1. Introduction
Flavanols, along with epicatechin and its oligomers, can be found in grape skins and seeds [
1,
2] immature apples [
3,
4], blueberries [
5], and cocoa [
6,
7]. These substances are brown and have a strong, astringent taste. In developed countries, 200-300 mg of these substances are ingested daily with food [
8,
9]. Numerous epidemiological studies carried out in developed countries have shown an inverse relationship between flavanol consumption and the risk of chronic diseases that are challenging to treat, such as cardiovascular disease [
10] and dementia [
11], and may delay the age-related damage to sensory organs [
12]. A study on over 21,000 older Americans revealed that the daily intake of 500mg of flavanols from cocoa led to a 27 % reduction in cardiovascular mortality over 3.6 years [
13]. Additionally, a meta-analysis of intervention trials utilizing grape extract has demonstrated its ability to enhance cardiovascular health by decreasing blood pressure [
14], improving vascular endothelial function [
15], and enhancing blood lipid profile [
16]. Blueberry extract has also been shown to assist with weight loss. [
17].
Conversely, flavanol bioavailability is minimized due to its low absorption rate in the body [
18] . Although a small percentage of catechin, a flavanol monomer, can be absorbed upon ingestion, it transforms into readily soluble compounds like glucuronic acid and sulfate conjugates while passing through the intestines and liver. Moreover, oligomeric procyanidins are not absorbed in the upper gastrointestinal tract. Consequently, the most of the ingested flavanols enter the lower gastrointestinal tract, and while intestinal bacteria break down some flavanols, the majority are excreted in the feces [
18]. Reports suggest that taking grape and blueberry extracts together increases the absorption of flavanols, but the impact is minimal [
19]. Despite the extremely low possibility of flavanols being distributed to their targeted organs, beneficial effects have been observed, making the mechanism challenging to understand.
We conducted various studies to elucidate the mechanism of flavanols' action on the cardiovascular systems. It was found that peripheral blood flow increased immediately after an oral dose of flavanol [
20] and that this effect was related to the sympathetic nervous system [
21,
22]. It has also been found that this acute hemodynamic change is observed only in polyphenols that exhibit astringent taste [
23]. We also found that polyphenols, which exhibit astringent taste, act as pro-oxidants and promote the production of O2- at a similar neutral pH as in the oral cavity and intestinal tract [
24]. This suggests that there is a relationship between the taste, redox properties and bioactivity of polyphenols.
In this investigation, polyphenol-rich extract from grape and blueberry (PEGB) was employed and a sensory evaluation of its flavor quality was performed using healthy volunteers. Furthermore, we investigated its redox properties in vitro under neutral pH conditions and confirmed its impact on cremaster arteriole blood flow in rats.
2. Material and Method
2.1. Materials
Polyphenol-rich extract from grape and blueberry (PEGB, Memophenol®) provided by Activ’Inside (Beychac et Caillau, France). Individual procyanidin standards from monomers to heptamers were obtained by the method of Shoji et al. Production of O2・- was measured using a chemiluminescent probe (2-methyl-6-p-methoxyphenylethynyl-imidazopyrazinone (MPEC, AB-2950, ATTO Co., Tokyo, Japan). Xanthine (X, 241-00013) and superoxide dismutase solution (SOD, 192-11281) were purchased from Fujifilm Wako Pure Chemical Corporation. Xanthine oxidase (XOD) was purchased from Toyobo Co., Ltd (XTO-212, Osaka, Japan). Caffein and quercetin hydrate were purchased from Tokyo Chemical Industry Co., Ltd.). Potassium aluminum sulfate was purchased from Kenkei Pharmaceutical Co., Ltd. (Osaka, Japan).
2.2. Analysis of Flavanol in PEGB by a Rapid HPLC with Fluorescence Detection
The HPLC equipment was used as described above. The column used was an Inertsil
® WP300 Diol (i.d. 4.6 × 250 mm; 5 µm particle size) was purchased from GL Science Inc. (Japan). The column temperature was maintained at 35 °C. A mixture of acetonitrile- acetic acid (mobile phase A, 98:2, v/v) and that of methanol-distilled water-acetic acid (mobile phase B, 95:3:2, v/v/v) were used as mobile phases. Flavan-3-ols and procyanidins were separated according to DP by using an increasing gradient of mobile phase B based on the method of Obara et al. [
25] . An isocratic elution using 7% B solution for 3 min, followed by a gradient elution using 30% B solution for 3-60 min, and then to 100% B solution over the next 10 min. The conditions were maintained using 7% B solution for the 7 min prior to the restart. The sample injection volume was 5 µL, and the flow rate was set at 1.0 mL/min. Fluorescence for flavan-3-ols and procyanidins was detected based on Ex and Em wavelengths of 230 and 321 nm, respectively. Individual flavan-3-ol and procyanidin standards from monomers to heptamers were obtained by using the method described by Shoji et al. [
26].
2.3. Evaluation of Sensory Perception of PEGB by Healthy Individuals
After the test protocol was approved by the Shibaura Institute of Technology Ethics Committee, the sensory test was registered in the Clinical Trial Registration System UMIN (
https://www.umin.ac.jp/ctr/new-registration.htm, UMIN000052854). The participants, 6 healthy people over the age of twenty (4 men and 2 women, 23 to 26 years old), were selected by the following preliminary test: one hour after consuming the specified meal, they were asked to drink three beverages (two water and one standard), which were held in the mouth for 10 seconds and then spat out to identify the standard. This three-point discrimination method was performed at three concentrations (0.1, 0.2 and 0.4 mg/ml). Caffeine was used as the standard for bitterness and potassium aluminum sulphate as the standard for astringency, and people who could identify bitterness at the second level and astringency at the third level were selected as participants. Similarly, one hour after ingesting the designated food, the participants held polyphenols diluted in three stages (concentrations: 0.1, 0.2, and 0.4 g/L) in their mouths for 10 seconds, then spit them out, and then answered the questionnaire. Quercetin hydrate and PEGB were used as test substances. The questionnaire used a visual analog scale (VAS). The VAS is a scale that rates intensity, with zero (no sensation at all) on the left and 100 (the strongest sensation imaginable) on the right. The terms are sweetness, saltiness, sourness, bitterness, umami, astringency, dryness, roughness, shrinkage, numbness, pungency, and oiliness. In the third step, in addition to the VAS, we also assessed how many minutes the aftertaste lasted.
