1. Introduction
In the animal production industry, it has become important to explore and research alternative feedstock for cost-effective animal production due to the rising cost of feed [
1]. It is necessary to find efficient alternative ingredients to replace traditional feed ingredients in swine diets. Soybean is one of the most expensive and limiting feed ingredients in diet formulations. However, soybean is the first choice among protein feeds due to its quality and accessibility. Especially for monogastric animals, post-extraction soybean cake represents the main source of protein [
2,
3]. The production and supply of soybean are critical due to their environmental impact and feed competition for land use [
4,
5]. There have been many attempts to find alternative feed ingredients as protein sources in pig diets [
6]. Most of the ingredients recently studied are vegetable resources, especially local species well-adapted to specific climatic conditions, such as lupin seed, canola cake, guar, sainfoin, taro, and others [
7,
8,
9,
10]. Different diet formulations and protein sources during fattening can affect growth performance as well as carcass and meat quality traits.
Walnut (Juglans regia L.) is an important economic forest tree in China [
11]. Yunnan province is the largest walnut-producing area in China (accounting for 27.17% of the national walnut production), and Fengqing County is the main walnut-producing area in Yunnan province [
11]. Walnuts contain substances such as lipids, proteins, fatty acids, carbohydrates, vitamins and trace elements. Walnut kernel cake is the protein-rich bio-waste derived from the major tree walnuts oil processing industry, and still contains some of the above substances after oil pressing [
12]. In line with a sustainable path, the concept of ‘waste to wealth’ leading to ‘green growth’ is a great opportunity to improve food security and is being adopted by many developed and developing countries [
13]. Currently, research into the use of walnut kernel cake for animal feed is the latest direction, as diversification and expansion of protein feed sources have been an ongoing problem in the pig industry. Research on walnut kernel cake may provide new feed options for the pig industry and improve the health and nutrition of animal husbandry [
12]. Partial replacement of soybean meal with walnut cake as the protein source had effect on meat quality of broiler breast meat [
14,
15]. However, the utilization of walnut cake in pig diet is rarely reported, particularly in the large Diqing Tibetan pig, a local breed in Yunnan province of China [
16].
This study aimed to investigate the effect of walnut kernel cake as a dietary protein substitute on adipose-deposition-related traits in Diqing Tibetan pigs, and to explore its molecular mechanism from the transcriptome level. In this study, we discovered that using walnut kernel cake as a substitute for soybean protein in feed could significantly alter adipose transcriptome levels, increase adipose deposition, and improve pork quality. This study provides new insights and references for research on feed protein substitution, pig breeding and genetic improvement of meat quality.
2. Materials and Methods
2.1. Experimental Treatment and Traits Recording
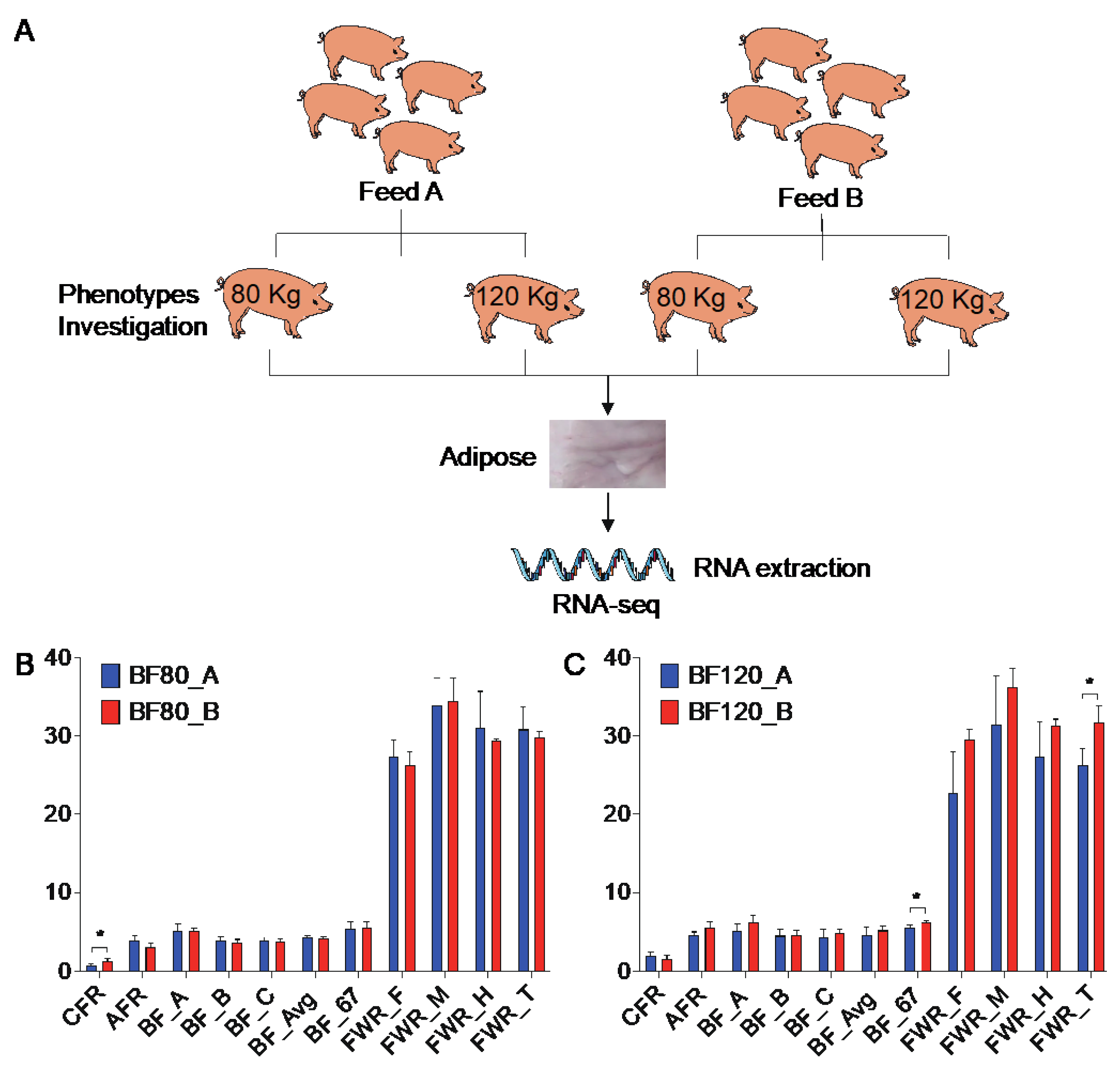
Twelve large Diqing Tibetan pigs at 90 days of age (initial body weight = 8.90 ± 1.85 kg) were used in this experiment conducted at the Yunnan (Yunnan Province, China). The 12 pigs were allotted to two dietary treatments and two stages (three pigs/diet/stage). Diets included a corn-soybean cake basal diet (Feed A), and walnut kernel cake diet (Feed B) containing 5% expeller-pressed walnut kernel cake substituted for corn and soybean cake. Pigs had ad libitum access to feed and water at all times. The nutritional value of walnut kernel cake is shown in
Table 1, which shows that walnut kernel cake is a valuable source of crude protein. The ingredients and nutritional value of the diets are shown in
Table 2.
The backfat tissues were collected from pigs with Feed A and Feed B when the body weight reached 80 kg and 120 kg, respectively. Samples were taken immediately after euthanasia, frozen in liquid nitrogen, and stored at -80 °C until used for extracting RNA. Meanwhile, the adipose-related traits were recorded according to technical regulation for testing of carcass traits in lean-type pig (NY/T 825-2004), containing caul fat rate (CFR), abdominal fat rate (AFR), backfat thickness at three positions (BF_A, BF_B, BF_C), average backfat thickness (BF_Avg), backfat thickness between the 6th and 7th ribs (BF_67), fat weight rate at forequarters, middle torso and hindquarters of pig (FWR_F, FWR_M, FWR_H) and total fat weight rate (FWR_T).
2.2. RNA Extraction, Library Construction, and Sequencing
We extracted total RNA from these 12 samples using Trizol reagent and treated the RNA with DNase I enzyme to remove DNA contamination. The purity, concentration and integrity of the RNA were tested by NanoDrop spectrometer, Agilent 2100 Bioanalyzer and Qubit 2.0 fluorescence analyzer. TruSeq Stranded Total RNA Sample Prep Kit was used for RNA library construction. First, poly(A)+ RNA is enriched using magnetic beads, and then the RNA is fragmented using fragmentation buffer. Next, first-strand cDNA was synthesized using SuperScript III reverse transcriptase, and DNA polymerase I and RNase H were used for second-strand synthesis. Afterwards, we performed end repair, A-tail modification and adapter ligation, and used PCR amplification to amplify the library to the desired concentration. Finally, the library was subjected to pair-end 150bp high-throughput sequencing using the Illumina HiSeq X Ten platform.
2.3. Sequencing Data Filtered and Differentially Expressed Genes (DEGs) Analysis
We first checked the quality of the sequencing data using FastQC (v0.11.8) and then used fastp (v0.20.0) software to remove low-quality sequences with default parameters to obtain clean data [
17,
18]. The clean data was further aligned to the reference genome (Sscrofa11.1.97) using STAR (v2.7.1) [
17,
19]. We used featureCounts (v2.0.0) to calculate gene expression level [
18,
20], and filtered out genes with an average count of less than 1 in all samples. And then used DESeq2 (v1.38.3) software to normalize gene expression levels, and further analyze differentially expressed genes (DEGs) [
21]. The genes with the criterion of |log
2(fold change)| > 1 (|log
2FC| > 1) and
P < 0.05 can be considered DEGs.
2.4. Functional Enrichment Analysis
Gene function enrichment analysis is a method used to discover gene functions associated with biological processes or pathways in a set of genes. In this study, we took the list of DEGs as input, and used the R package clusterProfiler (v4.6.2) to perform Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) functional enrichment analysis to explore the biological functions of DEGs [
22]. For GO analysis, we performed the analysis on three different GO categories (molecular function, biological process, and cellular component), setting
P < 0.05 as the significance threshold. At the same time, we also analyzed all KEGG pathways and set
P < 0.05 as the significance threshold. We used wordcloud (v2.6) package and enrichplot (v1.18.4) package in R language (v4.2.2) to visualize the results of GO and KEGG enrichment analysis. Significantly enriched pathways and GO terms were shown using barplot and dotplot. Frequency and classification of pathways and GO terms were shown using wordcloud and treeplot. Heatplot and cnetplot functions were also used to generate heatmaps and network diagrams to more intuitively display the relationship and interaction between DEGs and GO or KEGG pathways.
2.5. Gene Set Enrichment Analysis (GSEA)
GSEA (Gene Set Enrichment Analysis) is a method commonly used in transcriptome analysis, which can compare gene expression profiles between different samples and find gene sets related to certain biological processes, diseases, etc [
23]. In this study, GSEA analysis was performed using all genes for the two growth stages, respectively. First, all genes were sorted in descending order according to log
2FC, and then the gseGO and gseKEGG functions of the R package clusterProfiler were used to perform the GSEA of GO and KEGG, respectively. And we screened for significantly enriched gene sets with |NES| > 1,
P < 0.05,
P.adjust < 0.25.
