1. Introduction
Chronic inflammation is known to be provoked by the interaction of human organ interfaces with the environment, leading to dysfunctional conditions. This is assumed to be the cause of many chronic diseases that have become a widespread public health issue, especially in the western hemisphere. Highly prevalent examples of chronic inflammatory diseases (CIDs) include classic inflammatory diseases such as inflammatory bowel disease (IBD), Crohn's disease, and ulcerative colitis [
1,
2], as well as rheumatoid arthritis [
3], psoriasis, and psoriatic arthritis [
4]. In recent years, it has become increasingly clear that other systemic diseases should also be counted as inflammatory diseases. These include atherosclerosis and thus cardiovascular inflammation [
5] and metabolic inflammation [
6,
7]. Moreover, they are all associated with periodontitis, as well as other conditions [
8,
9,
10,
11,
12,
13,
14].
A review by Holmstrup et al. [
15] describes shared inflammatory pathways that could lead to a combination of all these diseases, implying that a targeted therapeutic intervention could also improve the clinical outcome of the other related diseases. CIDs represent a central challenge for modern medicine, and treating them is an example of modern precision medicine. Targeted anti-cytokine therapies are applied to modulate or suppress the disturbed immune response. To date, there has been a limited amount of studies examining the effects of these specific therapies on the oral interface, particularly the oral status and the periodontal microbiome. The aim of this pilot study was to investigate the influence of biological treatment for CID on the periodontal status and the oral microbiome.
2. Methods
2.1. Study Cohort
This monocentric, prospective, observational pilot study involved 21 patients with chronic inflammatory diseases, of which 8 patients dropped out after baseline. Patients received their first dose of biologic therapy according to guidelines and the decision of the interdisciplinary “inflammation conference“ during the study. They underwent dental examinations at two time points: the start of anti-cytokine therapy and approximately 14 weeks after initiation.
The average follow-up time was 98.8 days (14 weeks). Recruitment was carried out in the years 2016-2017 from the patient population of the Biological Follow Up (BFU) Study of the Comprehensive Center for Inflammation Medicine (CCIM) of the University Hospital Schleswig-Holstein (UKSH), Campus Kiel. Study participation was voluntary and occurred after written informed consent was obtained. The inclusion criteria were as follows:
-Diagnosed CID
-Upcoming targeted anti-cytokine therapy for the first time
-Participation in the Biological Follow Up Study of the CCIM
-Written informed consent
The exclusion criteria were defined as:
-Edentulousness
-Ongoing periodontal therapy
-Need for endocarditis prophylaxis
Participants were not selected based on whether they had been diagnosed with periodontitis. No information on their periodontal status was available prior to the start of the study. However, to prevent bias in the results, a questionnaire was used to ensure that the participants did not undergo periodontal therapy at any time during the study. In addition, detailed questions were asked about whether they had changed their oral hygiene habits in the period between the two examinations.
Eight participants were lost to follow-up. Their data were not included in the longitudinal analyses, but the European Federation of Periodontology/American Academy of Periodontology (EFP/AAP) case definition was also applied for these participants except for one participant who withdrew consent. The remaining seven participants who were lost to follow-up did not provide any information about why they did not participate further.
2.2. Serum Parameters and Dental Examination
The levels of C-reactive protein (CRP), leukocyte count, and platelet count were determined from fasting venous blood. Dental examinations were performed at the same time point in relation to the first intake of anti-cytokine therapy. The initial examination took place before the first dose (n = 6) or within 7 days after the first dose at the latest (n = 7). The follow-up examination took place after approximately 14 weeks and 16 weeks after the first administration the anti-cytokine therapy at the latest. Examinations were always performed in a standardized manner according to the same scheme and by the same investigator.
Diagnoses of reduced periodontium were determined according to the consensus report on periodontal health and gingival diseases and conditions of an intact and reduced periodontium [
16]. The given flowcharts of the EFP/AAP were used and suggested diagnoses of “healthy,” “healthy with reduced periodontium,” “gingivitis (localized/generalized),” and “gingivitis (localized/generalized) with reduced periodontium or periodontitis.” For cases classified as periodontitis, we followed the 2018 AAP/EFP guidelines to classify each case as stage I, II, III, or IV, and the extent was classified as localized or generalized [
17]. In detail, in the absence of radiographic bone loss, at least two non-adjacent teeth with interdental clinical attachment level (CAL) > 2 mm were used to assess a potential periodontitis case. All third molars or dental implants were excluded from diagnosis considerations.
Sites with 4-mm pocket depth (PD) and no bleeding on probing (BOP) were considered healthy when the patient did not have PD > 4 mm. Sites with 4-mm PD and BOP were included as contributing to the diagnosis of periodontitis. CAL ≥ 5 mm represented stage III/IV, CAL of 3-4 mm represented stage II, and CAL of 2 mm represented stage I. CAL < 2 mm was considered healthy. The presence of PD ≥ 6 mm in at least 2 or more adjacent teeth and furcation involvement of class II or III was used to assess potential cases of stage III or IV.