2.4. Redox Characterization of PEGB In Vitro using MPEC
Redox characterization of PEGB using MPEC, which specifically reacts with O
2・- , was carried out according to our previous study [
24]
. Briefly, a dilution series of test chemicals was prepared using 0.01 mM phosphate buffer (pH 2.8) because polyphenols are stable under acidic conditions [
27]. The test chemicals including 10 mU/mL xanthine oxidase (XOD) and 7.85μM MPEC were incubated at 37℃ in 0.1 mM phosphate buffer pH 7.0, as almost similar pH in gastrointestinal tract except stomach, for 45 min. Furthermore, the chemiluminescence of the reaction solution containing the test chemical dilution series was measured soon after adding 30μM xanthine (X). Each result represents three independent experiments, and each experiment was performed in triplicate. All measurements were accompanied by positive (X-XOD) or negative (10 mU/mL SOD) controls.
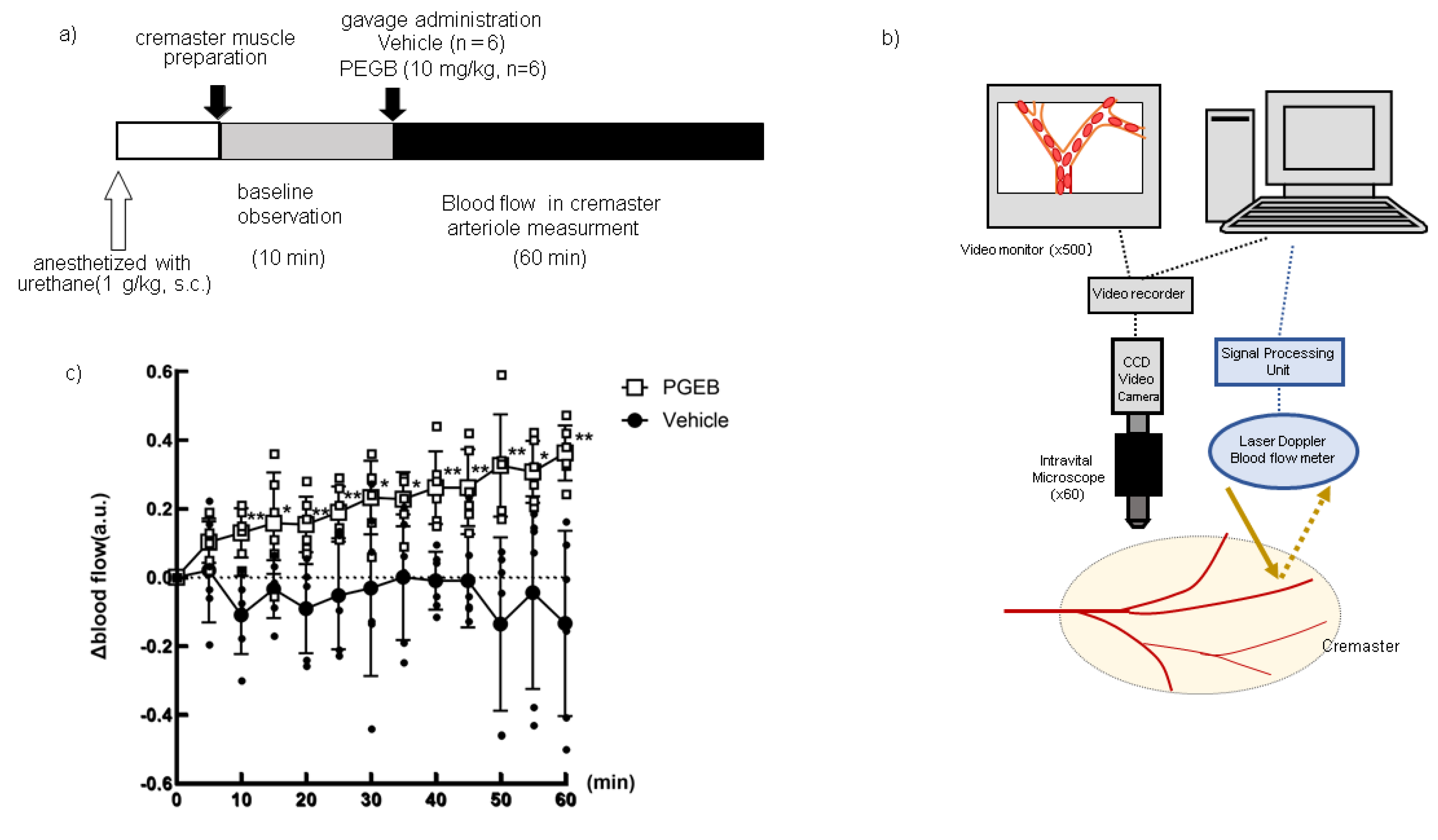
2.5. Hemodynamic Impact of PEGB on Rat Cremasteric Arteriole Blood Flow
Twelve rats were divided into two treatment groups, as follows: vehicle (4 mL/kg in 3% Tween80 solution, n=6), PGBE (10 mg/kg; n=6). All chemicals were dissolved in a 3% Tween 80 solution. Measurement of blood flow was performed according to Fushimi et al. [
28] , as shown in
Figure 4a. Briefly, rats were anesthetized with urethane (1 g/kg s.c.), and a gastric tube was inserted into the stomach. Each chemical was infused at a rate of 1.0 mL/min to avoid a circulatory reflex that can be induced by this procedure. The cremaster muscle was exteriorized, and the surface was perfused with phosphate-buffered saline. After a post-surgical equilibration period, baseline measurements of cremaster arteriole blood flow were conducted for 10 min. Each treatment was orally administered to animals through the gastric tube. Blood flow in the cremaster artery was monitored for 60 min before and after compound administration using a laser Doppler blood flow meter (Periscan PIM-2, Perimed Co. Ltd, Stockholm, Sweden)(
Figure 4c).
