1. Introduction
Diabetes is multifactorial complication often leads to major cardiovascular complications. Diabetes mellitus (DM) is brought on by high blood sugar levels that interfere with insulin metabolism and homeostasis [
1]. The metabolic condition with the clinical presentation of long-lasting hyperglycemia is diabetes mellitus (DM). When there is an impairment in a person's capacity to control the amount of glucose in their blood, it leads to diabetes which further can lead to both microvascular and macrovascular complications [
2,
3]. Globally, 1 in 11 adults suffer from diabetes mellitus. Among these, 90% suffer from type 2 diabetes mellitus [
4]. People nowadays lead unhealthy, sedentary lifestyles, eat unhealthy foods and beverages, consume inadequate amounts of fibre, have irregular sleep patterns, and engage in less physical activity. This contributes to the manifestation of a variety of pathologies including diabetes mellitus [
5]. Diabetes is a multifaceted illness with intricate organ-to-organ and target-to-target interaction. The current and conventional treatment options available to treat diabetes are focused on reducing hyperglycemia using targeted approaches [
6]. They are insufficient to treat the complex etiopathology, chronicity, and systemic consequences of diabetes, even while they effectively lower hyperglycemia. A multiple approach is needed to manage the complication of diabetes. Herbal medicines are thought to be the lead molecules in the current developments and the contribution of oxidative stress to the difficulties of diabetes mellitus. Utilizing medicinal plants, vitamins, and critical elements is a low-cost diabetic preventative and treatment technique [
7]. A comprehensive and systemic approach is therefore necessary for its effective management. Herbal therapy, the Indian system of medicine can be an important and effective option to fill this gap [
8].
The usage of herbal formulation is steadily increasing in the last few years and is receiving increased global attention. Single or multiple herbs polyherbal (PHF) are utilized for treatment in various disease manifestations [
9]. PHFs are considered more beneficial as they provide better therapeutic efficacy and less toxicity when compared to single herbal formulations [
10]. Through animal studies and human trials, a variety of polyherbal formulations have been demonstrated to have positive effects in the control of diabetes [
11]. PHMC Capsules is one such PHF which was found to possess antidiabetic and antioxidant properties. PHMC capsules consist of the following herbal extracts of Pterocarpus marsupium, Withania somnifera, Salacia reticulata, Gymnema Sylvestre extract, Curcuma longa, Vitis vinifera, and Piper nigrum. It possesses antidiabetic and antioxidant properties. Pterocarpus marsupium and Gymnema Sylvestre lower fasting glucose and postprandial blood glucose; additionally, the latter reduces glycosylated haemoglobin and serum lipid levels as well. Salacia reticulata is also known to lower blood glucose levels [
12]. Curcuma longa contains curcumin, which is the main ingredient and is known to lower blood glucose levels and serum lipid levels. Piper nigrum possesses hypolipidemic and hypoglycemic properties [
13]. Withania somnifera is well known herbal medicine which is known for its anti-diabetic and anti-inflammatory properties [
14]. Vitis vinifera contents like resveratrol is known to possess hypoglycemic and antioxidant properties [
15]. We have performed a Preclinical study conducted to ensure the impact of PHMC tablet [
16]. However, the safety and efficacy of PHMC capsules have not been established in humans. Thereby this study is aimed to evaluate the safety and efficacy of PHMC capsules in patients with type 2 diabetes mellitus.
2. Materials and Methods
2.1. Study Design
In this prospective, randomized, comparative, clinical, and pilot study conducted at the Interdisciplinary Institute of Indian System of Medicine (IIISM), SRM IST, Kattankulathur, all the participants were recruited at the general medicine outpatient clinic and the ayurvedic outpatient clinic at SRM Medical College Hospital and Research Centre, Tamil Nadu, India (conducted from April 2022–June 2022). All the laboratory investigations were collected at the Metabolic Ward, the Interdisciplinary Institute of Indian System of Medicine (IIISM), and SRM IST and sent for examination. The study protocol was approved by the Institutional Ethics Committee (2914/IEC/2021), followed by the study was registered in CTRI (CTRI/2022/05/042422) and participants (aged 18–60 years) who voluntarily signed the informed consent were included in the study.
2.2. Randomization and Blinding
Randomization was done based on computer random allocation software version 2.0. The concealment of the randomization code was done by a third party who was not involved in the trial. This was done to avoid selection bias. Each allocation was written on paper and concealed in a serially numbered, opaque envelope. There was no blinding involved.
2.3. Inclusion and Exclusion Criteria
2.3.1. Inclusion Criteria
Participants of both genders were included if they were in the age range of 18 to 60 years with T2DM being newly diagnosed or diagnosed within the last 10 years having FBS ≥ 126 mg/dl, RBS ≥ 200 mg/dl, and HbA1C between 7-9.5%, patients who is on treatment with metformin 500mg two times a day.
