Submitted:
24 March 2024
Posted:
26 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
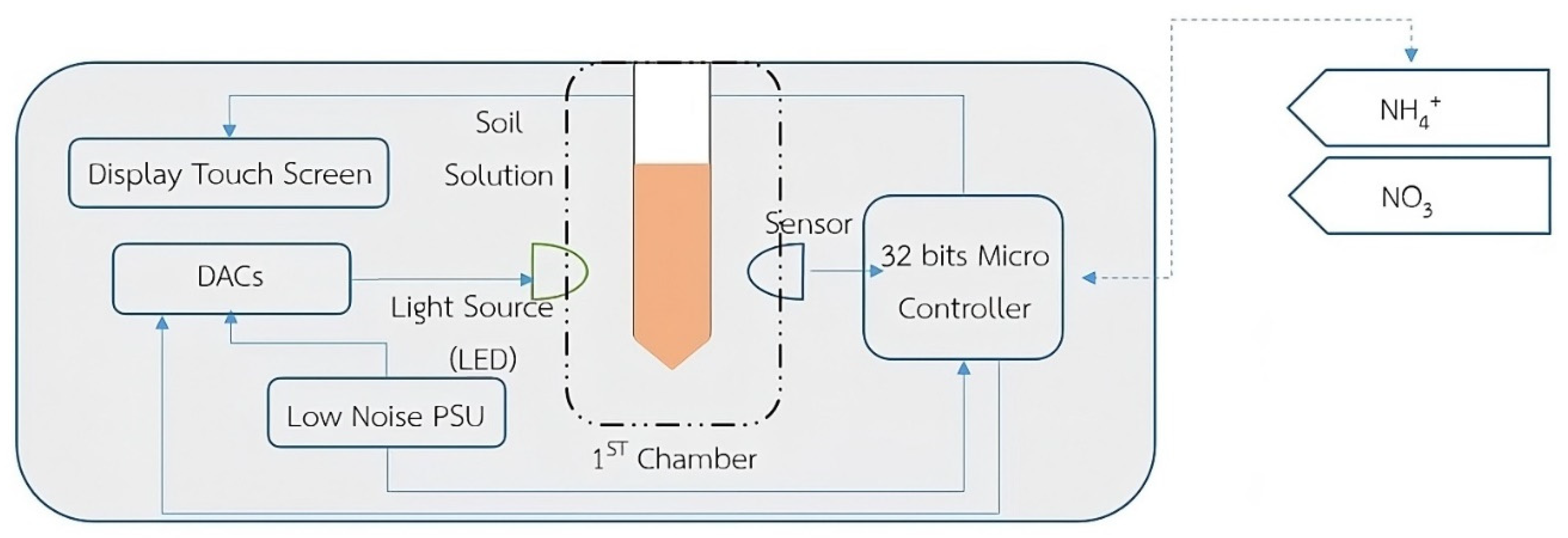
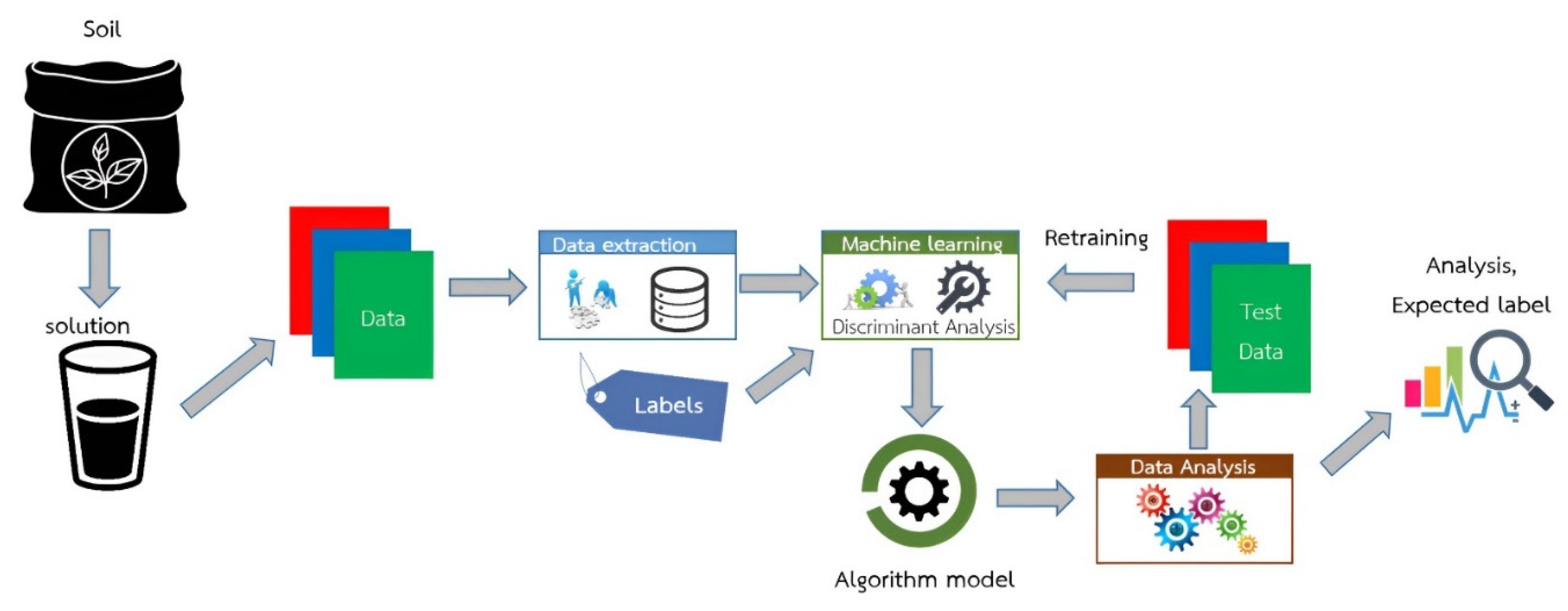