2.6. Data Analysis and Statistical Methods
All data were expressed as means and standard deviations except sensory test. In sensory test, were shown using boxplots with interquartile ranges and statistical analyses were carried out using Wilcoxon signed rank test. performed using one or two-way analysis of variance, and post hoc comparisons were made with the vehicle group using Dunnett’s test except sensory test. In sensory test, P < 0.05 was considered to indicate significance, and p < 0.1 was considered as a trend toward significance.
3. Results
3.1. Analysis of the Polyphenol in PEGB by a Rapid HPLC with Fluorescence Detection
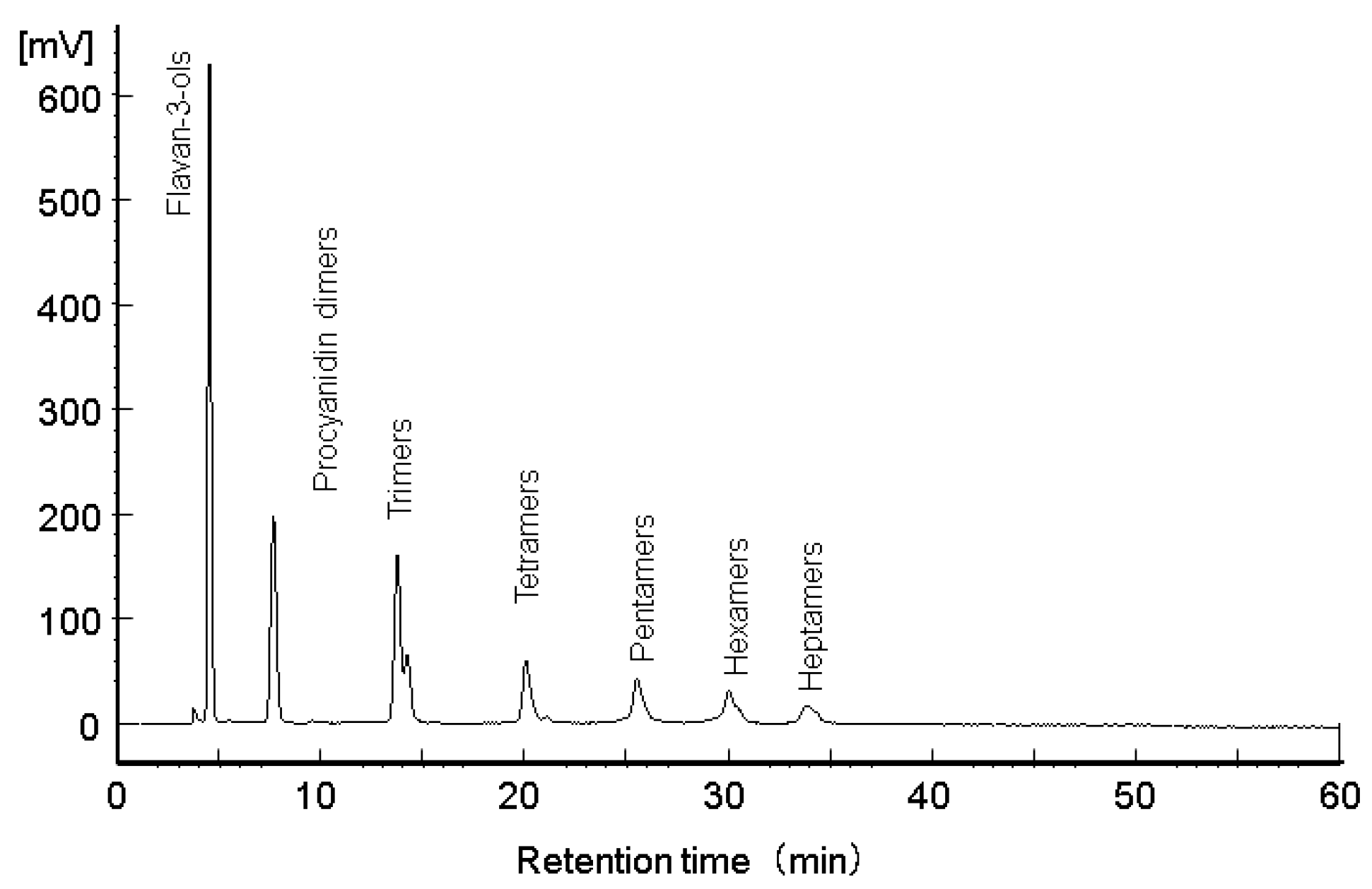
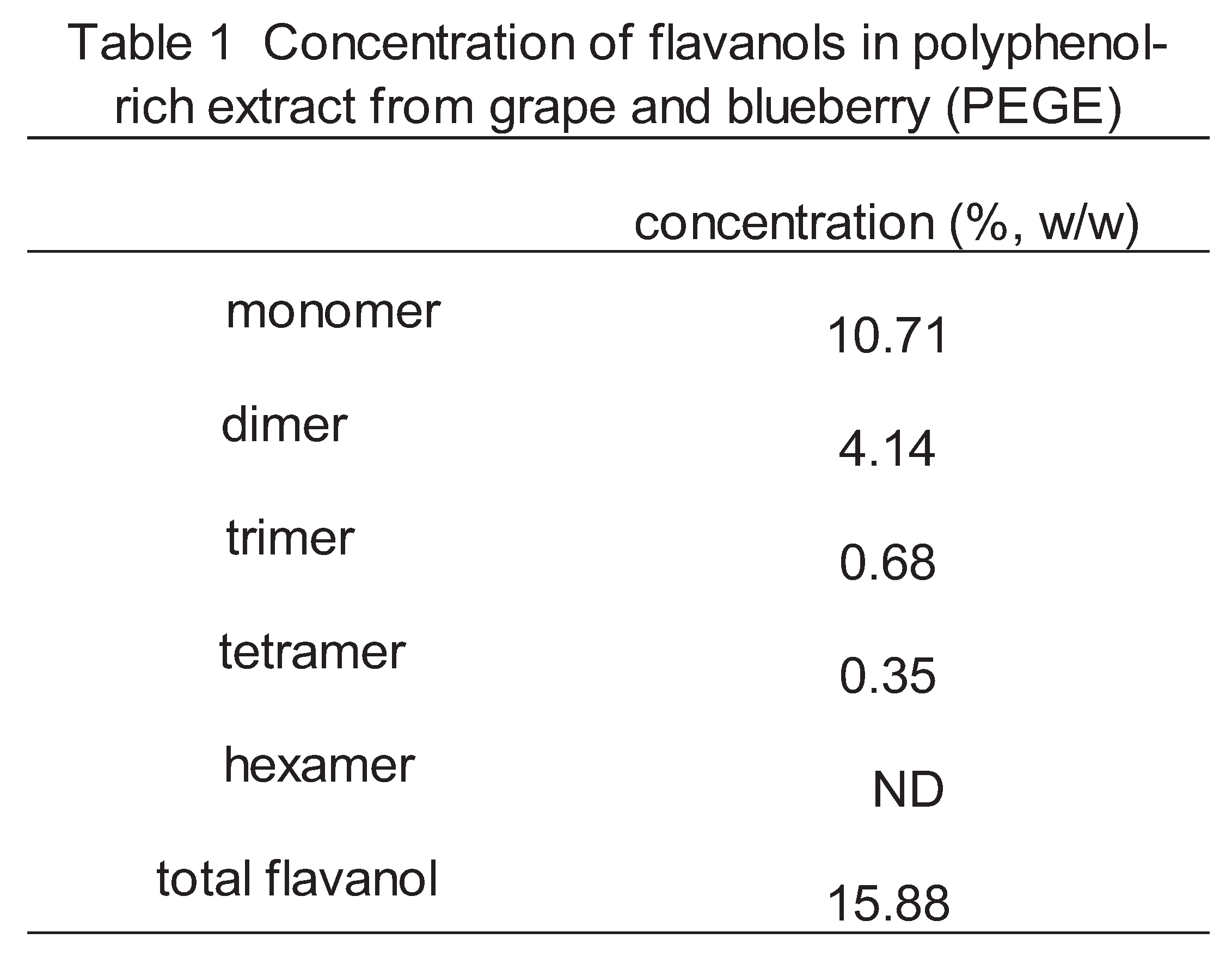
The percentage by weight of flavanols contained in PEGB is given in
Table 1 and the HPLC diagram of the standard material was shown in
Figure 1. PEGB contained approximately 16% total flavanols, of which approximately 11% were monomers.