2.3.2. Exclusion Criteria
Patients with T1DM, BMI >35 kg/m2 or less than 20 kg/m2,severe hyperglycemia (FBS > 234 mg/dl or PPBS > 360 mg/dl), abnormal lipid parameters such as cholesterol > 260 mg/dl, serum triglycerides > 300 mg/dl, HbA1C greater than 9.5%,AST and ALT levels greater than 2.5 times the upper normal limit, psychiatric disorder, a history of smoking and alcohol use, Severe renal, hepatic, cardiac, gastrointestinal, neurological, hematological or respiratory disorders, history of intake of any Ayurvedic/herbal/homeopathic/dietary supplements in the last two months, patients who have participated in any investigational study in the last 12 weeks, known hypersensitivity to the study drugs, women in childbearing age refusing to use contraceptive, pregnant and lactating women were excluded.
2.4. Intervention
In this clinical study, which was conducted on 60 patients suffering from type 2 diabetes mellitus, the patients were assigned to two groups: Group A (n = 30) with Metformin 500 mg twice a day after food and Group B (n = 30) with PHMC, which was given 500 mg twice a day before food. Laboratory investigations were collected at the time of screening and at the end of 90 days in both groups.
2.5. Molecular Docking
Molecular docking enables researchers to predict the binding modes of major bioactive compounds (
Table 1) with target proteins at the atomic level. This precision aids in understanding the specific interactions that contribute to the pharmacological activity. By simulating the docking process, researchers can identify critical amino acid residues and structural features crucial for ligand binding, offering a detailed map of the molecular landscape. Molecular docking is a computational technique used in drug discovery and structural biology to predict the binding mode and strength of a ligand (in this case, the listed compounds) to a target protein (in this case, the 1K3A target) [
17].
2.7. Statistical Analysis
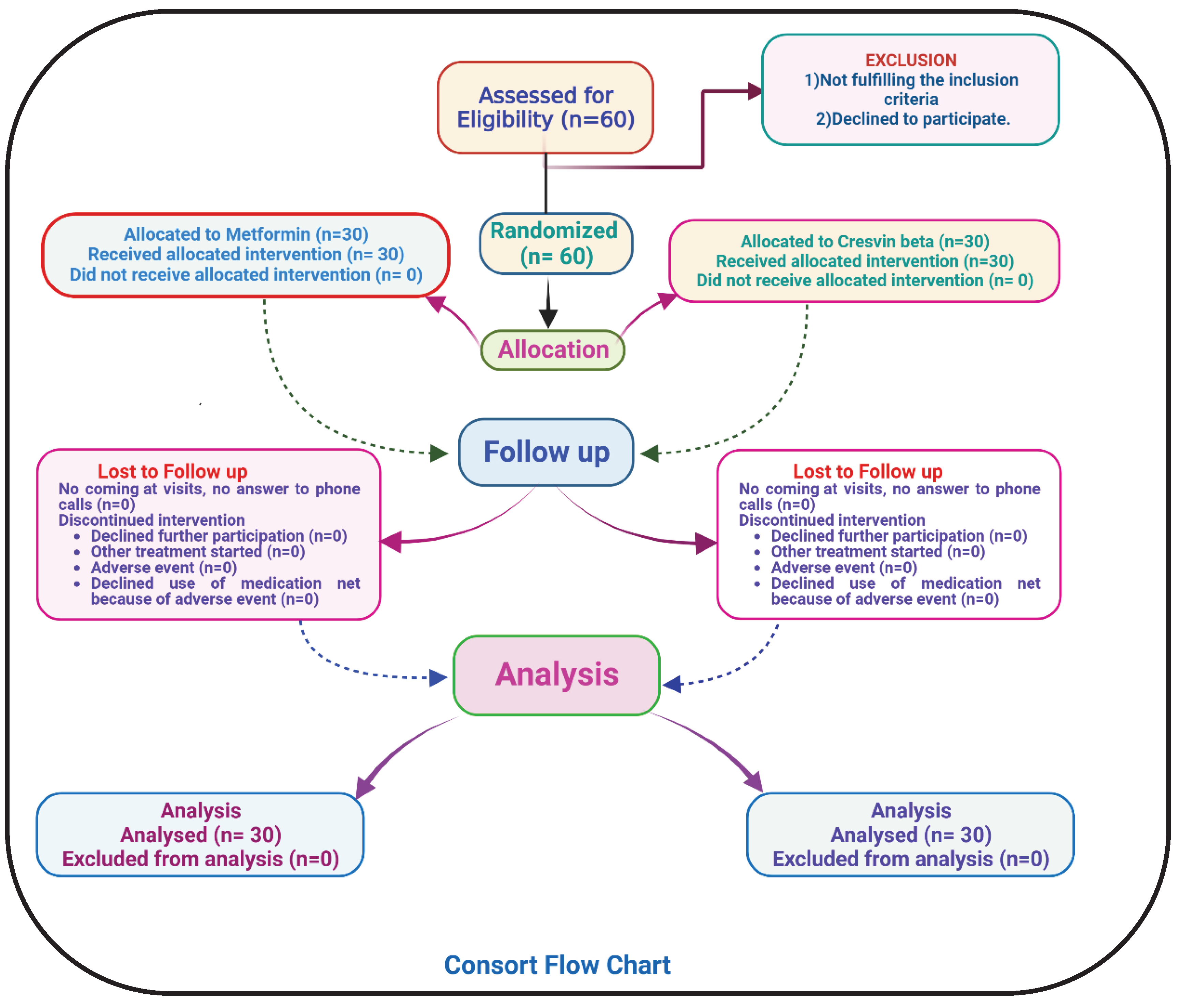
Statistical analysis of all the participants who took part from the starting till the end of the study were analyzed. Mean, Standard deviation and paired T-test were employed in statistical analysis of both Group A and Group B. The significance level was set to be 0.05 to compare the difference in efficacy between Group A and Group B. The statistical analysis was performed in GraphPad Prism version 8 by one-way ANOVA method. The consort flow chart of the study is illustrated in
Figure 1.