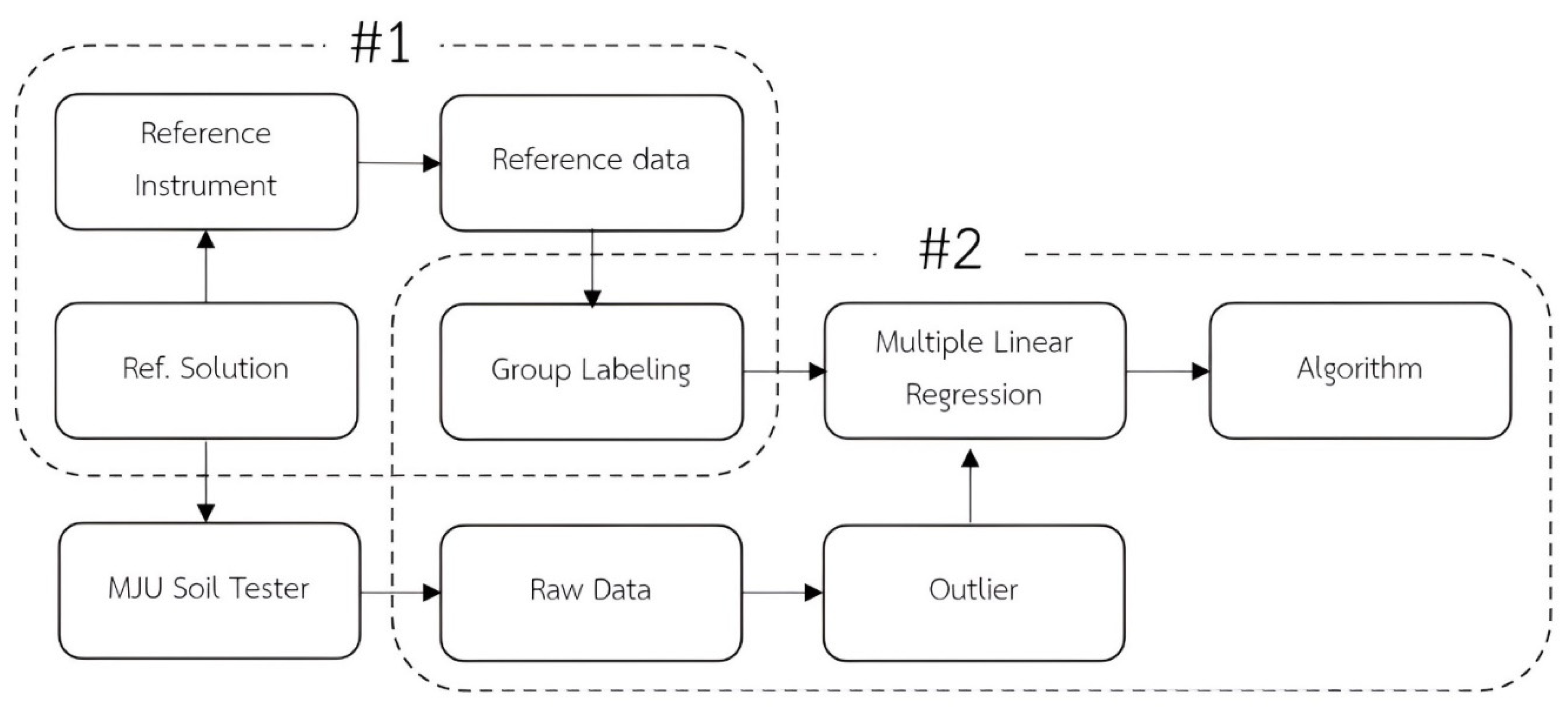
2.1. Conception Framework of MJU Soil Tester
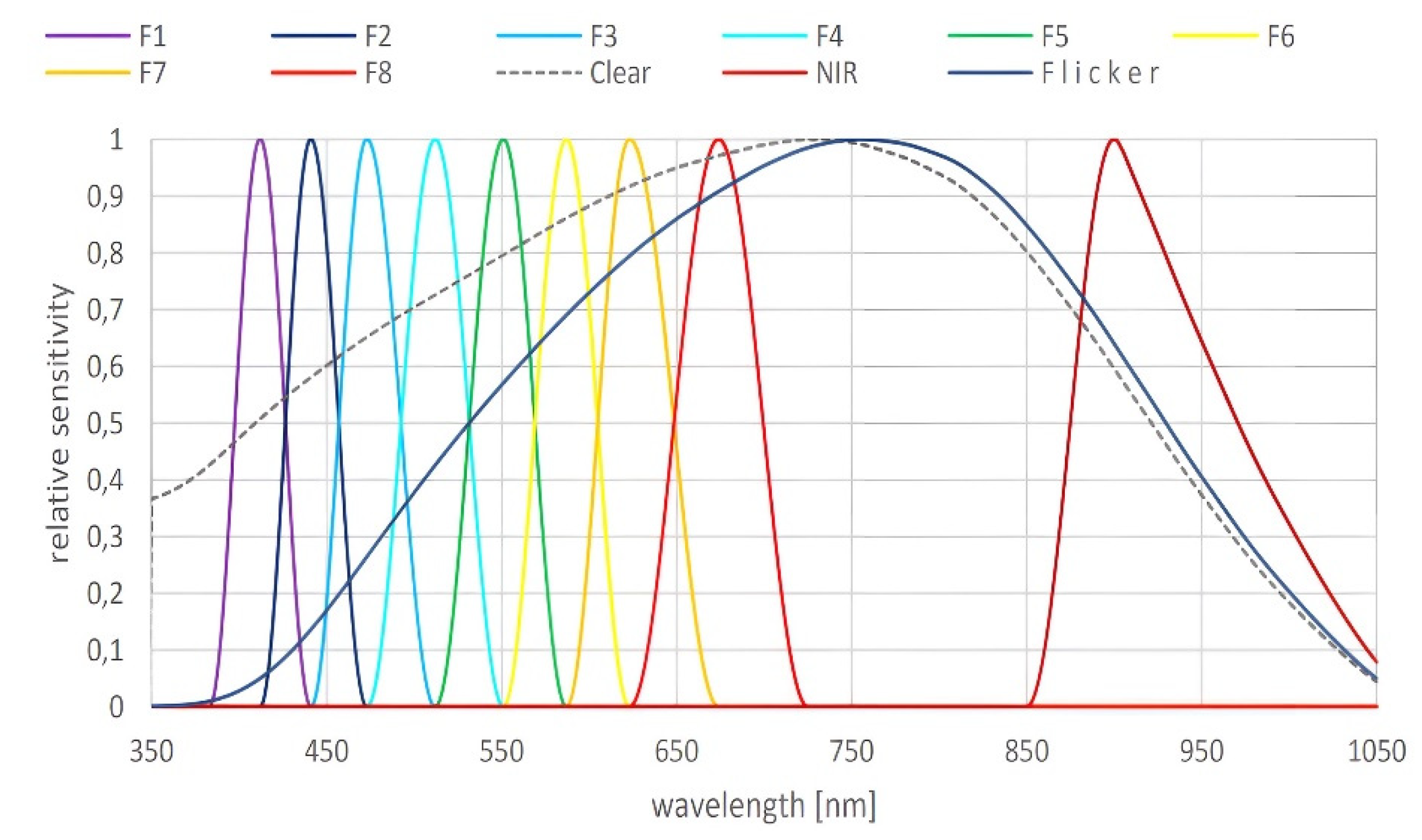
