Submitted:
21 March 2024
Posted:
23 March 2024
You are already at the latest version
Abstract
Keywords:
1. Background
2. Method
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources
2.4. Search Strategy
2.5. Selection Process
2.6. Data Collection Process
2.7. Data Items
2.8. Study Risk of Bias Assessment
2.9. Data Management and Synthesis Methods
3. Results
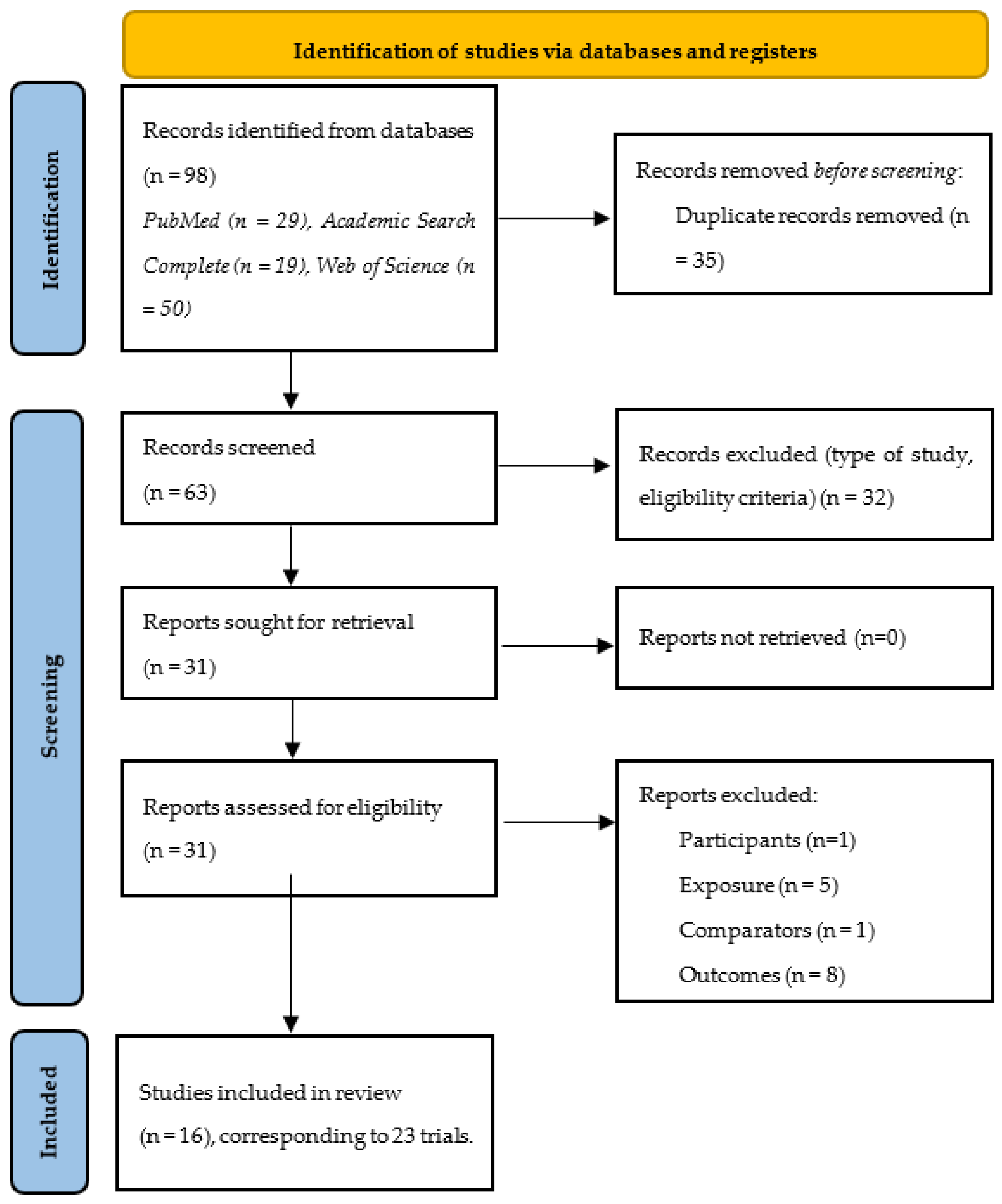
3.1. Study Identification and Selection
3.2. Study Characteristics and Context
3.3. Synthesis of Results
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Health, W. WHO European regional obesity report; 2022.
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Danaei, G.; Ding, E.L.; Mozaffarian, D.; Taylor, B.; Rehm, J.; Murray, C.J.L.; Ezzati, M. Correction: The Preventable Causes of Death in the United States: Comparative Risk Assessment of Dietary, Lifestyle, and Metabolic Risk Factors. PLoS Med. 2011, 8, e1000058. [Google Scholar] [CrossRef]
- Hubert, H.B.; Feinleib, M.; McNamara, P.M.; Castelli, W.P. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983, 67, 968–977. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.D.; Hinder, L.M.; Callaghan, B.C.; Feldman, E.L. Neurological consequences of obesity. Lancet Neurol. 2017, 16, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Prospective Studies Collaboration Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096. [CrossRef]
- Cellik-Guzel, E.; Bakkal, E.; Guzel, S.; Eroglu, H.E.; Acar, A.; Topcu, B.; Kucukyalcin, V. Can low brain-derived neurotrophic factor levels be a marker of the presence of depression in obese women? Neuropsychiatr. Dis. Treat. 2014, 10, 2079–86. [Google Scholar] [CrossRef] [PubMed]
- de Assis, G.G.; Murawska-Ciałowicz, E. Leptin—A Potential Bridge between Fat Metabolism and the Brain’s Vulnerability to Neuropsychiatric Disorders: A Systematic Review. J. Clin. Med. 2021, 10, 5714. [Google Scholar] [CrossRef]
- Chaldakov, G.N.; Fiore, M.; Ranćić, G.; Beltowski, J.; Tunçel, N.; Aloe, L. An Integrated View: Neuroadipocrinology of Diabesity. Serbian J. Exp. Clin. Res. 2014, 15, 61–69. [Google Scholar] [CrossRef]
- van Reedt Dortland, A.K.B.; Giltay, E.J.; van Veen, T.; Zitman, F.G.; Penninx, B.W.J.H. Longitudinal Relationship of Depressive and Anxiety Symptoms With Dyslipidemia and Abdominal Obesity. Psychosom. Med. 2013, 75, 83–89. [Google Scholar] [CrossRef]
- Ramos, L.W.F.; Murad, N.; Goto, E.; Antônio, E.L.; Silva, J.A.; Tucci, P.F.; Carvalho, A.C. Ischemia/reperfusion is an independent trigger for increasing myocardial content of mRNA B-type natriuretic peptide. Heart Vessels 2009, 24, 454. [Google Scholar] [CrossRef]
- Koizumi, M.; Watanabe, H.; Kaneko, Y.; Iino, K.; Ishida, M.; Kosaka, T.; Motohashi, Y.; Ito, H. Impact of obesity on plasma B-type natriuretic peptide levels in Japanese community-based subjects. Heart Vessels 2012, 27, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Vargas, H.; Martínez-Ezquerro, J.D.; Bienvenu, T. Brain-Derived Neurotrophic Factor, Food Intake Regulation, and Obesity. Arch. Med. Res. 2011, 42, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Krabbe, K.S.; Nielsen, A.R.; Krogh-Madsen, R.; Plomgaard, P.; Rasmussen, P.; Erikstrup, C.; Fischer, C.P.; Lindegaard, B.; Petersen, A.M.W.; Taudorf, S.; et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 2007, 50, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Lommatzsch, M.; Zingler, D.; Schuhbaeck, K.; Schloetcke, K.; Zingler, C.; Schuff-Werner, P.; Virchow, J.C. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol. Aging 2005, 26, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Murawska-Ciałowicz, E.; de Assis, G.G.; Clemente, F.M.; Feito, Y.; Stastny, P.; Zuwała-Jagiełło, J.; Bibrowicz, B.; Wolański, P. Effect of four different forms of high intensity training on BDNF response to Wingate and Graded Exercise Test. Sci. Rep. 2021, 11, 8599. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Poo, M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Quirié, A.; Hervieu, M.; Garnier, P.; Demougeot, C.; Mossiat, C.; Bertrand, N.; Martin, A.; Marie, C.; Prigent-Tessier, A. Comparative Effect of Treadmill Exercise on Mature BDNF Production in Control versus Stroke Rats. PLoS One 2012, 7, e44218. [Google Scholar] [CrossRef] [PubMed]
- Babaei, P.; Damirchi, A.; Mehdipoor, M.; Tehrani, B.S. Long term habitual exercise is associated with lower resting level of serum BDNF. Neurosci. Lett. 2014, 566, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.; Brassard, P.; Adser, H.; Pedersen, M. V.; Leick, L.; Hart, E.; Secher, N.H.; Pedersen, B.K.; Pilegaard, H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp. Physiol. 2009, 94, 1062–1069. [Google Scholar] [CrossRef]