Figure 1.
Normal phase HPLC chromatogram of flavanol standard.
Figure 1.
Normal phase HPLC chromatogram of flavanol standard.
3.2. Evaluation of Sensory Perception of PEGB by Healthy Individuals
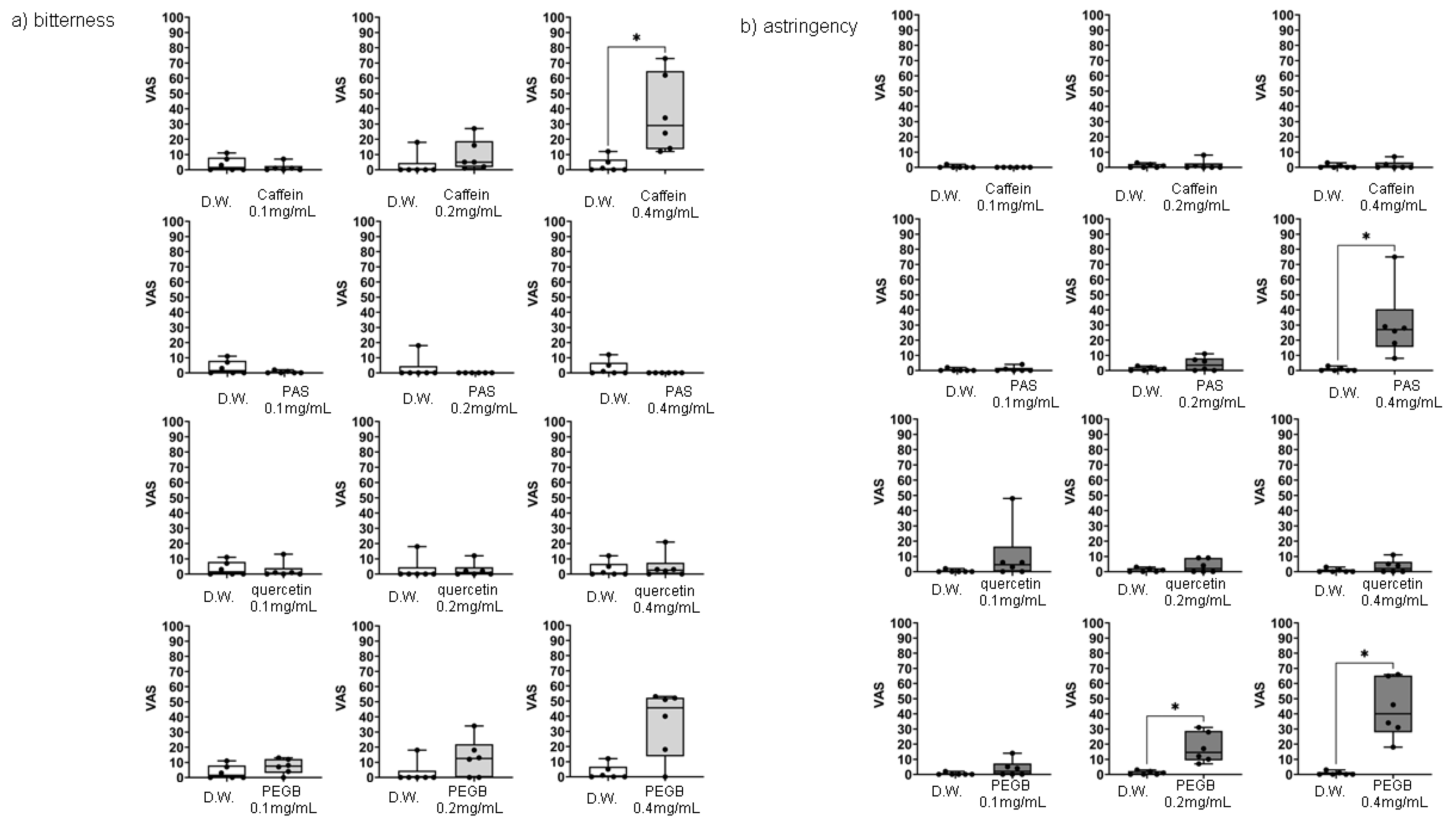
The sensory analysis results for PEGB and quercetin based on quantitative descriptive analysis using a visual analog scale are shown in
Figure 2. along with the results for caffeine, a bitter standard substance, and potassium aluminum sulfate, an astringent standard substance. Caffeine, a bitter standard substance, showed dose-dependent bitterness, there was a significant difference in bitter taste at 0.4mg/mL. Potassium aluminum sulfate showed significant astringency at 0.4 mg/mL. Quercetin did not exhibit any characteristic sensory properties at a concentration of 0.1-0.4 mg/mL, while PEGB showed a significant astringency at 0.2 and 0.4 mg/mL and slight bitterness at 0.4 mg/mL.