2.6. Weighted Gene Co-Expression Network Analysis (WGCNA)
We further performed WGCNA analysis in the R software environment using the WGCNA package (v1.72) [
24]. We filtered the raw data of all 12 RNA-seq samples in this study, eliminated low-expression genes, retained genes with a coefficient of variation greater than 0.4 and 30% of the samples had an expression level greater than 1 to obtain an expression matrix. After normalizing the expression matrix, we used the pickSoftThreshold function to estimate the soft threshold parameter, and selected the best parameter value that could achieve a high degree of modularity. Afterwards, using the blockwiseModules function (minModuleSize = 30, mergeCutHeight = 0.4), we clustered the gene expression data and identified co-expressed modules. We used the similarity based on module eigenvectors (Module Eigenegene) to calculate the similarity between modules. Through hierarchical clustering, we aggregated highly related modules together and assigned each module a color name. Finally, we imported adipose-related traits and performed correlation analysis with each module to identify key modules that affect the adipose traits.
2.7. Correlation Analysis of Adipose-Related Traits and Genes
In order to mine the genes that significantly affect adipose traits, we first extracted the expressed level of all 182 genes in the tan module that were highly correlated with adipose traits. And then Pearson correlation analysis was performed between the genes and the 11 adipose-related traits, such as Caul fat rate (CFR), Abdominal fat rate (AFR), Backfat thickness_A (BF_A), Backfat thickness_B (BF_B), Backfat thickness_C (BF_C), Backfat thickness_Avg (BF_Avg), Backfat thickness_67 (BF_67), Fat weight rate_Forequarters (FWR_F), Fat weight rate_Middle torso (FWR_M), Fat weight rate_Hindquarters (FWR_H) and Fat weight rate_Total (FWR_T). It was considered to be significantly correlated between genes and traits when P < 0.05. Furthermore, we also used the 80 kg and 120 kg stages of DEGs expression levels and adipose traits to conduct correlation analysis to explore the molecular mechanism of walnut kernel cake affecting pig adipose deposition in different periods.
2.8. scRNA-Seq Analysis of Adipose Tissue
To study the effect of walnut kernel cake on pig adipose deposition from the single-cell level, we downloaded the expression matrix of single-cell data (GSE193975) of pig adipose from the NCBI public database [
25]. We first used Seurat (v4.3.0) software to remove low-quality cells and genes (nCount_RNA < 1000 & nFeature_RNA < 500 & percent.mt > 20), then used decontX (v4.3.0) and DoubletFinder (v4.3.0) to remove ambient RNA and doublet, respectively [
26,
27,
28]. Subsequently, Seurat software was used for data dimensionality reduction and clustering. FindMarkers function in Seurat was used to find the DEGs among different clusters. The DEGs were determined according to the threshold of log
2FC > 0.25 and
P.adjust < 0.05. We annotated the cell types of clusters based on classic marker genes and online databases such as Cellmarker (
http://bio-bigdata.hrbmu.edu.cn/CellMarker/) and PanglaoDB (
https://panglaodb.se/index.html). Moreover, we used Seurat's DotPlot function to visualize the expression patterns of cell type-specific marker genes. The expression patterns of key genes significantly associated with adipose traits in different cell types were visualized using the VlnPlot function.
2.9. Statistical analysis
Differences in adipose-related traits across comparison combinations were analyzed using a two-tailed t-test. Pearson correlation analysis of traits and gene expression was performed using the “cor” function in the R package with the method of "Pearson". P less than 0.05 was considered statistically significant.
3. Results
3.1. Effects of Walnut Kernel Cake on Traits Related to Adipose Deposition
In order to study the effect of walnut kernel cake on adipose deposition in pigs, we measured 11 traits, such as CFR, AFR, BF_A, BF_B, BF_C, BF_Avg, BF_67, FWR_F, FWR_M, FWR_H and FWR_T at 80kg and 120kg, respectively (
Figure 1A). It was found that at the 80 kg stage, walnut kernel cake significantly increased the CFR of pigs (
P < 0.05,
Figure 1B). At the 120 kg stage, walnut kernel cake significantly increased the BF_67 and FWR_T of pigs (
P < 0.05,
Figure 1C). And there was an increasing trend for traits such as AFR, BF_A, BF_B, BF_C, BF_Avg, FWR_F, FWR_M, FWR_H at the 120 kg stage (
Figure 1C). The results showed that walnut kernel cake may promote adipose deposition and improve pork quality.
3.2. Walnut Kernel Cake Causes Significant Alteration in the Adipose Transcriptome
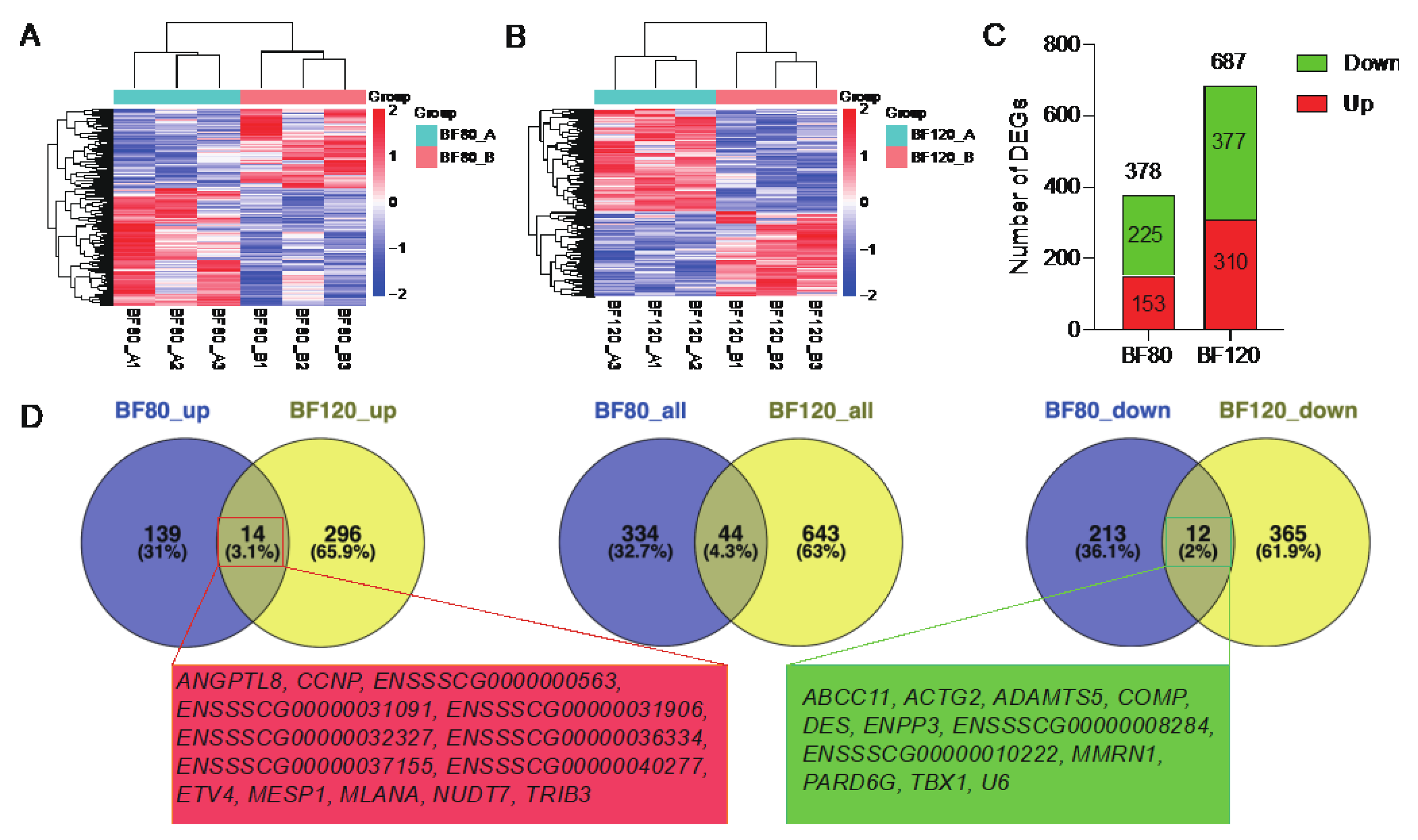
In order to study the effect of walnut kernel cake on pig adipose transcriptome, we collected adipose tissue from pigs at two stages of 80kg and 120kg for transcriptome sequencing. It was found that walnut kernel cake had a significant effect on pig adipose transcriptome at both stages (
Figure 2A, B). Using the criteria of
P < 0.05 and fold change > 2, we screened 378 and 687 differentially expressed genes (DEGs) at the two stages, respectively, among which there were 153 and 225 up-regulated and down-regulated genes at the 80 kg stage, and 310 and 377 up-regulated and down-regulated genes at the 120 kg stage (
Figure 2C). Furthermore, we found that there were 44 shared DEGs at the two stages of 80 kg and 120 kg, of which 14 shared up-regulated DEGs (
ANGPTL8,
CCNP,
ETV4,
MESP1,
MLANA,
NUDT7,
TRIB3, etc.) and 12 shared down-regulated DEGs (
ABCC11,
ACTG2,
DES,
ENPP3,
MMRN1,
TBX1, etc.) (
Figure 2D).