- Matthews, V.B.; Åström, M.-B.; Chan, M.H.S.; Bruce, C.R.; Krabbe, K.S.; Prelovsek, O.; Åkerström, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H.; et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef]
- Farinas, I. Neurotrophin actions during the development of the peripheral nervous system. Microsc. Res. Tech. 1999, 45, 233–242. [Google Scholar] [CrossRef]
- McAllister, A.K. Neurotrophins and neuronal differentiation in the central nervous system. Cell. Mol. Life Sci. 2001, 58, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Zagrebelsky, M.; Korte, M. Form follows function: BDNF and its involvement in sculpting the function and structure of synapses. Neuropharmacology 2014, 76, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, N.C.; Chinn, G.; Chou, M.; Kesslak, J.P.; Cotman, C.W. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience 2005, 133, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Intlekofer, K.A.; Berchtold, N.C.; Malvaez, M.; Carlos, A.J.; McQuown, S.C.; Cunningham, M.J.; Wood, M.A.; Cotman, C.W. Exercise and Sodium Butyrate Transform a Subthreshold Learning Event into Long-Term Memory via a Brain-Derived Neurotrophic factor-Dependent Mechanism. Neuropsychopharmacology 2013, 38, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.-A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, G.F.; Rhodes, J.S. Exercise Regulation of Cognitive Function and Neuroplasticity in the Healthy and Diseased Brain. In; 2015; pp. 381–406.
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise Induces Hippocampal BDNF through a PGC-1α/FNDC5 Pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef]
- Picard, M.; McEwen, B.S. Mitochondria impact brain function and cognition. Proc. Natl. Acad. Sci. 2014, 111, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Griffin, É.W.; Mullally, S.; Foley, C.; Warmington, S.A.; O’Mara, S.M.; Kelly, Á.M. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol. Behav. 2011, 104, 934–941. [Google Scholar] [CrossRef]
- Neeper, S.A.; Gómez-Pinilla, F.; Choi, J.; Cotman, C.W. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996, 726, 49–56. [Google Scholar] [CrossRef]
- Vaynman, S.; Ying, Z.; Gomez-Pinilla, F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur. J. Neurosci. 2004, 20, 2580–2590. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Dan, I.; Tsuzuki, D.; Kato, M.; Okamoto, M.; Kyutoku, Y.; Soya, H. Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with Stroop test. Neuroimage 2010, 50, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Higgins JPT, & Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Cochrane, 2011.
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg. Med. J. 2020, 37, 387–387. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.S.-L.; Wilczynski, N.L.; Haynes, R.B. ; Hedges Team Developing optimal search strategies for detecting clinically relevant qualitative studies in MEDLINE. Stud. Health Technol. Inform. 2004, 107, 311–6. [Google Scholar] [PubMed]
- Group CCCR Data extraction template for included studies; 2016.
- Higgins, J.D.J. Selecting studies and collecting data. In Cochrane Handbook for Systematic Reviews of Interventions Version 510; S.Green, J.P.T.H.&, Ed.; The Cochrane Collaboration, 2011; pp. 168–182.
- Lee, D.K.; In, J.; Lee, S. Standard deviation and standard error of the mean. Korean J. Anesthesiol. 2015, 68, 220. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Silva, A.F.; Afonso, J.; Sampaio, A.; Pimenta, N.; Lima, R.F.; Castro, H. de O.; Ramirez-Campillo, R.; Teoldo, I.; Sarmento, H.; González Fernández, F.; et al. Differences in visual search behavior between expert and novice team sports athletes: A systematic review with meta-analysis. Front. Psychol. 2022; 13. [Google Scholar] [CrossRef]
- Drevon, D.; Fursa, S.R.; Malcolm, A.L. Intercoder Reliability and Validity of WebPlotDigitizer in Extracting Graphed Data. Behav. Modif. 2017, 41, 323–339. [Google Scholar] [CrossRef]
- Claudino, J.G.; Afonso, J.; Sarvestan, J.; Lanza, M.B.; Pennone, J.; Filho, C.A.C.; Serrão, J.C.; Espregueira-Mendes, J.; Vasconcelos, A.L.V.; de Andrade, M.P.; et al. Strength Training to Prevent Falls in Older Adults: A Systematic Review with Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2021, 10, 3184. [Google Scholar] [CrossRef] [PubMed]
- Park, J, Lee Y, Seo H, Jang B, Son H, Kim S, Shin S, & H.S. Risk of Bias Assessment tool for Non-randomized Studies (RoBANS): Development and validation of a new instrument. In Proceedings of the 19th Cochrane Colloquium; 2011; pp. 19–22.
