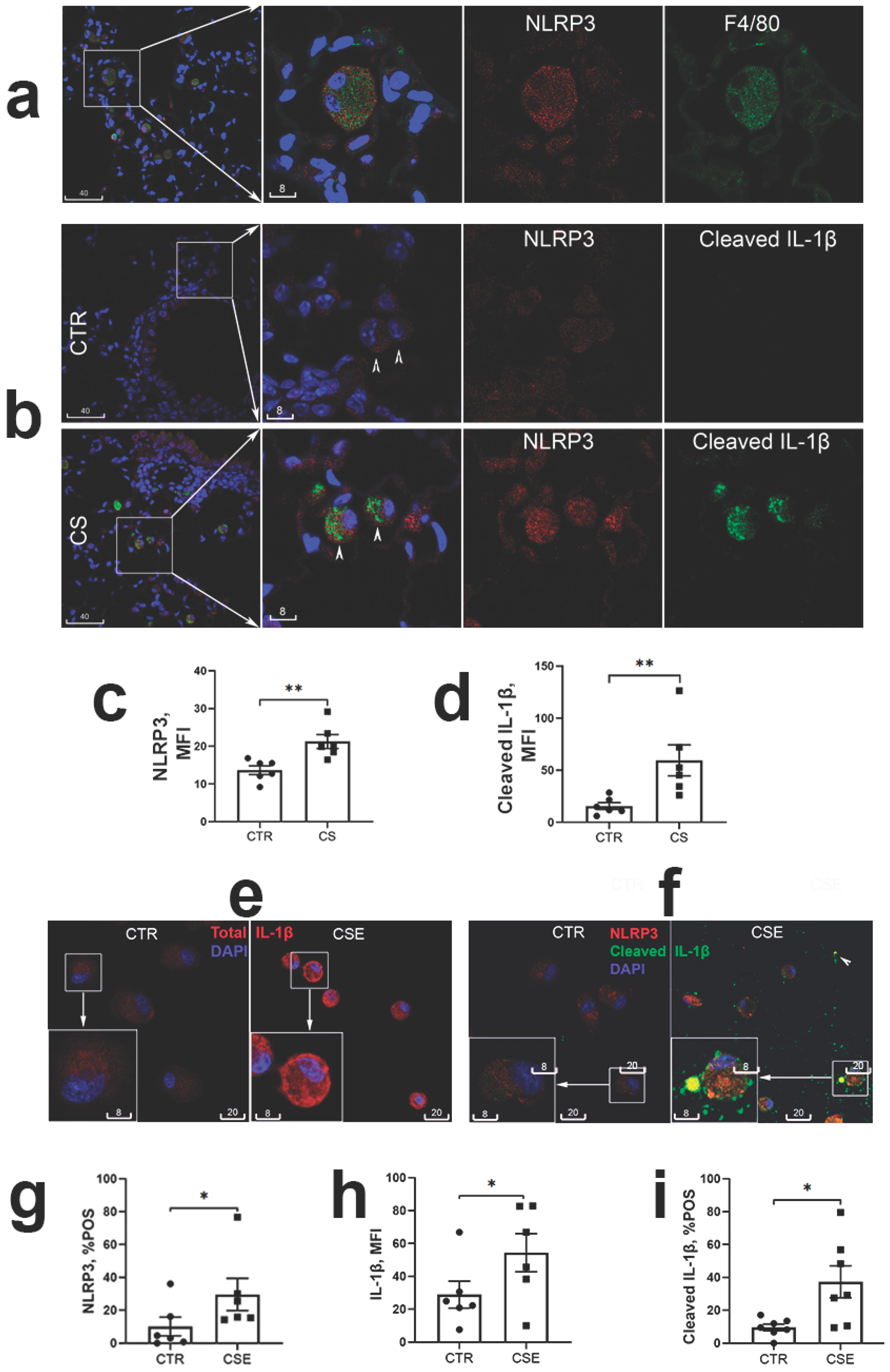
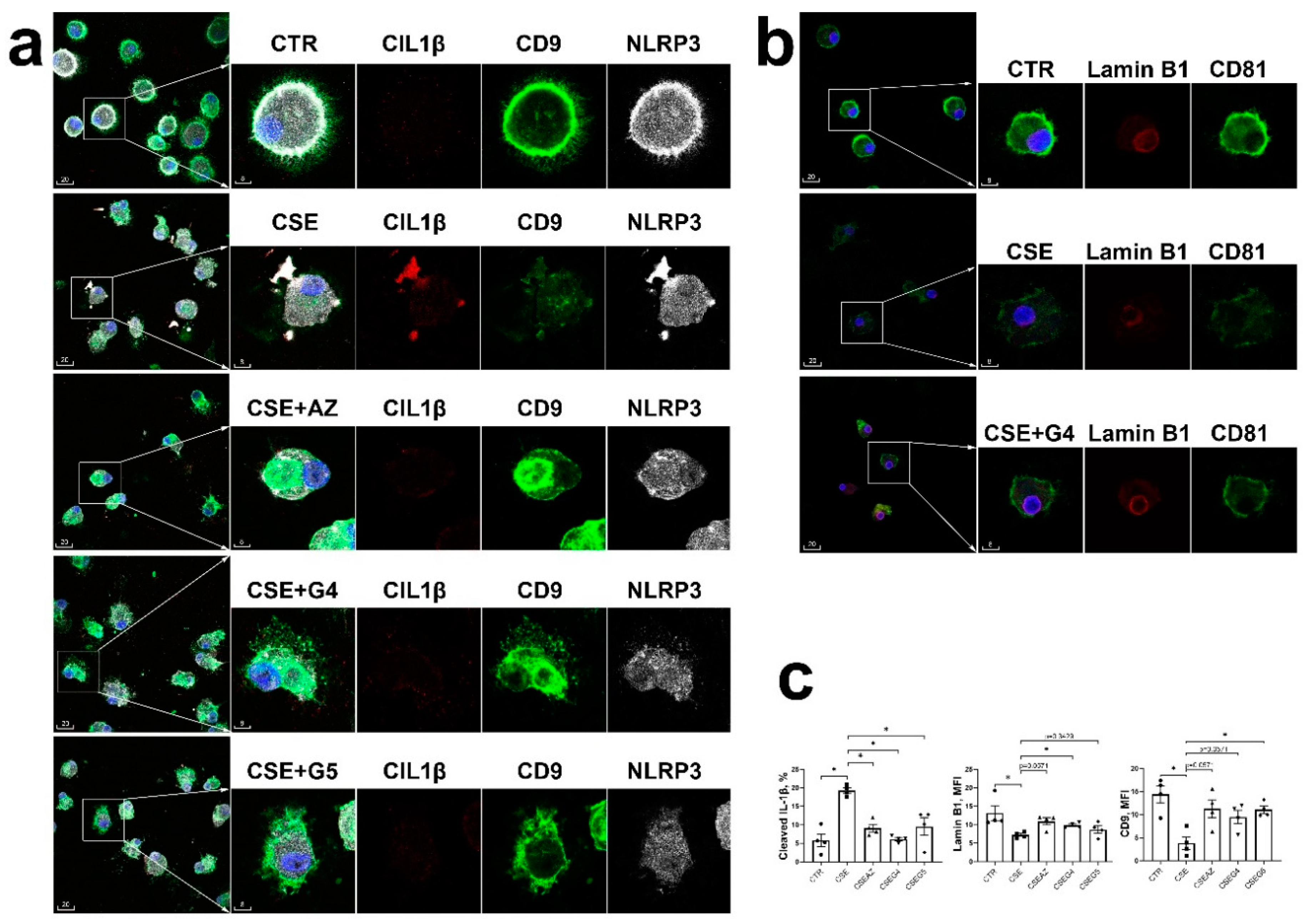
3.1. Cigarette Smoke Induced Macrophage NLRP3 Inflammasome Activation
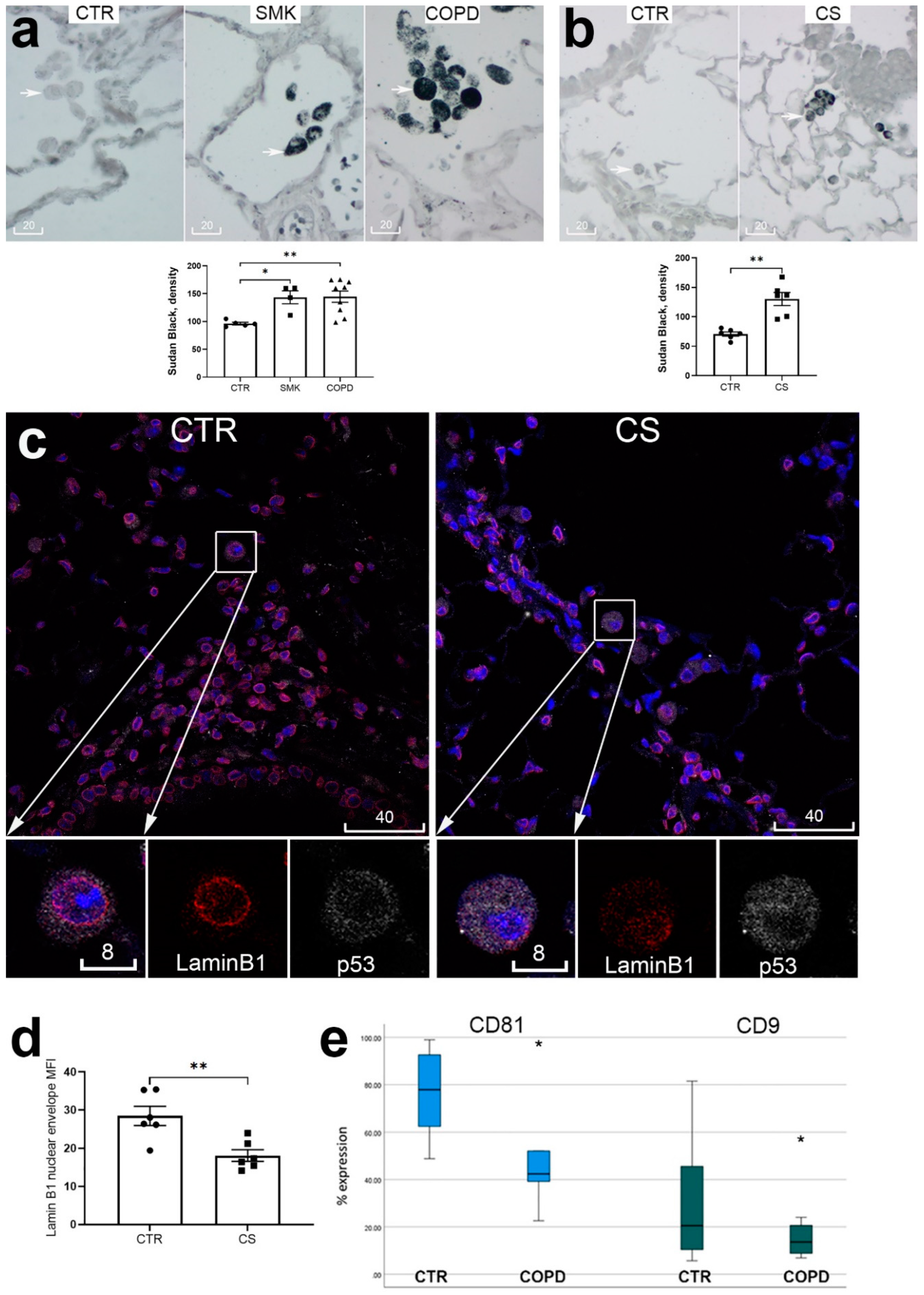
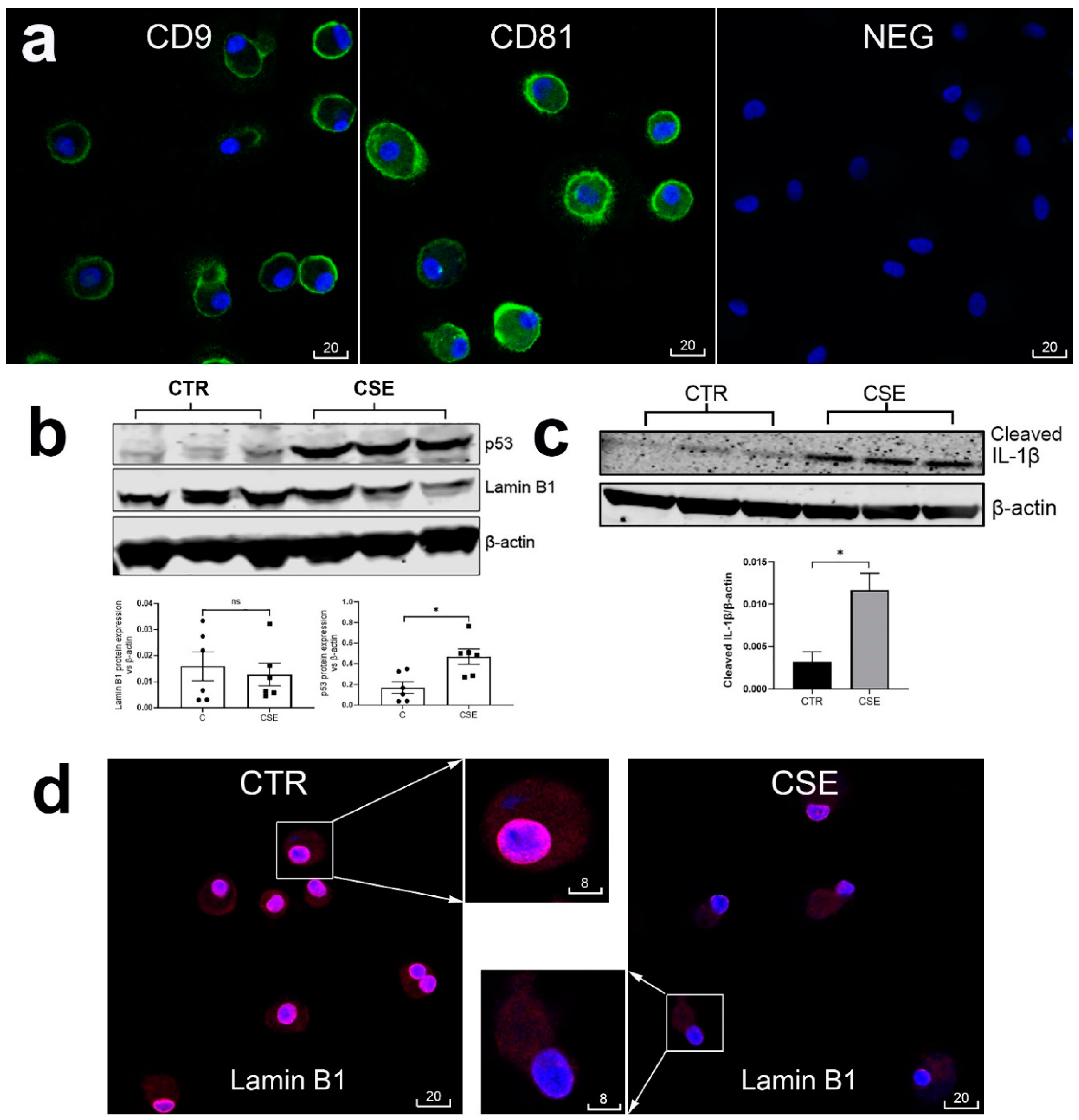
3.2. Accelerated Cellular Senescence of Alveolar Macrophages in COPD Patients and Mice Chronically Exposed to Cigarette Smoke
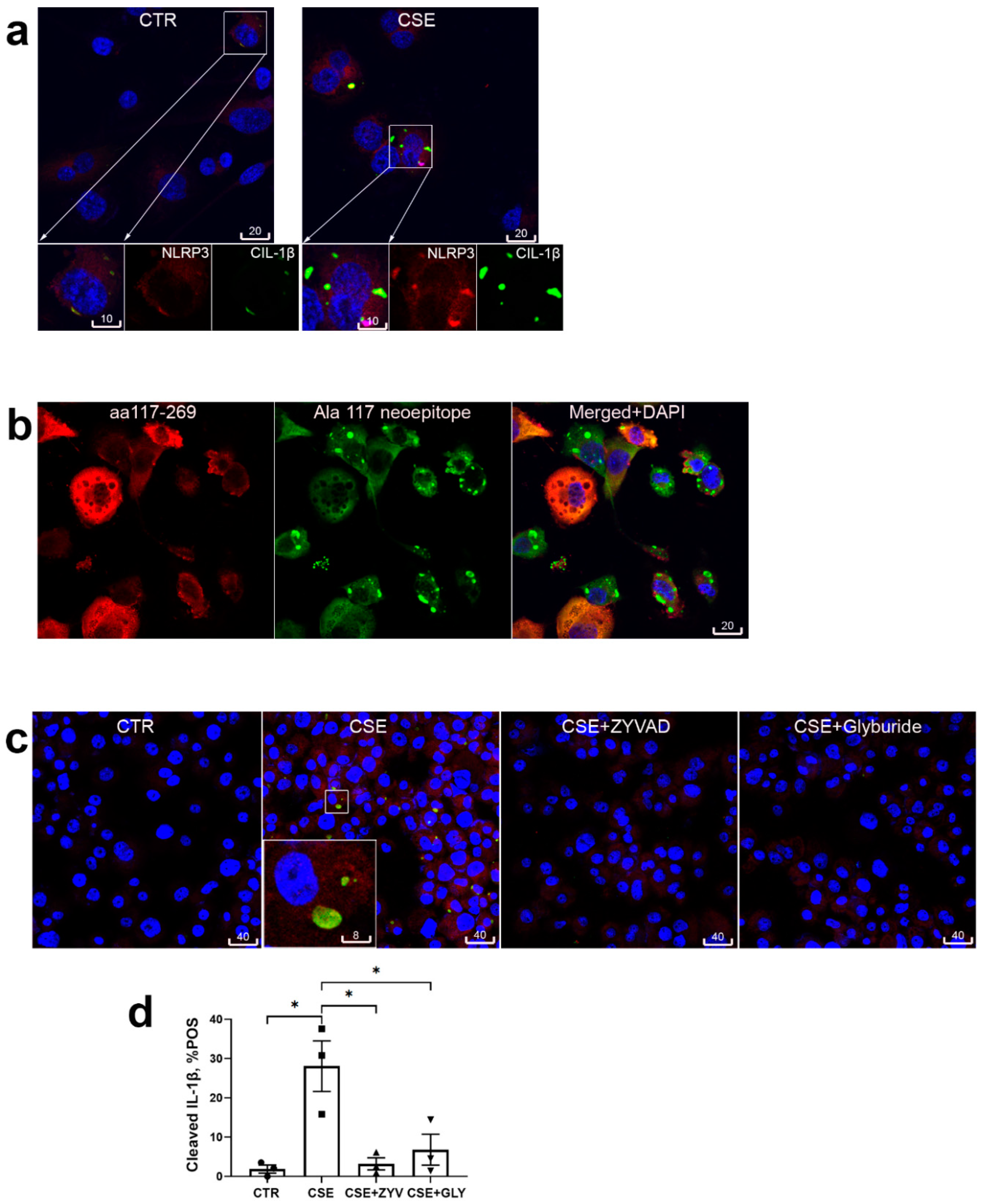
3.3. Short-Term Macrophage Cell Culture Model for Double Targeting NLRP3 Inflammasome Activation and Accelerated Cell Senescence
4. Discussion and Conclusion
Supplementary Materials
Acknowledgments
References
- Lindberg, A.; Lindberg, L.; Sawalha, S.; Nilsson, U.; Stridsman,C. ; Lundbäck, B.; Backman, H. Large underreporting of COPD as cause of death-results from a population-based cohort study. Respir. Med. 2021, 186. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Alveolar macrophages as orchestrators of COPD. COPD. 2004, 1, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Hodge, S.; Hodge, G.; Scicchitano, R.; Reynolds, P.N.; Holmes, M. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol. Cell Biol. 2003, 81, 289–96. [Google Scholar] [CrossRef] [PubMed]
- Hodge, S.; Hodge, G.; Ahern, J.; Jersmann, H.; Holmes, M.; Reynolds, P.N. Smoking alters alveolar macrophage recognition and phagocytic ability: implications in chronic obstructive pulmonary disease Am. J. Respir. Cell Mol. Biol. 2007, 37, 748–55. [Google Scholar] [CrossRef]
- Taylor, A.E.; Finney-Hayward, T.K.; Quint, J.K.; Thomas, C.M.; Tudhope, S.J.; Wedzicha, J.A.; Barnes, P.J.; Donnelly, L.E. Defective macrophage phagocytosis of bacteria in COPD. Eur. Respir. J. 2010, 35, 1039–47. [Google Scholar] [CrossRef] [PubMed]