3.3. GO Enrichment Analysis Reveals Adipose-Deposition-Related Biological Processes and Genes
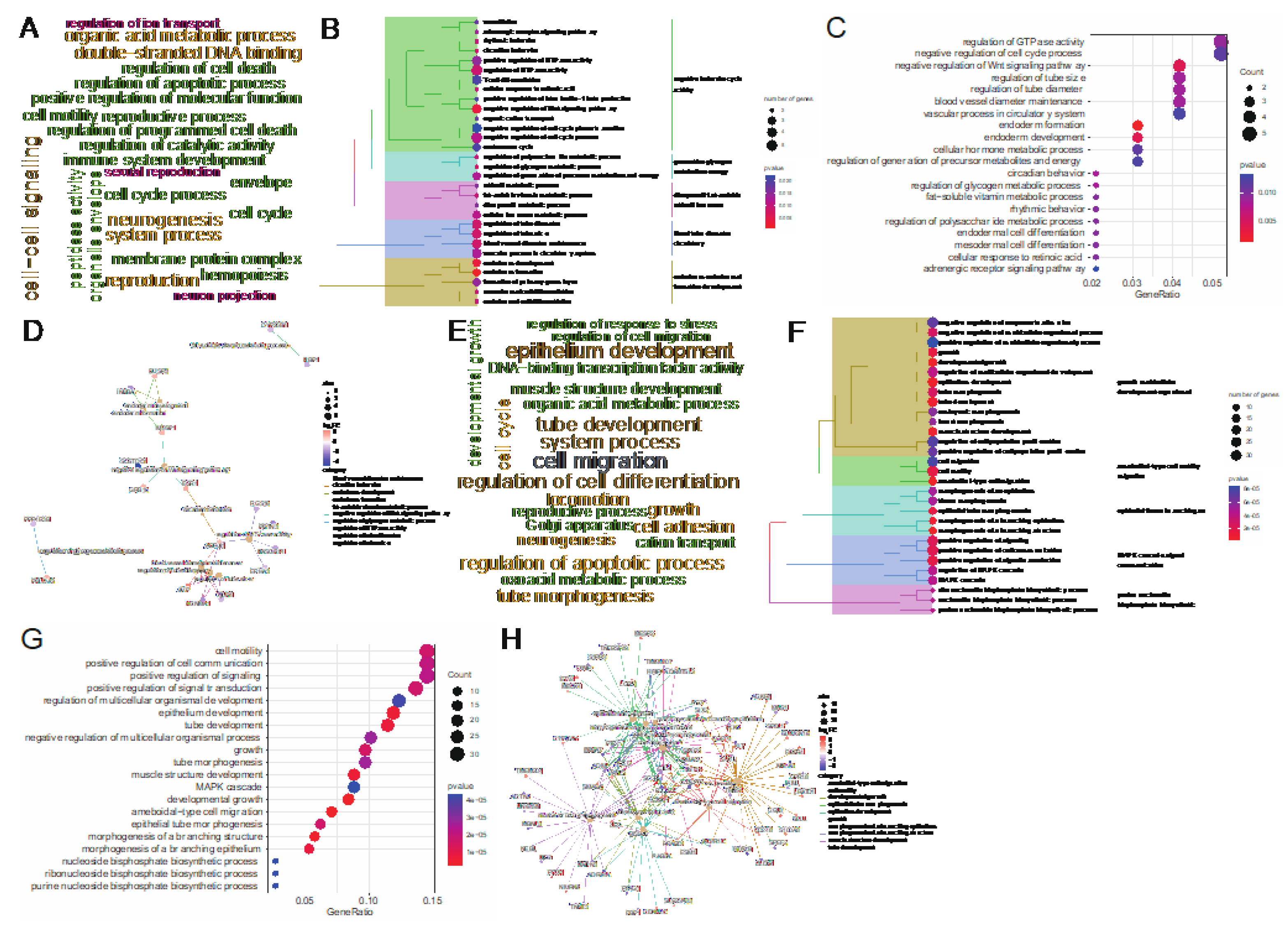
To study the function of DEGs at two body weight stages of 80 kg and 120 kg, we performed GO term enrichment analysis using DEGs. At the 80 kg body weight stage, we got 74 significantly enriched GO terms (
P < 0.05), and all these GO terms belonged to BP. The word cloud annotation shows that these GO terms are mainly enriched in cell-cell signaling, organic acid metabolic process, etc. (
Figure 3A). We classified the top 30 significantly enriched GO terms, which can be divided into five categories: negative behavior cycle activity, generation glycogen metabolites energy, diterpenoid fat−soluble retinoid hormone, blood tube diameter circulatory and endoderm endodermal formation development (
Figure 3B). We further listed the significant enrichment of top 20 GO terms and found that negative regulation of Wnt signaling pathway related to adipose deposition was significantly enriched. Moreover, Sugar and energy metabolism-related terms such as regulation of glycogen metabolic process, regulation of polysaccharide metabolic process and regulation of generation of precursor metabolites and energy were significantly enriched (
Figure 3C). It reveals that at this stage, walnut kernel cake may affect the metabolism of sugar and energy and further affect the deposition of adipose. In order to study the relationship between GO terms and genes, we further displayed the top 10 GO terms and the genes involved. We found that the genes
SOSTDC1,
GRB10 and
EGR1 were significantly enriched in the negative regulation of Wnt signaling pathway, and
PPP1R3B and
PHLDA2 were significantly enriched in the regulation of Glycogen metabolic process (
Figure 3D), suggesting that these genes may have an important role in adipose deposition.
At the 120 kg body weight stage, we got 546 significantly enriched GO terms (P<0.05), all of which belonged to BP. The word cloud annotation shows that these GO terms are mainly enriched in epithelium development, organic acid metabolic process and regulation of cell differentiation (
Figure 3E). We classified the top 30 significantly enriched GO terms, which can be divided into five categories: growth multicellular development organism, ameboidal−type cell motility migration, epithelial tissue branching, MAPK cascade signal communication and purine nucleotide bisphosphate biosynthetic (
Figure 3F). We further showed the significant enrichment of top 20 GO terms, and found that the adipose-related MAPK cascade term was significantly enriched. Besides, multiple epithelium-related terms were significantly enriched (
Figure 3G). In order to study the relationship between GO terms and genes, we further displayed the top 10 GO terms and the genes involved. We found that adipose deposition related genes such as
WNT2,
WNT11 and
SOX9 were significantly up-regulated (
Figure 3H). From the number of enriched terms, it can be seen that the number of significantly enriched terms at the 120 kg body weight stage was significantly higher than that at the 80 kg body weight stage, indicating that walnut kernel cake might have a greater impact on pigs at the later stage of growth than at the early stage.
3.4. KEGG Enrichment Analysis Reveals Adipose-Deposition-Related Pathways and Genes
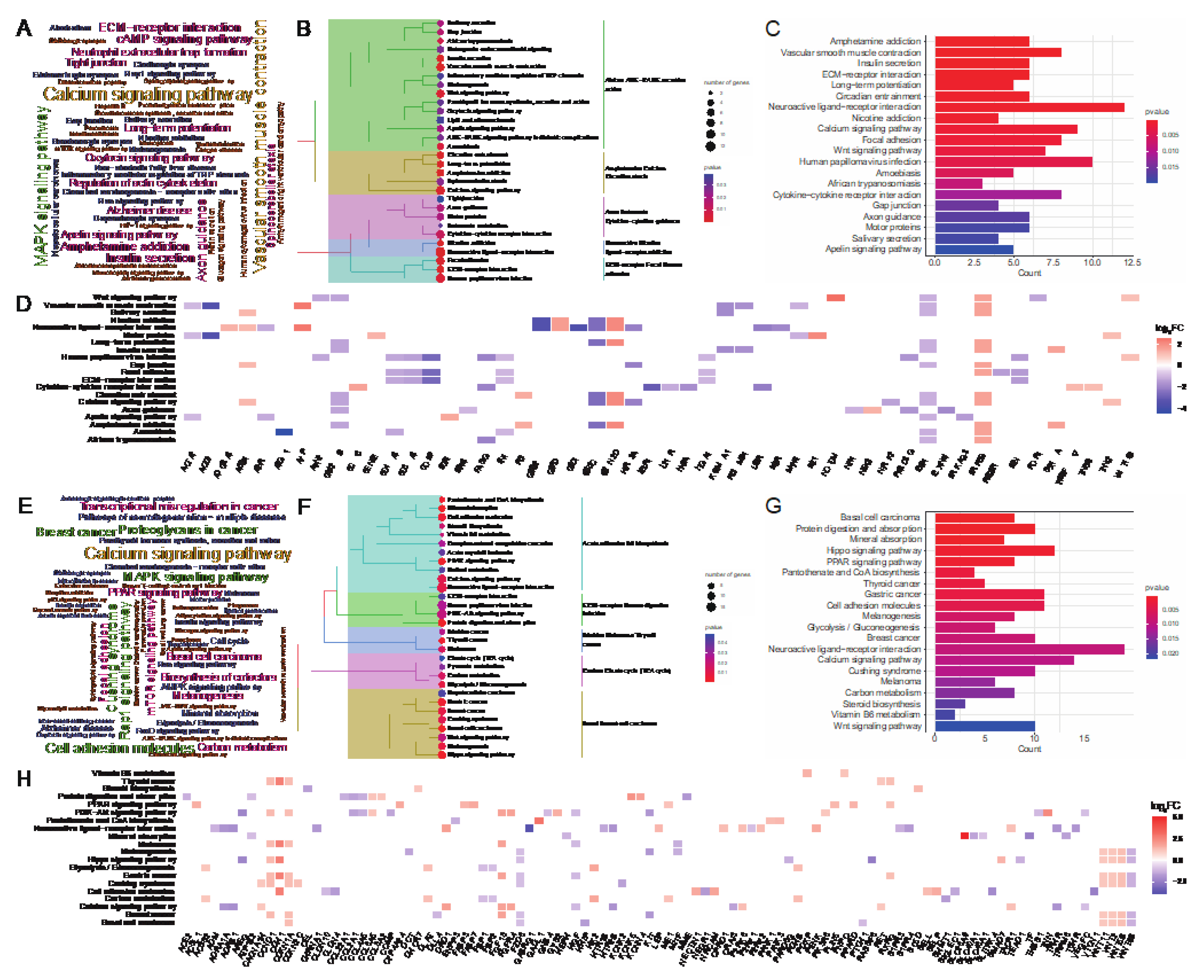
Further, we investigated the KEGG enrichment pathways of DEGs at two body weight stages of 80 kg and 120 kg. At the 80 kg body weight stage, we obtained 41 significantly enriched pathways (P<0.05). The word cloud annotation shows that these pathways are mainly enriched in Calcium signaling pathway, ECM-receptor interaction, MAPK signaling pathway, and Insulin secretion, and the latter three of which are related to adipose deposition (
Figure 4A). We classified top 30 significantly enriched pathways, which can be divided into five categories: African AGE−RAGE secretion action, Amphetamine Calcium Circadian ataxia, Axon Butanoate Cytokine−cytokine guidance, Neuroactive Nicotine ligand−receptor addiction and ECM−receptor Focal Human adhesion (
Figure 4B). We further showed the significantly enriched pathways of the top 20, and found some adipose-deposition related pathways such as Insulin secretion, ECM-receptor interaction, Wnt signaling pathway were significantly enriched (
Figure 4C). To study the relationship between pathways and genes, we further showed the top 10 pathways and the genes involved (
Figure 4D).
At the 120 kg body weight stage, we obtained 30 significantly enriched pathways (
P < 0.05). The word cloud annotation shows that these pathways are mainly enriched in Calcium signaling pathway, MAPK signaling pathway, PPAR signaling pathway, Insulin signaling pathway (
Figure 4E). We classified the top 30 significantly enriched pathways, which can be divided into five categories: Acute adhesion B6 biosynthesis, ECM−receptor Human digestion infection and Carbon Citrate cycle (TCA cycle) (
Figure 4F). We further showed the significantly enriched pathways of the top 20, and also found that adipose-deposition related pathways such as PPAR signaling pathway and Wnt signaling pathway were significantly enriched (
Figure 4G). In order to study the relationship between pathways and genes, we further displayed the top 10 pathways and the genes involved, and found that the genes in the PPAR signaling pathway were significantly up-regulated, such as
PPARD,
PLINNN5,
CYP4A24,
ACSL1,
FABP3,
FABP7,
ME1, etc. (
Figure 4H), indicating that walnut kernel cake may regulate adipose deposition by up-regulating PPAR signaling pathway.
