Introduction
Lower limb skeletal muscle ischemia-reperfusion (IR) injury can be seen in several clinical situations such as aortic aneurysm repairment, peripheral arterial surgery, vascular injury repairment, and shock and it
is associated with increased morbidity and mortality. Its precise mechanism is still unknown but it is generally accepted that oxidative stress mediators have a considerable role [
1]
.
Ischemia is the hypoperfusion of tissues induced by a limitation in arterial blood flow, and it plays a significant part in the pathogenesis of various disorders [
2,
3]. In addition to the cellular damage produced by ischemia alone, IR injury induced by reperfusion, which can be defined as the restoration of blood flow and reoxygenation of the ischemia-affected region [
2], causes a complex process. Reperfusion subsequent ischemia is known to aggravate skeletal muscle injury.
Ischemia-induced injury can be summarized as ATP inhibition by the transition of mitochondria from aerobic to anaerobic respiration, the development of apoptosis and necrosis with calcium-dependent proteolytic enzymes activated as a result of cell depolarization with an increase in intracellular sodium and extracellular potassium, and the inhibition of other ATP-dependent mechanisms at the cellular level. While oxidative stress caused by increased reactive oxygen derivatives in the cell with reperfusion could potentially be controlled by antioxidants under normal conditions, but this control ability reduces significantly during IR [
2].
Ischemia-reperfusion; releases reactive oxygen species (ROS) or reactive nitrogen species (RNS) like superoxide anion, hydroxyl radical, hydrogen peroxide, and peroxide nitrite. Additionally, IR induces neutrophils to cluster in location and become activated. Activated neutrophils contribute to ischemic injury by releasing cytotoxic free radicals and proteolytic enzymes. With high quantities of phospholipids and proteins, free radicals cause damage to cell membranes and subcellular structures, resulting in cell apoptosis and necrosis as a result of lipid peroxidation and, as a result, structural and metabolic alterations [
2,
4]. IR injury is characterized by structural and functional changes in the affected organ and damage to distant organs. This distant organ impact occurs as a result of free oxygen radicals released into the systemic circulation and various mediators.
Another point to consider is the oxidative stress caused by the imbalance between the production and elimination of oxidants during the release of reactive oxygen derivatives caused by IR [
1,
5]. Reactive oxygen derivatives cause severe damage to macromolecules due to increased oxidative stress. Membrane protein degeneration on proteins, lipid oxidation on lipids, and DNA degeneration on acids are samples of these impacts [
1]. Because lipids are a crucial part of the cell membrane, the harm they are subjected to is critical [
5]. Thiobarbituric Acid Reactive Substances (TBARS) assay is now employed as a marker to identify lipid oxidative damage and lipoprotein peroxidation [
4,
6]. It is based on the reaction of malondialdehyde (MDA) with thiobarbituric acid (TBA), which form a pink pigment with an absorption maximum at 532 nm [
7]. Catalase (CAT), which is in the enzymatic antioxidant class, has a higher antioxidant effect due to its ability to perform ROS resolution as a feature of the antioxidant class it is in. [
8]. The CAT enzyme, which is produced by various genes in plants such as sunflower, cotton, peas, cucumbers, rice, and pumpkins, is involved in hydrogen peroxide catalysis [
9].
Proanthocyanidin is a flavonoid discovered in 1947 by French researcher Jacques Masquelier [
10]. Because of their high antioxidant concentration, flavonoids, a highly essential phenol group in the human diet, have been the focus of several medical investigations. According to these researches, proanthocyanidins, which have been shown to be a more potent antioxidant than Vitamin C and Vitamin E [
11,
12], are present in the structure of many plants and are mostly found in fruits such as black grapes (Vitis vinifera) [
13] and white pine (Pinus maritima) [
14]. It is also possible to include black oak (Quercus marilandica), horse chestnut (Aesculus hippocastanum), hawthorn (Crataegus monogyna), and Vaccinium spp. to list proanthocyanidin is mainly found in. Besides its antioxidant content, its antibacterial, antiviral, anticarcinogenic, and antiallergenic effects [
11,
12] also exist and it has been used in research to prevent reperfusion injury. Studies have also shown that proanthocyanidin is effective in dentin demyelination [
15], catheter-related urinary tract infections [
16], and some types of cancer such as hepatocellular carcinoma. Although the effect of proanthocyanidin on IR injury in different tissues and systems such as cerebral tissue, intestinal tissue, gastric tissue, renal tissue, liver tissue, and genitourinary system, is being investigated, the number of research on skeletal muscle in this regard is few.
Material-Methods
Animals and Experimental Protocol
The present study was conducted at the Gazi University Animal Experiments Laboratory (Ankara, Turkey) in accordance with the ARRIVE guidelines. The study protocol was approved by the Animal Research Committee of Gazi University (G.Ü.ET-18.059). All of the animals were maintained in accordance with the recommendations of the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
The subjects in our study were 18 Wistar Albino rats weighing between 220 and 250 g, which were nurtured under the same habitat. The subjects were kept under 20-21°C within cycles of 12-hour daylight and 12-hour darkness. They were given free access to nutrition until 2 hours before the anesthesia procedure and randomly separated into three equal groups of 6 animals. Ketamine anesthesia was applied prior to midline laparotomy (100 mg/kg, intraperitoneally).
