Submitted:
22 March 2024
Posted:
25 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Dendrobium Production in Hawaii
1.2. Pests of Dendrobiums:
1.3. Current Management Challenges:
1.4. Silicon-Mediated Resistance
1.5. Objectives
2. Materials and Methods
2.1. Experiment Design:
2.2. Data Collection:
3. Results
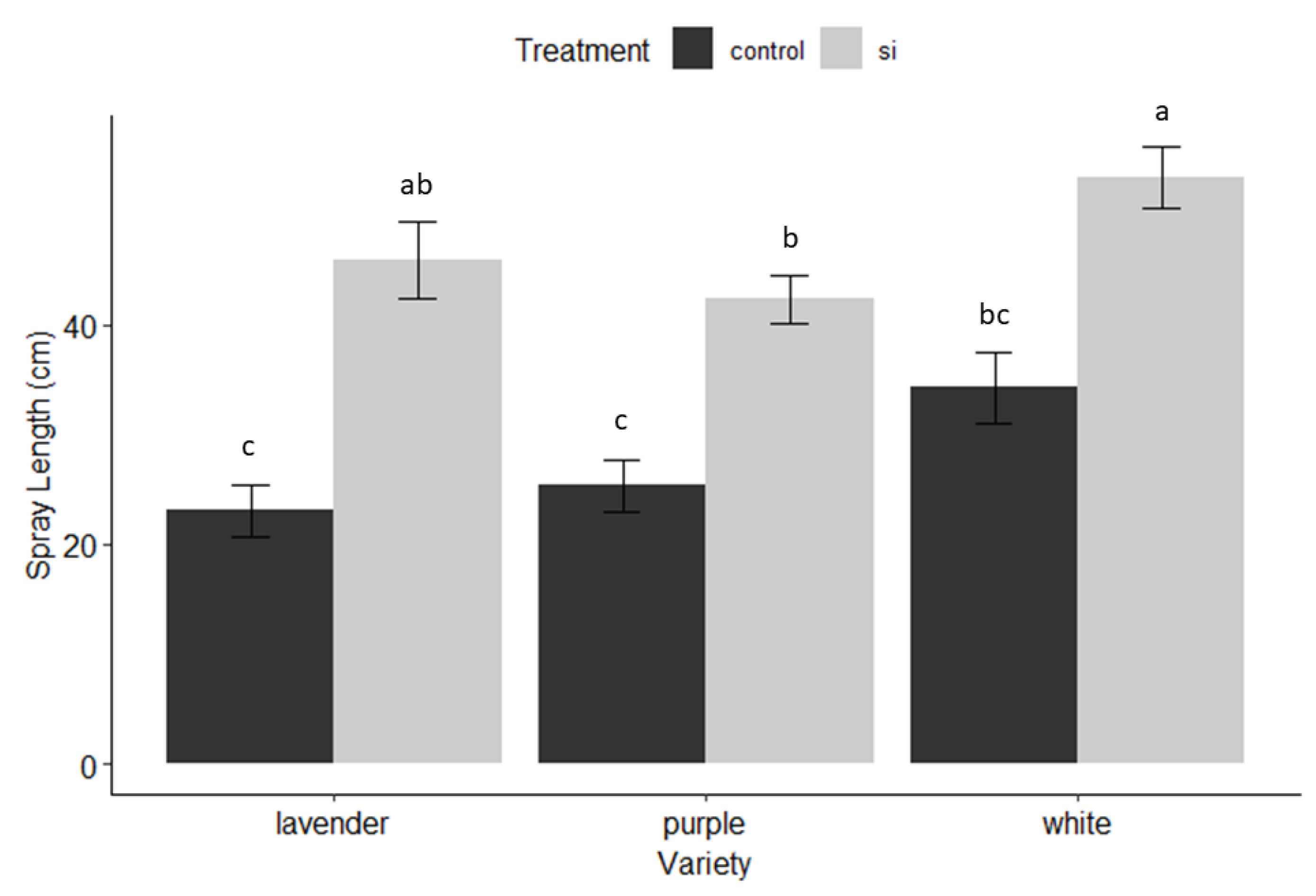
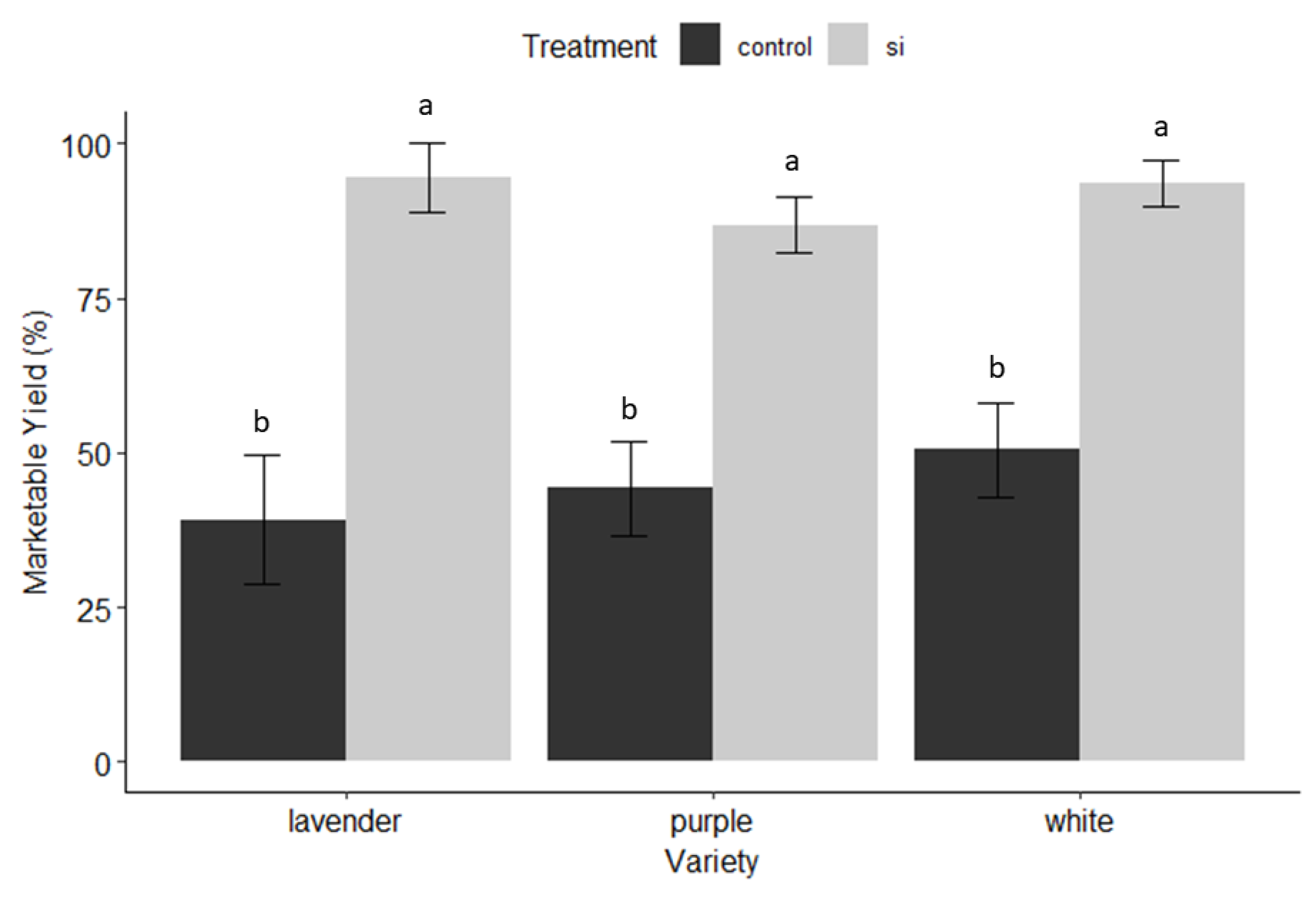
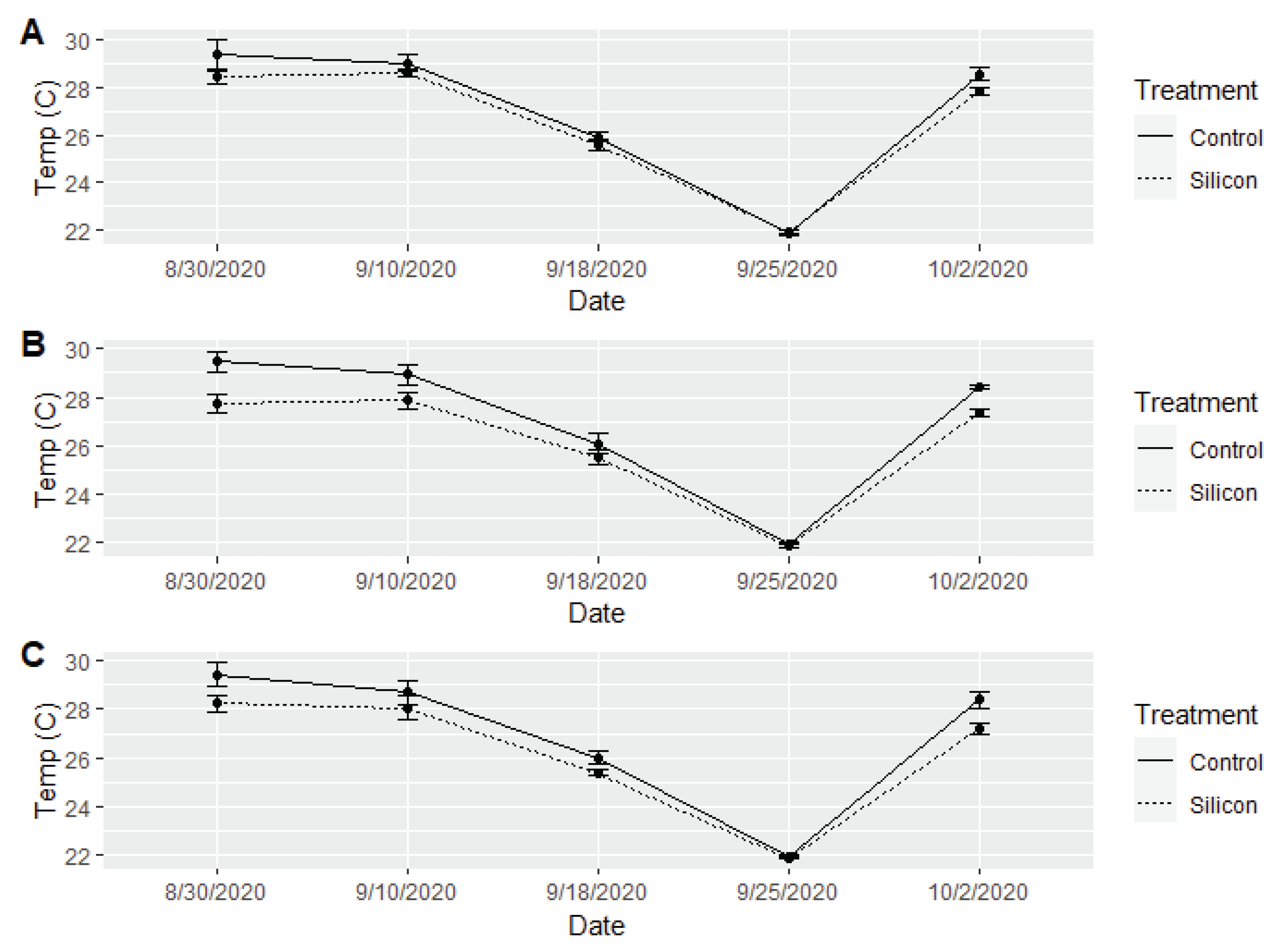
3.1. Harvest
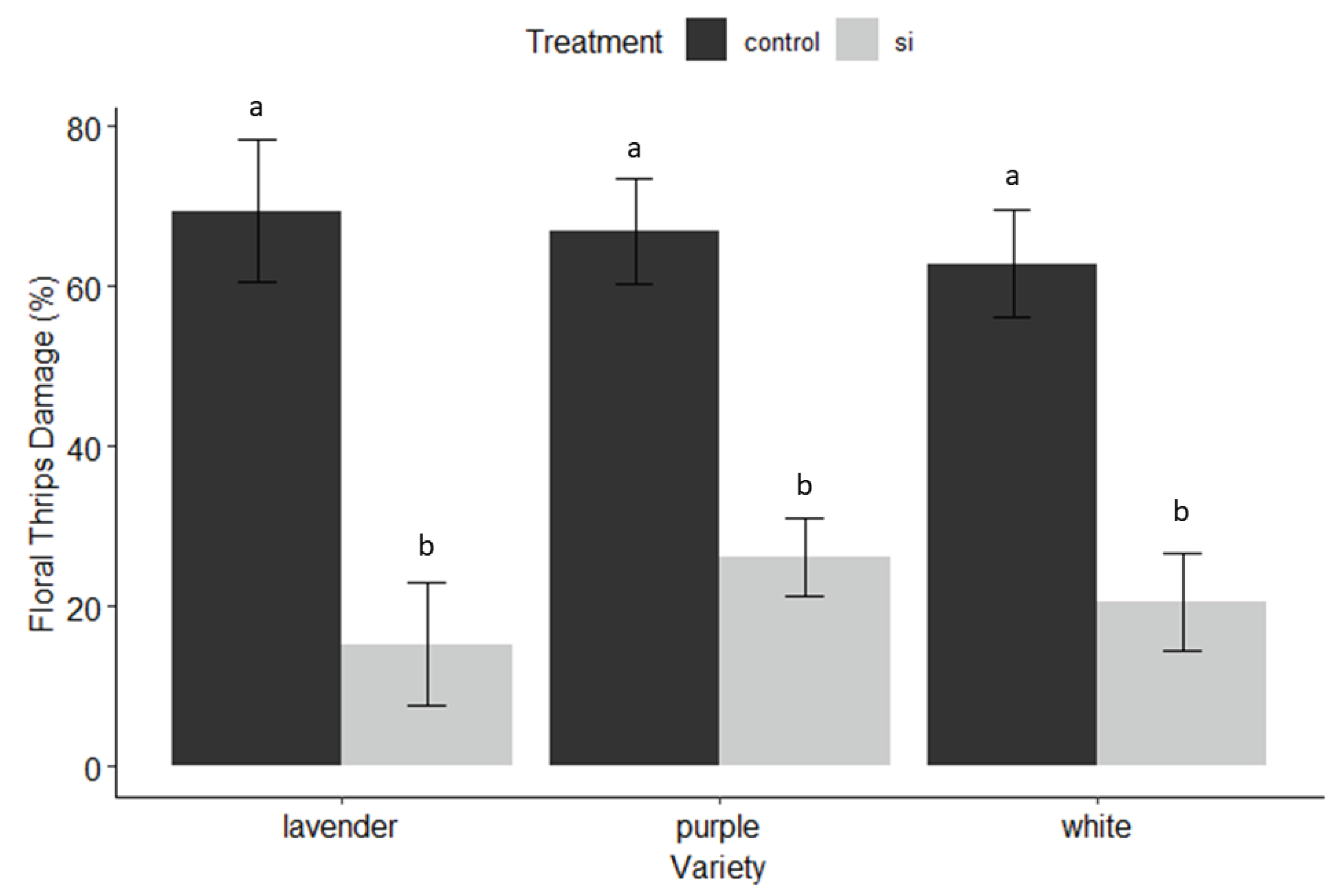
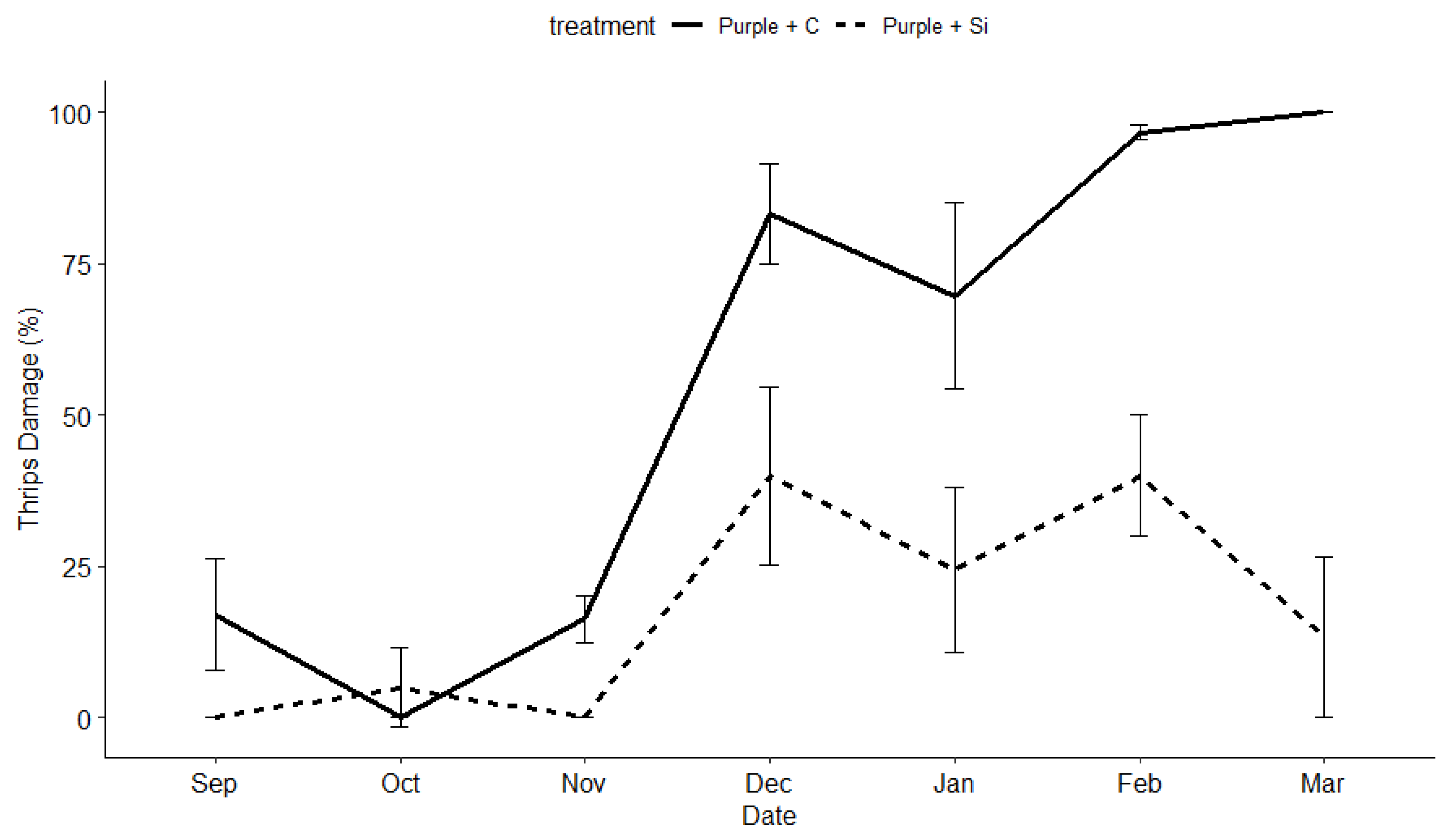
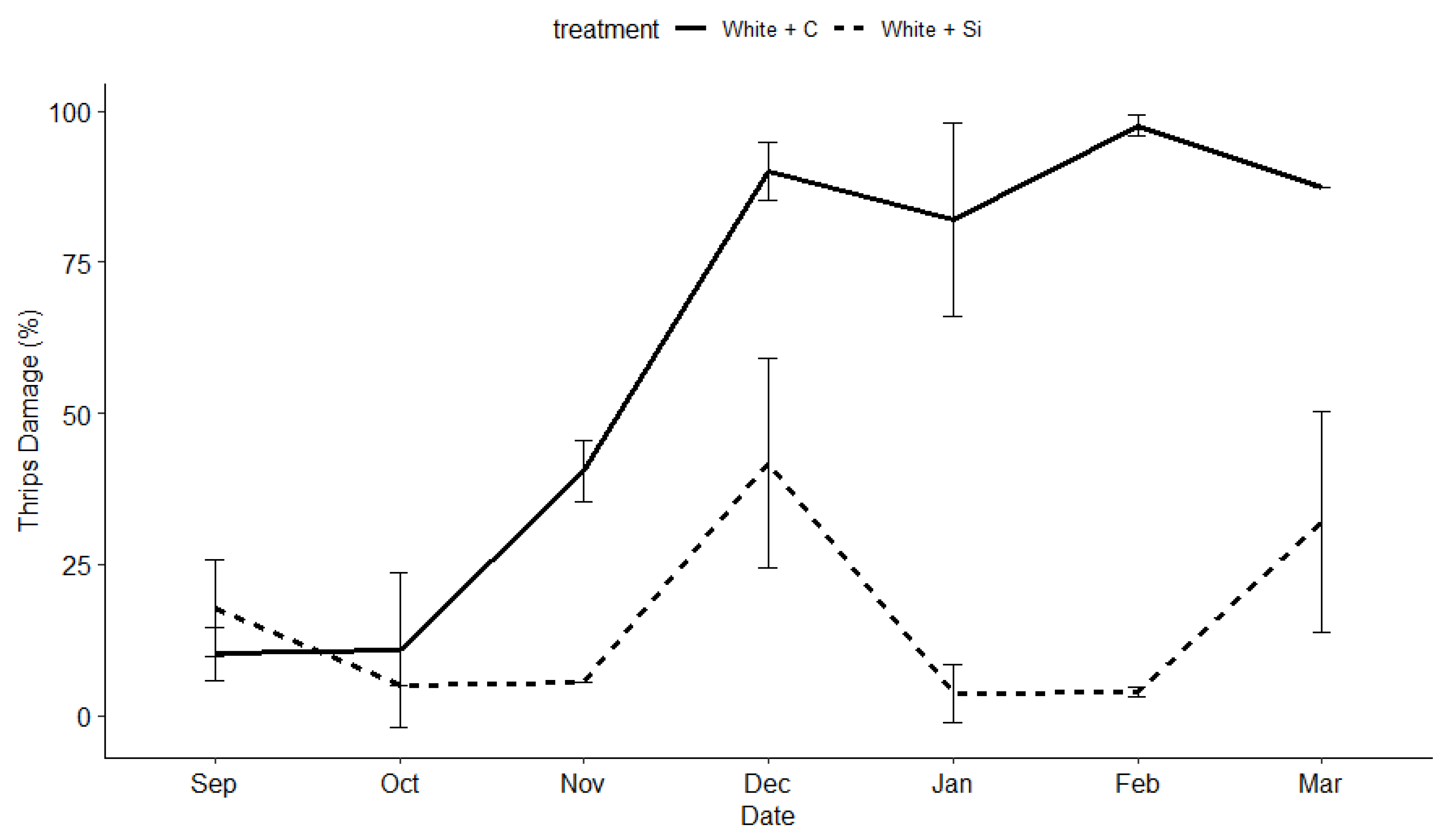
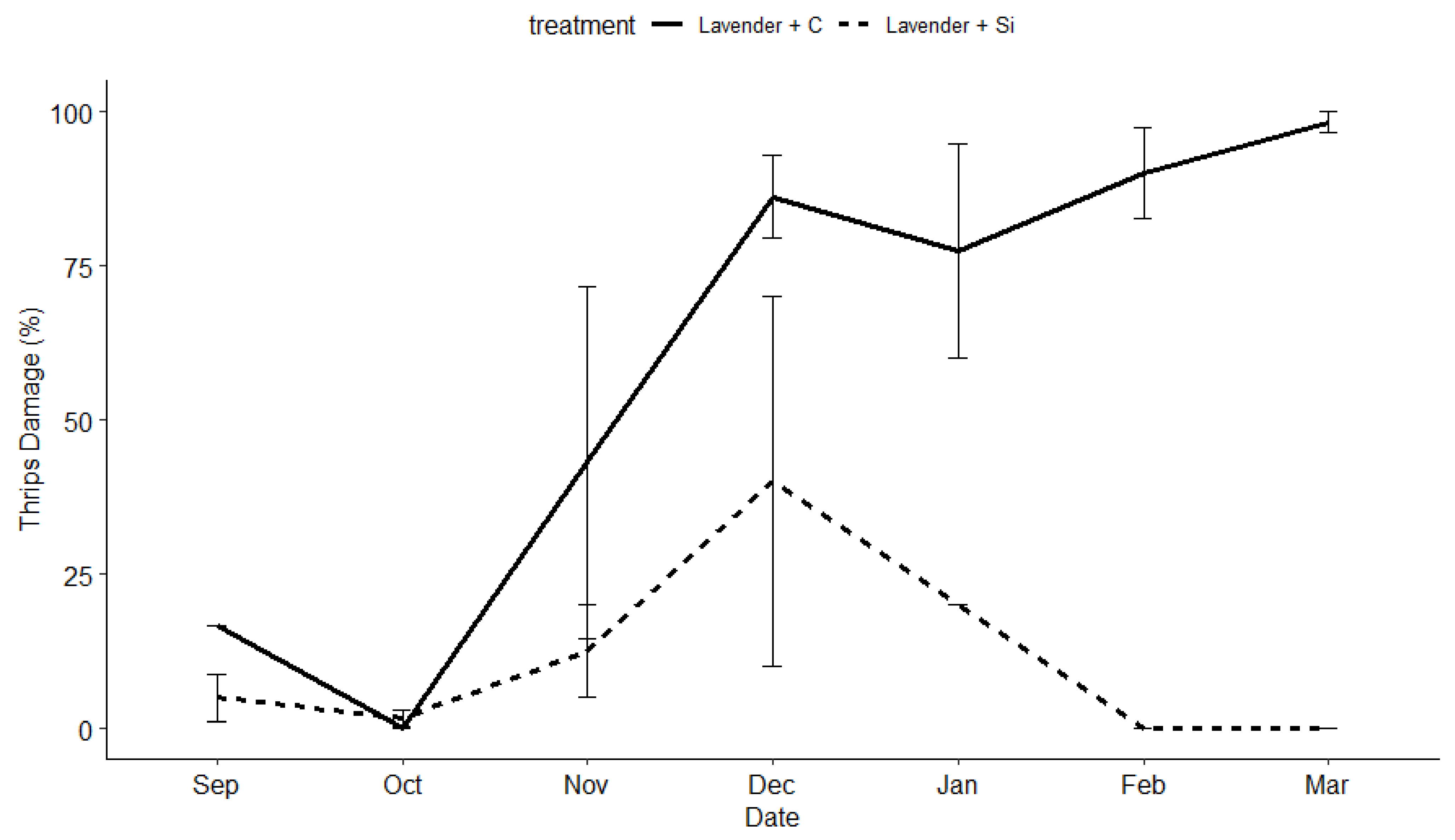
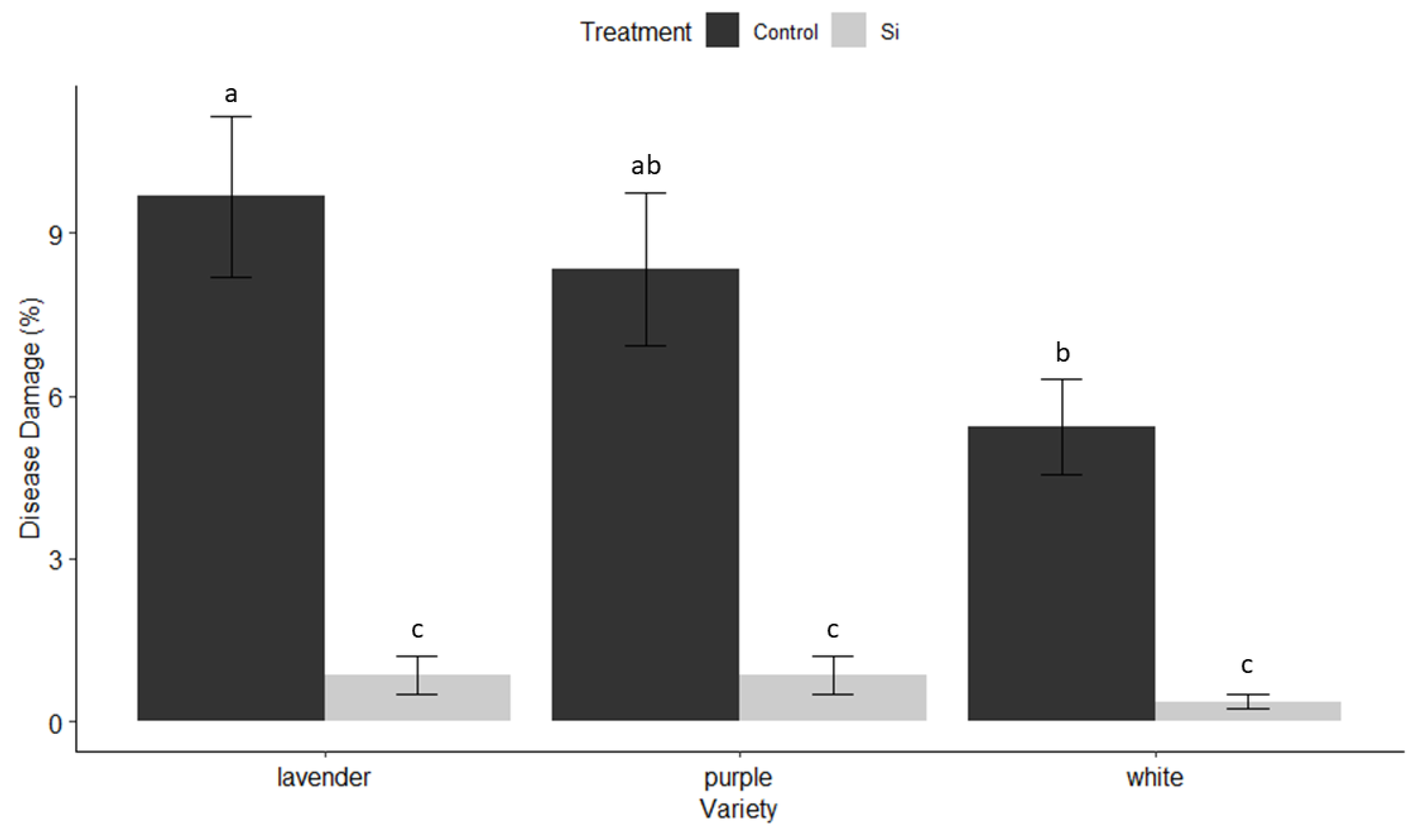
3.2. Insect and Disease Monitoring
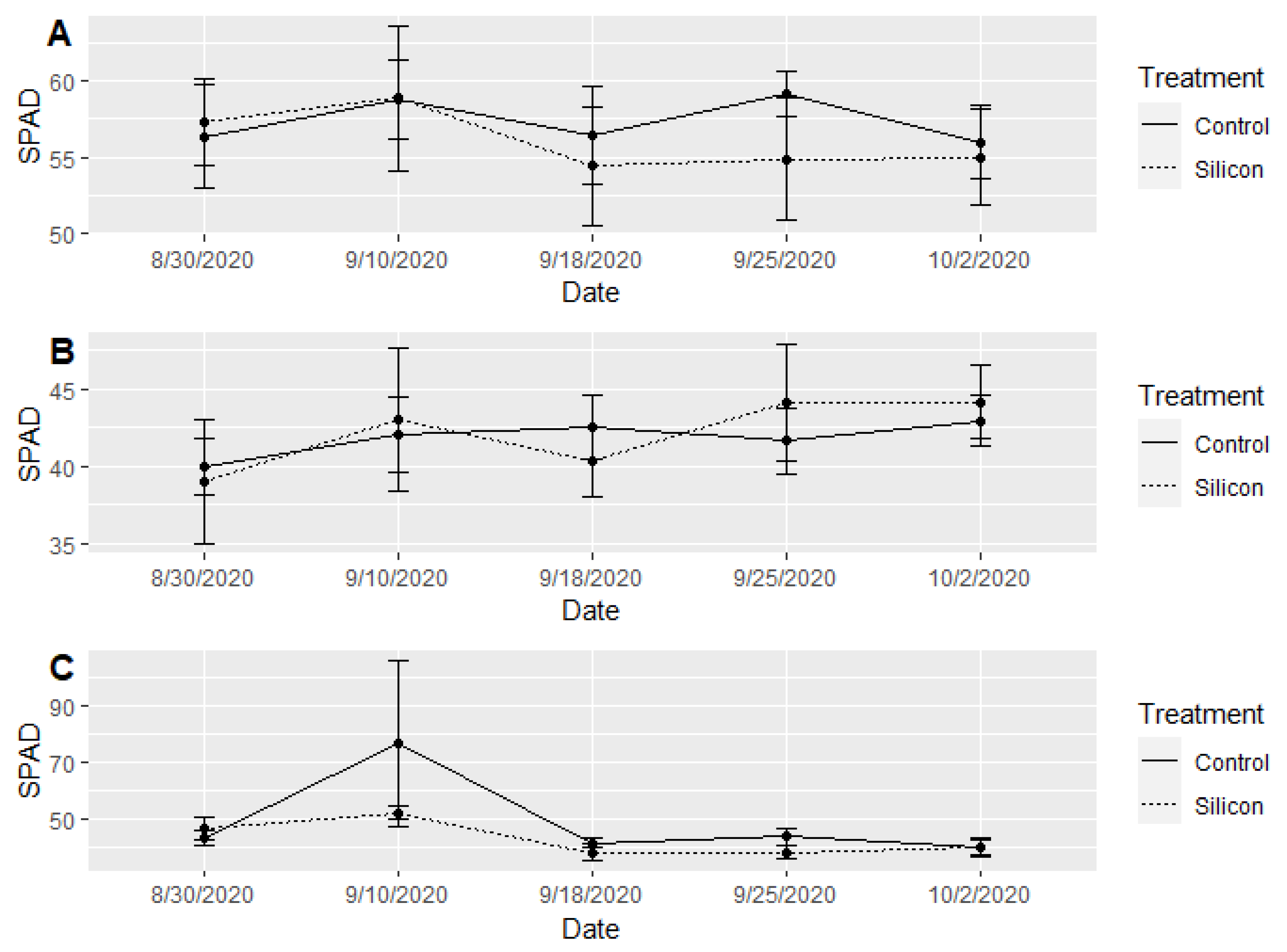