Furthermore, we found 8 common significantly enriched pathways in the two stages: Melanogenesis, Neuroactive ligand-receptor interaction, Calcium signaling pathway, Wnt signaling pathway, PI3K-Akt signaling pathway, Human papillomavirus infection, ECM-receptor interaction, Retinol metabolism. Among them, Wnt signaling pathway, PI3K-Akt signaling pathway and ECM-receptor interaction are related to adipose deposition, indicating that walnut kernel cake can further affect adipose deposition by significantly affecting these two pathways.
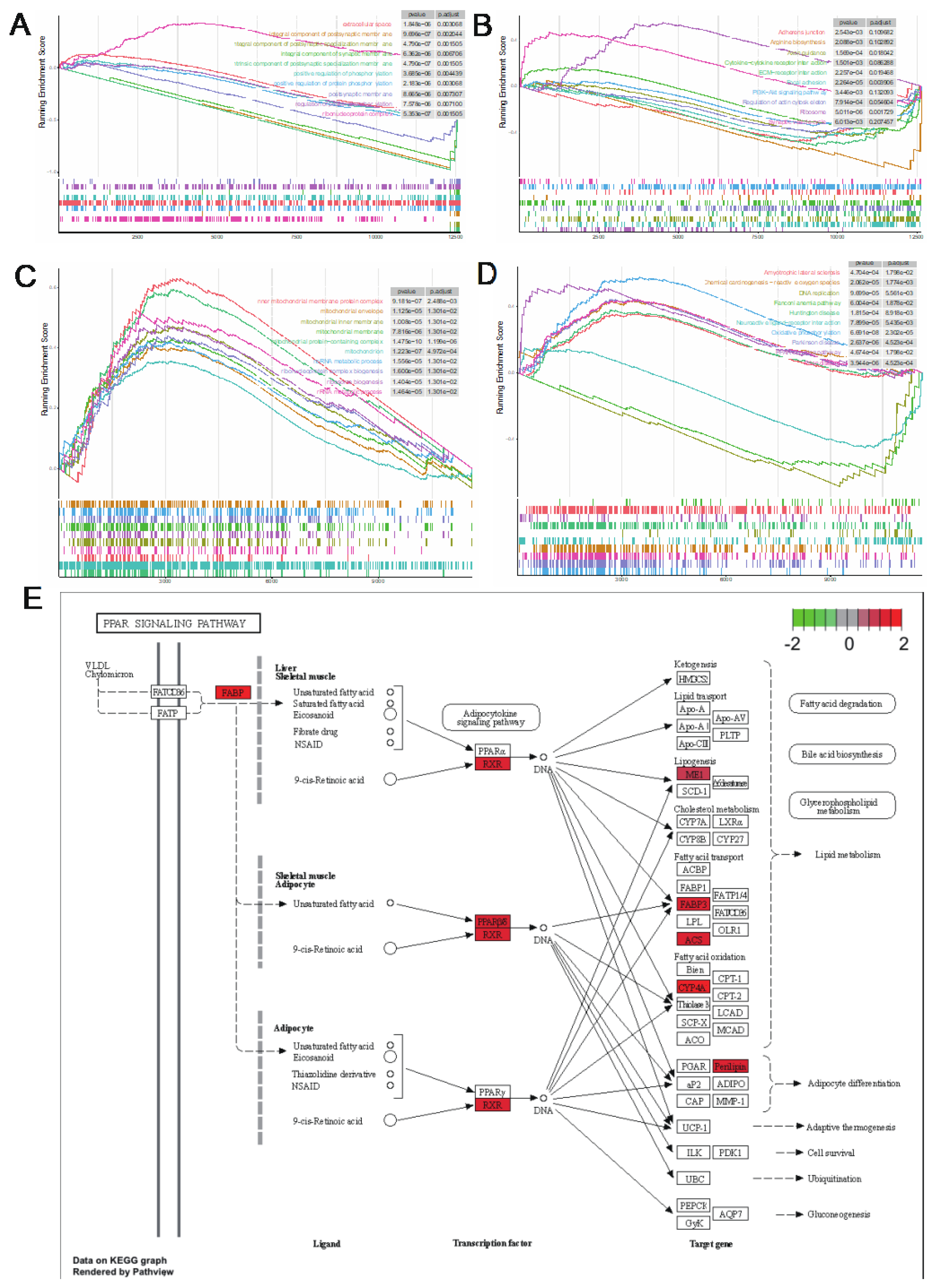
3.5. GSEA Reveals PPAR Signaling Pathway was Activated by Walnut Kernel Cake
To investigate gene set enrichment at both stages, we performed GSEA with all genes. At the 80 kg body weight stage, we obtained 41 and 11 significantly enriched GO terms and KEGG pathways, respectively (|NES| > 1,
P < 0.05,
P.adjust < 0.25). These include 9 up-regulated GO terms and 2 up-regulated KEGG pathways (|NES| > 1,
P < 0.05,
P.adjust < 0.25), 32 down-regulated GO terms and 9 down-regulated KEGG pathways (|NES| > 1,
P < 0.05,
P.adjust < 0.25). Among the top 10 GO terms, only ribonucleoprotein complex was up-regulated in the walnut kernel cake additional group (
Figure 5A). In the top 10 KEGG pathways, only Ribosome and Synaptic vesicle cycle were up-regulated in the walnut kernel cake additional group (
Figure 5B). At the 120 kg body weight stage, we obtained 205 and 38 significantly enriched GO terms and KEGG pathways, respectively (|NES| > 1,
P < 0.05,
P.adjust < 0.25). These include 145 up-regulated GO terms and 22 up-regulated KEGG pathways (|NES| > 1,
P < 0.05,
P.adjust < 0.25), 60 down-regulated GO terms and 16 down-regulated KEGG pathways (|NES| > 1,
P < 0.05,
P.adjust < 0.25). All the top 10 GO terms were up-regulated in the walnut kernel cake additional group, and many terms were related to mitochondria (
Figure 5C). Among the top 10 KEGG pathways, the PPAR signaling pathway was up-regulated in the walnut kernel cake additional group (
Figure 5D). The genes
ACSL1,
CYP4A24,
FABP3,
FABP7,
ME1,
PLIN5,
PPARD and
RXRG involved in this pathway are all related to adipose deposition, and all of them are significantly up-regulated (
Figure 5E). It shows that walnut kernel cake promotes adipose deposition in pigs by activating the PPAR signaling pathway and the related genes.
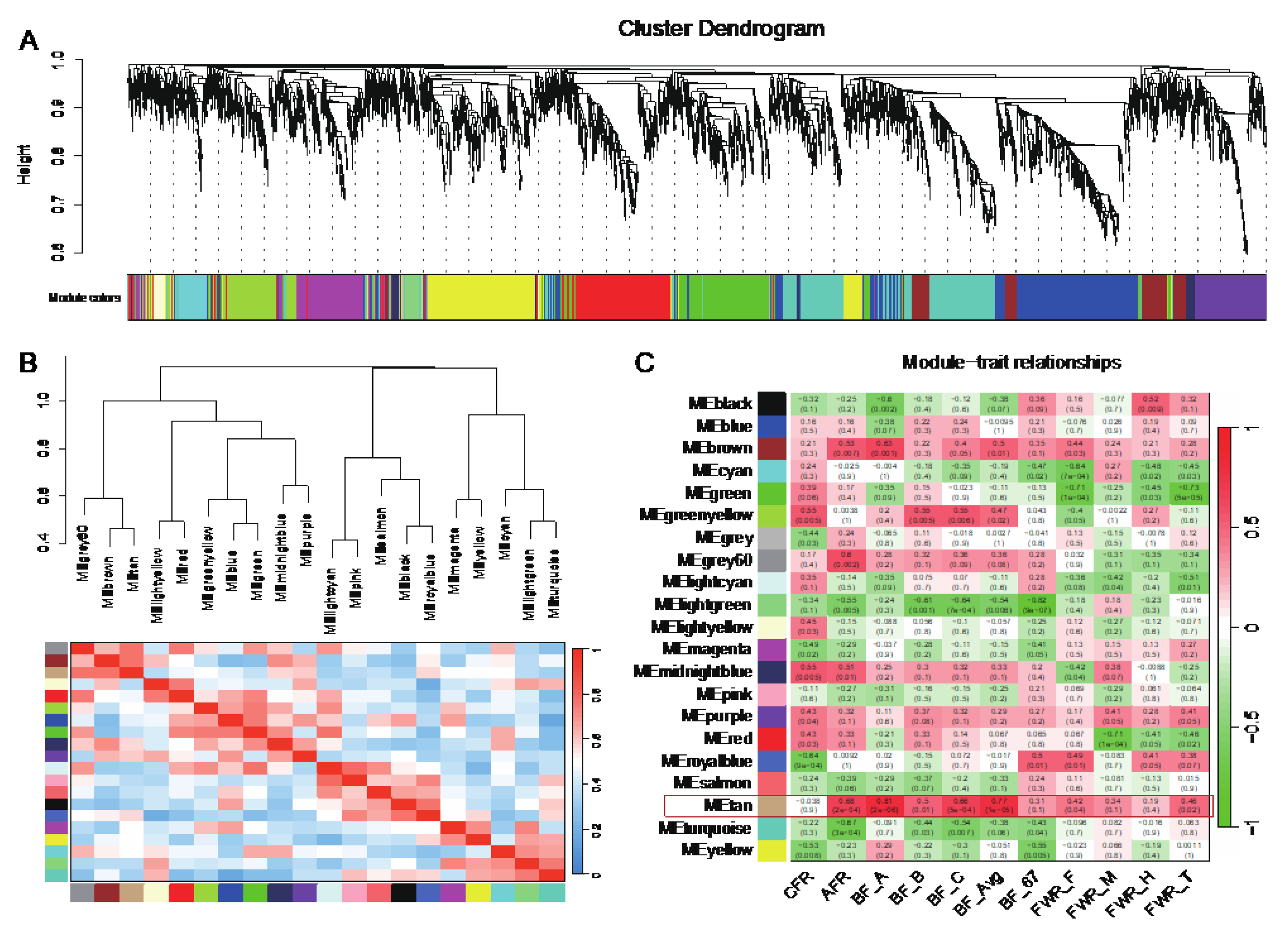
3.6. WGCNA Identifies Two Modules Significantly Associated with Adipose Traits
To study gene sets associated with traits, we performed WGCNA and obtained 21 modules (
Figure 6A). Using the hub genes in each module, we performed cluster analysis on these 21 modules (
Figure 6B). Further, we used the expression level of genes in each module to perform a correlation analysis with the 11 adipose-related traits. The result showed that the module tan was highly correlated with adipose traits, especially with BF and AFR (
Figure 6C). The correlation coefficients between the module tan and AFR, BF_A, BF_B, BF_C, BF_Avg were above 0.5 (
P < 0.05). It indicated that walnut kernel cake mainly affected traits such as BF and AF through the gene sets of this module.