Control group (Group C): Midline laparotomy was the sole surgical procedure without any additional intervention. After 2 hours of follow-up, skeletal tissue was collected and subjects were sacrificed. We obtained two samples from each subject for biochemical and histopathological analysis.
Ischemia-reperfusion group (Group IR): Midline laparotomy was done in a similar fashion. The infrarenal aorta was left clamped for 1 hour. After removing the clamp, reperfusion was established for another additional 1 hour. At the end of 2 hours, skeletal tissue was collected and subjects were sacrificed. We obtained two samples from each subject for biochemical and histopathological analysis.
Ischemia-reperfusion with Proanthocyanidin group (Group IR-PRO): After following the same steps in the IR group, proanthocyanidin (Procyanidin A1, PhytoLab, CAS:103883-03-0, 5mg) was given (10 mg/kg) intraperitoneally 30 minutes before the ischemia period. At the end of 2 hours, skeletal tissue was collected and subjects were sacrificed. We obtained two samples from each subject for biochemical and histopathological analysis.
A total of 18 different muscle tissues including control and experimental cases were processed for paraffin sections. Formalin fixation, dehydration, clearing with xylene, paraffin wax infiltration, and blocking steps were performed respectively. Sections of four-micron thickness were taken from paraffin blocks. Then hematoxylin and eosin stainings were performed.