Figure 2.
Sensory analysis of PEGB and quercetin based on quantitative descriptive analysis using visual analog scale. a) Caffeine (bitter standard), b) Potassium aluminum sulfate (APS; astringent standard), c) quercetin, d) PEGB. Each value represents the mean and standard deviation (n = 6, each). Statistical analyses were performed by Kruskal-Wallis test followed by Dunn’s multiple comparisons tests. Significantly different from water ;, *p < 0.05.
Figure 2.
Sensory analysis of PEGB and quercetin based on quantitative descriptive analysis using visual analog scale. a) Caffeine (bitter standard), b) Potassium aluminum sulfate (APS; astringent standard), c) quercetin, d) PEGB. Each value represents the mean and standard deviation (n = 6, each). Statistical analyses were performed by Kruskal-Wallis test followed by Dunn’s multiple comparisons tests. Significantly different from water ;, *p < 0.05.
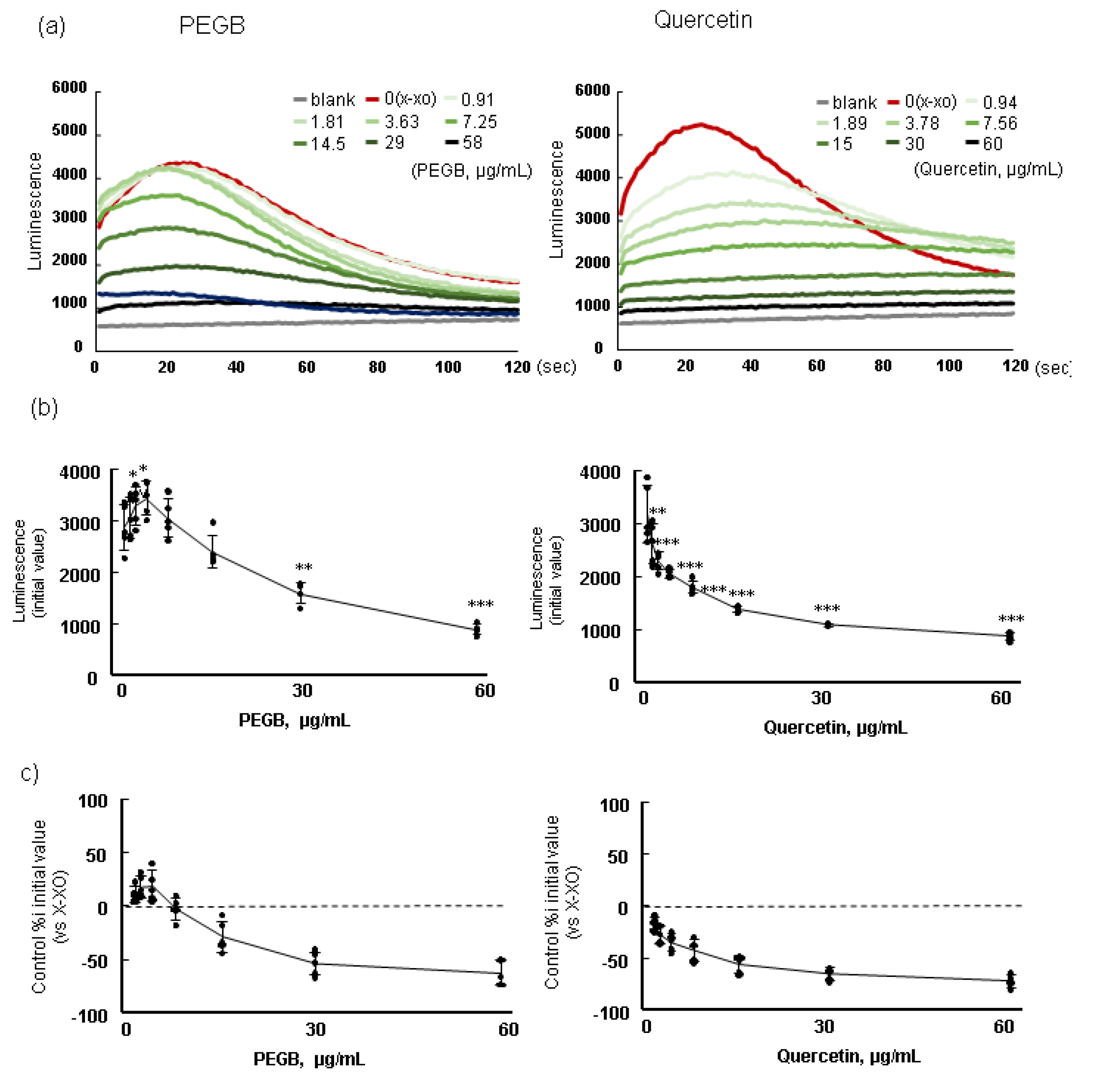
3.3. Redox Characterization of PEGB In Vitro using MPEC
A typical reaction in PEGB and quercetin as a polyphenol control with O
2・- produced by X-XOD at pH 7.0 is shown in
Figure 3. Results represent the average of three separate runs. These test chemicals had an immediate effect on the O
2・- produced by X-XOD, resulting in a change in chemiluminescence. The luminescence was increased by adding PEGB, especially at low concentrations, in contrast, it was reduced in high concentrations (
Figure 3a, left). A reduction in luminescence was observed at all concentrations upon adding quercetin, as shown in
Figure 3, right panel. The mean of the initial luminescence values for each concentration of PEGB and quercetin was shown in
Figure 3b. PEGB increased production of O
2・-- at 0.9 - 3.7 µg/mL and significantly scavenged O
2・- more than 30µg/mL (
Figure 3b left). Quercetin also scavenged O
2・- at all concentrations (
Figure 3b right). The reactivity of PEGB and quercetin to O
2・- production promotive activity or scavenging activity is shown in
Figure 3c. PEGB exhibited a biphasic response, promoting O
2・- production by more than 10% at low concentrations, and a prooxidant effect promoting O
2・- scavenging in a dose-dependent manner at high doses. On the other hand, quercetin only showed a dose-dependent O
2・-scavenging antioxidant effect.