3. Results
In this clinical study 80 patients were assessed for eligibility and 60 were randomized and enrolled in Group A (n=30) and Group B (n=30) respectively. All the participants took part till the end of the study and no participants withdrew from the study. The majority of patients 53.4%, were reported in the age group of 41–50 years, followed by 30% in the age group of 51–60 years, 13.3% in the age group of 31–40 years, and 3.3% in the age group of 18–30 year were found in the study. Among these 65% were male and 35% were female. Additionally, 10% of the patients were newly diagnosed with Type 2 diabetes mellitus, while 20% of the recruited subjects had a previous history of Type 2 diabetes mellitus. All the biochemical parameters of metformin treated group and investigational treated group were expressed in terms of Mean ±S.D. and the p value of all biological parameters are depicted in
Table 2.
3.1. Effect on Blood Glucose
The mean difference of FBS in PHMC from pre-treatment to post-treatment was 56.69, whereas that of Metformin was 31.9. Both PHMC and Metformin show extreme statistical significance (p<0.0001). Therefore, PHMC exhibits a similar effect on FBS in comparison to Metformin. The mean difference of PPBS in PHMC from pre-treatment to post-treatment was 48.62 (p=0.0001), whereas for Metformin it was 37.55(p=0.0003). PHMC indicates a high statistical significance and produces acceptable results same as Metformin. The mean difference in HbA1C from pre-treatment to post-treatment in PHMC group was 1.45 (p<0.0001), whereas it was 0.72 in Metformin (p-value -0.0002). PHMC indicates a high statistical significance for PPBS, HbA1C and shows similar results like Metformin.
3.2. Effect on Liver Function Tests
The mean difference of SGOT in PHMC group from pre-treatment to post-treatment was 2.17 (p=0.1425), whereas in Metformin it was 2.45(p=0.1425). Both Metformin and PHMC do not show significant differences nor have much beneficial activity in the management of SGOT. The mean difference of SGPT in PHMC from pre-treatment to post-treatment was 0.45(p=0.7019), whereas Metformin was 0.24 (p-value - 0.8349). The p-value of both Metformin and PHMC do not have much beneficial activity in the management of SGPT.
3.3. Effect on Haematological Parameters
The mean difference in total WBC in PHMC group from pre-treatment to post-treatment was 1755.17, whereas in Metformin group it was 1769. Both depict high statistical significance with p-value less than 0.001. Both PHMC and Metformin confer the same effect. The mean difference of haemoglobin in PHMC group from pre-treatment to post-treatment was -1.13(p=0.0004), whereas it was -1.23 (p=0.0003) in the Metformin group. PHMC shows fair statistical significance, whereas Metformin showed better improvement for Hb. However, PHMC doesn’t fall too far behind.
The mean difference of ESR in the PHMC group from pre-treatment to post-treatment was 2.38 (p<0.0001), whereas in Metformin it was 0.73(p=0.2132). PHMC shows high statistical significance and showed a beneficial effect in the improvement of ESR. The mean difference in platelet count in PHMC group from pre-treatment to post-treatment was -0.1(p=0.4671,), whereas Metformin group it was 0.12 (p=0.7366). Both PHMC and Metformin showed less beneficial activity in the management of platelets.
The mean difference of CRP in PHMC group from pretreatment to post treatment was 2.62(p=0.0001), whereas Metformin group it was 0.84(p=0.0230). Though Metformin has a beneficial activity in the management of CRP, PHMC has more acceptable results similar to that of Metformin by showing better statistical significance.
3.4. Effect on Renal Function
The mean difference of creatinine in the PHMC group from pre-treatment to post-treatment was 0.47(p<0.0001), whereas for Metformin it was 0.39 (p<0.0001). Both PHMC and Metformin show beneficial effects in the improvement of creatinine level.
3.5. Effect on Lipid Parameters
The mean difference of triglycerides in PHMC group from pre-treatment to post-treatment was 32.84(p=0.0096), whereas in the Metformin group it was 3.03 (p=0.9077). In terms of triglyceride management, PHMC outperforms Metformin. The mean difference of LDL in PHMC from pretreatment to posttreatment was 11.96 (p=0.1568), whereas for Metformin it was 5.45 (p=0.6715). Both PHMC and Metformin showed no significant value and have little effect on LDL levels.