- Ramirez-Campillo, R.; Castillo, D.; Raya-González, J.; Moran, J.; de Villarreal, E.S.; Lloyd, R.S. Effects of Plyometric Jump Training on Jump and Sprint Performance in Young Male Soccer Players: A Systematic Review and Meta-analysis. Sport. Med. 2020, 50, 2125–2143. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Campillo, R.; Sanchez-Sanchez, J.; Romero-Moraleda, B.; Yanci, J.; García-Hermoso, A.; Manuel Clemente, F. Effects of plyometric jump training in female soccer player’s vertical jump height: A systematic review with meta-analysis. J. Sports Sci. 2020, 38, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef]
- Huedo-Medina, T.B.; Sánchez-Meca, J.; Marín-Martínez, F.; Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods 2006, 11, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive Statistics for Studies in Sports Medicine and Exercise Science. Med. Sci. Sport. Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Sanchéz, M.A.; Bustos-Cruz, R.H.; Velasco-Orjuela, G.P.; Quintero, A.P.; Tordecilla-Sanders, A.; Correa-Bautista, J.E.; Triana-Reina, H.R.; García-Hermoso, A.; González-Ruíz, K.; Peña-Guzmán, C.A.; et al. Acute Effects of High Intensity, Resistance, or Combined Protocol on the Increase of Level of Neurotrophic Factors in Physically Inactive Overweight Adults: The BrainFit Study. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef]
- Roh, H.-T.; Cho, S.-Y.; So, W.-Y. Obesity promotes oxidative stress and exacerbates blood-brain barrier disruption after high-intensity exercise. J. Sport Heal. Sci. 2017, 6, 225–230. [Google Scholar] [CrossRef]
- Wheeler, M.J.; Green, D.J.; Ellis, K.A.; Cerin, E.; Heinonen, I.; Naylor, L.H.; Larsen, R.; Wennberg, P.; Boraxbekk, C.-J.; Lewis, J.; et al. Distinct effects of acute exercise and breaks in sitting on working memory and executive function in older adults: a three-arm, randomised cross-over trial to evaluate the effects of exercise with and without breaks in sitting on cognition. Br. J. Sports Med. 2020, 54, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Roh, H.T. Effects of aerobic exercise training on peripheral brain-derived neurotrophic factor and eotaxin-1 levels in obese young men. J. Phys. Ther. Sci. 2016, 28, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-Y.; So, W.-Y.; Roh, H.-T. Effects of aerobic exercise training and cranial electrotherapy stimulation on the stress-related hormone, the neurotrophic factor, and mood states in obese middle-aged women: a pilot clinical trial. Salud Ment. 2016, 39, 249–256. [Google Scholar] [CrossRef]
- Damirchi, A.; Tehrani, B.S.; Alamdari, K.A.; Babaei, P. Influence of Aerobic Training and Detraining on Serum BDNF, Insulin Resistance, and Metabolic Risk Factors in Middle-Aged Men Diagnosed With Metabolic Syndrome. Clin. J. Sport Med. 2014, 24, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Glud, M.; Christiansen, T.; Larsen, L.H.; Richelsen, B.; Bruun, J.M. Changes in Circulating BDNF in relation to Sex, Diet, and Exercise: A 12-Week Randomized Controlled Study in Overweight and Obese Participants. J. Obes. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Goldfield, G.S.; Kenny, G.P.; Prud’homme, D.; Holcik, M.; Alberga, A.S.; Fahnestock, M.; Cameron, J.D.; Doucette, S.; Hadjiyannakis, S.; Tulloch, H.; et al. Effects of aerobic training, resistance training, or both on brain-derived neurotrophic factor in adolescents with obesity: The hearty randomized controlled trial. Physiol. Behav. 2018, 191, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Gyorkos, A.; Baker, M.H.; Miutz, L.N.; Lown, D.A.; Jones, M.A.; Houghton-Rahrig, L.D. Carbohydrate-restricted Diet and Exercise Increase Brain-derived Neurotrophic Factor and Cognitive Function: A Randomized Crossover Trial. Cureus 2019, 11, e5604. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Yoo, J.H.; Kang, S.; Woo, J.H.; Shin, K.O.; Kim, K.B.; Cho, S.Y.; Roh, H.T.; Kim, Y. Il The Effects of 12 Weeks Regular Aerobic Exercise on Brain-derived Neurotrophic Factor and Inflammatory Factors in Juvenile Obesity and Type 2 Diabetes Mellitus. J. Phys. Ther. Sci. 2014, 26, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Levinger, I.; Goodman, C.; Matthews, V.; Hare, D.L.; Jerums, G.; Garnham, A.; Selig, S. BDNF, Metabolic Risk Factors, and Resistance Training in Middle-Aged Individuals. Med. Sci. Sport. Exerc. 2008, 40, 535–541. [Google Scholar] [CrossRef]
- Li, X.; Han, T.; Zou, X.; Zhang, H.; Feng, W.; Wang, H.; Shen, Y.; Zhang, L.; Fang, G. Long-term high-intensity interval training increases serum neurotrophic factors in elderly overweight and obese Chinese adults. Eur. J. Appl. Physiol. 2021, 121, 2773–2785. [Google Scholar] [CrossRef]
- Osali, A. Aerobic exercise and nano-curcumin supplementation improve inflammation in elderly females with metabolic syndrome. Diabetol. Metab. Syndr. 2020, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.-T.; So, W.-Y. The effects of aerobic exercise training on oxidant–antioxidant balance, neurotrophic factor levels, and blood–brain barrier function in obese and non-obese men. J. Sport Heal. Sci. 2017, 6, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Saleh V, Afroundeh R, Siahkohiyan M, & A.A. Anaerobic gymnastics exercises evoke systemic brain-derived neurotrophic factor in obese and normal-weight children. Int. J. Pediatr. 2020; 8, 12533–12544. [CrossRef]