For the assessment of the extent of periodontitis, teeth with PD < 4 mm were included based on the assessed CAL. These teeth represented previously treated sites if there was history of periodontal treatment according to a questionnaire. If patients stated that there had been no previous periodontal treatment, these teeth were considered healthy.
Probing depths were measured using a millimeter-scale periodontal probe (PCPUNC15, Hu-Friedy, Chicago, IL, USA) and six-point measurements (mesio-vestibular, vestibular, disto-vestibular, mesio-oral, oral, and disto-oral). Gingival recessions were determined using the same probe. For this purpose, the distance of the gingival margin to the enamel-cement interface was measured. From this, the CAL could be calculated by adding the probing depth and recession. If the enamel-cement interface was not accessible (e.g., due to a restoration), no value was recorded.
To calculate the interdental CAL, the mid-surface measurements of the teeth (oral and vestibular) were not considered. Instead, the calculation was performed using only the mesio-vestibular, disto-vestibular, mesio-oral, and disto-oral readings. During the examination, bleeding caused by probing was noted, and the BOP index was calculated by dividing the number of bleeding points by the total number of sites probed. The degree of tooth mobility was determined as 0-3 according to Lindhe for each tooth [
18].
Microbiome samples of the gingival crevicular fluid were obtained. The highest value of each participant's sulcus depth was used to define the deepest periodontal pockets. Additionally, a clinically healthy pocket was defined as a probing depth under 2 mm and negative BOP. The microbiome samples were taken from one sulcus each at both examination times.
To collect gingival crevicular fluid, sterile-packed paper tips (VDW, mtwo, absorbent paper tips, 29 mm) were inserted into the sulcus without pressure. They were left there for 5 seconds and then placed in a reaction tube. In case of visible contamination with blood, saliva, or pus, another sample was taken and labeled accordingly. This had to be performed a total of 4 times as 4 samples were likely contaminated with blood. All 4 samples were collected from the deepest measured pockets and taken at the second examination. Accordingly, additional sampling was performed in each case of pockets with a similar depth. The samples were immediately frozen at -80°C.
2.3. Statistical Analyses
R was used for statistical analyses and graphics [
19]. Intraindividual α-diversities were calculated using the R packages VEGAN, ADE4, and PHYLOSEQ. The calculation was done according to the distribution, which was tested using the Shapiro-Wilk test. A paired Student's t-test was used when a normal distribution was present, and the Wilcoxon signed rank test was used when a normal distribution was not present.
Interindividual β-diversity was determined as follows. Data from the available operational taxonomic units (OTUs) were merged with clinical information, information from the questionnaires, and taxonomic data to form an R-object called PHYLOSEQ [
20]. Further analyses were also performed using the MICROBIOME, VEGAN, and GGPLOT2 packages. A permutational multivariate analysis of variance (PermANOVA) was performed using the adonis2 function of the VEGAN package [
21]. The number of permutations was set to 10,000. Values of p < 0.05 were considered statistically significant.
2.4. Ethics
The study was approved by the local ethics committee (AZ: A 156/03). Each proband gave informed consent before inclusion in the study.
3. Results
3.1. Study Characteristics
In total, 21 participants were included. Eight participants were lost to follow-up, including one participant who withdrew consent. A total of 13 study participants completed the follow-up and were analyzed. This included 9 women (69.2%) and 4 men (30.8%). The median age was 48 years, and the median body mass index was 26.6 kg/m². The median sulcus depths in the deepest measured pockets of the test participants were 4.5 (4.0-5.0) mm in the first examination and 4.0 (4.0-5.0) mm in the second examination. The median sulcus depth of the clinically healthy pocket from which the microbiome probes were obtained was 2.0 (1.0-2.0) mm both before the start of anti-cytokine therapy and 15 weeks after.
At baseline, the median leukocyte value was 7.3 (6.5-8.3) x 103/µl, which is within the normal range (4-10 x 103/µl) (
Table 1). However, it decreased slightly between the first and second examinations from 7.3 (6.5-8.3) to 6.5 (6.0-7.1). The mean CRP value decreased significantly (p = 0.01) from 2.3 (1.5-8.9) mg/l to 2.2 (1.1-2.9) mg/l (
Table 1).
Remission of the underlying CID was defined as a subjectively perceived improvement in the patient's disease state, which was confirmed by a medical examination. In 9 of 13 cases, remission was detectable at the second examination.
In the TNF-α group, symptoms improved in 3 of 7 cases (43.9%), 2 additional cases experienced no significant change in disease activity, and one participant reported worsening. No information was available on the disease course of one participant in the TNF-α group. The remission rate for IL-17, IL-12/23, and IL-6 inhibitors was 100%, although it should be noted that the number of participants in these categories was lower than in the TNF-α group.