3.6. Effect on Inflammatory Mediator
The mean difference of IL6 in PHMC from pre-treatment to post-treatment was 0.8(p=0.0008), whereas in the Metformin group it was 0.68 (p=0.1037). PHMC shows better results when compared to the metformin group at lowering IL-6 levels. The mean difference of adiponectin in PHMC group from pre-treatment to post-treatment was -7.4 (p<0.0001); whereas in Metformin group it was -3.67(p<0.0001). Both PHMC and metformin produce acceptable results in regulating adiponectin levels.
The mean difference of endothelin in PHMC group from pre-treatment to post-treatment was 1.04 (p<0.0001), whereas in Metformin group it was 0.43 (p<0.0048). When it comes to managing endothelin levels, PHMC is beneficial as metformin by exhibiting fair statistical difference. The mean difference of TNF-alpha in PHMC group from pre-treatment to post-treatment was 2.11(p<0.0011), whereas Metformin was 1.45 (p<0.0073). Although the difference is less statistical significance, PHMC has acceptable results similar to Metformin for the management of TNF-alpha.
3.7. Molecular Docking Analysis
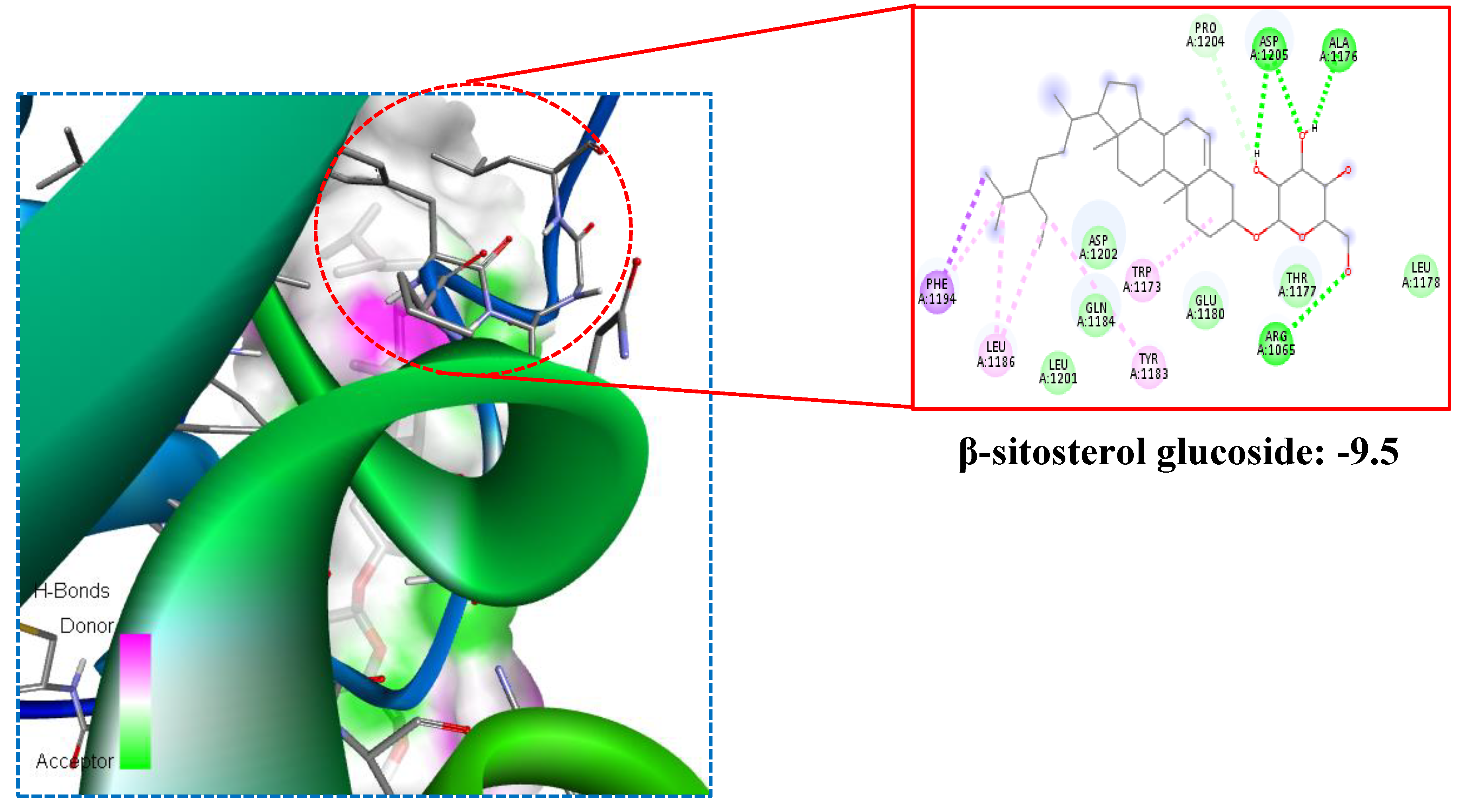
The docking scores provided in the
Table 3 represent the calculated binding affinities of each main compounds to the target protein, measured in kilocalories per mole (kcal/mol). The docking efficacy was represented in the
Figure 2.