- Asare, P.F.; Tran, H.B.; Hurtado, P.R.; Perkins, G.B; Nguyen, P.; Jersmann, H. Roscioli, E.; Hodge, S. Inhibition of LC3-associated phagocytosis in COPD and in response to cigarette smoke. Ther. Adv. Respir. Dis. 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Belchamber, K.B.R.; Singh, R.; Batista, C.M.; Whyte, M.K.; Dockrell, D.H.; Iain Kilty, I.; Robinson, M.J.; Wedzicha, J.A.; Barnes, P.J.; Donnelly, L.E. ; COPD-MAP consortium. Defective bacterial phagocytosis is associated with dysfunctional mitochondria in COPD macrophages. Eur. Respir. J, 1: 54, 1802. [Google Scholar]
- Barnawi, J.; Tran, H.; Jersmann, H.; Pitson, S.; Roscioli, E.; Hodge, G.; Meech, R.; Haberberger, R.; Hodge, S. Potential Link between the Sphingosine-1-Phosphate (S1P) System and Defective Alveolar Macrophage Phagocytic Function in Chronic Obstructive Pulmonary Disease (COPD). PLoS One, 0122. [Google Scholar] [CrossRef]
- Tran, H.B.; Barnawi, J.; Ween, M.; Hamon, R.; Roscioli, E.; Hodge, G.; Reynolds, P.N.; Pitson, S.M.; Davies, L.T.; Haberberger, R.; Hodge, S. Cigarette smoke inhibits efferocytosis via deregulation of sphingosine kinase signaling: reversal with exogenous S1P and the S1P analogue FTY720. J. Leukoc. Biol. 2016, 100, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Hamon, R.; Homan, C.C.; Tran, H.B.; Mukaro, V.R.; Lester, S.E.; Roscioli, E.; Bosco, M.D.; Murgia, C.M.; Ackland, M.L.; Jersmann, H.P.; Lang, C.; Zalewski, P.D.; Hodge, S.J. Zinc and zinc transporters in macrophages and their roles in efferocytosis in COPD. PLoS One. 2014, 9, e110056. [Google Scholar] [CrossRef]