3.2. Periodontal Status under Anti-Cytokine Therapy
Applying the EFP/AAP case definition [
17], 12 of 20 patients were classified as periodontitis cases at baseline, while 8 patients were classified as periodontally healthy. Out of the 13 patients who attended their second examination and were included in the statistical analysis, 8 patients were classified as periodontitis cases, and 5 five of them were characterized as having stage I periodontitis, 4 were grade A, and one was grade B. Two patients had stage II and grade A, and one patient had stage III and grade B periodontitis.
Out of the 7 patients who dropped out after baseline, 4 patients were considered periodontitis cases, of which 2 patients had stage I periodontitis (one grade A and the other grade B). The remaining 2 patients were classified as having stage II with grade C and stage III with grade C, respectively. The extent was “localized” in all patients.
Between the two examination time points, the median number of teeth with attachment loss per study participant decreased significantly from 3 (1-7) to 1 (0-3) teeth. In order to analyze whether the extent of pre-existing attachment loss influences changes in periodontal parameters after initiation of anti-cytokine treatment, the participants were divided into two groups based on their initial findings. Study participants with up to 5 teeth with attachment loss at the first examination were assigned to group A, and those with more than 5 teeth with attachment loss were assigned to group B. The four CIDs (rheumatoid arthritis, psoriasis, IBD, and ankylosing spondylitis) were represented in both groups. In group A, 5 participants received TNF-α antagonists, and 3 participants received IL-17 antagonists, while in group B, 2 participants received TNF-α antagonists, 2 participants received IL-12/23 antagonists, and one participant received an IL-6 receptor antagonist.
In group A, the median values of CAL and interdental CAL remained approximately constant, while the BOP index increased statistically significantly from 13.1 (6.6-18.8)% to 28.1 (20.0-31.4)% (p = 0.02). The median CRP level in this group decreased significantly (p = 0.02) from 1.9 (1.2-6.5) mg/l to 1.8 (0.3-2.7) mg/l between examinations. In group B, both the median CAL and interdental CAL decreased significantly. CAL decreased from 2.9 (2.3-3.4) mm to 2.4 (1.3-3.0) mm (p = 0.04), and interdental CAL decreased from 3.2 (2.5-3.3) mm to 2.8 (2.3-3.1) mm (p = 0.02). The BOP index in this group also dropped significantly (p = 0.02) from 19.4 (10.0-21.0) to 7.3 (6.9-9.9) (