A lower docking score generally indicates a stronger binding affinity. Maytenfolic acid is a natural compound that can be found in certain plant species. It belongs to the class of phytochemicals, which bioactive compounds are derived from seven plants. The present research such as Propterol (-7.8), β-sitosterol (-7.8), Stigmasterol (-7.2), β-sitosterol glucoside (-9.5), Xanthone (-6.5), Leucopelargonidin (-6.9), Iguesterin (-8.0), Maytenfolic acid (-8.1), Curcumin (-6.8), Gymnemic acid (-7.6), and Kaempferol (-7.1) showed high binding affinity on 1K3A target. These compounds are often investigated for their potential health benefits, including their impact on various diseases, such as diabetes. Anti-Diabetic Properties: Maytenfolic acid has been studied for its potential anti-diabetic properties. Some research suggests that it may exhibit effects that could be beneficial in managing diabetes. These effects may include improving insulin sensitivity, reducing blood glucose levels, and modulating key pathways involved in glucose metabolism. Anti-Inflammatory Effects: Chronic inflammation is closely linked to the development of diabetes and its complications. Maytenfolic acid, like many other phytochemicals, possesses anti-inflammatory properties.
By reducing inflammation, it may contribute to better glycemic control and overall metabolic health. Antioxidant Activity: Oxidative stress is another factor implicated in the progression of diabetes. Maytenfolic acid, with its antioxidant properties, may help neutralize free radicals and reduce oxidative stress, potentially providing protective effects against diabetes-related complications. Insulin Sensitization: Improving insulin sensitivity is a key aspect of diabetes management. Substances that enhance insulin sensitivity can contribute to better control of blood sugar levels. Maytenfolic acid may influence insulin signaling pathways, leading to improved insulin sensitivity.
4. Discussion
In this randomized, open label pilot study to evaluate the safety and efficacy of PHMC capsule, polyherbal metabolite formulation, administered at a dose of 500 mg twice daily before food to test group (Group B) to control group (Group A) taking Metformin 500 mg twice daily after food over a period of 3 months, we found that PHMC showed better effects compared to Metformin in the management of Diabetes Mellitus. This is the first randomized controlled trial conducted on human subjects to discern the safety and efficacy of PHMC capsules. The three main tests that are used to monitor chronic glycemia around the world are FBS, PPBS, and glycated haemoglobin (HbA1c), which particularly is considered as the gold standard test for assessing glycemic control during follow-up. FBS, PPBS, HbA1C were found to be improved with extreme statistical significance by PHMC. Metformin did not show any improvement in FBS but PHMC was found to effectively improve it besides outperforming Metformin in the improvement of latter two parameters. Numerous factors, such as an increase in the production of reactive oxygen species (ROS) and the development of advanced glycation end products (AGEs) as a result of chronic hyperglycemia, might contribute to alterations in haematological parameters in diabetic patients. These haematological abnormalities may cause complications such as anaemia and a hypercoagulable state, and they may also be a factor in the precipitation of CVD [
34,
35].
PHMC was found to improve WBC levels extremely and similar effect was also observed in metformin group. PHMC shows superiority in managing CRP levels even though metformin also showed same improvement. However, when it comes to ESR, PHMC showed improvement. One of the main causes of kidney failure and chronic kidney disease is T2DM.Serum creatinine is a reliable biomarker which indicates impaired kidney function [
36].
Both PHMC and metformin was found to improve creatinine in this study. Dyslipidemia frequently coexists with Diabetes Mellitus (T2DM). High total cholesterol, high triglycerides (TGL), and increased levels of small dense lipoprotein (LDL) are frequently found in diabetic patients with lipid abnormalities which increases the risk of developing cardiovascular disease (CVD) [
37]. In this study it was found that the Metformin and PHMC effect on LDL and TGL levels. However, PHMC have similar effect like metformin in the management of TGL. Similar results were seen when it comes to Interleukin-6 (IL-6) levels, which is a type of proinflammatory cytokine which is normally present in tissues but its irregular production and prolonged exposure causes inflammation, which in turn can cause overt insulin resistance resulting in T2DM [
38]. Reduced adiponectin, increased endothelin (ET)-1 expression and dysregulated production of adipocytokine TNF- α has been linked to a number of human disorders, including type 2 diabetes mellitus. These factors play a role in influencing the glucose metabolism by inhibiting the action of insulin [
39,
40,
41,
42]. Both PHMC and Metformin shows good effect in managing adiponectin [
43,
44]. However, PHMC additionally outshines metformin in managing ET-1 and TNF- α. On the other hand, when it comes to liver function tests such as SGOT and SGPT, both PHMC as well as Metformin was found to have little influence in their management. No adverse drug reactions or events were reported in both the groups along with no participants withdrawing during the course of this study. One of the limitations of the study is the number of participants included. No Adverse drug reaction was reported during the study period. The primary and secondary outcomes were assessed with the given number of participants.