- Žlibinaitė, L.; Solianik, R.; Vizbaraitė, D.; Mickevičienė, D.; Skurvydas, A. The Effect of Combined Aerobic Exercise and Calorie Restriction on Mood, Cognition, and Motor Behavior in Overweight and Obese Women. J. Phys. Act. Heal. 2020, 17, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, H.; Luo, Q.; Cui, S. The effect of physical exercise on circulating brain-derived neurotrophic factor in healthy subjects: A meta-analysis of randomized controlled trials. Brain Behav. 2022, 12, e2544. [Google Scholar] [CrossRef] [PubMed]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Lanctôt, K.L. The effect of acute exercise on blood concentrations of brain-derived neurotrophic factor in healthy adults: a meta-analysis. Eur. J. Neurosci. 2017, 46, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Larsen, K.T.; Ried-Larsen, M.; Møller, N.C.; Andersen, L.B. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: A review. Scand. J. Med. Sci. Sports 2014, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef]
- Marinus, N.; Hansen, D.; Feys, P.; Meesen, R.; Timmermans, A.; Spildooren, J. The Impact of Different Types of Exercise Training on Peripheral Blood Brain-Derived Neurotrophic Factor Concentrations in Older Adults: A Meta-Analysis. Sport. Med. 2019, 49, 1529–1546. [Google Scholar] [CrossRef]
- Knaepen, K.; Goekint, M.; Heyman, E.M.; Meeusen, R. Neuroplasticity – Exercise-Induced Response of Peripheral Brain-Derived Neurotrophic Factor. Sport. Med. 2010, 40, 765–801. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, R.; Álvarez-Bueno, C.; Martínez-Ortega, I.A.; Martínez-Vizcaíno, V.; Mesas, A.E.; Notario-Pacheco, B. Immediate effect of high-intensity exercise on brain-derived neurotrophic factor in healthy young adults: A systematic review and meta-analysis. J. Sport Heal. Sci. 2022, 11, 367–375. [Google Scholar] [CrossRef]
- Fujimura, H.; Altar, C.A.; Chen, R.; Nakamura, T.; Nakahashi, T.; Kambayashi, J.; Sun, B.; Tandon, N.N. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb. Haemost. 2002, 87, 728–34. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.I.; Smith, L.; Northey, J.; Rattray, B.; Cherbuin, N. Towards an understanding of the physical activity-BDNF-cognition triumvirate: A review of associations and dosage. Ageing Res. Rev. 2020, 60, 101044. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.J.; Scribbans, T.D.; Bentley, R.F.; Kellawan, J.M.; Gurd, B.; Tschakovsky, M.E. Neurotrophic growth factor responses to lower body resistance training in older adults. Appl. Physiol. Nutr. Metab. 2016, 41, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Bechara, R.G.; Lyne, R.; Kelly, Á.M. BDNF-stimulated intracellular signalling mechanisms underlie exercise-induced improvement in spatial memory in the male Wistar rat. Behav. Brain Res. 2014, 275, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.S.; Monteiro, P.A.; Gerosa-Neto, J.; Santana, P.R.; Peres, F.P.; Edwards, K.M.; Lira, F.S. Acute increases in brain-derived neurotrophic factor following high or moderate-intensity exercise is accompanied with better cognition performance in obese adults. Sci. Rep. 2020, 10, 13493. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Vaynman, S.; Akhavan, M.; Ying, Z.; Gomez-Pinilla, F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience 2006, 140, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Saucedo Marquez, C.M.; Vanaudenaerde, B.; Troosters, T.; Wenderoth, N. High-intensity interval training evokes larger serum BDNF levels compared with intense continuous exercise. J. Appl. Physiol. 2015, 119, 1363–1373. [Google Scholar] [CrossRef]
- Rios, M. BDNF and the central control of feeding: accidental bystander or essential player? Trends Neurosci. 2013, 36, 83–90. [Google Scholar] [CrossRef]
- Cordeira, J.; Rios, M. Weighing in the Role of BDNF in the Central Control of Eating Behavior. Mol. Neurobiol. 2011, 44, 441–448. [Google Scholar] [CrossRef]
- Xu, B.; Xie, X. Neurotrophic factor control of satiety and body weight. Nat. Rev. Neurosci. 2016, 17, 282–292. [Google Scholar] [CrossRef]
- Pandit, M.; Behl, T.; Sachdeva, M.; Arora, S. Role of brain derived neurotropic factor in obesity. Obes. Med. 2020, 17, 100189. [Google Scholar] [CrossRef]
- Alomari, M.A.; Khabour, O.F.; Alawneh, K.; Alzoubi, K.H.; Maikano, A.B. The importance of physical fitness for the relationship of BDNF with obesity measures in young normal-weight adults. Heliyon 2020, 6, e03490. [Google Scholar] [CrossRef]
- Lee, I.-T.; Wang, J.-S.; Fu, C.-P.; Lin, S.-Y.; Sheu, W.H.-H. Relationship between body weight and the increment in serum brain-derived neurotrophic factor after oral glucose challenge in men with obesity and metabolic syndrome. Medicine (Baltimore). 2016, 95, e5260. [Google Scholar] [CrossRef] [PubMed]
- Murawska-Ciałowicz, E.; Wiatr, M.; Ciałowicz, M.; Gomes de Assis, G.; Borowicz, W.; Rocha-Rodrigues, S.; Paprocka-Borowicz, M.; Marques, A. BDNF Impact on Biological Markers of Depression—Role of Physical Exercise and Training. Int. J. Environ. Res. Public Health 2021, 18, 7553. [Google Scholar] [CrossRef] [PubMed]
- Suwa, M.; Yamamoto, K.-I.; Nakano, H.; Sasaki, H.; Radak, Z.; Kumagai, S. Brain-Derived Neurotrophic Factor Treatment Increases the Skeletal Muscle Glucose Transporter 4 Protein Expression in Mice. Physiol. Res. 2010, 59, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.L.; Whitehurst, M.; Fico, B.G.; Dodge, K.M.; Ferrandi, P.J.; Pena, G.; Adelman, A.; Huang, C.-J. Acute high-intensity interval exercise induces greater levels of serum brain-derived neurotrophic factor in obese individuals. Exp. Biol. Med. 2018, 243, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Chaldakov, G.N.; Tonchev, A.B.; Aloe, L. NGF and BDNF: from nerves to adipose tissue, from neurokines to metabokines. Riv. Psichiatr. 2009, 44, 79–87. [Google Scholar] [PubMed]
- Magistretti, P.J.; Allaman, I. Lactate in the brain: from metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef]