- González-Gualda, E.; Baker, A.G.; Fruk, L.; Muñoz-Espín, D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J. 2021, 288, 56–80. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. [CrossRef]
- Prelog, M.; Schwarzenbrunner, N.; Sailer-Höck, M.; Kern, H.; Klein-Franke, A.; Ausserlechner, M.J.; Koppelstaetter, C.; Brunner, A.; Duftner, C.; Dejaco, C.; Strasak, A.M.; Müller, T.; Zimmerhackl, L.B.; Brunner, J. Premature aging of the immune system in children with juvenile idiopathic arthritis. Arthritis Rheum. 2008, 58, 2153–62. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Shao, L.; Colmegna, I.; Goronzy, J.J.; Weyand, C.M. Telomerase insufficiency in rheumatoid arthritis. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 4360–5. [Google Scholar] [CrossRef] [PubMed]
- Hodge, G.; Jersmann, H.; Tran, H.B.; Holmes, M,; Reynolds, P. N.; Hodge, S. Lymphocyte senescence in COPD is associated with loss of glucocorticoid receptor expression by pro-inflammatory/cytotoxic lymphocytes. Respir. Res. 2015, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Hodge, G.; Tran, H.B.; Reynolds, P.N.; Jersmann, H.; Hodge, S. Lymphocyte senescence in COPD is associated with decreased sirtuin 1 expression in steroid resistant pro-inflammatory lymphocytes. Ther. Adv. Respir. Dis. 1753. [Google Scholar] [CrossRef] [PubMed]