The plant compound maytenfolic acid is derived from natural sources, and its potential as a therapeutic agent for diabetes aligns with the growing interest in using plant-based compounds as complementary or alternative treatments [
45]. β-sitosterol glucoside is a plant-derived compound belonging to the group of phytosterols, which are structurally similar to cholesterol. It is often found in various plant sources, including fruits, vegetables, nuts, and seeds. Insulin Sensitivity: β-sitosterol glucoside may contribute to improved insulin sensitivity, allowing cells to respond more effectively to insulin and facilitating the uptake of glucose from the bloodstream. Glucose Metabolism: The compound may influence various pathways involved in glucose metabolism, potentially helping to regulate blood sugar levels. Inflammation Modulation: Chronic inflammation is associated with insulin resistance and diabetes. β-sitosterol glucoside, like other phytosterols, may have anti-inflammatory effects that could be beneficial in the context of diabetes [
46]. However future studies should be done with more participants over a long period of time with incorporation of additional parameters like HOMA-IR, HDL-C, VLDL-C, TC, and UACR etc.
5. Conclusion and Future Prospects
Clinical improvement of Type 2 Diabetes mellitus was observed over the course of a 3-month period following the administration of PHMC. Our research results express that the test drug PHMC shows good effects similar to metformin in the management of type 2 diabetes mellitus while pertaining excellent safety. PHMC can be considered as a safe and effective treatment option in the management of type 2 diabetes mellitus apart from the standard treatment. With this clinical evidence, we have concluded that the investigational product has a significant role in the management of diabetes mellitus. However, multi-centric trials are required to cover the impact of selection bias, race, ethnicity modification, and drug-food interaction.
Author Contributions
Conceptualization, N.K.N and V.P; methodology, N.K.N. and B.M.; software, P.S.; validation, N.K.N., N.M. and P.S.; formal analysis, N.K.N and B.M.; investigation, N.K.N., R.C.S.K. and B.M.; resources, R.C.S.K. and B.M.; data curation, N.K.N and P.S.; writing—original draft preparation, N.K.N and P.S.; writing-review and editing, N.K.N., P.S. and M.N.; visualization, N.K.N., P.S. and M.N.; supervision, V.P. and R.C.S.K.; project administration, N.K.N and B.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethical Committee of SRM Medical College Hospital and Research Centre (23 September 2021) followed by the study was registered in CTRI (2022/05).
Informed Consent Statement
Informed consent was obtained from all individual participants included in the study.
Data Availability Statement
No additional data beyond that published in this manuscript are available for sharing.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Yedjou, C.G.; Grigsby, J.; Mbemi, A.; Nelson, D.; Mildort, B.; Latinwo, L.; Tchounwou, P.B. The Management of Diabetes Mellitus Using Medicinal Plants and Vitamins. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Kaul, K.; Tarr, J.M.; Ahmad, S.I.; Kohner, E.M.; Chibber, R. Introduction to diabetes mellitus. Adv. Exp. Med. Biol. 2012, 771, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Duggal, N. Type 2 diabetes mellitus, its impact on quality of life and how the disease can be managed-a review. Obes. Med. 2022, 35, 100459. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Martin, S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Dhanasekaran, D.; Ganamurali, N.; Preethi, L.; Sabarathinam, S. Junk food-induced obesity-a growing threat to youngsters during the pandemic. Obes. Med. 2021, 26, 100364. [Google Scholar] [CrossRef] [PubMed]
- Modak, M.; Dixit, P.; Londhe, J.; Ghaskadbi, S.; Devasagayam, T.P. Indian herbs and herbal drugs used for the treatment of diabetes. J. Clin. Biochem. Nutr. 2007, 40, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Thottapillil, A.; Kouser, S.; Kukkupuni, S.K.; Vishnuprasad, C.N. An Ayurveda-Biology platform for integrative diabetes management. J. Ethnopharmacol. 2021, 268, 113575. [Google Scholar] [CrossRef] [PubMed]
- Sabarathinam, S.; Satheesh, S.; Raja, A. Plant-based medicines in the treatment of cardiometabolic disorders: A special view on sarcopenic obesity. Obes. Med. 2023, 41, 100497. [Google Scholar] [CrossRef]