- Proia, P.; Di Liegro, C.; Schiera, G.; Fricano, A.; Di Liegro, I. Lactate as a Metabolite and a Regulator in the Central Nervous System. Int. J. Mol. Sci. 2016, 17, 1450. [Google Scholar] [CrossRef]
- Müller, P.; Duderstadt, Y.; Lessmann, V.; Müller, N.G. Lactate and BDNF: Key Mediators of Exercise Induced Neuroplasticity? J. Clin. Med. 2020, 9, 1136. [Google Scholar] [CrossRef]
- Adeva-Andany, M.; López-Ojén, M.; Funcasta-Calderón, R.; Ameneiros-Rodríguez, E.; Donapetry-García, C.; Vila-Altesor, M.; Rodríguez-Seijas, J. Comprehensive review on lactate metabolism in human health. Mitochondrion 2014, 17, 76–100. [Google Scholar] [CrossRef]
- Bergersen, L.H. Is lactate food for neurons? Comparison of monocarboxylate transporter subtypes in brain and muscle. Neuroscience 2007, 145, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Dienel, G.A. The metabolic trinity, glucose–glycogen–lactate, links astrocytes and neurons in brain energetics, signaling, memory, and gene expression. Neurosci. Lett. 2017, 637, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Stern, S.A.; Bozdagi, O.; Huntley, G.W.; Walker, R.H.; Magistretti, P.J.; Alberini, C.M. Astrocyte-Neuron Lactate Transport Is Required for Long-Term Memory Formation. Cell 2011, 144, 810–823. [Google Scholar] [CrossRef] [PubMed]
- Steinman, M.Q.; Gao, V.; Alberini, C.M. The Role of Lactate-Mediated Metabolic Coupling between Astrocytes and Neurons in Long-Term Memory Formation. Front. Integr. Neurosci. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Jodeiri Farshbaf, M.; Ghaedi, K.; Megraw, T.L.; Curtiss, J.; Shirani Faradonbeh, M.; Vaziri, P.; Nasr-Esfahani, M.H. Does PGC1α/FNDC5/BDNF Elicit the Beneficial Effects of Exercise on Neurodegenerative Disorders? NeuroMolecular Med. 2016, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J. Neurosci. 2019, 1661–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, R.; Meng, Y.; Li, S.; Donelan, W.; Zhao, Y.; Qi, L.; Zhang, M.; Wang, X.; Cui, T.; et al. Irisin Stimulates Browning of White Adipocytes Through Mitogen-Activated Protein Kinase p38 MAP Kinase and ERK MAP Kinase Signaling. Diabetes 2014, 63, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Qiao, X.; Ma, Y.; Deng, H.; Xu, C.C.; Xu, L. Disordered metabolism in mice lacking irisin. Sci. Rep. 2020, 10, 17368. [Google Scholar] [CrossRef]
- Murawska-Cialowicz, E.; Wolanski, P.; Zuwala-Jagiello, J.; Feito, Y.; Petr, M.; Kokstejn, J.; Stastny, P.; Goliński, D. Effect of HIIT with Tabata Protocol on Serum Irisin, Physical Performance, and Body Composition in Men. Int. J. Environ. Res. Public Health 2020, 17, 3589. [Google Scholar] [CrossRef]
- Archundia-Herrera, C.; Macias-Cervantes, M.; Ruiz-Muñoz, B.; Vargas-Ortiz, K.; Kornhauser, C.; Perez-Vazquez, V. Muscle irisin response to aerobic vs HIIT in overweight female adolescents. Diabetol. Metab. Syndr. 2017, 9, 101. [Google Scholar] [CrossRef]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Liu, C.S.; Sherman, C.; Chan, S.; Lanctôt, K.L. The Effect of Exercise Training on Resting Concentrations of Peripheral Brain-Derived Neurotrophic Factor (BDNF): A Meta-Analysis. PLoS One 2016, 11, e0163037. [Google Scholar] [CrossRef] [PubMed]
- Shobeiri, P.; Karimi, A.; Momtazmanesh, S.; Teixeira, A.L.; Teunissen, C.E.; van Wegen, E.E.H.; Hirsch, M.A.; Yekaninejad, M.S.; Rezaei, N. Exercise-induced increase in blood-based brain-derived neurotrophic factor (BDNF) in people with multiple sclerosis: A systematic review and meta-analysis of exercise intervention trials. PLoS One 2022, 17, e0264557. [Google Scholar] [CrossRef]
- Ruiz-González, D.; Hernández-Martínez, A.; Valenzuela, P.L.; Morales, J.S.; Soriano-Maldonado, A. Effects of physical exercise on plasma brain-derived neurotrophic factor in neurodegenerative disorders: A systematic review and meta-analysis of randomized controlled trials. Neurosci. Biobehav. Rev. 2021, 128, 394–405. [Google Scholar] [CrossRef]
- Fleitas, J.C.; Hammuod, S.F.P.; Kakuta, E.; Loreti, E.H. A meta-analysis of the effects of physical exercise on peripheral levels of a brain-derived neurotrophic factor in the elderly. Biomarkers 2022, 27, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Begliuomini, S.; Lenzi, E.; Ninni, F.; Casarosa, E.; Merlini, S.; Pluchino, N.; Valentino, V.; Luisi, S.; Luisi, M.; Genazzani, A.R. Plasma brain-derived neurotrophic factor daily variations in men: correlation with cortisol circadian rhythm. J. Endocrinol. 2008, 197, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Gejl, A.K.; Enevold, C.; Bugge, A.; Andersen, M.S.; Nielsen, C.H.; Andersen, L.B. Associations between serum and plasma brain-derived neurotrophic factor and influence of storage time and centrifugation strategy. Sci. Rep. 2019, 9, 9655. [Google Scholar] [CrossRef]
- Roeh, A.; Holdenrieder, S.; Schoenfeld, J.; Haeckert, J.; Halle, M.; Falkai, P.; Scherr, J.; Hasan, A. Decreased Serum Brain-Derived Neurotrophic Factor Concentrations 72 Hours Following Marathon Running. Front. Physiol. 2021, 12. [Google Scholar] [CrossRef]
- Azevedo, K.P.M. de; de Oliveira, V.H.; Medeiros, G.C.B.S. de; Mata, Á.N. de S.; García, D.Á., Martínez, D.G., Leitão, J.C., Knackfuss, M.I., Eds.; Piuvezam, G. The Effects of Exercise on BDNF Levels in Adolescents: A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 6056. [Google Scholar] [CrossRef]
| Database | Specificities of the databases | Search Strategy |
|---|---|---|