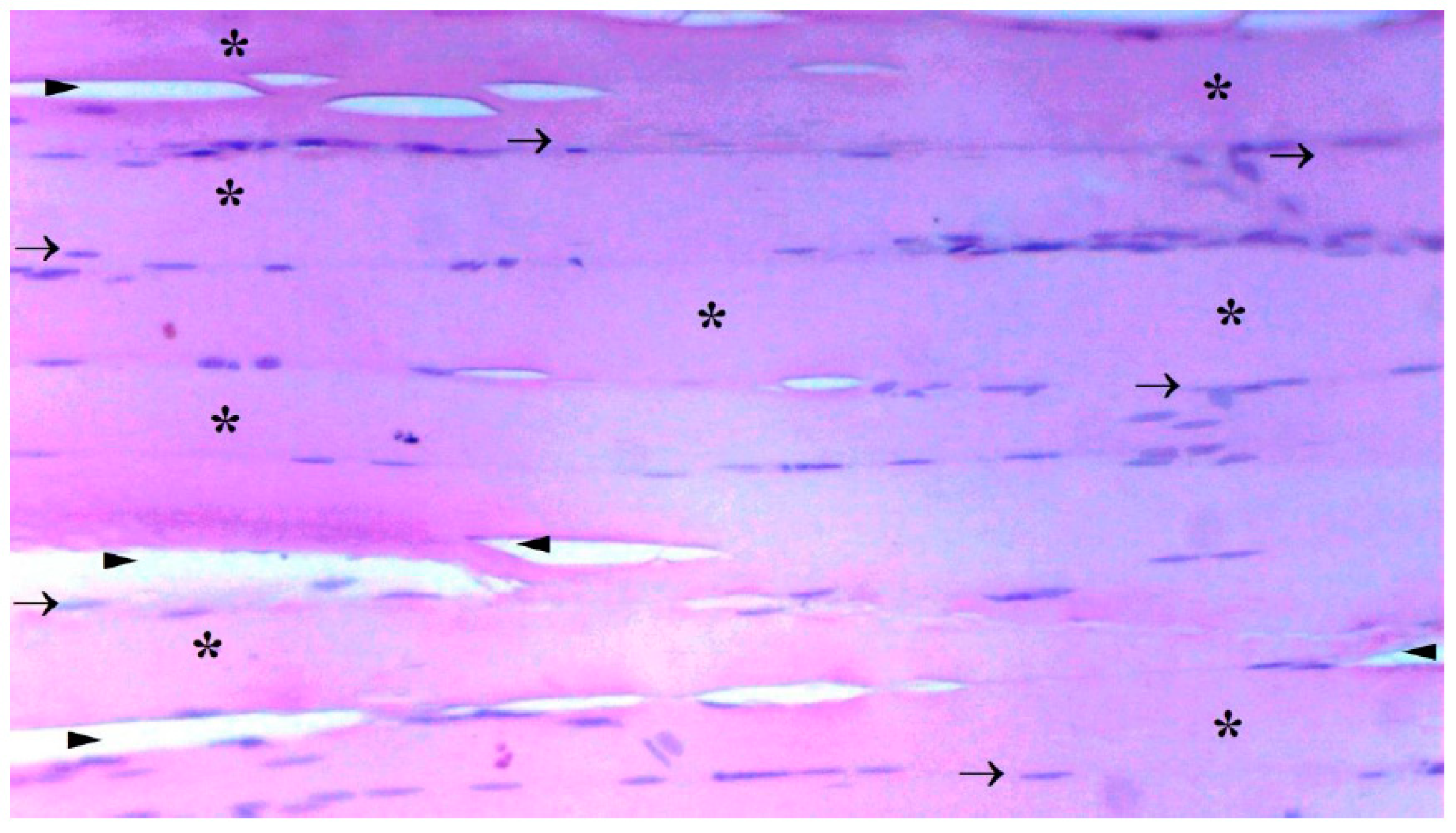
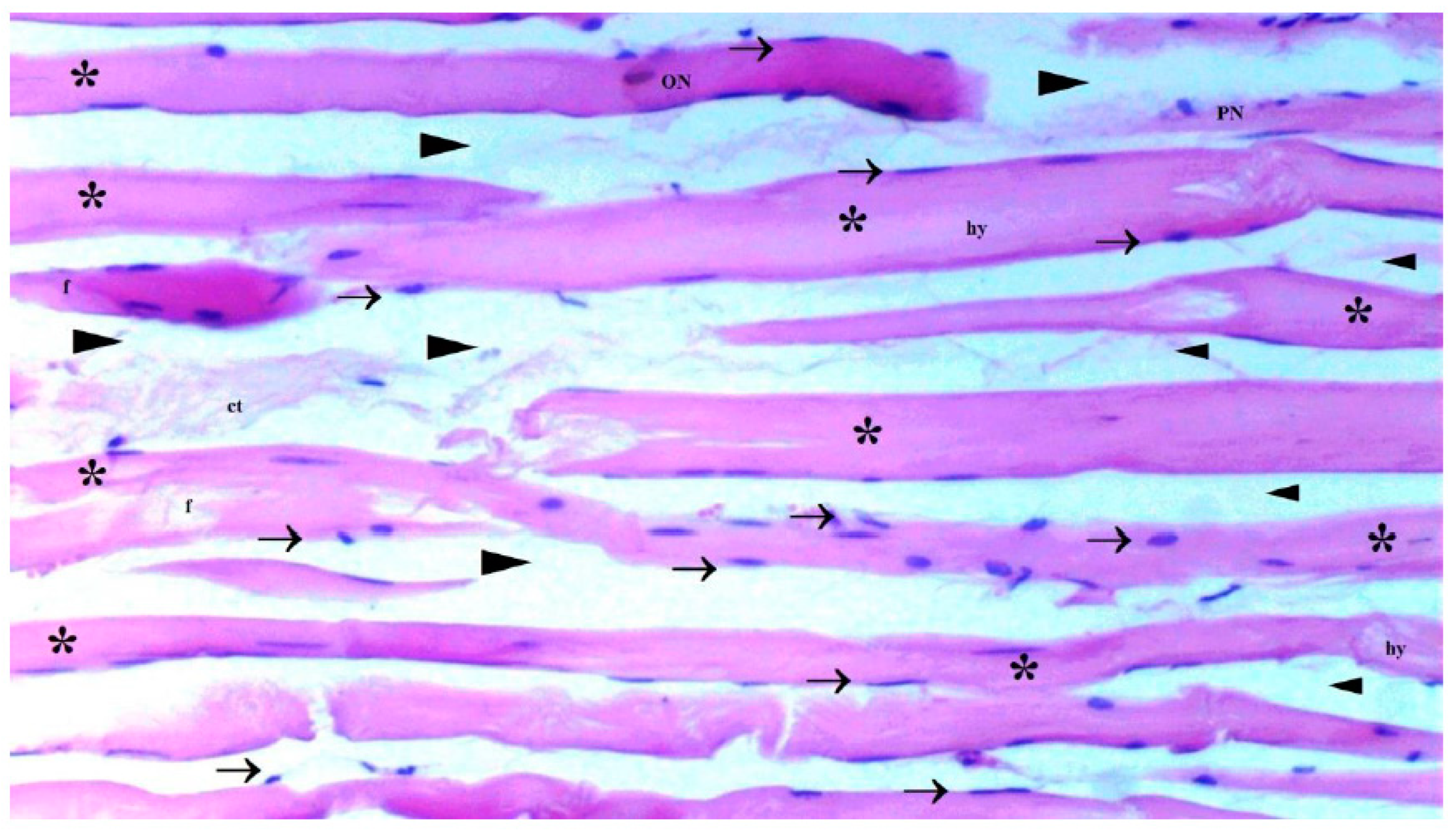
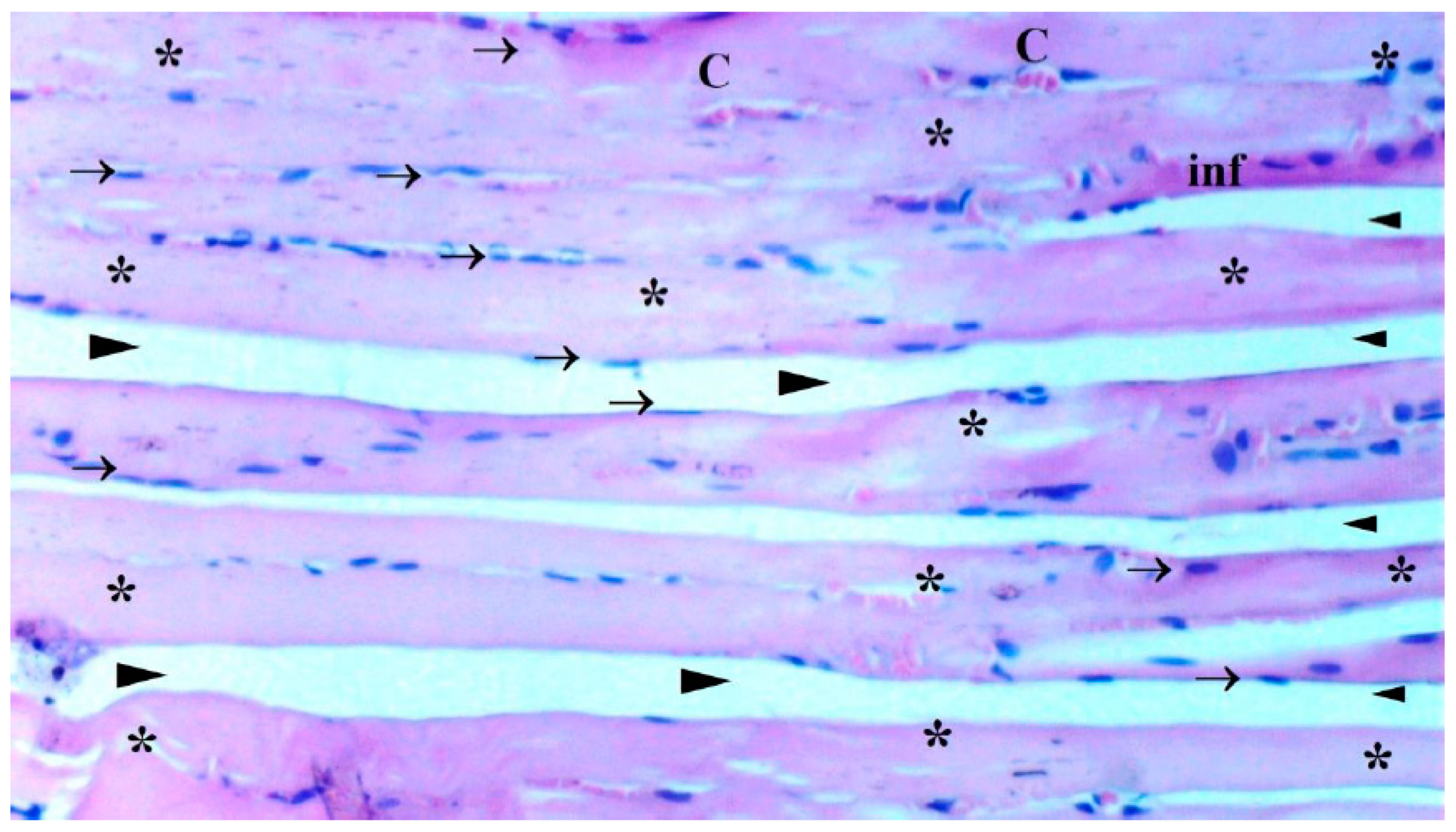
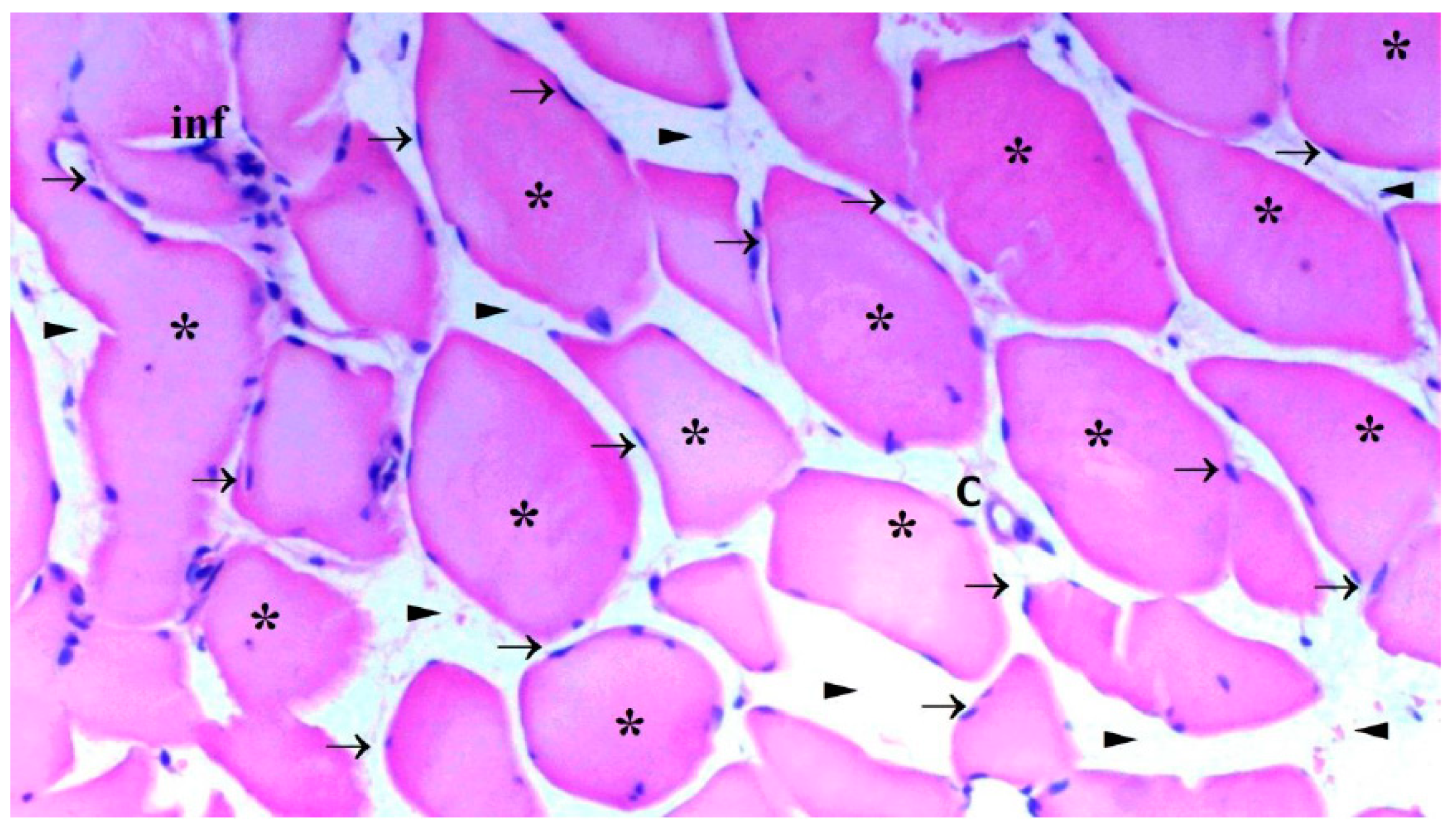
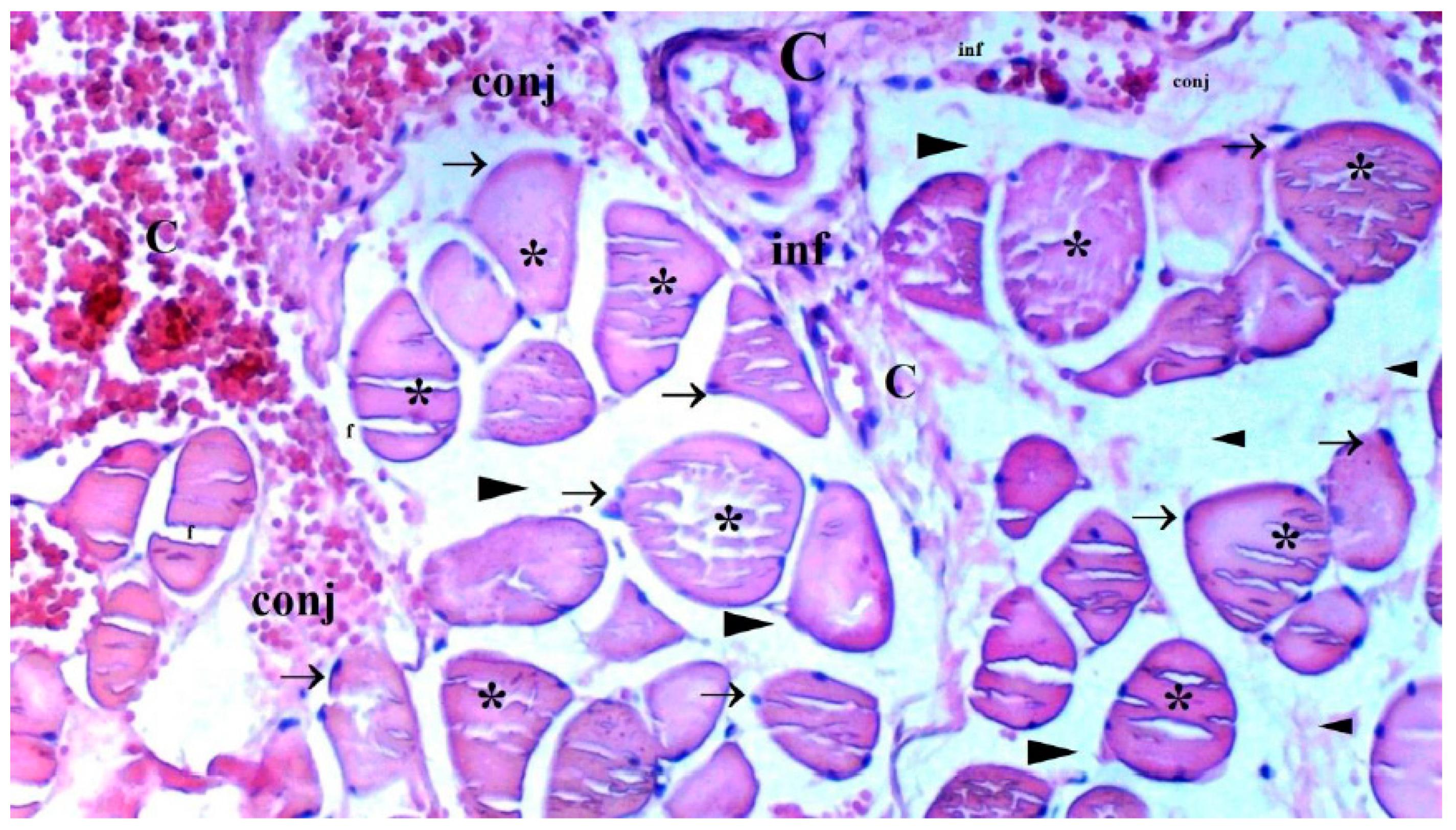
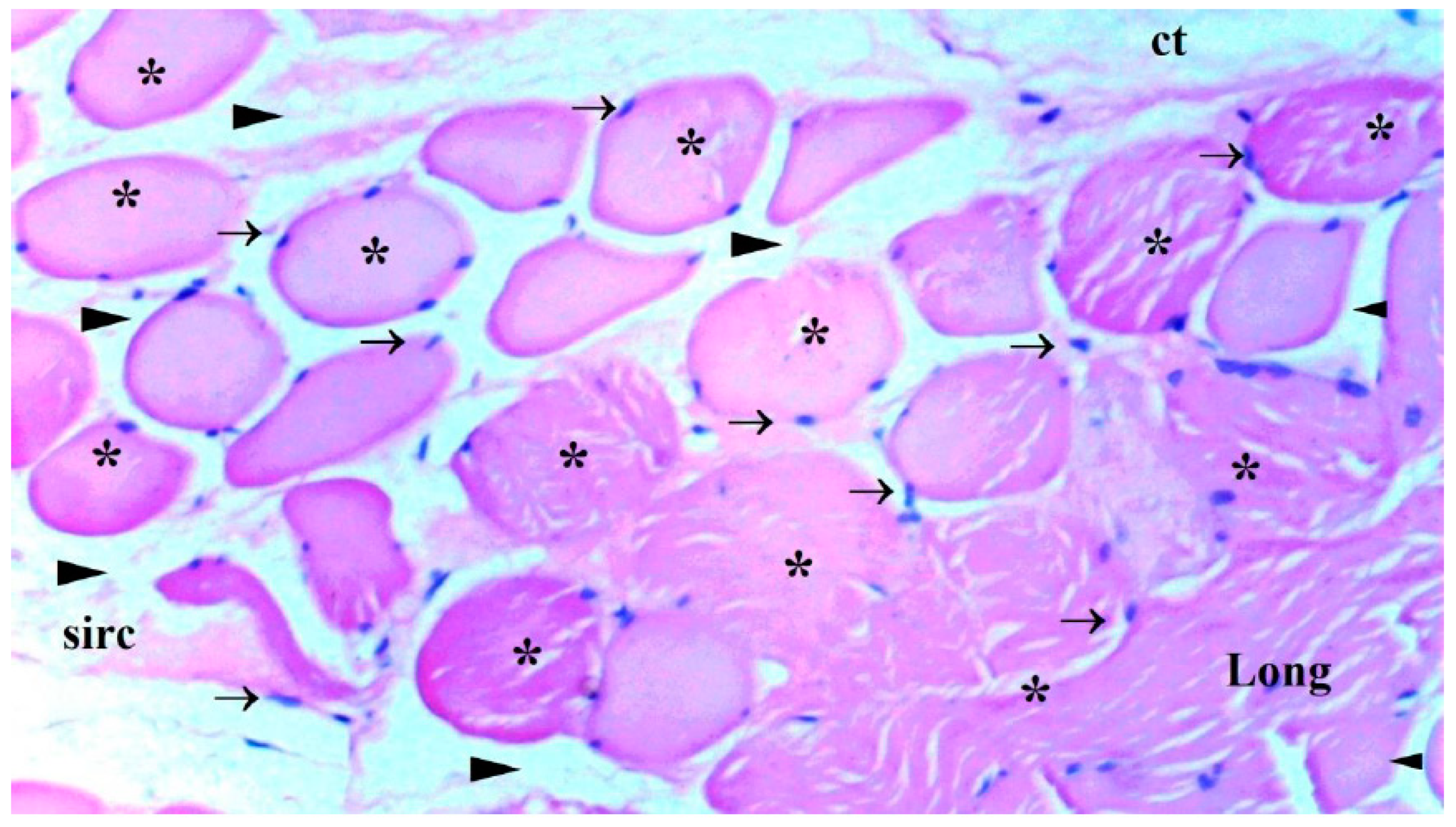
The presence of muscle atrophy hypertrophy, muscle degeneration-congestion, internalization of muscle nuclei-oval-central nucleus, leukocyte cell infiltration ve fragmentation-hyalinization are evaluated according to hematoxylin-eosin sections
For hematoxylin and eosin staining the sections were deparaffinized and hydrated. Then the sections were stained in hematoxylin for 3 minutes. Then we washed the sections in tap water until sections turn blue and differentiated in 1% acid alcohol. The sections were washed in tap water again and then stained in eosin solution for 10 minutes. After washing in tap water the sections were dehydrated and mounted.