Table 2).
3.3. Microbiological Status of the Periodontal Sulcus
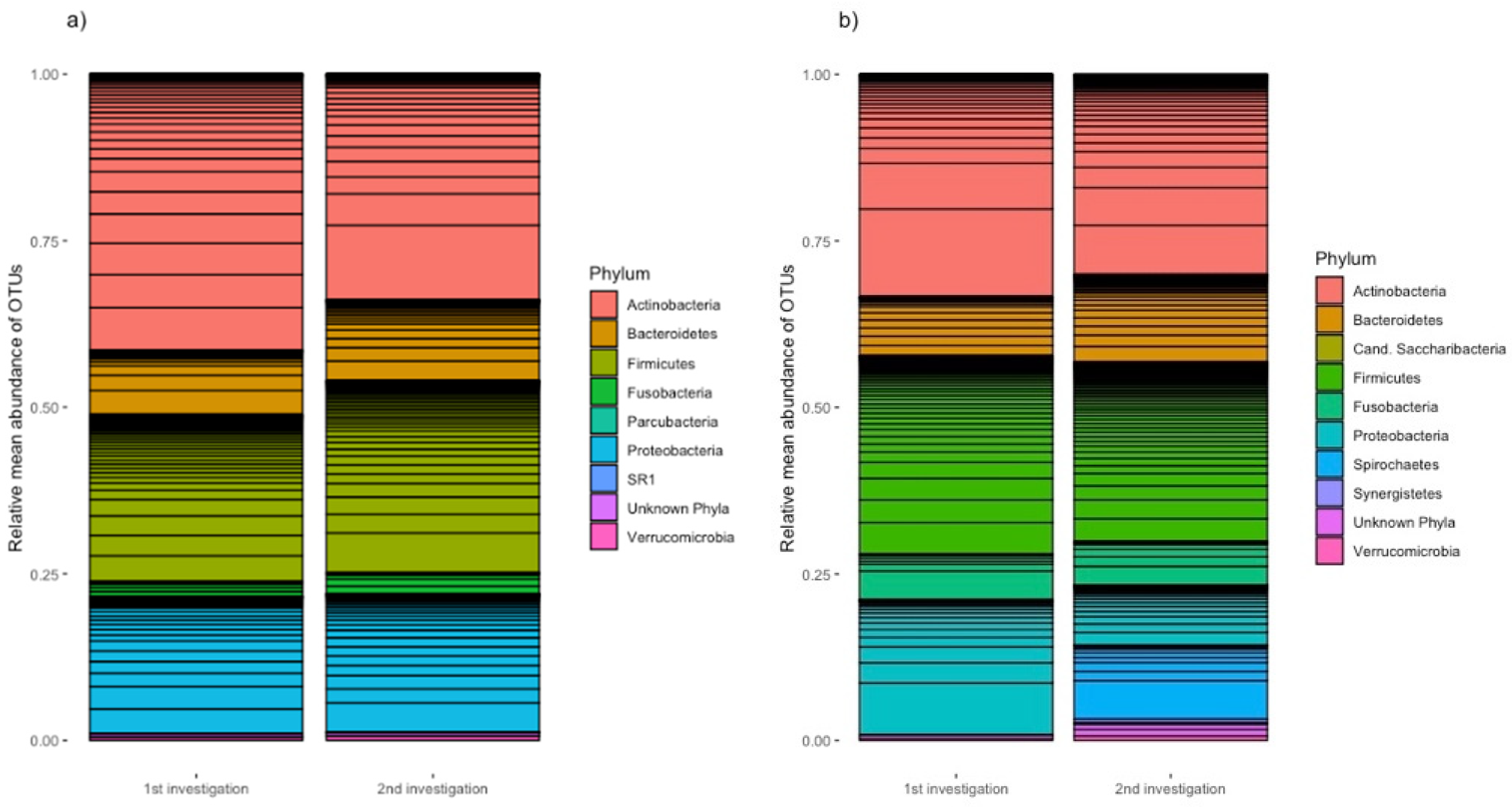
Gingival crevicular fluid was taken from each patient at two sites: their deepest periodontal pocket and a healthy pocket that did not show attachment loss or BOP. At the second examination, samples were collected at the same sites. Analysis of these microbiome samples indicated that the clinically healthy periodontal pockets of all probands showed a relative mean abundance of Actinobacteria of 41.5% at the first examination (
Figure 1a). Firmicutes accounted for the second most abundant proportion at 25.0%. Proteobacteria accounted for 20.5%, Bacteroidetes accounted for 9.7%, and Fusobacteria accounted for 2.3%.
At 13 weeks after the start of specific anti-cytokine therapy, there was a decreased proportion of Actinobacteria (33.9 %). Firmicutes represented 31.6%, Proteobacteria represented 20.8%, and Bacteroidetes represented 12.1%. Fusobacteria accounted for a relative mean abundance of 3.2%. The other phyla are shown in a graph, including unknown phyla, which made up less than 1% of the microbiome at both examinations.
The relative mean abundances of the samples from the deepest periodontal pockets are shown in
Figure 1b. Actinobacteria made up the largest proportion of phyla (33.4% at the first examination and 30.0% at the second examination). Firmicutes made up the second most abundant proportion of phyla at both examination time points (29.7% and 26.8%, respectively). The proportion of Bacteroidetes increased from 8.8% at the first examination to 13.1% at the second one. Fusobacteria occurred with similar abundance (6.9% in the first examination and 6.6% in the second examination). Proteobacteria was significantly more abundant in the first examination (20.3%) than in the second examination (8.9%).
Spirochetes was not found in any of the samples of the deepest measured pockets in the first examination, but in the second sampling, they accounted for 11.1%. The remaining observed phyla accounted for less than 1% with the exception of the unknown phyla at the second examination, which accounted for 1.9% (
Figure 1b).