3.3. Plant Growth and Health
3.4. Vase Life
3.5. Silicon Accumulation
4. Discussion
4.1. Role of Si in an IPM Program
4.2. Where and When Si Shines
5. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alhousari, F.; Greger, M. Silicon and Mechanisms of Plant Resistance to Insect Pests. Plants 2018, 7, 33. [Google Scholar] [CrossRef]
- Almeida, G.D.; Pratissoli, D.; Zanuncio, J.C.; Vicentini, V.B.; Holtz, A.M.; Serrão, J.C. Calcium silicate and organic mineral fertilizer applications reduce phytophagy by Thrips palmi Karny (Thysanoptera: Thripidae) on eggplants (Solanum melongena L. ). Interciencia 2008, 33, 835–838. [Google Scholar]
- Almeida, G.D.; Pratissoli, D.; Zanuncio, J.C.; Vicentini, V.B.; Holtz, A.M.; Serrão, J.E. Calcium silicate and organic mineral fertilizer increase the resistance of tomato plants to Frankliniella schultzei. Phytoparasitica 2009, 37, 225–230. [Google Scholar] [CrossRef]
- Ancheta D. 2020. Neighbor Islanders brace for higher prices after Young Brothers gets 46% rate increase. Hawaii News Now. Published: Aug. 18, 2020 at 10:07 AM HST. Updated: Aug. 18, 2020 at 10:09 AM HST. Retrieved June 2022, from https://www.hawaiinewsnow.com/2020/08/17/puc-approved-emergency-percent-rate-increase-young-brothers/.
- CABI. 2023. Thrips palmi. In: Invasive Species Compendium. Wallingford, UK: CAB International. Retrieved from https://www.cabi.org/isc/datasheet/53745#todistribution.