- Hodge, G.; Hodge, S.; Liu, H.; Nguyen, P.; Holmes-Liew, C.L.; Holmes, M. BOS Is Associated With Decreased SIRT1 in Peripheral Blood Proinflammatory T, NK, and NKT-like Lymphocytes. Transplantation. 2019, 103, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- De Maeyer, R.P. H; Chambers. E.S. The impact of ageing on monocytes and macrophages. Immunol. Lett. [CrossRef]
- Campbell, R.A. , Docherty, M-H.; Ferenbach, D.A.; Mylonas, K.J. The Role of Ageing and Parenchymal Senescence on Macrophage Function and Fibrosis. Front. Immunol. 7007. [Google Scholar] [CrossRef]
- Latz, E.; Duewell, P. NLRP3 inflammasome activation in inflammaging. Semin. Immunol. [CrossRef]
- Faner, R.; Sobradillo, P.; Noguera, A. , Gomez, C.; Cruz, T.; López-Giraldo, A.; Ballester, E.; Soler, N.; Arostegui, J.I; Pelegrín, P.; Rodriguez-Roisin, R.; Yagüe, J.; Cosio, B.G.; Juan, M.; Agustí, A. The inflammasome pathway in stable COPD and acute exacerbations. ERJ Open Res. 2016, 2, 00002–2016. [Google Scholar] [CrossRef]
- Colarusso, C.; Terlizzi, M.; Molino, A.; Pinto, A.; Sorrentino, R. Role of the inflammasome in chronic obstructive pulmonary disease (COPD). Oncotarget. 2017, 8, 81813–81824. [Google Scholar] [CrossRef] [PubMed]
- Colarusso, C.; Terlizzi, M.; Molino, A.; Imitazione, P.; Somma, P.; Rega, R.; Saccomanno, A.; Aquino, R.P.; Pinto, A.; Sorrentino, R. AIM2 Inflammasome Activation Leads to IL-1α and TGF-β Release From Exacerbated Chronic Obstructive Pulmonary Disease-Derived Peripheral Blood Mononuclear Cells. Front. Pharmacol, 2: 10. [CrossRef]