Figure 3.
Redox properties of PEGB and quercetin pH 7. Typical luminescence change after addition of with PEGB (a, left) or quercetin (a, right); mean of initial luminescence value PEGB (b, left) or quercetin (b, right); ratio of luminescence intensity to control (X-XOD) upon addition of PEGB(c, left) or quercetin (c, right). Each values represent mean ± standard deviation; * p < 0.05, ** p < 0.01, *** p < 0.001, one-way ANOVA, followed by the post hoc Dunnett’s test, compared with the positive control (X-XOD).
Figure 3.
Redox properties of PEGB and quercetin pH 7. Typical luminescence change after addition of with PEGB (a, left) or quercetin (a, right); mean of initial luminescence value PEGB (b, left) or quercetin (b, right); ratio of luminescence intensity to control (X-XOD) upon addition of PEGB(c, left) or quercetin (c, right). Each values represent mean ± standard deviation; * p < 0.05, ** p < 0.01, *** p < 0.001, one-way ANOVA, followed by the post hoc Dunnett’s test, compared with the positive control (X-XOD).
3.4. Hemodynamic Impact of PEGB on Rat Cremasteric Arteriole Blood Flow
Figure 4 represented the results of blood flow the change in the cremasteric arteriole following a single oral administration of the was 10 mg/kg PEGB shown in
Figure 4d. Blood flow increased significantly at 10 min after PEGB administration, which was maintained during the 60 min observation period.
Figure 4.
Changes in cremaster arteriole blood flow following a single oral dose of 10 mg/kg PEGB. Experimental procedure, a); scheme for measurement,b): change of blood flow after administration of PEGB. The values represent the mean ± standard deviation. # p < 0.1, * p < 0.05, ** p < 0.01, two-way ANOVA, followed by the post hoc Dunnett’s test, compared with vehicle.
Figure 4.
Changes in cremaster arteriole blood flow following a single oral dose of 10 mg/kg PEGB. Experimental procedure, a); scheme for measurement,b): change of blood flow after administration of PEGB. The values represent the mean ± standard deviation. # p < 0.1, * p < 0.05, ** p < 0.01, two-way ANOVA, followed by the post hoc Dunnett’s test, compared with vehicle.
4. Discussion
PEGB was found to contain 16% flavanols, of which 11% monomeric catechin and 4% procyanidin dimers (
Table 1). This study compared the sensory properties between PEGB and quercetin hydrate (
Figure 2). It was found that PEGB exhibits a bitterness like that of caffeine, which is a target for bitterness. Similarly, PEGB had an astringent taste like that of the positive control potassium aluminum sulfate. But quercetin did not have bitterness or astringency at comparable concentrations. Previous papers have reported interactions between flavanol monomers catechins and oligomers procyanidin and the bitter taste receptor taste receptor 2 (T2R). There are 25 types of T2R in humans, and it has been reported that (+)-catechin interacts with T2R14 and 39, and (-)-epicatechin interacts with T2R4, 5, and 39 [
29]. It was also reported that dimer procyanidin B1 interacted with T2R5 and 7, procyanidin B4, B7 and C2 was interacted T2R5 [
29]. PEGE may have a bitter taste due to its interactions with T2Rs.
Flavanol oligomers are known to exhibit astringent taste, although there is limited understanding of how this taste is perceived in mammals The sensation of astringency is suggested to be caused by particles interacting with proline-rich proteins in saliva, causing friction between the particles and the oral mucosa [
30]. Nonetheless, recent findings indicate that polyphenols can activate G protein-coupled receptors [
31] or transient receptor potential (TRP) [
32,
33] autonomously without requiring saliva. [
34]. TRP channels are known to be a family of six related proteins known as control responses to stimuli, including temperature, touch, pain, and osmolarity in the sensory systems of mammals [
35]. Among TRPs, potential vanilloid (TRPV)1 and ankyrin (TRPA)1 show primary expression in the sensory neurons of the oral and gastrointestinal sensory nervous system [
36]. These TRP channels can be activated by ROS reactions on cysteine residues near the C- and N-termini on the cytosolic side [
37]. In reactivity to O
2・-, PEGB promoted production at low concentrations and scavenged O
2・- at high concentrations at the neutral pH range similar to oral cavity and intestine. In contrast, quercetin was observed to scavenge O
2・ at any concentration (
Figure 3). This difference in redox properties, namely the promotion of O
2・ production in the gastrointestinal tract and the sensation of astringency, may be related.