- Cai, K.; Gao, D.; Luo, S.; Zeng, R.; Yang, J.; Zhu, X. Physiological and cytological mechanisms of silicon-induced resistance in rice against blast disease. Physiol. Plant 2008, 134, 324–333. [Google Scholar] [CrossRef]
- Chubachi, T.; Asano, I.; Oikawa, T. The diagnosis of nitrogen nutrition of rice plants using chlorophyll meter. Soil Sci. Plant Nutr. 1986, 57, 190–193. [Google Scholar]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Belanger, R.R. The Controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef]
- Dogramaci, M.; Arthurs, S.P.; Chen, J.; Osborne, L. Silicon Applications Have Minimal Effects on Scirtothrips dorsalis (Thysanoptera: Thripidae) Populations on Pepper Plant, Capsicum annum L. Fla. Entomol. 2013, 96, 48–54. [Google Scholar] [CrossRef]
- Eng, S.; Khun, T.; Esquivel, M.; Ooki, N.; Bloese, J.; Sand, S.; Lincoln, N. Farmers’ Perceived Needs of Extension’ Support During Covid-19 in Hawai’i. Journal of Extension 2021, 59, 15. [Google Scholar] [CrossRef]
- Fateux, F.; Remus-Borel, W.; Menzies, J.G.; Belanger, R.R. Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol. Lett. 2005, 249, 1–6. [Google Scholar] [CrossRef]
- Fortunato, A.A.; Rodrigues, F.A.; Baroni, J.C.P.; Soares, G.C.B.; Rodriguez, M.A.D.; Pereira, O.L. Silicon suppresses Fusarium wilt development in banana plants. J. Phytopathol. 2012, 160, 674–679. [Google Scholar] [CrossRef]
- Frantz, J.M.; Locke, J.C.; Datnoff, L.; Omer, M.; Widrig, A.; Strutz, D.; Horst, L.; Krause, C.R. Detection, distribution, and quantification of silicon in floriculture crops utilizing three distinct analytical methods. Communications in Soil Science and Plant Analysis 2008, 39, 2734–2751. [Google Scholar] [CrossRef]
- Gao, D.; Cai, K.; Chen, J.; Luo, S.; Zeng, R.; Yang, J.; Zhu, X. Silicon enhances photochemical efficiency and adjusts mineral nutrient absorption in Magnaporthe oryzae infected rice plants. Acta Physiol. Plant. 2011, 33, 675–682. [Google Scholar] [CrossRef]
- Gardener, W.D. 1991. Pest-related flower shipment rejections, pp. 49–51. In K. W. Leonhardt, D.O. Evans and J.M. Halloran [eds.], The Hawaii Tropical Cut Flower Industry Conference, 29-31 March 1990, Hilo, Hawaii, College of Tropical Agriculture and Human Resources, Honolulu.