- Tran, H.B.; Hamon, R.; Jersmann, H.; Ween, M.P.; Asare, P.; Haberberger, R.; Pant, H.; Hodge, S.J. AIM2 nuclear exit and inflammasome activation in chronic obstructive pulmonary disease and response to cigarette smoke. J. Inflamm. (Lond). 2021, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Pinkerton, J.W.; Kim, R.Y.; Robertson, A.A.B.; Hirota, J.A.; Wood, L.G.; Knight, D.A.; Cooper, M.A.; O'Neill, L.A.J.; Horvat, J.C.; Hansbro, P.M. Inflammasomes in the lung. Mol. Immunol. [CrossRef]
- Barnes, P.J. Targeting cellular senescence as a new approach to chronic obstructive pulmonary disease therapy. Curr. Opin. Pharmacol. [CrossRef]
- Hodge, S.; Tran, H.B.; Hamon, R.; Roscioli, E.; Hodge, G.; Jersmann, H.; Ween, M.; Reynolds, P.N.; Yeung, A. Treiberg, J.; Wilbert, S. Nonantibiotic macrolides restore airway macrophage phagocytic function with potential anti-inflammatory effects in chronic lung diseases. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L678–L687. [Google Scholar] [CrossRef] [PubMed]
- Cordts, F.; Pitson, S.; Tabeling, C.; Gibbins, I.; Moffat, D.F.; Jersmann, H.; Hodge, S.; Haberberger, R.V. Expression profile of the sphingosine kinase signalling system in the lung of patients with chronic obstructive pulmonary disease. Life Sci. 2011, 89, 806–11. [Google Scholar] [CrossRef]
- Ween, M.P.; White, J.B.; Tran, H.B.; Mukaro, V.; Jones, C.; Macowan, M.; Hodge, G.; Trim, P.J.; Snel, M.F.; Hodge, S., J. The role of oxidised self-lipids and alveolar macrophage CD1b expression in COPD. Sci. Rep. 2021, 11, 4106. [Google Scholar] [CrossRef]
- Hodge, S.; Hodge, G.; Jersmann, H.; Matthews, G.; Ahern, J.; Holmes, M.; Reynolds, P.N. Azithromycin improves macrophage phagocytic function and expression of mannose receptor in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med.