| PubMed | None to report | ("brain derived neurotrophic factor"[MeSH Terms] OR ("brain derived"[All Fields] AND "neurotrophic"[All Fields] AND "factor"[All Fields]) OR "brain derived neurotrophic factor"[All Fields] OR "bdnf"[All Fields] OR ("brain derived neurotrophic factor"[MeSH Terms] OR ("brain derived"[All Fields] AND "neurotrophic"[All Fields] AND "factor"[All Fields]) OR "brain derived neurotrophic factor"[All Fields] OR ("brain"[All Fields] AND "derived"[All Fields] AND "neurotrophic"[All Fields] AND "factor"[All Fields]) OR "brain derived neurotrophic factor"[All Fields])) AND ("aerobic*"[All Fields] OR "HIIT"[All Fields] OR ("high intensity interval training"[MeSH Terms] OR ("high intensity"[All Fields] AND "interval"[All Fields] AND "training"[All Fields]) OR "high intensity interval training"[All Fields] OR ("high"[All Fields] AND "intensity"[All Fields] AND "interval"[All Fields] AND "training"[All Fields]) OR "high intensity interval training"[All Fields]) OR "anaerobic*"[All Fields]) AND ("obeses"[All Fields] OR "obesity"[MeSH Terms] OR "obesity"[All Fields] OR "obese"[All Fields] OR "obesities"[All Fields] OR "obesity s"[All Fields] OR ("overweight"[MeSH Terms] OR "overweight"[All Fields] OR "overweighted"[All Fields] OR "overweightness"[All Fields] OR "overweights"[All Fields])) |
| Academic Search Complete | Search for title and abstract also includes keywords | "(BDNF OR brain-derived neurotrophic factor) AND (aerobic* OR HIIT OR high intensity interval training OR anaerobic*) AND (obesity OR overweight) |
| Web of Science | Search for title and abstract also includes keywords and its designated “topic” | BDNF OR brain-derived neurotrophic factor (All Fields) and aerobic* OR HIIT OR high intensity interval training OR anaerobic* (All Fields) and obesity OR overweight (All Fields) |
| Study | Study design | Population (n) | Sex | Sample characteristics | Mean age (years) | BMI (kg/m2) | Training Status |
|---|---|---|---|---|---|---|---|
| Dominguez-Sanchez 2018a,b,c [56] | RCT (parallel-group | 51 Overweight adults (BMI ≥25 and ≤30 kg/m2) | Men | -HIIT group: 14 -RT group: 12 -CT group: 12 -Non-exercising control: 12 |
- HIIT group: 24.5±3.7 - RT group: 22.8±3.1 - CT group: 22.2±3.4 -Non-exercising control: 24.7±3.4 |
-HITT group: 27.4±1.7 -RT group: 27.8±1.3 -CT group: 28.1±1.2 -Non-exercising control: 28.7±2.0 |
Physically inactive |
| Roh et al. 2017a [57] | RCT (parallel-group | 24 obese and non-obese healthy | Men | -Obese group: 12 -Non-obese group: 12 |
-Obese group: 22.9±2.2 -Non-obese group: 22.9±2.2 |
-Obese group: 29.3±3.0 -Non-obese group: 21.5±1.6 |
Untrained |
| Wheeler et al. 2020 a,b [58] | RCT (parallel-group | 65 overweight/obese older adults with normal cognitive function | Men and postmenopausal women (≥55 to ≤80 years; BMI ≥25 kg/m2 to <45 kg/m2) | -Sitting: 22 - EX+SIT: 23 - EX+BR: 20 |
67±7 years | 31.2±4.1 | Sedentary |
| Study | Physical Exercise protocol | Comparators | Main outcome | Brand/company name of BDNF kits | Main Results |
|---|---|---|---|---|---|
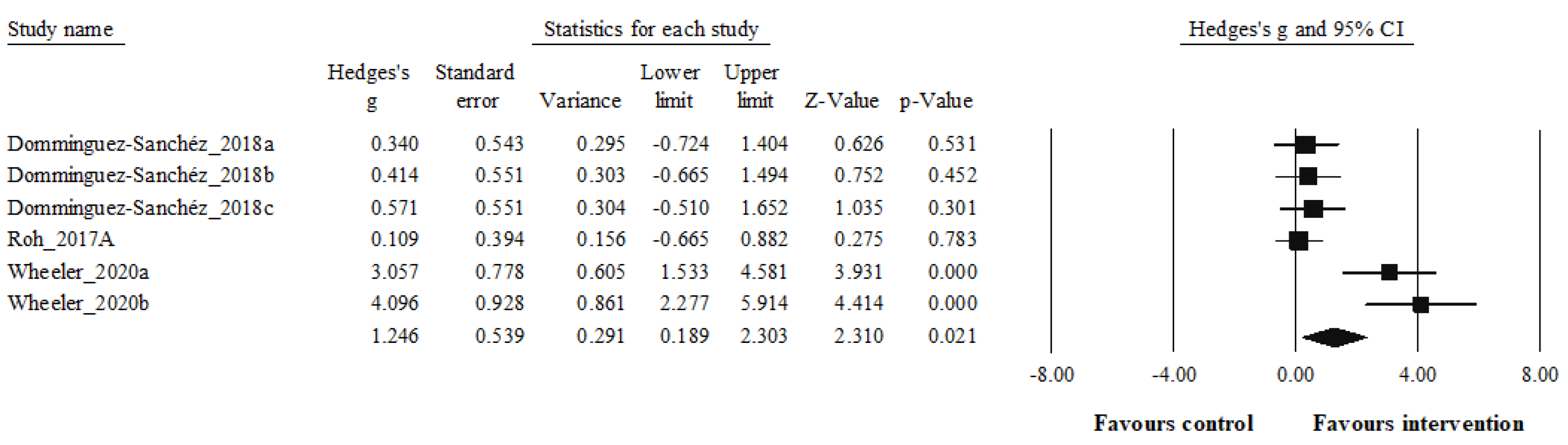
| Dominguez-Sanchez 2018a,b,c [56] | -HIIT group: 5-min warm-up and ended with a 4-min cooldown at a 65% HRmax; HIIT: four bouts of 4-min intervals at 85– 95% HRmax interspersed with 4 min of active recovery at 75–85% HRmax -RT group: initiated with ≈12–15 repetitions per set of six exercises that targeted all the major muscle groups at high intensity included both upper and lower body large-muscle exercises using weight machines (bicep screw curl, triceps extension, dumbbell side lateral raise, military press, dumbbell squat, and dumbbell front) CG group: underwent both the HIIT and RT protocols, as described above, in that order. |
Non-exercising control: Without exercise training | Plasma BDNF (ng/mL) | SPR Biosensors methods, an amino-coupling chemistry kit KAN-50 Coupling Kit (GE Healthcare, Uppsala, Sweden) | -HIIT group: BDNF increased (+6.8 %, p= 0.134) -RT group: BDNF increased (+9.3 %, p= 0.066) -CT: BDNF increased +11.6 %, p=0.029) -CG: BDNF increased +0.6 %, p=0.804) -Compared to the CG, the greatest increase was as follows: CG (+20.8 %), RT (14.3 %), HIIT (9.8 %) |
| Roh et al. 2017a [57] | Acute exercise on the treadmill was applied to both groups. Treadmill run of 20 min at an intensity of 85% of the anthropometrically measured VO2max (Bruce protocol) | - | Serum BDNF (pg/mL) | Human BDNF enzyme-linked immunosorbent assay (ELISA) kit (cat. no. DBD00; R&D Systems, Minneapolis, MN, USA) |
-BDNF increased in both groups (obese and non-obese) after acute exercise. -Compared to the non-obese, BDNF increased more in the obese group after exercise. |
| Wheeler et al. 2020a,b [58] | -EX+SIT: sitting (1 hour), moderate-intensity walking (30 min, the speed was set at 3.2 km/hour, between 65% and 75% of age predicted HRmax), uninterrupted sitting (6.5 hours), that is, 30 min. aerobic exercise, 109±12 bpm, 71%±8% HRmax, 11±2 RPE -EX+BR: sitting (1 hour), moderate-intensity walking (30 min, the speed was set at 3.2 km/hour, HR between 65% and 75% of age predicted HRmax), sitting interrupted every 30 min with 3 min of light-intensity walking (6.5 hours), that is, 30 min. aerobic exercise 109±12 bpm, 71%±8% HRmax, 11±2 RPE. |
SIT: uninterrupted sitting (8 hours, control) |
Serum BDNF (ng/mL) | Human BDNF ELISA Kits (R&D Systems, Wiesbaden, Germany | -Serum BDNF was increased in both EX+SIT, +171 (−449 to +791, p=0.03 vs SIT), and EX+BR, +139 (−481 to +759, p=0.045 vs SIT), relative to SIT, −227 (−851 to +396). -No significant differeence was observed between EX+SIT and EX+BR |
| Study | Study design | Population (n) | Sex | Sample characteristics | Mean age (years) | BMI (kg/m2) | Training Status |
|---|---|---|---|---|---|---|---|
| Cho et al. 2016a [59] | RCT (parallel-group) | 16 obese young men with a BMI greater than 25 kg/m2. | Men | -EG: 8 -CG: 8 |
-EG: 22.9±2.5 -CG: 22.3±2.1 |
-EG: 28.7±2.5 -CG: 27.7±2.2 |
No participation in regular physical activity programs |