3.7. Correlation Analysis Identifies Adipose-Deposition-Related Genes
In order to study the genes that significantly affect adipose-related traits, we extracted the 182 genes in the tan module and performed correlation analysis with the 11 traits. Among the 2002 gene-trait pairs, 432 (21.58%) were significantly correlated (
P < 0.05, |
r| > 0.576), of which 169 were |
r| > 0.7, involving 88 genes, such as
ABTB2,
ADAMTS18,
AHSP,
ALAS2 and
ATP6V1G2 (
Figure 7A). In which
ALAS2,
DUSP4,
ENSSSCG00000007978,
ENSSSCG00000036334,
HK2,
KCNS3 and
LRATD1 are the DEGs in the 80 kg stages. And a total of 17 genes such as
ABTB2,
DUSP4,
ENSSSCG00000001458,
ENSSSCG00000025367,
ENSSSCG00000036334,
EVPL,
HK2,
HMCN1,
HSPA12A,
KCNH3,
KIF17,
LRATD1,
NR1I2,
RGS7,
SMAD7,
TNN and
U6 are the DEGs in the 120 kg stages.
At the same time, we also used the DEGs of the two stages of 80 kg and 120 kg to conduct correlation analysis with these 11 traits and found that there were 147 pairs and 735 pairs of significant correlations in the two stages (
P < 0.05, |
r| > 0.576). Among them, there are 35 gene-trait pairs with |
r| > 0.7 in the 80 kg stage, involving 30 genes, in which three genes such as
ALAS2,
ADRB1 and
ENSSSCG00000016467 have correlation coefficients greater than 0.8 (
Figure 7B). In the 120 kg stage, there are 185 gene-trait pairs involving 154 genes with |
r| > 0.7. Among them, 35 gene-trait pairs with 30 genes have correlation coefficients greater than 0.8 (
Figure 7C).
Notably, genes in 96% (24/25) negatively correlated gene-trait pairs are significantly down-regulated, and genes in 80% (8/10) positively correlated gene-trait pairs are significantly up-regulated. Namely, 91.43% (32/35) gene-trait pairs promoted adipose deposition, involving 90% (27/30) of the genes. Among the 32 gene-trait pairs that promote adipose deposition, 25 are related to FWR, involving the genes ADRB1, CCDC173, CDC45, DCLK3, DUSP10, ENSSSCG00000001081, ENSSSCG00000001458, ENSSSCG00000013869, ENSSSCG00000016467, ENSSSCG00000038037, FBP2, GATM, GPR18, LEP, MAP3K15, NECTIN1, PLA2G10, RNASE4, SLC25A45, ZGRF1. There are 6 gene-trait pairs related to backfat thickness, such as CKB, EEF1A2, ENSSSCG00000033248, ENSSSCG00000040134, PER2 and PTGES. It was further explained that walnut kernel cake mainly affected adipose-related traits such as FWR and BF at the stage of 120 kg.
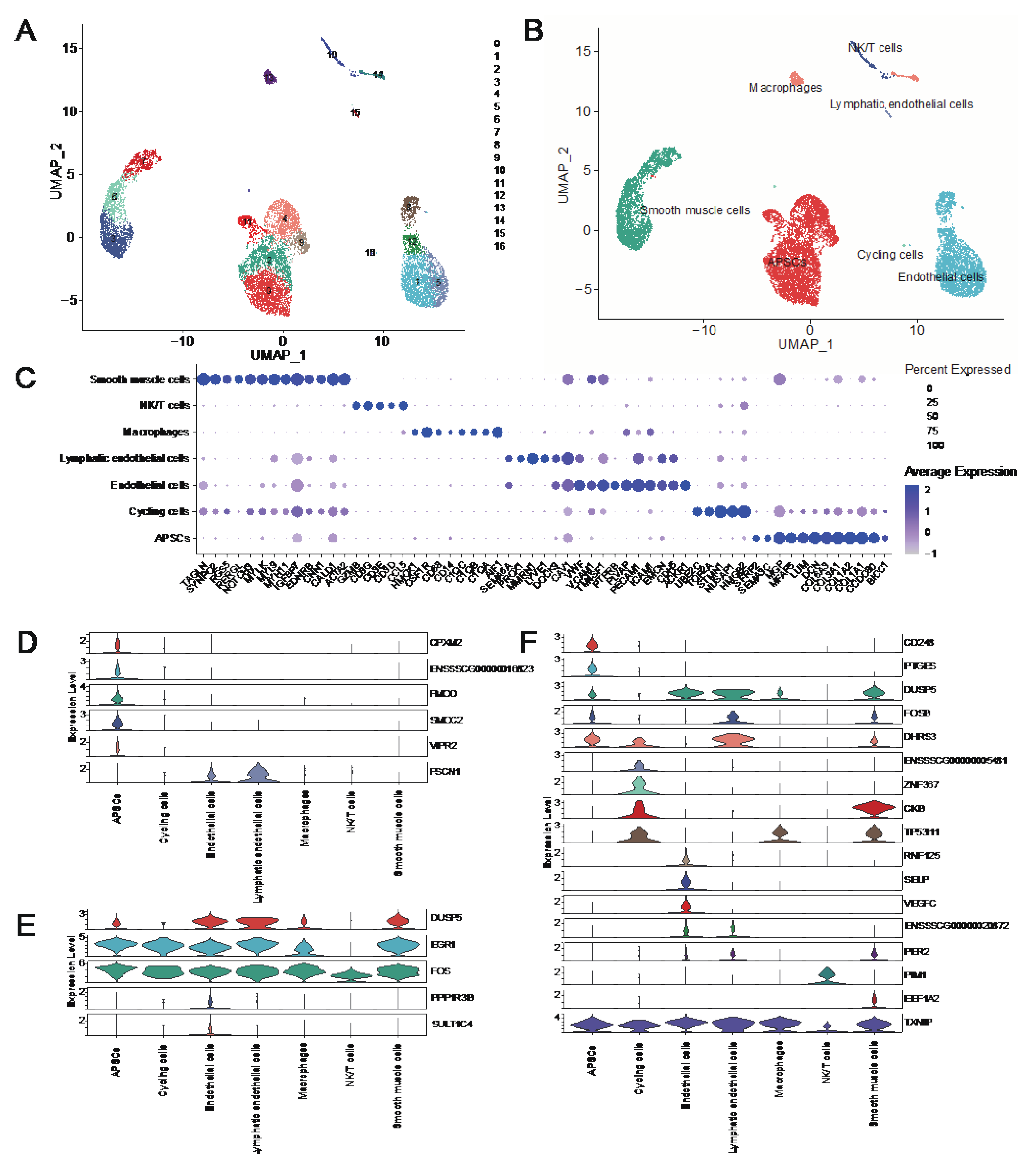
3.8. Single-Cell Transcriptome Analysis Reveals Key Genes for Adipose Deposition
In order to explore the regulatory mechanism of walnut kernel cake on adipose deposition at single-cell level, we used the published single-cell transcriptome data of porcine adipose tissue to conduct an in-depth analysis of genes significantly related to adipose traits. We initially obtained data with 10890 cells covering 15490 genes. After quality control, we finally retained 9941 cells, and after further dimensionality reduction, a total of 17 clusters were obtained (
Figure 8A). Combining CellMarker, PanglaoDB and the classic adipose tissue marker genes in the literatures, we annotated these 17 clusters as seven cell types, including adipocyte progenitor/stem cells (APSCs), cycling cells, endothelial cells, lymphatic endothelial cells, macrophages, NK/T cells and smooth muscle cells (
Figure 8B).
Figure 8C shows the marker genes we used to annotate cell types (
Figure 8C). Next, we investigated genes expression that were significantly associated with the traits in each cell type. The results showed that these genes were mainly expressed in non-immune cell types such as APSCs, cycling cells, endothelial cells and smooth muscle cells. Among them,
CPXM2,
FMOD,
SMOC2,
VIPR2,
CD248 and
PTGES were specifically expressed in APSCs.
ZNF367 and
ENSSSCG00000005481 genes are specifically expressed in cycling cells.
PPP1R3B,
SULT1C4,
RNF125,
SELP and
VEGFC genes were specifically expressed in endothelial cells (
Figure 8D-F). The above genes, especially the cell type-specific expression genes of APSCs, may play an important role in regulating adipogenesis and deposition. In addition, we found that
EGR1,
FOS and
TXNIP genes are relatively highly conserved in various cell types and may also play an important role in regulating adipose deposition.
4. Discussion
In recent years, with the global population and the increasing demand for animal protein, feed protein substitutes have received extensive attention [
19,
20]. Feed protein substitution aims to reduce the farming industry's dependence on traditional protein sources (such as fish meal, soybean meal, etc.) and farming costs while improving farming efficiency and environmental sustainability [
21]. In past studies, many alternative protein sources have been proposed and widely used, including plant protein [
22,
23], insect protein [
24], single-cell protein [
25], and algae protein [
26], etc. Walnut meal is a by-product of walnut processing and is usually used as a feed additive [
15,
27]. However, there are few reports on walnut kernel cake as a feed protein substitute on adipose deposition in pigs. In this study, substituting walnut kernel cake for part of soybean protein in feed was found to have an effect on adipose deposition in large Diqing Tibetan pigs and altered the gene expression profile of adipose tissue.
In this study, walnut kernel cake significantly promoted adipose deposition and improved pork quality in pigs. Specifically, it significantly increased the caul fat rate of pigs at the 80 kg stage, and significantly increased the backfat thickness and FWR of pigs at the 120 kg stage. Some studies have shown that walnut functional food or walnut meal will not cause obesity or adipose deposition [
28,
29,
30,
31]. However, the walnut kernel cake in this study caused adipose deposition in pigs, probably because the walnut kernel cake contained more lipids compared to the partially replaced soybean meal, which is consistent with the experimental results of Untea et al. in chicken [
15,
27].
Walnut kernel cake also significantly altered the transcriptome level of porcine adipose tissue. Among them, the number of DEGs in the 120kg stage was 1.82 times that in the 80kg stage, indicating that the walnut kernel cake played an important role in the adipose deposition of pigs, especially in the late fattening period. We found a total of 26 shared genes in the two stages, among which
ANGPTL8 is an adipocytokine known to play an important regulatory role in fat metabolism. The study found that
ANGPTL8 can promote the maturation of adipocytes and the release of fatty acids, while regulating insulin sensitivity and glucose metabolism [
32,
33,
34,
35].
ETV4 may play a role in regulating of adipocyte differentiation and metabolism [
36,
37].
TRIB3 is involved in regulating cell proliferation, differentiation and apoptosis [
38]. In adipocytes,
TRIB3 may interact with the insulin signaling pathway to affect fat metabolism and insulin sensitivity [
39]. These genes may play an important regulatory role in adipose deposition.