- Tran, H.B.; Jersmann, H.; Truong, T.T.; Hamon, R.; Roscioli, E.; Ween, M.; Pitman, M.R.; Pitson, S.M.; Hodge, G.; Reynolds, P.N.; Hodge, S. Disrupted epithelial/macrophage crosstalk via Spinster homologue 2-mediated S1P signaling may drive defective macrophage phagocytic function in COPD. PLoS One. 2017, 12, e0179577. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.B.; Lewis, M.D.; Tan, L.W.; Lester, S.E.; Baker, L.M.; Ng, J.; Hamilton-Bruce, M.A. , Hill, C.L.; Koblar, S.A.; Rischmueller, M.; Ruffin, R.E.; Wormald, P.J.; Zalewski, P.D.; Lang, C.J. Immunolocalization of NLRP3 Inflammasome in Normal Murine Airway Epithelium and Changes following Induction of Ovalbumin-Induced Airway Inflammation J Allergy (Cairo). 2012;2012:819176. [CrossRef]
- Tran, H.B.; Jakobczak, R.; Abdo, A.; Asare, P.; Reynolds, P.; Beltrame, J.; Hodge, S.; Zalewski, P. Immunolocalization of zinc transporters and metallothioneins reveals links to microvascular morphology and functions. Histochem. Cell Biol. 2022, 158, 485–496. [Google Scholar] [CrossRef]
- Hodge, S.J.; Hodge, G.L.; Holmes, M.; Reynolds, P.N. Flow cytometric characterization of cell populations in bronchoalveolar lavage and bronchial brushings from patients with chronic obstructive pulmonary disease. Cytometry B Clin. Cytom.
- Hodge, S.; Hodge, G.; Ahern, J.; Jersmann, H.; Holmes, M.; Reynolds, P.N. Smoking alters alveolar macrophage recognition and phagocytic ability: implications in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol.
- Hodge, S.; Matthews, G.; Mukaro. V.; Ahern, J.; Shivam, A.; Hodge, G.; Holmes, M.; Jersmann, H.; Reynolds, P.N. Cigarette smoke-induced changes to alveolar macrophage phenotype and function are improved by treatment with procysteine. Am. J. Respir. Cell. Mol. Biol. 2011, 44, 673–81. [Google Scholar] [CrossRef]
- Tsuji, T.; Aoshiba, K.; Nagai, A. Alveolar cell senescence in patients with pulmonary emphysema. Am. J. Respir. Crit. Care Med. 2006, 174, 886–93. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Araya, J.; Kurita, Y.; Kobayashi, K.; Takasaka, N.; Yoshida, M.; Hara, H.; Minagawa, S.; Wakui, H.; Fujii, S.; Kojima, J.; Shimizu, K.; Numata, T.; Kawaishi, M.; Odaka, M.; Morikawa, T.; Harada, T.; Nishimura, S.L.; Kaneko, Y.; Nakayama, K.; Kuwano, K. . PARK2-mediated mitophagy is involved in regulation of HBEC senescence in COPD pathogenesis. Autophagy. 2015, 11, 547–59. [Google Scholar] [CrossRef]
- 40. Jang, J-H.; Chand, H.S.; Bruse, S.; Doyle-Eisele, M.; Royer, C.; McDonald, J.; Qualls, C.; Klingelhutz, A.J.; Lin, Y.; Mallampalli, R.; Tesfaigzi, Y.; Nyunoya, T. Connective Tissue Growth Factor Promotes Pulmonary Epithelial Cell Senescence and Is Associated with COPD Severity. COPD. [CrossRef]
- Saito, N.; Araya, J.; Ito, S.; Tsubouchi, K.; Minagawa, S. ; Hara. H.; Ito, A.; Nakano, T.; Hosaka, Y.; Ichikawa, A.; Kadota, T.; Yoshida, M.; Fujita, Y.; Utsumi, H.; Kurita, Y.; Kobayashi, K.; Hashimoto, M.; Wakui, H.; Numata, T.; Kaneko, Y.; Asano, H.; Odaka, M.; Ohtsuka, T.; Morikawa, T.; Nakayama, K.; Kuwano, K. Involvement of Lamin B1 Reduction in Accelerated Cellular Senescence during Chronic Obstructive Pulmonary Disease Pathogenesis J. Immunol. 2019, 202, 1428–1440. [Google Scholar] [CrossRef]
- Sarker, R.S.J.; Conlon, T.M.; Morrone, C.; Srivastava, B.; Konyalilar, N.; Verleden, S.E.; Bayram, H.; Fehrenbach, H.; Yildirim, A.Ö. CARM1 regulates senescence during airway epithelial cell injury in COPD pathogenesis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 317, L602–L614. [Google Scholar] [CrossRef]
- de Vries, M.; Nwozor, K.O.; Muizer, K.; Wisman, M.; Timens, W.; van den Berge, M.; Faiz, A.; Hackett, T-L. ; Heijink, I.H.; Brandsma, C.A. The relation between age and airway epithelial barrier function. Respir. Res. 2022, 23, 43. [Google Scholar] [CrossRef]
- Hodge, G.; Jersmann, H.; Tran, H.B.; Asare, P.F.; Jayapal, M.; Reynolds, P.N.; Holmes, M.; Hodge, S. COPD is associated with increased pro-inflammatory CD28null CD8 T and NKT-like cells in the small airways. Clin. Exp. Immunol. 2022, 207, 351–359. [Google Scholar] [CrossRef]
- Fernandes, J.R.; Pinto, T.N.C.; Arruda, L.B.; da Silva, C.C.B.M.; de Carvalho, C.R.F.; Pinto, R.M.C.; da Silva Duarte, A.J.; Benard, G. Age-associated phenotypic imbalance in TCD4 and TCD8 cell subsets: comparison between healthy aged, smokers, COPD patients and young adults. Immun. Ageing. 2022, 19, 9. [Google Scholar] [CrossRef]
- Jung, T.; Bader, N.; Grune, T. Lipofuscin: formation, distribution, and metabolic consequences. Ann N Y Acad Sci. 2007;1119:97-111. [CrossRef]
- Leuenberger, P.; Vonmoos, S.; Vejdovsky, R. Morphologic changes of alveolar macrophages in smoking sarcoidosis patients. Eur. J. Respir. Dis. Suppl.