3.4. α-Diversity under Anti-Cytokine Treatment
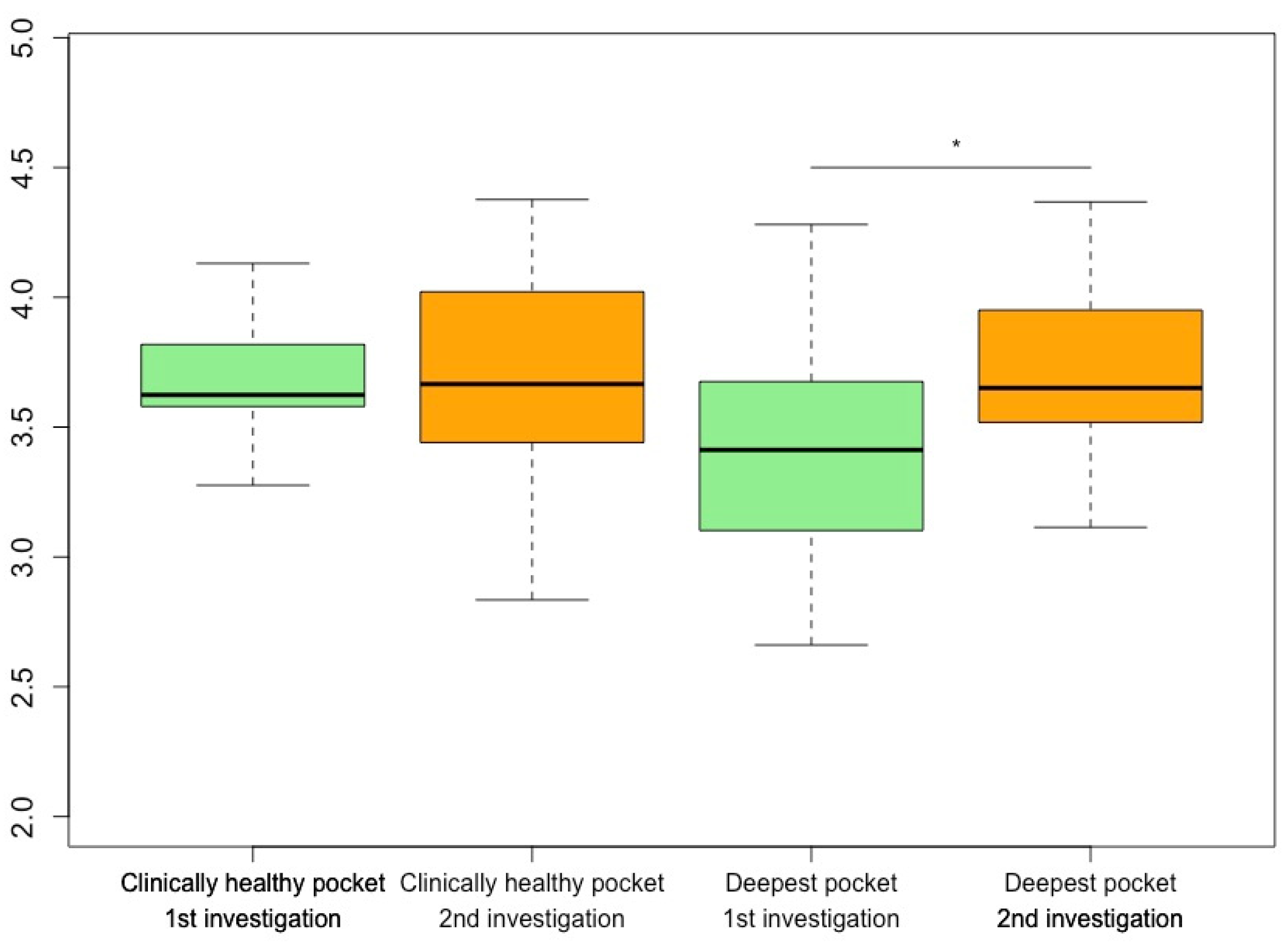
The analysis of α-diversity showed a significant increase in the Shannon index of the deepest measured periodontal pockets between the first and second examinations (p = 0.039). With one exception, an increase in α-diversity in terms of the Shannon Index could be seen in all samples taken from the deepest periodontal pockets of all participants (
Figure 2). An analysis of the α-diversity between drug groups showed no significant changes.
3.5. β-Diversity of Periodontal Pockets under Anti-Cytokine Therapy
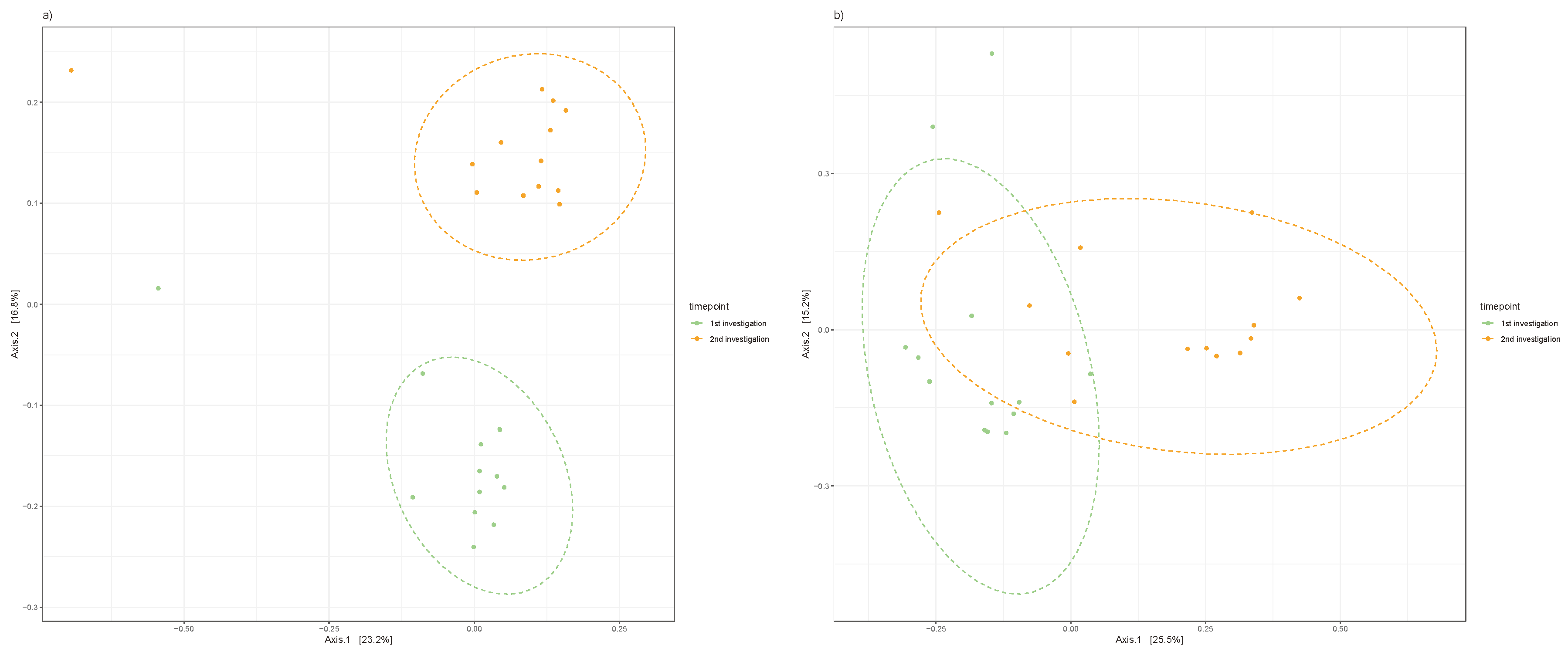
When distinguishing between clinically healthy pockets, principal coordinate analysis of the Jaccard index explained the variance in β-diversities of the clinically healthy pockets (p<0.001; R² = 11.7) and the deepest measured pockets of the participants (p<0.001; R² = 12.7). There was a clear difference in β-diversity after the initiation of anti-cytokine therapy, as displayed in
Figure 3.
4. Discussion
Peddis et al. [
22] conducted a systematic review of 35 publications addressing the interactions of anti-cytokine therapies and periodontitis. They included 15 non-randomized clinical trials, 12 in vivo animal models, and 7 case reports. The most studied disease was rheumatoid arthritis, followed by ankylosing spondylitis, psoriatic arthritis, systemic sclerosis, and Crohn's disease. Most studies dealt with TNF-α blockers. The authors concluded that the evidence clearly supports positive effects of anti-TNF-α therapy on periodontal health . Statements on other anti-cytokine approaches could not clearly been made. An examination of the oral microfilm is still lacking.
In the present study, we periodontally characterized participants with CID during the first 15 weeks of a targeted anti-cytokine therapy. The initial prevalence of periodontitis was above average. The median number of teeth with attachment loss decreased significantly. In patients with more than 5 teeth with attachment loss at baseline, parameters of periodontal damage improved significantly. This improvement occurred without concomitant manual periodontal therapy or change in oral hygiene habits.