- Gill, S.; Dutky, E.; Raupp, M.; Davidson, J.; Nakahara, S. 2012. Thrips Management in Greenhouses. University of Maryland Extension, FS-762-2012.
- Hara, A.H.; Hata, T.Y. 1999. Pests and pest management. Pg. 29-45. In: K. Leonhardt, and K. Sewake [eds.], Growing dendrobium orchids in Hawaii: Production and management guide. University of Hawaii, Honolulu, HI.
- Hata, T.Y.; Hara, A.H.; Hansen, J.D. Feeding preference of melon thrips on orchids in Hawaii. Hortsci. 1991, 26, 1294–1295. [Google Scholar] [CrossRef]
- Hata, T.Y.; Hara, H.A. Anthurium thrips, Chaetanaphothrips orchidii (Moulton): Biology and insecticidal control on Hawaiian anthuriums. Trop. Pest Manag. 1992, 38, 230–233. [Google Scholar] [CrossRef]
- Hata, T.Y.; Hara, A.H.; Hu, B.K.S.; Kaneko, R.T.; Tenbrink, V.L. Field sprays and insecticidal dips after harvest for pest management of Frankliniella occidentalis and Thrips palmi (Thysanoptera: Thripidae) on orchids. Journal of Economic Entomology 1993, 86, 1483–1789. [Google Scholar] [CrossRef]
- He, J.; Khoo, G.H.; Hew, C.S. Susceptibility of CAM Dendrobium leaves and flowers to high light and high temperature under natural tropical conditions. Environmental and Experimental Botany 1998, 40, 255–264. [Google Scholar] [CrossRef]
- Hogenhout, S.A.; Bos, J.I.B. Effector proteins that modulate plant–insect interactions. Curr. Opin. in Plant Biol. 2011, 14, 422–428. [Google Scholar] [CrossRef]
- Hollingsworth, R.G.; Hara, A.H.; Sewake, K.T. Pesticide use and grower perceptions of pest problems on ornamental crops in Hawaii. J. Ext. 2000, 38. [Google Scholar]
- Hollingsworth, R.G.; Hara, A.H.; Sewake, K.T. Scouting for thrips in orchid plants. CTAHR Publication IP-3, College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa. 2001.
- Huang, C.H.; Roberts, P.D.; Datnoff, L.E. Silicon suppresses Fusarium crown and root rot of tomato. J. Phytopathol. 2011, 159, 546–554. [Google Scholar] [CrossRef]
- Immaraju, J.; Paine, T.D.; Bethke, J.A. Western Flower Thrips (Thysanoptera: Thripidae) Resistance to Insecticides in Coastal California Greenhouses. J. Econ. Entomology. 1992, 85, 9–14. [Google Scholar] [CrossRef]
- Ito, J.S.; Aragaki, M. Botrytis blossom blight of Dendrobium. Phytopathology 1977, 67, 820–824. [Google Scholar] [CrossRef]
- Jana, S.; Jeong, B.R. Silicon: The most under-appreciated element in horticulture crops. Trends Hortic. Res. 2014, 4, 1–19. [Google Scholar]
- Johnson, M. Population trends of a newly introduced species, Thrips palmi (Thysanoptera: Thripsidae), on commercial watermelon plantings in Hawai’i. J. Econ. Entomology. 1986, 79, 718–720. [Google Scholar] [CrossRef]
- Jones, L.H.P.; Handreck, K.A. Silica in soils, plants, and animals. Adv. Agron. 1967, 19, 107–149. [Google Scholar]
- Keeping, M.G.; Miles, N.; Sewpersad, C. Silicon reduces impact of plant nitrogen in promoting stalk borer (Eldana saccharina) but not sugarcane thrips (Fulmekiola serrata) infestations in sugarcane. Front. Plant. Sci. 2014, 4, 289. [Google Scholar] [CrossRef] [PubMed]
- Kirk, E.; Sand, S.; Bloese, J.; Gutierrez-Coarite, R.; Keach, J.; Eng, S. 2021. COVID-19 Hawaii agriculture survey: Initial and on-going impacts. Cooperative Extension. College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa.
- Kvedaras, O.L.; An, M.; Choi, Y.S.; Gurr, G.M. Silicon enhances natural enemy attraction and biological control through induced plant defences. Bull. Entomol. Res. 2010, 100, 367–371. [Google Scholar] [CrossRef]
- Li, Y.; Bi, Y.; Ge, Y.; Sun, X.; Wang, Y. Antifungal activity of sodium silicate on Fusarium sulphureum and its effect on dry rot of potato tubers. J. Food Sci. 2009, 74, 213–218. [Google Scholar] [CrossRef]
- Liang, Y.; Nikolic, M.; Belanger, R.R.; Gong, H.; Song, A. 2015. Silicon in agriculture: From theory to practice. Dordrecht, the Netherlands: Springer.
- Loke, M. 2018. Farm disaster survey results Kilauea East rift zone eruptions, 2018 report. Cooperative Extension Service. College of Tropical Agriculture and Natural Resources. University of Hawaii at Manoa.
- Ma, J.F.; Tamai, K.; Yamaji, N.; Mitani, N.; Konishi, S.; Katsuhara, M.; Ishiguro, M.; Murata, Y.; Yano, M. A silicon transporter in rice. Nature 2006, 440, 688–691. [Google Scholar] [CrossRef]
- Mantovani, C.; Prado, R.M.; Pivetta, K.F.L. Silicon foliar application on nutrition and growth of Phalaenopsis and Dendrobium orchids. Sci. Hortic. 2018, 241, 83–92. [Google Scholar] [CrossRef]
- Mau, R.F.; Kessing, J.L. 1993. Frankliniella occidentalis (Pergande). Retrieved from http://www.extento.hawaii.edu/Kbase/crop/Type/f_occide.htm.
- Nagata, T.; Almeida, A.C.L.; Resende, R.O.; Avila, A.C. 2002. The transmission specificity and efficiency of tospoviruses. Pg. 45-52. In: R. Marullo, and L. Mound [eds.], Thrips and Tospoviruses: Proc. 7th Intl. Symp. Thysanoptera. Australian Nat. Insect Collection, Canberra, Australia.
- Nakahara, L.M. 1985. Thrips palmi on dendrobium, pp 29-31. In Proc. 1985 Hawaii Commercial Dendrobium Growers Conf., 11-12 Oct., Cooperative Extension Service. University of Hawaii, Honolulu.