- Parasuraman, S.; Thing, G.S.; Dhanaraj, S.A. Polyherbal formulation: Concept of ayurveda. Pharmacogn. Rev. 2014, 8, 73–80. [Google Scholar] [CrossRef]
- Zarvandi, M.; Rakhshandeh, H.; Abazari, M.; Shafiee-Nick, R.; Ghorbani, A. Safety and efficacy of a polyherbal formulation for the management of dyslipidemia and hyperglycemia in patients with advanced-stage of type-2 diabetes. Biomed. Pharmacother. 2017, 89, 69–75. [Google Scholar] [CrossRef]
- Grover, J.K.; Yadav, S.; Vats, V. Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacolog. 2022, 81, 81–100. [Google Scholar] [CrossRef]
- Khaliq, T.; Sarfraz, M.; Ashraf, M.A. Recent Progress for the Utilization of Curcuma longa, Piper nigrum and Phoenix dactylifera Seeds against Type 2 Diabetes. West Indian Med. J. 2015, 64, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Mandlik Ingawale, D.S.; Namdeo, A.G. Pharmacological evaluation of Ashwagandha highlighting its healthcare claims, safety, and toxicity aspects. J. Diet. Suppl. 2021, 18, 183–226. [Google Scholar] [CrossRef]
- Orhan, N.; Aslan, M.; Orhan, D.D.; Ergun, F.; Yeşilada, E. In-vivo assessment of antidiabetic and antioxidant activities of grapevine leaves (Vitis vinifera) in diabetic rats. J. Ethnopharmacol. 2006, 108, 280–286. [Google Scholar] [CrossRef]
- Kn, K.; Chandra, R.C.S.; Balasubramanian, D.; Balasundar, S.; Mothiswaran, D.; Narayanan, J.; Vishakan, S.; Chitra, V.; Venkataraman, P. Acute and subacute toxicity assessment of polyherbal metabolites of PHMC tablets. J Hunan University (Natural Science Edition). 2022, 49, 1779. [Google Scholar]
- Favelyukis, S.; Till, J.H.; Hubbard, S.R.; Miller, W.T. Structure and autoregulation of the insulin-like growth factor 1 receptor kinase. Nature structural Biol. 2001, 8, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Badkhane, Y.; Yadav, A.S.; Sharma, A.K.; Raghuwanshi, D.K.; Uikey, S.K.; Mir, F.A. Pterocarpus marsupium Roxb-Biological activities and medicinal properties. Inter J Advances in Pharma Sci. 2010, 1, 350–357. [Google Scholar] [CrossRef]
- Tiwari, M.A.; Sharma, M.A.; Khare, H.N. Chemical constituents and medicinal uses of Pterocarpus marsupium Roxb. Flora and Fauna. 2015, 21, 55–59. [Google Scholar]
- Katiyar, D.; Singh, V.; Ali, M. Phytochemical and pharmacological profile of Pterocarpus marsupium: A review. The Pharma Innovation. 2016, 5, 31. [Google Scholar]
- Rahman, M.S.; Mujahid, M.D.; Siddiqui, M.D.; Rahman, M.A.; Arif, M.; Eram, S.; Khan, A.; Azeemuddin, M.D. Ethnobotanical uses, phytochemistry and pharmacological activities of Pterocarpus marsupium: a review. Pharmacog, J. 2018, 10, 1–8. [Google Scholar]
- Misra, L.; Mishra, P.; Pandey, A.; Sangwan, R.S.; Sangwan, N.S.; Tuli, R. Withanolides from Withania somnifera roots. Phytochem. 2008, 69, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Rautela, I.; Sharma, M.D.; Sharma, N.; Kishor, K.; Singh, K.; Sharma, N. Comparative GC-MS analysis of leaf and root extract of medicinal plant Withania somnifera. World J Pharm Res. 2008, 7, 956–972. [Google Scholar]
- Yuhao, L.; Huang, T.H.; Yamahara, J. Salacia root, a unique Ayurvedic medicine, meets multiple targets in diabetes and obesity. Life Sci. 2008, 82, 1045–1049. [Google Scholar]
- Yoshikawa, M.; Nishida, N.; Shimoda, H.; Takada, M. Polyphenol constituents from Salacia species: quantitative analysis of mangiferin with alpha-glucosidase and aldose reductase inhibitory activities. Yakugaku Zasshi. 2001, 121, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Arunakumara, K.K.I.U.; Subasinghe, S. Salacia reticulata Wight: A review of botany, phytochemistry and pharmacology. Tropical Agri Res Exten. 2010, 13, 41–47. [Google Scholar] [CrossRef]
- Saneja, A.; Sharma, C.; Aneja, K.R.; Pahwa, R. Gymnema sylvestre (Gurmar): A review Der Pharmacia Lettre. 2010, 2, 275–284. [Google Scholar]
- Ibrahim, D.S. Role of Gymnema sylvestre leaf extract on hepatotoxicity induced by cisplatin in rats. Egyptian J Exp Biol (Zoology). 2022, 18, 163–170. [Google Scholar] [CrossRef]
- Li, S.; Yuan, W.; Deng, G.; Wang, P.; Yang, P.; Aggarwal, B. Chemical composition and product quality control of turmeric (Curcuma longa L.). Pharmaceutical Crops. 2011, 2, 28–54. [Google Scholar] [CrossRef]