The functional enrichment results of differentially expressed genes showed that multiple fat-related pathways were significantly enriched, including the PPAR signaling pathway, Insulin signaling pathway, PI3K-Akt signaling pathway, Wnt signaling pathway, MAPK signaling pathway, etc. These pathways have key regulatory roles in adipocyte differentiation, proliferation and fatty acid synthesis, which can promote adipocyte maturation, increase adipocyte number and adipose deposition [
40,
41,
42,
43,
44,
45]. GSEA analysis showed that the walnut kernel cake activated the PPAR signaling pathway at the 120 kg stage. It plays a key regulatory role in adipocyte differentiation and fatty acid synthesis. The PPAR signaling pathway can promote the differentiation process of adipose stem cells into adipocytes, and promote the differentiation and maturation of adipocytes [
46,
47]. In addition, it can also promote fatty acid synthesis and triacylglycerol synthesis, thereby increasing adipose deposition and storage [
48].
PPAR signaling pathway is very important for the regulation of insulin sensitivity. Insulin is an important metabolic hormone that promotes glucose uptake and utilization and inhibits fatty acid release. Activating the PPAR signaling pathway can improve the sensitivity of cells to insulin, and promote the uptake and metabolism of glucose by adipose cells, thereby reducing the release of fatty acids and inhibiting the decomposition of adipose [
49,
50]. This pathway may play an important regulatory role in the process of walnut kernel cake promoting adipose deposition. All DEGs involved in this pathway such as
ACSL1,
CYP4A24,
FABP3,
FABP7,
ME1,
PLIN5,
PPARD and
RXRG were related to adipose deposition, and were significantly up-regulated in the walnut kernel cake supplemented group. In addition, we also found mitochondrial and energy metabolism-related pathways and the role of these pathways in adipose deposition in this study needs further study.
We screened some DEGs significantly associated with adipose traits through WGCNA and correlation analysis. In the 120 kg stage, we found many genes highly correlated with adipose traits (|
r| > 0.8,
P < 0.05). Among them, 96% of DEGs negatively correlated with adipose traits were down-regulated, and 80% of DEGs positively correlated with adipose traits were up-regulated. The above results further indicated that walnut kernel cake feeding can promote adipose deposition in pigs. Among them,
Per2 and
Cry2 interact to promote adipogenesis by inhibiting the Wnt signaling pathway in mice [
51]. The increase of
PTGES may promote the synthesis of prostaglandin E2, which in turn regulates the metabolic activity of adipocytes. Prostaglandin E2 is considered to be a biologically active substance that can promote adipocyte proliferation and fat synthesis. It can promote the differentiation and proliferation of adipocytes and increase the synthesis and deposition of fat by activating
PGE2 receptors in adipocytes [
52]. The up-regulation of these genes may play an important regulatory role in the process of walnut kernel cake promoting adipose deposition. In addition, we also found some other DEGs that regulated adipose deposition, such as CKB and LEP [
53,
54]. How the above genes and pathways interact to promote adipose deposition in the present study needs further exploration.
There are few studies exploring adipose deposition at the single-cell level. This study further explored the expression of these key genes in different adipose tissue cell types using single-cell transcriptome data. It was found that
CPXM2,
FMOD,
SMOC2,
VIPR2,
CD248,
PTGES and other genes were specifically expressed in APSCs. The APSCs are adipocyte precursors and stem cells that can differentiate into mature adipocytes and promote adipose deposition [
55]. Among them,
CD248 and
PTGES were significantly up-regulated at the 120 kg stage, and these two genes were highly positively correlated with backfat thickness, indicating that these two genes may mainly act on APSCs to promote the differentiation of adipose precursor stem cells and then promote the formation of adipocytes and adipose deposition.
5. Conclusions
In the present study, we found that incorporating walnut kernel cake into feed can efficiently replace a portion of soybean cake, offering pigs high-quality protein and fostering adipose deposition. Some adipose-deposition-related genes (ACSL1, ANGPTL8, CCNP, CD248, CKB, CYP4A24, ETV4, FABP3, FABP7, ME1, PER2, PLIN5, PPARD, PTGES, RXRG, TRIB3) and pathways (PPAR signaling pathway, Insulin signaling pathway, PI3K-Akt signaling pathway, Wnt signaling pathway, MAPK signaling pathway) may contribute to adipose deposition. Adipose deposition is a complex biological process involving the combined action of multiple genes and regulatory networks. An in-depth study of the functions of these genes and pathways in adipose metabolism and deposition will help to better understand their roles in animal breeding and adipose-metabolism-related diseases in human. This study provides a theoretical basis for feed protein replacement, pig genetic breeding and meat quality improvement, and also provides reference materials for the study of human fat metabolism and related diseases.
Author Contributions
Conceptualization, X.D. and Y.B.; Data Curation, L.L. and L.M.; Formal Analysis, L.L., X.S. and L.M.; Funding Acquisition, X.D., Y.B. and L.L.; Investigation, X.S., D.Y. and A.A.; Project administration, X.D. and Y.B.; Resources, D.Y.; Supervision, X.D. and Y.B.; Visualization, L.L. and X.S.; Writing—Original Draft, L.L.; Writing— Review and Editing, L.M., D.Y., A.A., Y.B. and X.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2022YFD1601903); Hebei Province Natural Science Foundation (C2021402038); National Natural Science Foundation of China (32002151); Major Science and Technology Projects in Yunnan Province (202202AE090005 and 202302AE090015) and National Transgenic Project of China (2018ZX0800928B).
Institutional Review Board Statement
This study adhered to the guidelines set by the Ministry of Agriculture and Rural Affairs of China for the ethical treatment and utilization of experimental animals. Approval for the research was obtained from the ethics committee of Yunnan Agricultural University (YNAU, Kunming, China).
Informed Consent Statement
Not applicable.
Data Availability Statement
The RNA-seq datasets supporting the conclusions of this article are available in the National Center for Biotechnology Information (NCBI) database. The corresponding accession number is PRJNA1003490.
Acknowledgments
We are very grateful to Shangri-La Lvyuan Ecological Breeding Professional Cooperative for providing us with experimental animals. Thanks to Zhengjun Zhang and Lihua Chen for their help in the process of raising experimental animals. Thanks to Shouzhang Sun, Rui Zhang, Tao Lin and Guoxiang Lan for their help in the process of slaughtering, phenotype determination and samples collection.
Conflicts of Interest
The authors declare that there are no conflicts of interest to disclose.
References
- Kim, S.; Cho, J. H.; Kim, H. B.; Song, M. Rice as an alternative feed ingredient in swine diets. J Anim Sci Technol 2021, 63, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Sońta, M.; Łukasiewicz-Mierzejewska, M.; Puppel, K.; Rekiel, A.; Więcek, J.; Batorska, M. Influence of raw pea (Pisum sativum) or blue lupin seeds (Lupinus angustifolius) on the level of selected bioactive substances in pork meat. Annals of Animal Science 2022, 22, 701–709. [Google Scholar] [CrossRef]
- Wilkinson, J. M.; Lee, M. R. F. Review: Use of human-edible animal feeds by ruminant livestock. Animal 2018, 12, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Swiatkiewicz, M.; Olszewska, A.; Grela, E. R.; Tyra, M. The Effect of Replacement of Soybean Meal with Corn Dried Distillers Grains with Solubles (cDDGS) and Differentiation of Dietary Fat Sources on Pig Meat Quality and Fatty Acid Profile. Animals (Basel) 2021, 11. [Google Scholar]
- van Zanten, H. H. E.; Bikker, P.; Meerburg, B. G.; de Boer, I. J. M. Attributional versus consequential life cycle assessment and feed optimization: alternative protein sources in pig diets. The International Journal of Life Cycle Assessment 2017, 23, 1–11. [Google Scholar] [CrossRef]
- Parrini, S.; Aquilani, C.; Pugliese, C.; Bozzi, R.; Sirtori, F. Soybean Replacement by Alternative Protein Sources in Pig Nutrition and Its Effect on Meat Quality. Animals (Basel) 2023, 13. [Google Scholar] [CrossRef]
- Yun, H. M.; Lei, X. J.; Lee, S. I.; Kim, I. H. Rapeseed meal and canola meal can partially replace soybean meal as a protein source in finishing pigs. Journal of Applied Animal Research 2017, 46, 195–199. [Google Scholar] [CrossRef]
- Hasan, I.; Khan, R. A.; Alharbi, W.; Alharbi, K. H.; Abu Khanjer, M.; Alslame, A. Synthesis, characterization and photo-catalytic activity of guar-gum-g-aliginate@silver bionanocomposite material. RSC Adv 2020, 10, 7898–7911. [Google Scholar] [CrossRef] [PubMed]
- Seoni, E.; Battacone, G.; Ampuero Kragten, S.; Dohme-Meier, F.; Bee, G. Impact of increasing levels of condensed tannins from sainfoin in the grower-finisher diets of entire male pigs on growth performance, carcass characteristics, and meat quality. Animal 2021, 15, 100110. [Google Scholar] [CrossRef]
- Degola, L.; Jonkus, D. The influence of dietary inclusion of peas, faba bean and lupin as a replacement for soybean meal on pig performance and carcass traits. 2018.