- Parthiban, P.; Chinniah, C.; Murali Baskaran, R.K.; Suresh, K.; Karthick, S. Influence of calcium silicate application on the population of sucking pests of groundnut (Arachis hypogaea L. ). Silicon. 2018, 11, 1687–1692. [Google Scholar] [CrossRef]
- Peris-Felipo, F.J.; Benavent-Gil, Y.; Hernandez-Apaolaza, L. Silicon beneficial effects on yield, fruit quality, and shelf-life of strawberries grown in different culture substrates under different iron status. Plant Physiol. Biochem. 2020, 152, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283. [Google Scholar] [CrossRef]
- Resende, R.S.; Rodrigues, F.A.; Cavatte, P.C.; Martins, S.C.V.; Moreira, W.R.; Chaves, A.R.M.; DaMatta, F.M. Leaf gas exchange and oxidative stress in sorghum plants supplied with silicon and infected by Colletotrichum sublineolum. Phytopathology 2012, 102, 892–898. [Google Scholar] [CrossRef]
- Reynolds, M.G.; Keeping, J.H.; Meyer, E.H. Silicon-augmented resistance of plants to herbivorous insects: A review. Ann. Appl. Biol. 2009, 155, 171–186. [Google Scholar] [CrossRef]
- Rodrigues, F.A.; McNally, D.J.; Datnoff, L.E.; Jones, J.B.; Labb, E.C.; Benhamou, N.; Menzies, J.G.; Belanger, R.R. Silicon enhances the accumulation of diterpenoid phytoalexins in rice: A potential mechanism for blast resistance. Phytopathology 2004, 94, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Sakimura, K.; Nakahara, L.M.; Denmark, H.A. A thrips, Thrips palmi Karny (Thysanoptera: Thripsidae) Fla. Dept. Agri. Consumer Serv., Division of Plant Ind., Entomol. Circ. 1986, 280.
- Samuels, A.; Glass, A.; Ehret, D.; Menzies, J. Distribution of silicon in cucumber leaves during infection by powdery mildew fungus (Sphaerotheca fuliginea). Canad. J. of Bot. 1991, 69, 140–146. [Google Scholar] [CrossRef]
- Seal, D.R.; Kumar, V.; Kakkar, G.; Mello, S.C. Abundance of Adventive Thrips palmi (Thysanoptera: Thripidae) Populations in Florida During the First Sixteen Years. Fla. Entomol. 2013, 96, 789–796. [Google Scholar] [CrossRef]
- Srivastava, S.; Kadooka, C.; Uchida, J.Y. Fusarium species as pathogen on orchids. Microbiol. Res. 2018, 207, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Snyder, G.H. Chapter 11 Methods for silicon analysis in plants, soil, and fertilizers. Studies in Plant Science. Elsevier 2001, 8, 185–196. [Google Scholar] [CrossRef]
- Tsai, J.H.; Yue, B.; Webb, S.E.; Funderburk, J.E.; Hsu, H.T. Effects of Host Plant and Temperature on Growth and Reproduction of Thrips palmi (Thysanoptera: Thripidae). Environ. Entomol. 1995, 24, 1598–1603. [Google Scholar] [CrossRef]
- Uchida, J.Y.; Aragaki, M. Etiology of necrotic flecks on Dendrobium blossoms. Phytopathology 1979, 69, 1115–1117. [Google Scholar] [CrossRef]
- USDA NASS. 2020. Floriculture Crops 2019 Summary (December 2020). USDA, National Agricultural Statistics Service. Retrieved from chrome- https://www.nass.usda.gov/Publications/Todays_Reports/reports/floran20.pdf.
- USDA NASS. 2021. Pacific Region – Hawaii, Hawaii Horticulture and Nursery Products Annual Summary 2020. Retrieved from https://www.nass.usda.gov/Statistics_by_State/Hawaii/Publications/Flowers_and_Nursery_Products/2021 Hawaii Whole Flower.pdf.
- Vendrame, W.A.; Palmateer, A.J.; Pinares, A.; Moore, K.A.; Datnoff, L.E. Silicon fertilization affects growth of hybrid Phalaenopsis orchid liners. Horttechnology. 2010, 20, 603–607. [Google Scholar] [CrossRef]
- Wagner, F. The importance of silicic acid for the growth of some cultivated plants, their metabolism, and their susceptibility to true mildews. Phytopathol. Zeitschrift. 1940, 12, 427–479. [Google Scholar]
- Waterhouse, D.F.; Norris, K.R. 1989. Chapter 4 Frankliniella occidentalis (Pergande). pp. 24–35. In: Biological Control Pacific Prospects - Supplement 1. Australian Centre for International Agriculture Research: Canberra. 123 pages.
- Wang, C.L.; Chu, Y.I. Rearing method of southern yellow thrips, Thrips palmi Karny, in the laboratory. Plant Prot. Bull. (Taiwan, R.O.C) 1986, 28, 411. [Google Scholar]

| Scientific Name | Common Name | Categorization |
|---|---|---|
| Chaetanaphothrips orchidii* | Anthurium thrips | Thrips |
| Frankliniella occidentalis* | western flower thrips | Thrips |
| Frankliniella shultzei | yellow flower thrips | Thrips |
| Haplothrips gowdeyi | black flower thrips | Thrips |
| Chaetanaphothrips signipennis* | banana rust thrips | Thrips |
| Heliothrips haemorrhoidalis | greenhouse thrips | Thrips |
| Hercinothrips femoralis | banded greenhouse thrips | Thrips |
| Thrips hawaiiensis | Hawaiian flower thrips | Thrips |
| Thrips palmi* | melon thrips | Thrips |
| Thrips tabaci | onion thrips | Thrips |
| Botrytis cinerea* | gray mold | Fungus |
| Fusarium proliferatum* | Fusarium leaf spot | Fungus |
| Fusarium solani* | Fusarium leaf spot | Fungus |
| Phyllosticta capitalensis | Phyllosticta leaf spot | Fungus |
| TOTAL SPRAYS (N) | ||
| VARIETY | sILICON | cONTROL |
|
PURPLE DENDROBIUM CV ‘UNIWAI ROYALE’ |
155 ± 2.00a | 156 ± 2.40a |
|
WHITE DENDROBIUM CV ‘UNIWAI MIST’ |
99 ± 1.46a | 108 ± 1.97 a |
|
LAVENDER DENDROBIUM CV ‘UNIWAI SUPREME’ |
49 ± 1.86 a | 51 ± 1.20 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