- Lukášová, E.; Kovařík, A.; Kozubek, S. . Consequences of lamin B1 and lamin B receptor downregulation in senescence cells. Cells. 2018, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of p53 in the Regulation of Cellular Senescence. Biomolecules. 2020, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Siganaki, M.; Koutsopoulos, A.V.; Neofytou, E.; Vlachaki, E.; Psarrou, M.; Soulitzis, N.; Pentilas, N.; Schiza, S.; Siafakas, N.M.; Tzortzaki, E.G. Deregulation of apoptosis mediators' p53 and bcl2 in lung tissue of COPD patients. Respir. Res. 2010, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Morissette, M.C.; Vachon-Beaudoin, G.; Parent, J.; Chakir, J.; Milot, J. Increased p53 level, Bax/Bcl-x(L) ratio, and TRAIL receptor expression in human emphysema. Am. J. Respir. Crit. Care Med. 2008, 178, 240–7. [Google Scholar] [CrossRef] [PubMed]
- Nyunoya, T.; Monick, M.M.; Klingelhutz, A.; Yarovinsky, T.O.; Cagley, J.R.; Hunninghake, G.W. Cigarette smoke induces cellular senescence. Am. J. Respir. Cell. Mol. Biol. 2006, 35, 681–8. [Google Scholar] [CrossRef] [PubMed]
- Damico, R.; Simms, T.; Kim, B.S.; Tekeste, Z.; Amankwan, H.; Damarla, M.; Hassoun, P.M. p53 mediates cigarette smoke-induced apoptosis of pulmonary endothelial cells: inhibitory effects of macrophage migration inhibitor factor. Am. J. Respir. Cell. Mol. Biol. 2011, 44, 323–32. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Kim, E.C.; Son, Y.; Lee, D.W.; Park, Y.S.; Choi, J.H.; Cho, K.H.; Kwon, K.S.; Kim, J.R. CD9 induces cellular senescence and aggravates atherosclerotic plaque formation. Cell Death Differ. 2020, 27, 2681–2696. [Google Scholar] [CrossRef]
- Kim, J.R.; Choi, J.H. CD9 expression in vascular aging and atherosclerosis. Histol. Histopathol. 2020, 35, 1449–1454. [Google Scholar] [CrossRef]
- Takeda, Y.; He, P.; Tachibana, I.; Zhou, B.; Miyado, K.; Kaneko, H.; Suzuki, M.; Minami, S.; Iwasaki, T.; Goya, S.; Kijima, T.; Kumagai, T.; Yoshida, M.; Osaki, T.; Komori, T.; Mekada, E.; Kawase, I. Double deficiency of tetraspanins CD9 and CD81 alters cell motility and protease production of macrophages and causes chronic obstructive pulmonary disease-like phenotype in mice. J. Biol. Chem. 2008, 283, 26089–97. [Google Scholar] [CrossRef]
- Jin, Y.; Takeda, Y.; Kondo, Y.; Tripathi, L.P.; Kang, S.; Takeshita, H.; Kuhara, H.; Maeda, Y.; Higashiguchi, M.; Miyake, K.; Morimura, O.; Koba, T.; Hayama, Y.; Koyama, S.; Nakanishi, K.; Iwasaki, T.; Tetsumoto, S.; Tsujino, K.; Kuroyama, M.; Iwahori, K.; Hirata, H.; Takimoto, T.; Suzuki, M.; Nagatomo, I.; Sugimoto, K.; Fujii, Y.; Kida, H.; Mizuguchi, K.; Ito, M.; Kijima, T.; Rakugi, H.; Mekada, E.; Tachibana, I.; Kumanogoh, A. Double deletion of tetraspanins CD9 and CD81 in mice leads to a syndrome resembling accelerated aging. Sci. Rep. 2018, 8, 5145. [Google Scholar] [CrossRef]
- Suzuki, M.; Tachibana, I.; Takeda, Y.; He, P.; Minami, S.; Iwasaki, T.; Kida, H.; Goya, S.; Kijima, T.; Yoshida, M.; Kumagai, T.; Osaki, T. , Kawase, I. Tetraspanin CD9 negatively regulates lipopolysaccharide-induced macrophage activation and lung inflammation. J. Immunol. 2009, 182, 6485–93. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, N.S.; Bracke, K.R.; Dupont, L.L.; Van Pottelberge, G.R.; Provoost, S.; Vanden Berghe, T.; Vandenabeele, P.; Lambrecht, B.N.; Joos, G.F.; Brusselle, G.G. Role of IL-1α and the Nlrp3/caspase-1/IL-1β axis in cigarette smoke-induced pulmonary inflammation and COPD. Eur. Respir. J. 2011, 38, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Eltom, S.; Belvisi, M.G.; Stevenson, C.S.; Maher, S.A.; Dubuis, E.; Fitzgerald, K.A.; Birrell, M.A. Role of the inflammasome-caspase1/11-IL-1/18 axis in cigarette smoke driven airway inflammation: an insight into the pathogenesis of COPD. PLoS One. 2014, 9, e112829. [Google Scholar] [CrossRef] [PubMed]
- Huot-Marchand, S; Nascimento, M.; Culerier, E.; Bourenane, M.; Savigny, F.; Panek, C.; Serdjebi, C.; Le Bert, M.; Quesniaux, V., F., J.; Ryffel, B.; Broz, P.; Riteau, N.; Gombault, A.; Couillin, I. Cigarette smoke-induced gasdermin D activation in bronchoalveolar macrophages and bronchial epithelial cells dependently on NLRP3 Front. Immunol. 2022;13:918507. [CrossRef]
- Di Stefano, A.; Caramori, G.; Barczyk, A.; Vicari, C.; Brun, P.; Zanini, A.; Cappello, F.; Garofano, E.; Padovani, A.; Contoli, M.; Casolari, M.; Durham, A.L.; Chung, K.F.; Barnes, P.J.; Papi, A.; Adcock, I.; Balbi, B. Innate immunity but not NLRP3 inflammasome activation correlates with severity of stable COPD. Thorax. 2014, 69, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Wang, X.; Ge, S.; Chen, W.; Wang, W. Han, X. Long-term cigarette smoking suppresses NLRP3 inflammasome activation in oral mucosal epithelium and attenuates host defense against Candida albicans in a rat model. Biomed. Pharmacother, 1085. [Google Scholar] [CrossRef]