- Zhou, X.; Peng, X.; Pei, H.; Chen, Y.; Meng, H.; Yuan, J.; Xing, H.; Wu, Y. An overview of walnuts application as a plant-based. Front Endocrinol (Lausanne) 2022, 13, 1083707. [Google Scholar] [CrossRef]
- Danilov, A.; Donică, I. The use of nut kernel cake in the feeding of young pigs. Scientific Papers. Series D. Animal Science 2022, 65, 110–116. [Google Scholar]
- Sari, T.; Sirohi, R.; Krishania, M.; Bhoj, S.; Samtiya, M.; Duggal, M.; Kumar, D.; Badgujar, P. C. Critical overview of biorefinery approaches for valorization of protein rich tree nut oil industry by-product. Bioresource Technology 2022, 127775. [Google Scholar] [CrossRef]
- Untea, A. E.; Varzaru, I.; Saracila, M.; Panaite, T. D.; Oancea, A. G.; Vlaicu, P. A.; Grosu, I. A. Antioxidant Properties of Cranberry Leaves and Walnut Meal and Their Effect on Nutritional Quality and Oxidative Stability of Broiler Breast Meat. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Untea, A. E.; Turcu, R. P.; Saracila, M.; Vlaicu, P. A.; Panaite, T. D.; Oancea, A. G. Broiler meat fatty acids composition, lipid metabolism, and oxidative stability parameters as affected by cranberry leaves and walnut meal supplemented diets. Scientific Reports 2022, 12, 21618. [Google Scholar] [CrossRef]
- Cai, Y.; Quan, J.; Gao, C.; Ge, Q.; Jiao, T.; Guo, Y.; Zheng, W.; Zhao, S. Multiple domestication centers revealed by the geographical distribution of Chinese native pigs. Animals 2019, 9, 709. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C. A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T. R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G. K.; Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Tantikitti, C. Feed palatability and the alternative protein sources in shrimp feed. Songklanakarin J. Sci. Technol 2014, 36, 51–55. [Google Scholar]
- Wang, J.; Chen, L.; Xu, J.; Ma, S.; Liang, X.; Wei, Z.; Li, D.; Xue, M. C1 gas protein: A potential protein substitute for advancing aquaculture sustainability. Reviews in Aquaculture 2023, 15, 1179–1197. [Google Scholar] [CrossRef]
- Chojnacka, K.; Mikula, K.; Izydorczyk, G.; Skrzypczak, D.; Witek-Krowiak, A.; Gersz, A.; Moustakas, K.; Iwaniuk, J.; Grzędzicki, M.; Korczyński, M. Innovative high digestibility protein feed materials reducing environmental impact through improved nitrogen-use efficiency in sustainable agriculture. Journal of Environmental Management 2021, 291, 112693. [Google Scholar] [CrossRef]
- Chakraborty, P.; Mallik, A.; Sarang, N.; Lingam, S. S. A review on alternative plant protein sources available for future sustainable aqua feed production. Int. J. Chem. Stud 2019, 7, 1399–1404. [Google Scholar]
- Montoya-Camacho, N.; Marquez-Ríos, E.; Castillo-Yáñez, F. J.; Cárdenas López, J. L.; López-Elías, J. A.; Ruíz-Cruz, S.; Jiménez-Ruíz, E. I.; Rivas-Vega, M. E.; Ocaño-Higuera, V. M. Advances in the use of alternative protein sources for tilapia feeding. Reviews in Aquaculture 2019, 11, 515–526. [Google Scholar] [CrossRef]
- DiGiacomo, K.; Leury, B. Insect meal: a future source of protein feed for pigs? Animal 2019, 13, 3022–3030. [Google Scholar] [CrossRef]
- Hamidoghli, A.; Yun, H.; Won, S.; Kim, S.; Farris, N. W.; Bai, S. C. Evaluation of a single-cell protein as a dietary fish meal substitute for whiteleg shrimp Litopenaeus vannamei. Fisheries science 2019, 85, 147–155. [Google Scholar] [CrossRef]
- Øverland, M.; Mydland, L. T.; Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. Journal of the Science of Food and Agriculture 2019, 99, 13–24. [Google Scholar] [CrossRef]
- Untea, A. E.; Varzaru, I.; Saracila, M.; Panaite, T. D.; Oancea, A. G.; Vlaicu, P. A.; Grosu, I. A. Antioxidant Properties of Cranberry Leaves and Walnut Meal and Their Effect on Nutritional Quality and Oxidative Stability of Broiler Breast Meat. Antioxidants 2023, 12, 1084. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Zhong, D.-Y.; Wang, G.-L.; Zhang, R.-G.; Zhang, Y.-L. Effect of walnut meal peptides on hyperlipidemia and hepatic lipid metabolism in rats fed a high-fat diet. Nutrients 2021, 13, 1410. [Google Scholar] [CrossRef]
- Weschenfelder, C.; Schaan de Quadros, A.; Lorenzon dos Santos, J.; Bueno Garofallo, S.; Marcadenti, A. Adipokines and adipose tissue-related metabolites, nuts and cardiovascular disease. Metabolites 2020, 10, 32. [Google Scholar] [CrossRef]
- Tindall, A. M.; Petersen, K. S.; Lamendella, R.; Shearer, G. C.; Murray-Kolb, L. E.; Proctor, D. N.; Kris-Etherton, P. M. Tree nut consumption and adipose tissue mass: mechanisms of action. Current developments in nutrition 2018, 2, nzy069. [Google Scholar] [CrossRef]
- Rock, C. L.; Flatt, S. W.; Barkai, H.-S.; Pakiz, B.; Heath, D. D. A walnut-containing meal had similar effects on early satiety, CCK, and PYY, but attenuated the postprandial GLP-1 and insulin response compared to a nut-free control meal. Appetite 2017, 117, 51–57. [Google Scholar] [CrossRef]
- Tang, J.; Ma, S.; Gao, Y.; Zeng, F.; Feng, Y.; Guo, C.; Hu, L.; Yang, L.; Chen, Y.; Zhang, Q.; Yuan, Y.; Guo, X. ANGPTL8 promotes adipogenic differentiation of mesenchymal stem cells: potential role in ectopic lipid deposition. Front Endocrinol (Lausanne) 2022, 13, 927763. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, J.; Hong, B. S.; Ke, W.; Huang, M.; Li, Y. Circulating betatrophin/ANGPTL8 levels correlate with body fat distribution in individuals with normal glucose tolerance but not those with glucose disorders. BMC endocrine disorders 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Vatner, D. F.; Goedeke, L.; Camporez, J.-P. G.; Lyu, K.; Nasiri, A. R.; Zhang, D.; Bhanot, S.; Murray, S. F.; Still, C. D.; Gerhard, G. S. Angptl8 antisense oligonucleotide improves adipose lipid metabolism and prevents diet-induced NAFLD and hepatic insulin resistance in rodents. Diabetologia 2018, 61, 1435–1446. [Google Scholar] [CrossRef]
- Wei, X.; Han, S.; Wang, S.; Zheng, Q.; Li, X.; Du, J.; Zhao, J.; Li, F.; Ma, Y. ANGPTL8 regulates adipocytes differentiation and adipogenesis in bovine. Gene 2019, 707, 93–99. [Google Scholar] [CrossRef]
- Park, K. W.; Waki, H.; Choi, S.-P.; Park, K.-M.; Tontonoz, P. The small molecule phenamil is a modulator of adipocyte differentiation and PPARγ expression [S]. Journal of lipid research 2010, 51, 2775–2784. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Wu, Z.; Xiong, X.; Zhang, J.; Ma, J.; Xiao, S.; Huang, L.; Yang, B. Subcutaneous and intramuscular fat transcriptomes show large differences in network organization and associations with adipose traits in pigs. Science China Life Sciences 2021, 1–15. [Google Scholar] [CrossRef]
- Hernández-Quiles, M.; Campesino, L. M.; Morris, I.; Ilyas, Z.; Alcaraz, P. S.; Varga, Á.; Varga, J.; van Es, R.; Vos, H.; Wilson, H. L. The pseudokinase TRIB3 regulates adipose tissue homeostasis and adipocyte function. Proteomics approaches for the study of adipose tissue biology TRIB3 and beyond.
- Lee, S. K.; Park, C. Y.; Kim, J.; Kim, D.; Choe, H.; Kim, J.-H.; Hong, J. P.; Lee, Y. J.; Heo, Y.; Park, H. S. TRIB3 is highly expressed in the adipose tissue of obese patients and is associated with insulin resistance. The Journal of Clinical Endocrinology & Metabolism 2022, 107, e1057–e1073. [Google Scholar]
- Janani, C.; Kumari, B. R. PPAR gamma gene–a review. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2015, 9, 46–50. [Google Scholar]
- Ahmed, B.; Sultana, R.; Greene, M. W. Adipose tissue and insulin resistance in obese. Biomedicine & Pharmacotherapy 2021, 137, 111315. [Google Scholar]
- Czech, M. P. Fat targets for insulin signaling. Molecular cell 2002, 9, 695–696. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Huang, Y.; Yang, Z.; Ma, Y.; Chaogetu, B.; Zhuoma, Z.; Chen, H. RNA-Seq analysis identifies differentially expressed genes in subcutaneous adipose tissue in qaidaford cattle, cattle-yak, and angus cattle. Animals 2019, 9, 1077. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Sun, T.; Yang, Z.; Xu, W.; Wang, J.; Zeng, L.; Deng, J.; Yang, X. Transcriptome landscapes of differentially expressed genes related to fat deposits in Nandan-Yao chicken. Functional & integrative genomics 2021, 21, 113–124. [Google Scholar]
- Morigny, P.; Boucher, J.; Arner, P.; Langin, D. Lipid and glucose metabolism in white adipocytes: pathways, dysfunction and therapeutics. Nat Rev Endocrinol 2021, 17, 276–295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yang, X.; Zhao, Q.; Li, Z.; Fu, F.; Zhang, H.; Zheng, M.; Zhang, S. Molecular mechanism of stem cell differentiation into adipocytes and adipocyte differentiation of malignant tumor. Stem Cells International 2020, 2020. [Google Scholar] [CrossRef]
- Xu, C.; Wang, J.; Zhu, T.; Shen, Y.; Tang, X.; Fang, L.; Xu, Y. Cross-talking between PPAR and WNT signaling and its regulation in mesenchymal stem cell differentiation. Current stem cell research & therapy 2016, 11, 247–254. [Google Scholar]
- Liu, L.; Cui, H.; Xing, S.; Zhao, G.; Wen, J. Effect of divergent selection for intramuscular fat content on muscle lipid metabolism in chickens. Animals 2019, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fu, M.; Cui, T.; Xiong, C.; Xu, K.; Zhong, W.; Xiao, Y.; Floyd, D.; Liang, J.; Li, E. Selective disruption of PPARγ2 impairs the development of adipose tissue and insulin sensitivity. Proceedings of the National Academy of Sciences 2004, 101, 10703–10708. [Google Scholar] [CrossRef] [PubMed]
- Haag, M.; Dippenaar, N. G. Dietary fats, fatty acids and insulin resistance: short review of a multifaceted connection. Medical Science Monitor 2005, 11, RA359. [Google Scholar]
- Li, W.; Xiong, X.; Kiperman, T.; Ma, K. Transcription repression of Cry2 via Per2 interaction promotes adipogenesis. bioRxiv 2023. [Google Scholar] [CrossRef]
- Vianello, E.; Dozio, E.; Bandera, F.; Froldi, M.; Micaglio, E.; Lamont, J.; Tacchini, L.; Schmitz, G. Correlative study on impaired prostaglandin E2 regulation in EAT and maladaptive cardiac remodeling via EPAC2 and ST2 signaling in overweight CVD subjects. International Journal of Molecular Sciences 2020, 21, 520. [Google Scholar] [CrossRef]
- Rahbani, J. F.; Roesler, A.; Hussain, M. F.; Samborska, B.; Dykstra, C. B.; Tsai, L.; Jedrychowski, M. P.; Vergnes, L.; Reue, K.; Spiegelman, B. M. Creatine kinase B controls futile creatine cycling in thermogenic fat. Nature 2021, 590, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Auger, C.; Kajimura, S. Adipose tissue remodeling in pathophysiology. Annual Review of Pathology: Mechanisms of Disease 2023, 18, 71–93. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Zhou, H.; Deng, T. The composition, function, and regulation of adipose stem and progenitor cells. Journal of Genetics and Genomics 2022, 49, 308–315. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Experimental design and traits determination. (A) Experimental Design Flowchart. (B, C) Phenotypes investigation at 80 kg (B) and 120 kg (C) body weight stages, including 11 traits such as caul fat rate (CFR), abdominal fat rate (AFR), backfat thickness at three positions (BF_A, BF_B, BF_C), average backfat thickness (BF_Avg), backfat thickness between the 6th and 7th ribs (BF_67), fat weight rate at three positions (FWR_F, FWR_M, FWR_H), and total fat weight rate (FWR_T).