- Colombo, F.; Di Lorenzo, C.; Regazzoni, L.; Fumagalli, M.; Sangiovanni, E.; Peres de Sousa, L.; Bavaresco, L.; Tomasi, D.; Bosso, A.; Aldini, G.; Restani, P.; DellAgli, M. Phenolic profiles and anti-inflammatory activities of sixteen table grape (Vitis vinifera L.) varieties. Food Funct. 2019, 10, 1797–1807. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Nowicka, P.; Turkiewicz, I.; Golis, T. Characterization in vitro potency of biological active fractions of seeds, skins and flesh from selected Vitis vinifera L. cultivars and interspecific hybrids. J Func Foods. 2019, 56, 353–363. [Google Scholar] [CrossRef]
- Cotoras, M.; Vivanco, H.; Melo, R.; Aguirre, M.; Silva, E.; Mendoza, L. In Vitro and in Vivo Evaluation of the Antioxidant and Prooxidant Activity of Phenolic Compounds Obtained from Grape (Vitis vinifera) Pomace. Molecules. 2014, 19, 21154–21167. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Ke Ha, L.; Nguyen, D.C.; Dao, T.P.; Thi Hong Nhan, L.; Nguyen, D.H.; Nguyen, T.D.N.; Vo, D.V.; Tran, Q.T.; Bach, L.G. The study on extraction process and analysis of components in essential oils of black pepper (Piper nigrum L.) seeds harvested in Gia Lai Province. Vietnam. Processes. 2019, 7, 56. [Google Scholar]
- Arkew, M.; Yemane, T.; Mengistu, Y.; Gemechu, K.; Tesfaye, G. Hematological parameters of type 2 diabetic adult patients at Debre Berhan Referral Hospital, Northeast Ethiopia: A comparative cross-sectional study. PloS one. 2021, 16, 0253286. [Google Scholar] [CrossRef]
- Tiwari Pandey, A.; Pandey, I.; Kanase, A.; Verma, A.; Garcia-Canibano, B.; Dakua, S.P.; Balakrishnan, S.; Singh, M.P. Validating Anti-Infective Activity of Pleurotus Opuntiae via Standardization of Its Bioactive Mycoconstituents through Multimodal Biochemical Approach. 2021, 11, 484. [Google Scholar] [CrossRef]
- Kene, K.; Wondimnew, T.; Welde, M.; Mateos, T.; Adugna, T.; Gerema, U.; Abdisa, D.; Abera, D. Prevalence and determinants of Impaired Serum Creatinine and Urea among type 2 diabetic patients of Jimma Medical Center, Jimma, Southwestern Ethiopia. Endocrine and Metabolic Science. 2021, 3, 100096. [Google Scholar] [CrossRef]
- Athyros, V.G.; Doumas, M.; Imprialos, K.P.; Stavropoulos, K.; Georgianou, E.; Katsimardou, A.; Karagiannis, A. Diabetes and lipid metabolism. Hormones (Athens, Greece). 2018, 17, 61–67. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S.H.; Liaqat, A.; Kamal, S.; Qadir, M.I.; Rasul, A. Role of Interleukin-6 in Development of Insulin Resistance and Type 2 Diabetes Mellitus. Critical reviews in eukaryotic gene expression. 2017, 27, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. International journal of molecular sciences. 2017, 18, 1321. [Google Scholar] [CrossRef] [PubMed]
- Idris-Khodja, N.; Ouerd, S.; Mian, M.O.R.; Gornitsky, J.; Barhoumi, T.; Paradis, P.; Schiffrin, E.L. Endothelin-1 Overexpression Exaggerates Diabetes-Induced Endothelial Dysfunction by Altering Oxidative Stress. American journal of hypertension. 2016, 29, 1245–1251. [Google Scholar] [CrossRef]
- Swaroop, J.J.; Rajarajeswari, D.; Naidu, J.N. Association of TNF-α with insulin resistance in type 2 diabetes mellitus. The Indian journal of medical research. 2012, 135, 127–130. [Google Scholar] [CrossRef]
- TaheriChadorneshin, H.; Cheragh-Birjandi, S.; Goodarzy, S.; Ahmadabadi, F. The impact of high intensity interval training on serum chemerin, tumor necrosis factor-alpha and insulin resistance in overweight women. Obesity Medicine. 2019, 14, 100101. [Google Scholar] [CrossRef]
- Pandey, A.T.; Pandey, I.; Kerkar, P.; Singh, M.P. Antimicrobial activity and mycochemical profile of methanol extract from Pleurotus flabellatus. Vegetos. 2021, 34, 619–629. [Google Scholar] [CrossRef]
- AbdelMassih, A.F.; Ye, J.; Kamel, A.; Mishriky, F.; Ismail, H.A.; Ragab, H.A.; El Qadi, L.; Malak, L.; Abdu, M.; El-Husseiny, M. A multicenter consensus: A role of furin in the endothelial tropism in obese patients with COVID-19 infection. Obesity Medicine 2020, 19, 100281. [Google Scholar] [CrossRef] [PubMed]
- Oboh, M.; Govender, L.; Siwela, M.; Mkhwanazi, B.N. Anti-diabetic potential of plant-based pentacyclic triterpene derivatives: Progress made to improve efficacy and bioavailability. Molecule. 2021, 26, 7243. [Google Scholar] [CrossRef]
- Ramalingam, S.; Packirisamy, M.; Karuppiah, M.; Vasu, G.; Gopalakrishnan, R.; Gothandam, K.; Thiruppathi, M. Effect of β-sitosterol on glucose homeostasis by sensitization of insulin resistance via enhanced protein expression of PPRγ and glucose transporter 4 in high fat diet and streptozotocin-induced diabetic rats. Cytotechnology 2020, 72, 357–366. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).