Furthermore, changes in relative abundances of the subgingival microbiome at the phylum level were detected during specific anti-cytokine therapy. It is commonly known that a the microbiome of a patient with periodontitis differs from that of a periodontally healthy patient [
14]. In addition, differences in microbial composition between healthy and diseased sulci can be observed in intraindividual comparisons [
16].
In the healthy samples and those from the deepest periodontal pockets, Actinobacteria was found to be the most abundant phylum, closely followed by Firmicutes. They accounted for about 1/3-1/4 of the phyla in each of the samples, and proportionally more Actinobacteria occurred in the clinically healthy sulci. Shi et al. [
23] also described similar observations when examining samples in participants who were healthy, those with chronic periodontitis, and those with aggressive periodontitis (according to the 1999 classification). They described a dominance of Firmicutes, Actinobacteria, and Proteobacteria in the samples of periodontally healthy participants. Periodontitis showed fewer Actinobacteria and relative increases in Fusobacteria, Spirochetes, and Saccharibacteria [
23].
It is interesting to note in this context that the samples that we examined were from participants without periodontitis, but some had increased pocket depths and CIDs. This suggests that there are early changes in the subgingival microbiome in such patients that might favor the development of periodontal inflammation. This would provide an explanation for the frequently described associations between periodontitis and chronic inflammatory diseases, such as rheumatoid arthritis [
9,
12] , ankylosing spondylitis[
24], psoriasis[
25], and IBDs [
11]. For IBDs, positive correlations between periodontal and intestinal inflammation have been described [
26,
27].