Figure 1.
Experimental design and traits determination. (A) Experimental Design Flowchart. (B, C) Phenotypes investigation at 80 kg (B) and 120 kg (C) body weight stages, including 11 traits such as caul fat rate (CFR), abdominal fat rate (AFR), backfat thickness at three positions (BF_A, BF_B, BF_C), average backfat thickness (BF_Avg), backfat thickness between the 6th and 7th ribs (BF_67), fat weight rate at three positions (FWR_F, FWR_M, FWR_H), and total fat weight rate (FWR_T).
Figure 2.
Differential expression analysis of adipose transcriptome at 80 kg and 120 kg body weight stages. (A) Heatmap of differentially expressed genes (DEGs) at 80 kg stage. (B) Heatmap of DEGs at 120 kg stage. (C) Statistics on the number of DEGs. (D) Venn diagram of DEGs at the two stages. The left is a Venn diagram of up-regulated DEGs. The middle is a Venn diagram of all DEGs. The right is the Venn diagram of down-regulated DEGs. The genes in the red and green boxes below are the up-regulated and down-regulated DEGs shared by the two stages, respectively.
Figure 2.
Differential expression analysis of adipose transcriptome at 80 kg and 120 kg body weight stages. (A) Heatmap of differentially expressed genes (DEGs) at 80 kg stage. (B) Heatmap of DEGs at 120 kg stage. (C) Statistics on the number of DEGs. (D) Venn diagram of DEGs at the two stages. The left is a Venn diagram of up-regulated DEGs. The middle is a Venn diagram of all DEGs. The right is the Venn diagram of down-regulated DEGs. The genes in the red and green boxes below are the up-regulated and down-regulated DEGs shared by the two stages, respectively.
Figure 3.
GO term enrichment analysis of DEGs at 80 kg and 120 kg body weight stages. (A) GO term word cloud annotation at 80 kg body weight stage. (B) Taxonomic summary of the GO term at 80 kg body weight stage. (C) Top 20 GO terms at the 80 kg body weight stage. (D) Top 10 GO terms and their enriched gene interactions at the 80 kg body weight stage. (E) GO term word cloud annotation at 120 kg body weight stage. (F) Taxonomic summary of the GO term at 120 kg body weight stage. (G) Top 20 GO terms at the 120 kg body weight stage. (H) Top 10 GO terms and their enriched gene interactions at the 120 kg body weight stage.
Figure 3.
GO term enrichment analysis of DEGs at 80 kg and 120 kg body weight stages. (A) GO term word cloud annotation at 80 kg body weight stage. (B) Taxonomic summary of the GO term at 80 kg body weight stage. (C) Top 20 GO terms at the 80 kg body weight stage. (D) Top 10 GO terms and their enriched gene interactions at the 80 kg body weight stage. (E) GO term word cloud annotation at 120 kg body weight stage. (F) Taxonomic summary of the GO term at 120 kg body weight stage. (G) Top 20 GO terms at the 120 kg body weight stage. (H) Top 10 GO terms and their enriched gene interactions at the 120 kg body weight stage.
Figure 4.
KEGG enrichment analysis of DEGs at 80 kg and 120 kg body weight stages. (A) KEGG pathway word cloud annotation at 80 kg body weight stage. (B) Taxonomic summary of the pathways at 80 kg body weight stage. (C) Top 20 pathways at the 80 kg body weight stage. (D) Top 10 pathways and their enriched gene interactions at the 80 kg body weight stage. (E) KEGG pathway word cloud annotation at 120 kg body weight stage. (F) Taxonomic summary of the pathways at 120 kg body weight stage. (G) Top 20 pathways at the 120 kg body weight stage. (H) Top 10 pathways and their enriched gene interactions at the 120 kg body weight stage. Red and blue squares indicate gene up- and down-regulation, respectively.
Figure 4.
KEGG enrichment analysis of DEGs at 80 kg and 120 kg body weight stages. (A) KEGG pathway word cloud annotation at 80 kg body weight stage. (B) Taxonomic summary of the pathways at 80 kg body weight stage. (C) Top 20 pathways at the 80 kg body weight stage. (D) Top 10 pathways and their enriched gene interactions at the 80 kg body weight stage. (E) KEGG pathway word cloud annotation at 120 kg body weight stage. (F) Taxonomic summary of the pathways at 120 kg body weight stage. (G) Top 20 pathways at the 120 kg body weight stage. (H) Top 10 pathways and their enriched gene interactions at the 120 kg body weight stage. Red and blue squares indicate gene up- and down-regulation, respectively.
Figure 5.
Gene set enrichment analysis of 80 kg and 120 kg body weight stages. (A) GSEA of GO term at 80 kg body weight stage. (B) GSEA of KEGG pathway at 80 kg body weight stage. (C) GSEA of GO term at 120 kg body weight stage. (D) GSEA of KEGG pathway at 120 kg body weight stage. (E) PPAR signaling pathway and its related DEGs at 120 kg body weight stage.
Figure 5.
Gene set enrichment analysis of 80 kg and 120 kg body weight stages. (A) GSEA of GO term at 80 kg body weight stage. (B) GSEA of KEGG pathway at 80 kg body weight stage. (C) GSEA of GO term at 120 kg body weight stage. (D) GSEA of KEGG pathway at 120 kg body weight stage. (E) PPAR signaling pathway and its related DEGs at 120 kg body weight stage.
Figure 6.
Construction of co-expression modules based on genes in all samples. (A) Cluster dendrogram of genes. Each branch represents one gene, and every color below represents one co-expression module. (B) Hierarchical clustering and heatmap of modules. The grey module contains genes that are not significantly correlated with genes in other modules and is removed here. (C) Heatmap of the correlation between module eigengenes and adipose-related traits. The grey60 and tan modules were the most positively correlated with traits.
Figure 6.
Construction of co-expression modules based on genes in all samples. (A) Cluster dendrogram of genes. Each branch represents one gene, and every color below represents one co-expression module. (B) Hierarchical clustering and heatmap of modules. The grey module contains genes that are not significantly correlated with genes in other modules and is removed here. (C) Heatmap of the correlation between module eigengenes and adipose-related traits. The grey60 and tan modules were the most positively correlated with traits.
Figure 7.
Correlation analysis of genes and adipose-related traits. (A) Heatmap of the correlation coefficients between genes and adipose-related traits in tan module. (B) Heatmap of correlation coefficients between DEGs and adipose-related traits at 80 kg body weight stage. (C) Heatmap of correlation coefficients between DEGs and adipose-related traits at 120 kg body weight stage. Each row represents a gene, and each column represents a trait. Gene names with correlation coefficients greater than 0.8 for traits are displayed.
Figure 7.
Correlation analysis of genes and adipose-related traits. (A) Heatmap of the correlation coefficients between genes and adipose-related traits in tan module. (B) Heatmap of correlation coefficients between DEGs and adipose-related traits at 80 kg body weight stage. (C) Heatmap of correlation coefficients between DEGs and adipose-related traits at 120 kg body weight stage. Each row represents a gene, and each column represents a trait. Gene names with correlation coefficients greater than 0.8 for traits are displayed.
Figure 8.
Single-cell transcriptome analysis of porcine adipose tissue. (A) UMAP plot of cell clusters in pig adipose tissue. (B) UMAP plot of cell types in adipose tissue. (C) Dotplot of marker genes in different cell types. (D) Violin plots of genes that are significantly associated with adipose-related traits in the grey60 and tan modules. (E) Violin plot of DEGs that are significantly associated with adipose-related traits at the 80 kg body weight stage. (F) Violin plot of DEGs that are significantly associated with adipose-related traits at the 120 kg body weight stage.
Figure 8.
Single-cell transcriptome analysis of porcine adipose tissue. (A) UMAP plot of cell clusters in pig adipose tissue. (B) UMAP plot of cell types in adipose tissue. (C) Dotplot of marker genes in different cell types. (D) Violin plots of genes that are significantly associated with adipose-related traits in the grey60 and tan modules. (E) Violin plot of DEGs that are significantly associated with adipose-related traits at the 80 kg body weight stage. (F) Violin plot of DEGs that are significantly associated with adipose-related traits at the 120 kg body weight stage.
Table 1.
The nutritional value of walnut cake.
Table 1.
The nutritional value of walnut cake.
| Nutritional value/% |
Value* |
SE* |
| Water |
8.1320419 |
0.0290737 |
| Crude protein |
22.61978519 |
0.450726075 |
| Ca |
0.4539975 |
0.00827276 |
| TP |
0.5211185 |
0.3548774 |
| Ash |
2.7819314 |
0.049862 |
| Crude fiber |
33.7111167 |
0.5357425 |
| Neutral detergent fiber |
49.2587963 |
0.8416535 |
| Acid detergent fiber |
33.1360225 |
0.7875224 |
| Crude fat |
8.1654504 |
0.1081171 |
Table 2.
Composition and nutritional value of experimental diets.
Table 2.
Composition and nutritional value of experimental diets.
| Ingredients/% |
8-15kg |
15-30kg |
30-60kg |
60-120kg |
| Feed A |
Feed B |
Feed A |
Feed B |
Feed A |
Feed B |
Feed A |
Feed B |
| Corn |
63.61 |
61.7 |
44.9 |
40.4 |
47.7 |
43.3 |
50.4 |
45.7 |
| Soybean meal |
23.54 |
21.4 |
13.39 |
9.7 |
10.7 |
7.3 |
8 |
3.9 |
| Wheat bran |
3 |
3 |
36.3 |
39.4 |
36.3 |
39 |
36.3 |
40 |
| Stone powder |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
| Soybean oil |
1.4 |
0.4 |
|
|
|
|
|
|
| Fish meal |
3 |
3 |
|
|
|
|
|
|
| Walnut cake |
|
5 |
|
5 |
|
5 |
|
5 |
| Nacl |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
| Lys(78.5%) |
0.15 |
0.2 |
0.11 |
0.2 |
|
0.1 |
|
0.1 |
| Premix |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Total |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
| Nutritional value |
|
|
|
|
|
|
|
|
| DE(MJ/kg) |
13.60 |
13.60 |
11.70 |
11.77 |
11.72 |
11.76 |
11.72 |
11.76 |
| CP |
18.22 |
18.23 |
15.02 |
15.09 |
14.07 |
14.07 |
13.11 |
13.11 |
| CF |
2.62 |
4.15 |
3.87 |
5.45 |
3.75 |
5.36 |
3.64 |
5.25 |
| Met+Cys |
0.62 |
1.11 |
0.54 |
1.00 |
0.51 |
0.98 |
0.49 |
0.96 |
| Lys |
1.07 |
1.07 |
0.78 |
0.78 |
0.63 |
0.63 |
0.56 |
0.57 |
| Ca |
0.76 |
0.77 |
0.64 |
0.65 |
0.63 |
0.64 |
0.62 |
0.63 |
| P |
0.62 |
0.62 |
0.72 |
0.74 |
0.71 |
0.74 |
0.70 |
0.73 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).