18 skeletal muscle tissue samples separated for biochemical evaluation were first washed with cold NaCl solution (0.154 M) to discard blood contamination and then homogenized in a Diax 900; Heidolph Instruments GmbH&Co KG, Schwabach, Germany at 1000 U for about 3 min. After centrifugation at 10,000 g for about 60 min, the upper clear layer was taken. TBARS assay and CAT activity were quantified, afterward.
Statistical Analysis
The Statistical Package for the Social Sciences (SPSS, Chicago, IL, USA) 20.0 for Windows was used. The Kolmogorov-Smirnov test was used for analyzing each category variable. Biochemical and histopathological parameters were tested by using the Kruskal-Wallis test, Bonferroni Correction test and Mann-Whitney U test. A statistical value of less than 0.05 was considered significant. All values were expressed as mean± standard error (Mean ± SE).
Results
Histopathological Results
The histological parameters muscle atrophy-hypertrophy, muscle degeneration-congestion, the internalization of muscle nuclei oval-central nucleus, leukocyte cell infiltration, and fragmentation-hyalinization varied considerably across groups. (p=0.003, p=0.002, p=0.001, p=0.006, p=0.004, respectively), (
Table 1,
Figure 1,
Figure 2,
Figure 3,
Figure 4,
Figure 5 and
Figure 6). Muscle atrophy-hypertrophy was significantly higher in the IR group than in the C and IR-PRO groups. (p=0.001, p=0.011, respectively). Muscle degeneration-congestion of IR group was substantially greater than in the C and IR-PRO groups. (p=0.001, p=0.005, respectively). In the IR group, the internalisation of muscle nuclei oval-central nucleus was notably higher than in the C and IR-PRO groups (p<0.0001, p=0.024, respectively). Additionally, the internalisation of muscle nuclei oval-central nucleus was extensively elevated in the IR-PRO group than in the C group (p=0.024). Leukocyte cell infiltration and fragmentation-hyalinization were also considerably higher in the IR group than in the C and IR-PRO groups.(p=0.002, p=0.023, respectively), (p=0.002, p=0.006, respectively) (
Table 1,
Figure 1,
Figure 2,
Figure 3,
Figure 4,
Figure 5 and
Figure 6).
Biochemical Results
When the levels of TBARS in muscle tissue were measured within the groups, there was a substantial variance. The IR group’s TBARS level was found to be considerably higher than the control group’s (p<0.0001). TBARS level was found to be significantly lower in IR-PRO group compared to the IR group (p<0.0001), (
Table 2).
In terms of CAT enzyme activity in muscle tissue, there was a remarkable difference between the groups (p<0.0001). The IR group’s CAT enzyme activity was found to be considerably decreased compared to the control group’s (p<0.0001). CAT enzyme activity was notably increased in the IR-PRO group than in the IR group (p<0.0001), although it was similar in the C and IR-PRO groups (p=0.284) (
Table 2).
Discussion
Due to nonignorable clinical outcomes of IR injury, various substances, including antioxidants, statins, and anesthetics, have been investigated and suggested for the preventative therapeutic use of IR injury in studies [
17]. According to our knowledge, the number of investigations of proanthocyanidins’ effect on lower extremity skeletal muscle IR injury is merely rare. Our study hypothesized that proanthocyanidin would have a protective effect on IR injury in skeletal muscle of the lower extremities. Our conclusions support our theoretical basis.
As mentioned before, IR injury increases ROS and RNS and decreases antioxidant enzyme levels and expression, according to several studies [
18]. Numerous enzymes perform as intracellular antioxidants to protect cells from IR-induced oxidative damage. [
19] In this manuscript, we determined CAT enzyme activity for cellular antioxidant defense functions. In this regard, our study supported the literature by increasing the CAT enzyme in Group IR-PRO and Group C compared to Group IR. Based on our observations, another affirming finding is the difference in TBARS level for determined lipid peroxidation, a process by which free radicals destroy lipids’ carbon-carbon double bonds [
7], elevated in Group IR and reduced in Group C and Group IR-PRO.
Histological evaluation was in line with biochemical findings. Intraperitoneal proanthocyanidin treatment before ischemia in rats reversed IR injury in skeletal muscle histology. Our data verified proanthocyanidin’s protection. According to histological inspection, IR damage was associated with muscle degeneration-congestion, fragmentation-hyalinization, leukocyte cell infiltration, muscle atrophy-hypertrophy, and internalization of oval-central nuclei and proanthocyanidin administration prior to ischemic injury inhibited these changes and preserved skeletal muscle.
These changes demonstrated that proanthocyanidin can minimize IR-induced destruction of cells. Hence, it can protect muscles from IR-induced damage.
In summary, we believe our research showed intraperitoneal proanthocyanidin has a protective effect on IR injury of skeletal muscle with a possible mechanism by reducing lipid peroxidation and increasing antioxidant enzyme activity. Examining the literature reveals that there are numerous research on proanthocyanidin. The existence of several types of proanthocyanidin, as well as changes in the duration of administration, dosage, and form, restrict our ability to provide a precise analysis. Future research is required to determine the IR injury effect and proanthocyanidin efficacy.