| Cho et al. 2016b [60] | RCT (parallel-group) | 36 obese middle-aged Korean women | Women | -AE group: 12 -AE+CES group: 12 -CG: 12 |
-AE group: 54.83± 2.79 -AE+CES group : 54.75±2.45 -CG: 54.67±3.03 |
-AE group: 26.87±2.15 -AE+CES group: 26.90±2.82 -CG: 26.28±1.67 |
No participation in regular physical activity programs |
| Damirchi et al. 2014 [61] | RCT (parallel-group) | 21 middle-aged men diagnosed with MetS | Men | -EG: 11 -CG: 10 |
-EG: 54.12±2.77 -CG: 55.57±3.45 |
-EG: 29.8±3.75 -CG: 27.04±2.89 |
None of the subjects followed a training program within the past 2 year |
| Glud et al. 2019ab [62] | RCT (parallel-group) | 50 overweight or obese (26 women, 24 men) but healthy individuals | Men and Women | -EXO: 7 women, 9 men -DIO: 8 women, 6 men -DEX: 11 women, 9 men |
-EXO: 31.2±2.9- 35±1.3 -DIO: 32.4±2.4- 37.2±2.7 -DEX: 33.4±2.1-35.1 ± 3.9 |
-EXO: 31.2±2.9- 35±1.3 -DIO: 32.4±2.4- 37.2±2.7 -DEX: 33.4±2.1-35.1 ±3.9 |
Physically inactive |
| Goldfield et al. a,b,c 2018 [63] | RCT (parallel-group) | 282 adolescents with overweight and obesity (84 boys, 198 girls) |
Boys and girls | -AE group: n=69 -RT group: n=70 -CT group: n=74 -CG: n=69 |
-AE group: 15.5±1.3 -RT group: 15.8±1.5 -CT group: 15.5±1.3 -CG: 15.6±1.3 |
-AE group= 34.6±4.2 -RT group= 35.3±4.8 -CT group= 34.5±4.1 -CG= 34.3±5.0 |
Irregularly active |
| Gyorkos et al. 2019 [64] | RCT (parallel-group) | 12 obese with MetS | Free-Living İndividuals | -CRPD-Sed: 5 -CRPD-Ex: 7 |
40.9±20.2 years of age | - | Sedentary (defined as not engaged in physical activity at least 3 days/week for three months) |
| Study | Study design | Population (n) | Sex | Sample characteristics | Mean age (years) | BMI (kg/m2) | Training Status |
|---|---|---|---|---|---|---|---|
| Lee et al. 2014 [65] | RCT (parallel-group) | 26 juveniles (boys = 15, girls = 9) | Boys and girls | -Obesity group (OG, n= 8) -T2DM group (TG, n=7) -CG (CG, n = 11) |
-Obesity group= 16.37±0.91 -T2DM group= 15.57±2.14 -CG= 16.45±1.36 |
-Obesity group= 27.47±2.51 -T2DM group= 23.72±4.47 -CG= 22.35±3.94 |
Physically inactive |
| Levinger et al. 2008a [66] | RCT (parallel-group) | 49 men (N = 25) and women (N = 24) | Men and women | -HiMF training: 15 -HiMF control: 14 -LoMF training: 10 -LoMF control: 10 |
-HiMF training: 51.0±7.0 -HiMF control: 51.9±5.8 -LoMF training: 50.3±4.1 -LoMF control : 48.9±7.4 |
-HiMF training: 31.6±4.6 -HiMF control: 30.3±3.7 -LoMF training: 23.9±3.4 -LoMF control: 23.8±3.0 |
Physically inactive |
| Li et al. 2021a,b [67] | RCT (parallel-group) | 29 (18 males and 11 females) older adults | Male and female | -HIIT (group: 10 -VICT group: 10 -CG: 9 |
-HIIT group: 64.9±3.45 -VICT group: 66.4±4.50 -CG: 63.9±3.95 |
-HIIT group: 27.8±1.04 -VICT group: 27.7±2.84 -CG: 27.1±1.50 |
Physically inactive |
| Osali et al. 2020 [68] | RCT (parallel-group) | 44 women with Mets | Women | -MetS exercise+Nano-Curcumin: 11 -MetS exercise: 11 -MetS Nano-Curcumin: 11 -MetS control: 11 |
62.3±1.23 years | MetS exercise+Nano-Curcumin: 31.24±3.12 MetS exercise: 32.22±2.46 MetS Nano-Curcumin: 29.54±2.67 MetS control: 29.02±1.56 |
Pysically inactive (<30 min of physical activity or exercise training per day) |
| Roh et al. 2017b [69] | RCT (parallel-group) | 20 healthy |
Men | -Obese group:10 -Non-obese group: 10 |
-Obese group: 23.00±2.36 -Non-obese group: 22.80±2.35 |
-Obese group: 29.74±3.12 -Non-obese group: 22.00±1.22 |
Physically inactive |
| Saleh et al. 2020 [70] | RCT (parallel-group) | 60 subjects with age range of 8 to 12 years old | -Obese experimental group: 15 -Obese control group: 15 -Normal weight experimental group: 15 -Normal weight control group: 15 |
-Obese experimental group: 10.13±1.35 -Obese control group: 9.80±1.32 -Normal weight experimental group: 9.80±1.47 -Normal weight control group: 9.86±1.30 |
Body fat (%) -Obese experimental group: 27.33±0.67 -Obese control group: 27.03±0.69 -Normal weight experimental group: 6.74±0.35 -Normal weight control group: 6.79±0.45 |
Participants who enrolled in the elementary level of gymnastics | |
| Zibinaite et al. 2019 [71] | RCT (parallel-group) | 26 overweight and obese participants | Women | EG: 13 CG: 13 |
EG: 44.2±8.7 CG: 44.1±5.8 |
EG: 31.9±3.4 CG: 32.5±3.6 |
Women with a sedentary lifestyle (regular exercise ≤1 h/week) |
| Study | Physical Exercise protocol | Comparators | Main outcome | Brand/company name of BDNF kits | Main Results |
|---|---|---|---|---|---|
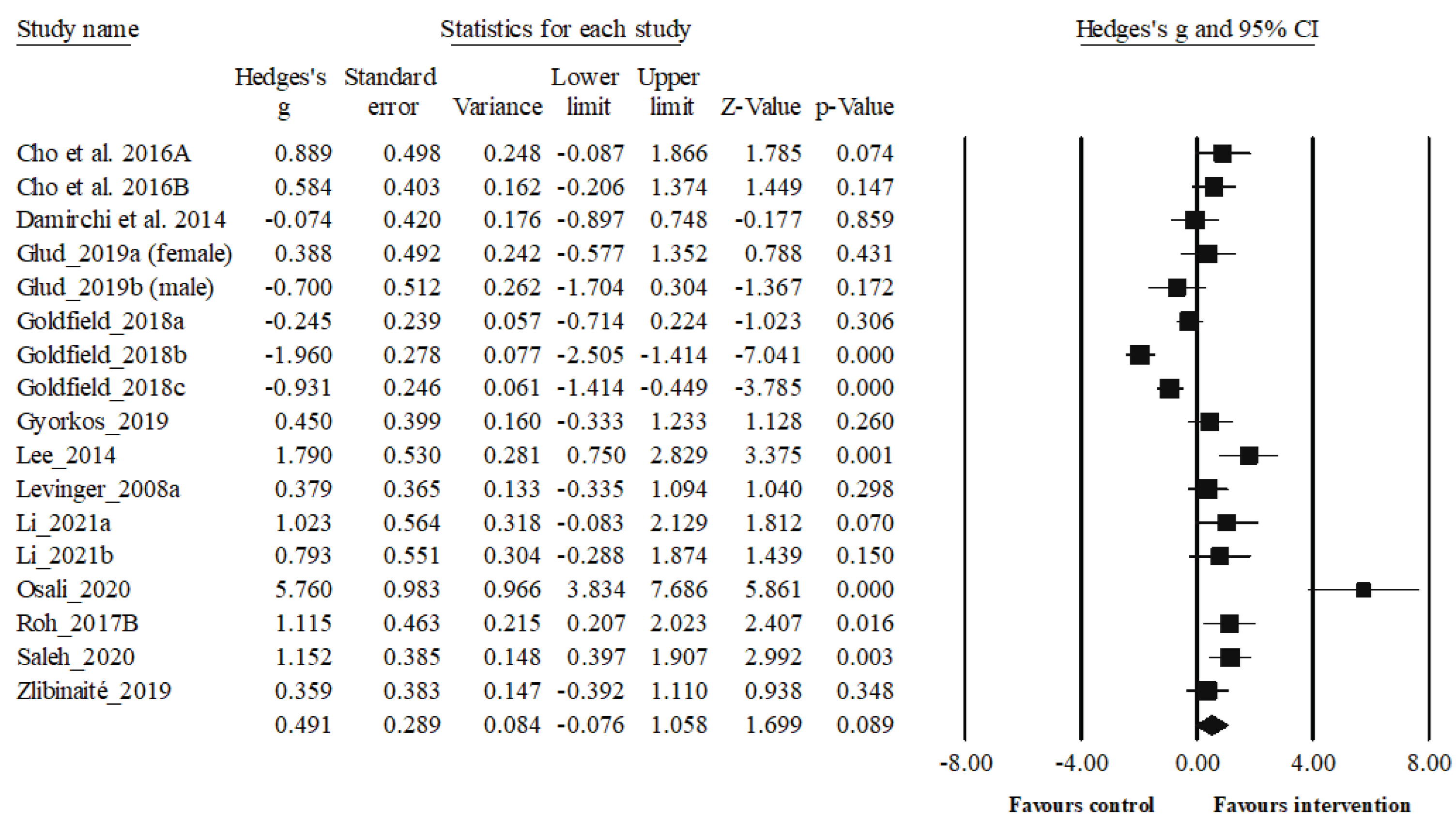
| Cho et al. 2016a [59] | EG had undergone the supervised treadmill running exercise [70% of the HRR, 40 min each] sessions 3 times a week for 8 weeks | CG just maintained their usual lifestyles | Serum BDNF (ng/mL) | A human BDNF ELISA Kit (R&D Systems, Minneapolis, MN, USA). | After intervention, BDNF increased significantly only in EG (20.56 %) |
| Cho et al. 2016b [60] | The AE group was subjected to three sessions of 40-minute treadmill running a week for 8 weeks. The program consisted of 10-minutes of warm-up stretching before the exercise and 10-minutes of cool-down stretching after the exercise. Exercise intensity of treadmill running was set at 70% of HRR determined using Karvonen’s formula. | CG maintained their own lifestyles with no intervention. | Serum BDNF (pg/mL) | A human BDNF ELISA Kit (Cat. no. DBD00; R&D Systems, Minneapolis, MN, USA) |
Serum BDNF levels were significantly increased in the AE group compared to CG (p <.05). |
| Damirchi et al. 2014 [61] | The participants followed a 6-week aerobic training Programs (3 sessions per week; 25-40 minutes walking, running by 50%-60% of V̇O2peak adjusted by heart rate determined by a graded exercise test. There were 20 minutes for warm-up and calisthenics at the beginning of each session and also a 10-minute cool down period at the end. |