- Han, S-H. ; Jerome, J.A.; Gregory, A., D.; Mallampalli R., K. Cigarette smoke destabilizes NLRP3 protein by promoting its ubiquitination Respir Res. 2017, 18, 2. [CrossRef]
- Buscetta, M.; Di Vincenzo, D.; Miele, M.; Badami, E.; Pace, E.; Cipollina, C. Cigarette smoke inhibits the NLRP3 inflammasome and leads to caspase-1 activation via the TLR4-TRIF-caspase-8 axis in human macrophages FASEB J. 2020, 34, 1819–1832. 34. [CrossRef]
- Buscetta, M.; Cristaldi, M.; Cimino, M.; La Mensa, A.; Dino, P.; Bucchieri, F.; Rappa, F.; Amato, S.; Aronica, T., S.; Pace, E.; Bertani, A.; Cipollina, C. Cigarette smoke promotes inflammasome-independent activation of caspase-1 and -4 leading to gasdermin D cleavage in human macrophages FASEB J. 2022, 36, e22525. 36. [CrossRef]
- Nanda, S.K.; Vollmer, S.; Perez-Oliva, A.B. Posttranslational regulation of inflammasomes, its potential as biomarkers and in the Identification of novel drugs targets. Front. Cell. Dev. Biol. 8875. [Google Scholar] [CrossRef]
- Lopez-Castejon, G. Control of the inflammasome by the ubiquitin system. FEBS J. 2020, 287, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Zangiabadi, S.; Abdul-Sater, A.A. Regulation of the NLRP3 Inflammasome by Posttranslational Modifications. J. Immunol. 2022, 208, 286–292. [Google Scholar] [CrossRef]
- Fleischmann, M; Andrew G Jarnicki, A. G.; Brown, A.S.; Yang, C.; Anderson, G.P.; Garbi, N.; Hartland, E.L.; van Driel, I.R.; Ng, G.Z. Cigarette smoke depletes alveolar macrophages and delays clearance of Legionella pneumophila. Am J Physiol Lung Cell Mol Physiol 2023, 324, L373–L384. [CrossRef]
- Ferrucci, L.; Fabbri, E. . Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Albert, R.K.; Connett, J.; Bailey, W.C.; Casaburi, R.; Cooper, J.A. Jr; Criner, G.J.; Curtis, J.L.; Dransfield, M.T.; Han, M.K.; Lazarus, S.C.; Make, B.; Marchetti, N.; Martinez, F.J.; Madinger, N.E.; McEvoy, C.; Niewoehner, D.E.; Porsasz, J.; Price, C.S.; Reilly, J.; Scanlon, P.D.; Sciurba, F.C.; Scharf, S.M.; Washko, G.R.; Woodruff, P.G.; Anthonisen, N.R.; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N. Engl. J. Med. 2011, 365, 689–98. [Google Scholar] [CrossRef]
- Hodge, S.; Reynolds, P.N. Low-dose azithromycin improves phagocytosis of bacteria by both alveolar and monocyte-derived macrophages in chronic obstructive pulmonary disease subjects. Respirology. 2012, 17, 802–7. [Google Scholar] [CrossRef] [PubMed]
- Vermeersch, K.; Gabrovska, M.; Aumann, J.; Demedts, I. K; Corhay, J-L.; Marchand, E.; Slabbynck, H.; Haenebalcke, C.; Haerens, M.; Hanon, S.; Jordens, P.; Peché, R.; Fremault, A.; Lauwerier, T.; Delporte, A.; Vandenberk, B.; Willems, R.; Everaerts, S.; Belmans, A.; Bogaerts, K.; Verleden, G.M.; Troosters, T.; Ninane, V.; Brusselle, G.G.; Janssens, W. Azithromycin during acute Chronic Obstructive Pulmonary Disease exacerbations requiring hospitalization (BACE). A multicenter, randomized, double-blind, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 2019, 200, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Lendermon, E.A.; Coon, T.A.; Bednash, J.S.; Weathington, N.M.; McDyer, J.F.; Mallampalli, R.K. Azithromycin decreases NALP3 mRNA stability in monocytes to limit inflammasome-dependent inflammation. Respir Res. 2017, 18, 131. [Google Scholar] [CrossRef] [PubMed]
- Haydar, D.; Cory, T.J.; Birket, S.E.; Murphy, B.S.; Pennypacker, K.R.; Sinai, A.P.; Feola, D.J. Azithromycin polarizes macrophages to an M2 phenotype via inhibition of the STAT1 and NF-κB signaling pathways. J. Immunol. 2019, 203, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Persson, H.L.; Vainikka, L.K.; Sege, M.; Wennerström, U.; Dam-Larsen, S.; Persson, J. Leaky lysosomes in lung transplant macrophages: azithromycin prevents oxidative damage. Respir. Res. 2012, 13, 83. [Google Scholar] [CrossRef]
- Taylor, S.L.; Leong, L.E.X; Mobegi, F.M.; Choo, J.M.; Wesselingh, S.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; Peters, M.J.; Baraket, M.; Marks, G.B.; Gibson, P.G.; Rogers, G.B.; Simpson, J.L. Long-term azithromycin reduces Haemophilus influenzae and increases antibiotic resistance in severe asthma. Am. J. Respir. Crit. Care Med. 2019, 200, 309–317. [Google Scholar] [CrossRef]
- Kricker, J.A.; Page, C.P.; Gardarsson, F.R.; Baldursson, O.; Gudjonsson, T.; Parnham, M.J. Nonantimicrobial Actions of Macrolides: Overview and Perspectives for Future Development. Pharmacol. Rev. 2021, 73, 233–262. [Google Scholar] [CrossRef]
- Mencarelli, A.; Distrutti, E.; Renga, B.; Cipriani, S.; Palladino, G.; Booth, C.; Tudor, G. ; Guse, J-H.; Hahn, U.; Burnet, M.; Fiorucci, S. Development of non-antibiotic macrolide that corrects inflammation-driven immune dysfunction in models of inflammatory bowel diseases and arthritis. Eur. J. Pharmacol. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