It is well known that capsaicin, a TRPV1 ligand, activates TRPV1 expressed in the gastrointestinal tract, including the oral and nasal cavities, and that its stimulation increases sympathetic nerve activity and affects the circulatory and metabolic systems. In the present results, a single dose of PEGB significantly increased blood flow to the cremaster arteriole (
Figure 4). Our previous findings indicated that flavanols' impact on the circulatory system results from increased sympathetic nerve activity [
21,
22]. In addition, recent our research using TRP channel inhibitors has shown that flavanols enhance peripheral blood flow by increasing sympathetic nerve activity through TRP channels in the gastrointestinal tract [
24]. Furthermore, it has been confirmed that this impact was eliminated by simultaneous administration of N-acetylcysteine, an O2・- inhibitor [
24].These findings suggest that flavanols produce O
2・- at neutral pH, which in turn activates TRP channels, and the resulting stimulation is then transmitted to the brain via interneurons, ultimately activating sympathetic nerve activity. No bitter or astringent taste was observed with quercetin at similar concentrations, indicating that the taste is highly dependent on the chemical structure or bioactivity of the polyphenol.
However, this study is limited by the fact that PEGB also contains other polyphenols [
38], thus it is needed to consider the physiological effects observed after PEGB administration due to substances other than flavanols. Furthermore, a thorough analysis of the constituents within PEGB is imperative, with consideration given to non-flavanol components and their contribution.
5. Conclusions
In conclusion, PEGB containing flavanols displays a potent astringent flavor, increases O2・- production at low concentrations in a neutral pH environment, and considerably enhances blood flow to skeletal muscles after a single dose in rats. It has been postulated that these impacts may be attributed to the association between O2・- and TRP channels, which takes place upon the exposure of flavanols to a neutral pH environment in the gastrointestinal tract, excluding the stomach. The perception of astringency resulting from the interaction between O2・- and TRP channels is a possibility, but additional research is necessary to fully comprehend the specifics.
Authorship
Performed literature search and drafted the manuscript: NO, VC; experiment : TS, KO, CH, KH, TF; interpretation of the data: TF, YF, NO; provided critical review for the manuscript: UJ, ASA, RP, SC; gave final approval for the publication of this manuscript: VC, NO.
Acknowledgments
This research has been conducted with funds from “Piano di incentivi per la Ricerca, Linea Intervento 2 PIACERI, 2020–2022”, University of Catania, Italy, and funds from Research Innovation, Ministry of Education in Saudi Arabia Project number (IFKSUOR3-524-1) King Saud University, Riyadh, Saudi Arabia”.
Disclosure of stated of COI
The authors declare that they have no conflict of interest.
References
- Muñoz-González C, Criado C, Pérez-Jiménez M, Pozo-Bayón M. Evaluation of the Effect of a Grape Seed Tannin Extract on Wine Ester Release and Perception Using In Vitro and In Vivo Instrumental and Sensory Approaches. Foods. 2021, 10. [Google Scholar]
- Rajakumari R, Volova T, Oluwafemi OS, Rajesh Kumar S, Thomas S, Kalarikkal N. Grape seed extract-soluplus dispersion and its antioxidant activity. Drug Dev Ind Pharm. 2020, 46, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Shoji T, Yanagida A, Kanda T. Gel permeation chromatography of anthocyanin pigments from Rosé cider and red wine. J Agric Food Chem. 1999, 47, 2885–2890. [Google Scholar] [CrossRef] [PubMed]
- Shoji T, Obara M, Takahashi T, Masumoto S, Hirota H, Miura T. The Differences in the Flavan-3-ol and Procyanidin Contents of the Japanese 'Fuji' and 'Orin' Apples Using a Rapid Quantitative High-Performance Liquid Chromatography Method: Estimation of the Japanese Intake of Flavan-3-ols and Procyanidins from Apple as Case Study. Foods. 2021, 10. [Google Scholar]
- Rodriguez-Mateos A, Cifuentes-Gomez T, Tabatabaee S, Lecras C, Spencer JP. Procyanidin, anthocyanin, and chlorogenic acid contents of highbush and lowbush blueberries. J Agric Food Chem. 2012, 60, 5772–5778. [Google Scholar] [CrossRef]
- Hatano T, Miyatake H, Natsume M, Osakabe N, Takizawa T, Ito H, et al. Proanthocyanidin glycosides and related polyphenols from cacao liquor and their antioxidant effects. Phytochemistry. 2002, 59, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Natsume M, Osakabe N, Yamagishi M, Takizawa T, Nakamura T, Miyatake H, et al. Analyses of polyphenols in cacao liquor, cocoa, and chocolate by normal-phase and reversed-phase HPLC. Bioscience, biotechnology, and biochemistry. 2000, 64, 2581–2587. [Google Scholar] [CrossRef]
- Huang Q, Braffett BH, Simmens SJ, Young HA, Ogden CL. Dietary Polyphenol Intake in US Adults and 10-Year Trends: 2007-2016. J Acad Nutr Diet. 2020, 120, 1821–1833. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros R, Knaze V, Rothwell JA, Hémon B, Moskal A, Overvad K, et al. Dietary polyphenol intake in Europe: the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Nutr. 2016, 55, 1359–1375. [Google Scholar] [CrossRef]
- Dugo L, Tripodo G, Santi L, Fanali C. Cocoa Polyphenols: Chemistry, Bioavailability and Effects on Cardiovascular Performance. Curr Med Chem. 2018, 25, 4903–4917. [Google Scholar]
- Holland TM, Agarwal P, Wang Y, Leurgans SE, Bennett DA, Booth SL, et al. Dietary flavonols and risk of Alzheimer dementia. Neurology. 2020, 94, e1749–e56. [Google Scholar]
- Tang D, Tran Y, Shekhawat GS, Gopinath B. Dietary Flavonoid Intake and Chronic Sensory Conditions: A Scoping Review. Antioxidants (Basel, Switzerland). 2022, 11. [Google Scholar]