In one study, Atarashi et al. collected oral microbiome samples from patients with IBDs and transferred them into germ-free mice. They showed that proliferation of TH1 cells was associated with the abundance of oral
Klebsiella bacteria in the colon [
28], suggesting a direct involvement of oral flora in intestinal inflammation. However, other explanations for associations of CIDs with periodontitis, such as shared genetic risk factors between rheumatoid arthritis and periodontitis [
29] or altered parainflammatory conditions [
30], have also been described.
However, it has to be discussed whether this improvement can be directly attributed to the given medication, possibly also through a change in the subgingival microfilm, or the improvement of the underlying CID. So far, treatment of classical inflammatory targets, including IL-1 and TNF-α, have failed to meet expectations in periodontal therapy [
31]. Our data imply that fundamental changes in the subgingival microbiota occur under targeted anti-cytokine therapy, leading to an improved periodontal status.
Nevertheless, there are some limitations to this study, that was designed as a pilot study to develop hypotheses for further studies. The main limiting factors here are the small number of participants and the heterogeneous study group, with different systemic conditions, that are linked through inflammation. We characterized them in detail, but we did not have a healthy control group, and a study with a case-control design will be needed in the future. However, we attempted to address this problem by using intra-individual microbiome samples from clinically healthy and less healthy tooth pockets. Furthermore, we tried to control for external influences such as changes in grooming habits or diet by distributing questionnaires. Nevertheless, biases due to the longitudinal study design are also conceivable.
5. Conclusion
Within the study limitations, the investigated data demonstrate a relationship between anti-cytokine treatment and the oral status in patients with CIDs. In addition, anti-cytokine therapies could improve the microbiome diversity of the tooth pocket. This could be especially of interest for patients who have a higher tendency to develop periodontitis. However, it is still unclear whether periodontitis drives systemic inflammation, (or vice versa) or whether there is a mutual influence (which is more likely). Additional investigations are needed to analyze microbiome composition and metabolism directly in the tooth pocket and whether the systemic or local administration of specific biologicals can improve periodontal conditions in patients with CID and without CID.
Author Contributions
Conceptualization, Juliane Wagner, André Franke, Matthias Laudes, Christof Dörfer, Jörg Wiltfang, Christian Graetz and Dominik Schulte; Formal analysis, Luisa Haker, Malte Rühlemann, Hendrik Naujokat, Johannes Spille and Wolfgang Lieb; Investigation, Juliane Wagner, Louisa Mewes, Corinna Bang and Wolfgang Lieb; Methodology, Juliane Wagner, Luisa Haker, Louisa Mewes, Corinna Bang, Christian Graetz and Dominik Schulte; Project administration, André Franke, Stefan Schreiber, Matthias Laudes, Christof Dörfer, Jörg Wiltfang, Christian Graetz and Dominik Schulte; Resources, Corinna Bang, Wolfgang Lieb, Stefan Schreiber and Matthias Laudes; Software, Wolfgang Lieb; Supervision, André Franke, Stefan Schreiber, Matthias Laudes, Christof Dörfer, Jörg Wiltfang, Christian Graetz and Dominik Schulte; Validation, Christian Graetz; Visualization, Juliane Wagner, Luisa Haker and Hendrik Naujokat; Writing – original draft, Juliane Wagner; Writing – review & editing, Luisa Haker, Louisa Mewes, Corinna Bang, Malte Rühlemann, Johannes Spille, Wolfgang Lieb, André Franke, Stefan Schreiber, Matthias Laudes, Christof Dörfer, Jörg Wiltfang, Christian Graetz and Dominik Schulte.
Funding
No Funding: not applicable.
Institutional Review Board Statement
The project received infrastructure support from the PopGen Biobank (Kiel, Germany). It was approved by the local ethics committee (AZ: A 156/03) in Kiel. The study was registered at the German Clinical Trial Register (DRKS): DRKS00032473
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All samples and information on their corresponding phenotypes were obtained from the PopGen Biobank (Schleswig-Holstein, Germany) and can be accessed through a Material Data Access Form. Information about the Material Data Access Form and how to apply can be found at “
http://www.uksh.de/p2n/Information+for+Researchers.html.”
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no potential conflict of interest with respect to the authorship and/or publication of this article.
References
- Kim DH, Cheon JH. Pathogenesis of Inflammatory Bowel Disease and Recent Advances in Biologic Therapies. Immune Netw. 2017;17:25–40. [CrossRef]
- Zhang Y-Z, Li Y-Y. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91–9.
- Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. The Lancet. 2010;376:1094–108.
- Coates LC, FitzGerald O, Helliwell PS, Paul C. Psoriasis, psoriatic arthritis, and rheumatoid arthritis: Is all inflammation the same? Seminars in Arthritis and Rheumatism. 2016;46:291–304.