Author Contributions
MA AÖ, and BK designed the study, and analyzed and interpreted data. AÖ, BK and MA performed the experiments. MA, AÖ and BK confirm the authenticity of all the raw data. MA, AÖ, MA and BK provided scientific and technical assistance, and critically revised the article for important intellectual content. AÖ and BK collected samples. ŞCS and MK performed cellular and molecular experiments. All authors have read and approved the final manuscript.
Funding
This study was supported by the Gazi University BAP coordination unit within the scope of this project numbered TGA-2021-7075.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
Ethical approval for the study was obtained from Animal Research Committee of Gazi University (Ankara, Turkey; approval no. G.Ü.ET-18-059).
Patient Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
References
- Bochenek, L.d.M.S.; Parisotto, E.B.; Salomão, E.d.A.; Maldonado, M.J.M.; Silva, I.S. Characterization of oxidative stress in animal model of neonatal hypoxia. Acta Cir. Bras. 2021, 36, e361108. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.-F.; Tuo, Q.-Z.; Yin, Q.-Z.; Lei, P. The pathological role of ferroptosis in ischemia/reperfusion-related injury. Zool. Res. 2020, 41, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; Yiang, G.-T.; Liao, W.-T.; Tsai, A.P.Y.; Cheng, Y.-L.; Cheng, P.-W.; Li, C.-Y.; Li, C.J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell. Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Draganovic, D.; Lucic, N.; Jojic, D. Oxidative Stress Marker and Pregnancy Induced Hypertension. Med Arch. 2016, 70, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Slopovsky, J.; Kucharska, J.; Obertova, J.; Mego, M.; Kalavska, K.; Cingelova, S.; Svetlovska, D.; Gvozdjakova, A.; Furka, S.; Palacka, P. Plasma thiobarbituric acid reactive substances predicts survival in chemotherapy naïve patients with metastatic urothelial carcinoma. Transl. Oncol. 2021, 14, 100890. [Google Scholar] [CrossRef] [PubMed]
- Dzięgielewska-Gęsiak, S.; Stołtny, D.; Brożek, A.; Muc-Wierzgoń, M.; Wysocka, E. Are insulin-resistance and oxidative stress cause or consequence of aging. Exp. Biol. Med. 2020, 245, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- De Leon, J.A.D.; Borges, C.R. Evaluation of Oxidative Stress in Biological Samples Using the Thiobarbituric Acid Reactive Substances Assay. J. Vis. Exp. 2020, e61122. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Weseler, A.R.; Bast, A. Masquelier’s grape seed extract: from basic flavonoid research to a well-characterized food supplement with health benefits. Nutr. J. 2017, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.C.; Yin, J.; Zhou, B.; Liu, Y.T.; Yu, Y.; Li, G.Q. Grape seed proanthocyanidin protects liver against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress. World J Gastroenterol. 2015, 21, 7468–7477. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A.A.R. Proanthocyanidin protects against cisplatin-induced nephrotoxicity. Phytotherapy Res. 2009, 23, 1738–1741. [Google Scholar] [CrossRef] [PubMed]
- Guler, A.; Sahin, M.A.; Yucel, O.; Yokusoglu, M.; Gamsizkan, M.; Ozal, E.; Demirkilic, U.; Arslan, M. Proanthocyanidin prevents myocardial ischemic injury in adult rats. J. Pharmacol. Exp. Ther. 2011, 17, BR326–BR331. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Rimbach, G.; Virgili, F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (pinus maritima) bark, pycnogenol. Free. Radic. Biol. Med. 1999, 27, 704–724. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, J.; Yang, Z.; Sui, R.; Miao, Q.; Li, Y.; Yu, J.; Liu, C.; Zhang, G.; Xiao, B.; et al. Therapeutic effect of oligomeric proanthocyanidin in cuprizone-induced demyelination. Exp. Physiol. 2019, 104, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Asma, B.; Vicky, L.; Stephanie, D.; Yves, D.; Amy, H.; Sylvie, D. Standardised high dose versus low dose cranberry Proanthocyanidin extracts for the prevention of recurrent urinary tract infection in healthy women [PACCANN]: a double blind randomised controlled trial protocol. BMC Urol. 2018, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Cearra, I.; Herrero de la Parte, B.; Ruiz Montesinos, I.; Alonso-Varona, A.; Moreno-Franco, D.I.; García-Alonso, I. Effects of Folinic Acid Administration on Lower Limb Ischemia/Reperfusion Injury in Rats. Antioxidants 2021, 10, 1887. [Google Scholar] [CrossRef]
- Yildirim, B.A.; Albayrak, S. Effect of crocin on experimental gastrocnemius muscle ischemia/reperfusion injury in rat. Vet Res Forum. 2023, 14, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Gobut, H.; Erel, S.; Ozdemir, C.; Mortas, T.; Arslan, M.; Kucuk, A.; Kasapbasi, E.; Kavutcu, M. Effects of cerium oxide on liver tissue in liver ischemia-reperfusion injury in rats undergoing sevoflurane anesthesia. Exp. Ther. Med. 2023, 25, 164. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).