- Sesso HD, Manson JE, Aragaki AK, Rist PM, Johnson LG, Friedenberg G, et al. Effect of cocoa flavanol supplementation for the prevention of cardiovascular disease events: the COcoa Supplement and Multivitamin Outcomes Study (COSMOS) randomized clinical trial. The American journal of clinical nutrition. 2022, 115, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Ashoori M, Soltani S, Kolahdouz-Mohammadi R, Moghtaderi F, Clayton Z, Abdollahi S. The effect of whole grape products on blood pressure and vascular function: A systematic review and meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2023, 33, 1836–1848. [Google Scholar] [CrossRef] [PubMed]
- Foshati S, Nouripour F, Sadeghi E, Amani R. The effect of grape (Vitis vinifera) seed extract supplementation on flow-mediated dilation, blood pressure, and heart rate: A systematic review and meta-analysis of controlled trials with duration- and dose-response analysis. Pharmacol Res. 2022, 175, 105905. [Google Scholar] [CrossRef]
- Lupoli R, Ciciola P, Costabile G, Giacco R, Minno M, Capaldo B. Impact of Grape Products on Lipid Profile: A Meta-Analysis of Randomized Controlled Studies. J Clin Med. 2020, 9. [Google Scholar]
- Miraghajani M, Momenyan S, Arab A, Hasanpour Dehkordi A, Symonds ME. Blueberry and cardiovascular disease risk factors: A systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2020, 53, 102389. [Google Scholar] [CrossRef]
- Osakabe N, Terao J. Possible mechanisms of postprandial physiological alterations following flavan 3-ol ingestion. Nutr Rev. 2018, 76, 174–186. [Google Scholar] [CrossRef]
- Dudonné S, Dal-Pan A, Dubé P, Varin TV, Calon F, Desjardins Y. Potentiation of the bioavailability of blueberry phenolic compounds by co-ingested grape phenolic compounds in mice, revealed by targeted metabolomic profiling in plasma and feces. Food Funct. 2016, 7, 3421–3430. [Google Scholar] [CrossRef]
- Ingawa K, Aruga N, Matsumura Y, Shibata M, Osakabe N. Alteration of the systemic and microcirculation by a single oral dose of flavan-3-ols. PloS one. 2014, 9, e94853. [Google Scholar]
- Koizumi R, Fushimi T, Sato Y, Fujii Y, Sato H, Osakabe N. Relationship between hemodynamic alteration and sympathetic nerve activation following a single oral dose of cinnamtannin A2. Free radical research. 2021, 55, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Saito A, Inagawa K, Ebe R, Fukase S, Horikoshi Y, Shibata M, et al. Onset of a hypotensive effect following ingestion of flavan 3-ols involved in the activation of adrenergic receptors. Free radical biology & medicine. 2016, 99, 584–592. [Google Scholar]
- Aruga N, Toriigahara M, Shibata M, Ishii T, Nakayama T, Osakabe N. Responses to a single dose of different polyphenols on the microcirculation and systemic circulation in rats. Journal of Functional Foods. 2014, 10, 355–363. [Google Scholar] [CrossRef]
- Fushimi T, Hirahata C, Hiroki K, Fujii Y, Calabrese V, Suhara Y, et al. Activation of transient receptor potential channels is involved in reactive oxygen species (ROS)-dependent regulation of blood flow by (-)-epicatechin tetramer cinnamtannin A2. Biochemical pharmacology. 2023, 214, 115682. [Google Scholar]
- Obara M, Masumoto S, Ono Y, Ozaki Y, Shoji T. Procyanidin Concentrations and H-ORAC of Apples Cultivated in Japan. Food Science and Technology Research. 2016, 22, 563–568. [Google Scholar] [CrossRef]
- Shoji T, Masumoto S, Moriichi N, Kanda T, Ohtake Y. Apple (Malus pumila) procyanidins fractionated according to the degree of polymerization using normal-phase chromatography and characterized by HPLC-ESI/MS and MALDI-TOF/MS. J Chromatogr A. 2006, 1102, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Friedman M, Jürgens HS. Effect of pH on the stability of plant phenolic compounds. J Agric Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Fushimi T, Fujii Y, Koshino H, Inagawa K, Saito A, Koizumi R, et al. Method for detecting hemodynamic alterations following a single gavage in rats. Exp Anim. 2021, 70, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Osakabe N, Shimizu T, Fujii Y, Fushimi T, Calabrese V. Sensory Nutrition and Bitterness and Astringency of Polyphenols. Biomolecules. 2024, 14. [Google Scholar]
- Soares S, Brandão E, Guerreiro C, Soares S, Mateus N, de Freitas V. Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste. Molecules (Basel, Switzerland). 2020, 25. [Google Scholar]
- Schöbel N, Radtke D, Kyereme J, Wollmann N, Cichy A, Obst K, et al. Astringency is a trigeminal sensation that involves the activation of G protein-coupled signaling by phenolic compounds. Chem Senses. 2014, 39, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Takahashi S, Kurogi M, Saitoh O. The diversity in sensitivity of TRPA1 and TRPV1 of various animals to polyphenols. Biomed Res. 2021, 42, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Kurogi M, Kawai Y, Nagatomo K, Tateyama M, Kubo Y, Saitoh O. Auto-oxidation products of epigallocatechin gallate activate TRPA1 and TRPV1 in sensory neurons. Chem Senses. 2015, 40, 27–46. [Google Scholar] [CrossRef] [PubMed]
- 34. Osakabe N, Fushimi T, Fujii Y, Calabrese V. Procyanidins and sensory nutrition; Do procyanidins modulate homeostasis via astringent taste receptors? Bioscience, biotechnology, and biochemistry, 2023.
- Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell. 2002, 108, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Yu X, Yu M, Liu Y, Yu S. TRP channel functions in the gastrointestinal tract. Seminars in immunopathology. 2016, 38, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Kozai D, Ogawa N, Mori Y. Redox regulation of transient receptor potential channels. Antioxidants & redox signaling. 2014, 21, 971–986. [Google Scholar]
- Bensalem J, Dudonné S, Etchamendy N, Pellay H, Amadieu C, Gaudout D, et al. Polyphenols From Grape and Blueberry Improve Episodic Memory in Healthy Elderly with Lower Level of Memory Performance: A Bicentric Double-Blind, Randomized, Placebo-Controlled Clinical Study. The journals of gerontology Series A, Biological sciences and medical sciences. 2019, 74, 996–1007. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).