- Ross R. Atherosclerosis — An Inflammatory Disease. New England Journal of Medicine. 1999;340:115–26. [CrossRef]
- Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177–85. [CrossRef]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. [CrossRef]
- Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, et al. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55:21–31. [CrossRef]
- Araújo VMA, Melo IM, Lima V. Relationship between Periodontitis and Rheumatoid Arthritis: Review of the Literature. Mediators Inflamm [Internet]. 2015 [cited 2020 Jan 29];2015. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4539505/.
- Christophers E. Periodontitis and risk of psoriasis: another comorbidity. Journal of the European Academy of Dermatology and Venereology. 2017;31:757–8.
- Papageorgiou SN, Hagner M, Nogueira AVB, Franke A, Jäger A, Deschner J. Inflammatory bowel disease and oral health: systematic review and a meta-analysis. Journal of Clinical Periodontology. 2017;44:382–93. [CrossRef]
- Potempa J, Mydel P, Koziel J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat Rev Rheumatol. 2017;13:606–20. [CrossRef]
- Slots J. Periodontitis: facts, fallacies and the future. Periodontol 2000. 2017;75:7–23. [CrossRef]
- Ungprasert P, Wijarnpreecha K, Wetter DA. Periodontitis and Risk of Psoriasis: A Systematic Review and Meta-analysis. J Eur Acad Dermatol Venereol. 2017;31:857–62. [CrossRef]
- Holmstrup P, Damgaard C, Olsen I, Klinge B, Flyvbjerg A, Nielsen CH, et al. Comorbidity of periodontal disease: two sides of the same coin? An introduction for the clinician. J Oral Microbiol [Internet]. 2017 [cited 2018 Jan 21];9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5508374/.
- Chapple ILC, Mealey BL, Van Dyke TE, Bartold PM, Dommisch H, Eickholz P, et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. Journal of Periodontology [Internet]. 2018 [cited 2023 Oct 30];89. Available from: https://aap.onlinelibrary.wiley.com/doi/10.1002/JPER.17-0719.
- Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. Journal of Periodontology [Internet]. 2018 [cited 2023 Oct 30];89. Available from: https://aap.onlinelibrary.wiley.com/doi/10.1002/JPER.18-0006.
- Lindhe J, Nyman S. The role of occlusion in periodontal disease and the biological rationale for splinting in treatment of periodontitis. Oral Sci Rev. 1977;10:11–43.
- Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecology. 2001;26:32–46.
- McMurdie PJ, Holmes S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS One [Internet]. 2013 [cited 2019 Dec 20];8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3632530/.
- Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, et al. vegan: Community Ecology Package. R package version 2.5-6. https://CRAN.R-project.org/package=vegan. 2019.
- Peddis N, Musu D, Ideo F, Rossi-Fedele G, Cotti E. Interaction of biologic therapy with apical periodontitis and periodontitis: a systematic review. Australian Dental Journal. 2019;64:122–34. [CrossRef]
- Shi M, Wei Y, Hu W, Nie Y, Wu X, Lu R. The Subgingival Microbiome of Periodontal Pockets With Different Probing Depths in Chronic and Aggressive Periodontitis: A Pilot Study. Front Cell Infect Microbiol. 2018;8:124. [CrossRef]
- Ratz T, Dean LE, Atzeni F, Reeks C, Macfarlane GJ, Macfarlane TV. A possible link between ankylosing spondylitis and periodontitis: a systematic review and meta-analysis. Rheumatology. 2015;54:500–10. [CrossRef]
- Woeste S, Graetz C, Gerdes S, Mrowietz U. Oral Health in Patients with Psoriasis-A Prospective Study. J Invest Dermatol. 2019. [CrossRef]
- Figueredo CM, Martins AP, Lira-Junior R, Menegat JB, Carvalho AT, Fischer RG, et al. Activity of inflammatory bowel disease influences the expression of cytokines in gingival tissue. Cytokine. 2017;95:1–6. [CrossRef]
- Lira-Junior R, Figueredo CM. Periodontal and inflammatory bowel diseases: Is there evidence of complex pathogenic interactions? World J Gastroenterol. 2016;22:7963–72.
- Atarashi K, Suda W, Luo C, Kawaguchi T, Motoo I, Narushima S, et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science. 2017;358:359.
- Schulz S, Pütz N, Jurianz E, Schaller H-G, Reichert S. Are There Any Common Genetic Risk Markers for Rheumatoid Arthritis and Periodontal Diseases? A Case-Control Study. Mediators of Inflammation. 2019;2019:1–11. [CrossRef]
- Schulz J, Knappe C, Graetz C, Mewes L, Türk K, Black AK, et al. Secreted frizzled-related protein 5 serum levels in human periodontitis—A nested case–control study. Journal of Clinical Periodontology. 2019;46:522–8.
- Cotti E, Schirru E, Acquas E, Usai P. An overview on biologic medications and their possible role in apical periodontitis. J Endod. 2014;40:1902–11. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).