The CG was asked not to change their sedentary lifestyle during the study | Serum BDNF (pg/mL) | A human BDNF ELISA Kit (R&D Systems, Minneapolis, MN, USA) | High BDNF level in patients with MetS decreased significantly after AE. |
| Glud et al. 2019ab [62] | -EXO; 12 weeks of aerobic exercise and isocaloric diet= The exercise consisted of supervised aerobic exercise three times per week with a duration of 60–75 min per training session, an estimated energy expenditure of 500–600 kcal per session, and an intensity of 70% of HRR -DEX; 12 weeks of aerobic exercise in parallel with 8 weeks of VLED (800 kcal/day) followed by a 4-week weight maintenance diet) |
DIO; 8 weeks of VLED 600 kcal/day followed by a 4-week weight maintenance diet | Serum BDNF (ng/mL) | Quantikine ELISA Human Free BDNF immunoassay (DBD00, R&D Systems, Abingdon OX14, UK) |
EXO: BDNF decreased significantly 22.4% in women, and 22.1% in men DIO: BDNF decreased significantly 29.9% in women, and 4.2% in men DEX: BDNF decreased significantly 32.5% in women. |
| Goldfield et al. a,b,c 2018 [63] | -AE group: Aerobic group exercised on treadmills, elliptical machines and/or bicycle ergometers (6 months intervention, twice a week, 20–45 min, 65–85% HRmax) -RT group: 6 months intervention, twice a week, RT group also progressed from 20 to 45 min per session, performing 7 exercises using weight machines or free weights, and progressing from 2 sets of 15 repetitions at moderate intensity to 3 sets of 8 repetitions at the maximum resistance that could be moved 8 times (8-RM)) -CT group: aerobic+resistance |
The CG received only dietary counselling with no exercise prescription | Serum BDNF (ng/mL) | Human Free BDNF Quantikine ELISA kit, R&D systems, Cat# DBD00) | No significant within- or between-group changes in BDNF level. Although not significant, after 6 months intervention; AE group: (+1.80) RT group: (−2.00) CT group: (−1.70) |
| Study | Physical Exercise protocol | Comparators | Main outcome | Brand/company name of BDNF kits | Main Results |
|---|---|---|---|---|---|
| Gyorkos et al. 2019 [64] | Each session consisted of HIIT on a cycle ergometer, including a 3-min warm-up, 10 x 60s cycling intervals interspersed with 60s of active recovery, ~90% HRmax, and a 3-min cool down, 3 day/week for four-week. | -CRPD-Sed: (<50gCHO) | Serum BDNF (ng/mL) |
Human BDNF Elisa Kit (#DBD00, Thermo Fisher Scientific) | -Basal BDNF protein content was significantly increased following CRPD-Sed (+20%; P < 0.05) and CRPD-Ex (+38%; p< 0.05) when compared to baseline. -The addition of HIIT exercise to the diet resulted in a significant increase in circulating serum BDNF when compared to CRPD-Sed. |
| Lee et al. 2014 [65] | Aerobic exercise was conducted for a total of 40–60 minutes per session at a VO2max of 50~60%, 3 sessions a week, for 12 weeks. |
-CG maintained their own lifestyles with no intervention. |
Serum BDNF (pg/mL) | A human BDNF Elisa Kit (R&D Systems, Minneapolis, MN, USA) | -There was an increase in BDNF level after 12 weeks of regular aerobic exercise program, only in the obese group. |
| Levinger et al. 2008a [66] | 10 weeks, two sets of 15–20 repetitions at 40–50% 1RM. From weeks 2–10, participants performed each exercise for three sets, 8–20 repetitions, at 50–85% 1RM. | CG: did not perform regular exercise during the study period. | Serum BDNF (pg/mL) | Human BDNF Elisa Kit (Catalog number: DY248; Minneapolis, MN) |
-Resistance training had no effect on BDNF levels or other risk factors for MetS. |
| Li et al. 2021a,b [67] | HIIT: 4 × 3 min at 90% VO2max interspersed with 3 min at 60% VO2max, about 45 min., 3 training sessions per week for 12 week, VICT: 25 min at 70% VO2max, about 45 min.,3 training sessions per week for 12 week |
The subjects in the CG lived normally and did not participate in any training | Serum BDNF (pg/mL) | Human BDNF Elisa Kit (Abcam Inc., Cambridge, UK) | -BDNF increased significantly in both HIIT and VICT group. -There was no significant difference between HIIT and VICT in terms of BDNF. |
| Osali et al. 2020 [68] | Exercise with moderate intensity: Each subject walked or ran at 65–75% HRR on a treadmill for 3×12–17 min, 6 weeks. | continued the regular daily life | Serum BDNF (pg/mL) | Human BDNF Elisa Kits (Adipo Bioscience, USA) | BDNF increased significantly in EG |
| Roh et al. 2017b [69] | Subjects performed treadmill exercise for 40 min 3 times weekly for 8 weeks at 70% HRR, totally 60 min.exercise program | Non-obese | Serum BDNF (pg/mL) | Human BDNF ELISA Kit (#DBD00; R&D Systems, Minneapolis, MN, USA) |
BDNF increased significantly only in obese group, not non-obese group. |
| Saleh et al. 2020 [70] | 45 minutes anaerobic gymnastics exercise including 10-minute warm up, 30- minutes of main exercises, and 5 minutes cool down, 3 times per week for 8 weeks. | Normal weight and obese control groups continued the regular daily life | Saliva BDNF (pg/mL) | Human BDNF PicoKine™ ELISA Kit (Catalog No. EK0307; R&D Systems, Austria) |
BDNF increased in obese group (+33.80%, p=0.002), and normal-weight group (+31.36%, p=0.003). |
| Zibinaite et al. 2019 [71] | Participants underwent 72 supervised aerobic exercise training sessions on cycle ergometers during 6 months, including 3 sessions of training per week. A 50 min aerobic training 80 at an intensity between 60% and 70% of the HRmax was performed. | CG did not undergo any intervention and were instructed to maintain their regular physical activity and diet regime for 6 months | Serum BDNF (pg/mL) | Human BDNF Elisa Kits (Gemini; Stratec Biomedical, Birkenfeld, Germany). | BDNF level remained unchanged (p>0.05) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