2.2. LED Light Source and Sensor Detector
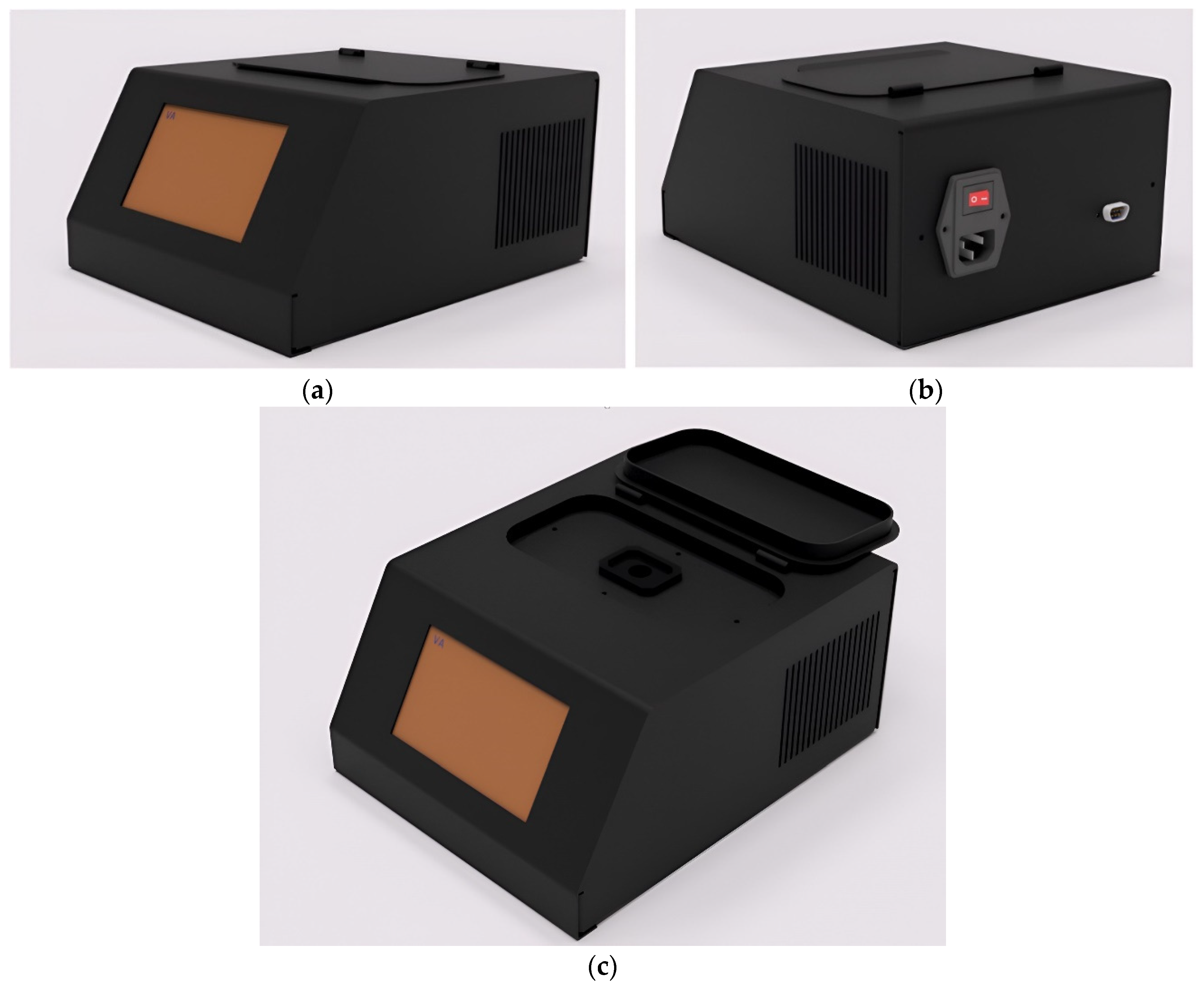
2.3. External Construction of MJU Soil Tester Device
2.4. User Interface on Touchscreen
2.5. Algorithm Development
2.6. Soil Sampling for Soil Sample
2.7. Testing of MJU Soil Tester
3. Results
3.1. Structure and Components of MJU Soil Tester Houseware
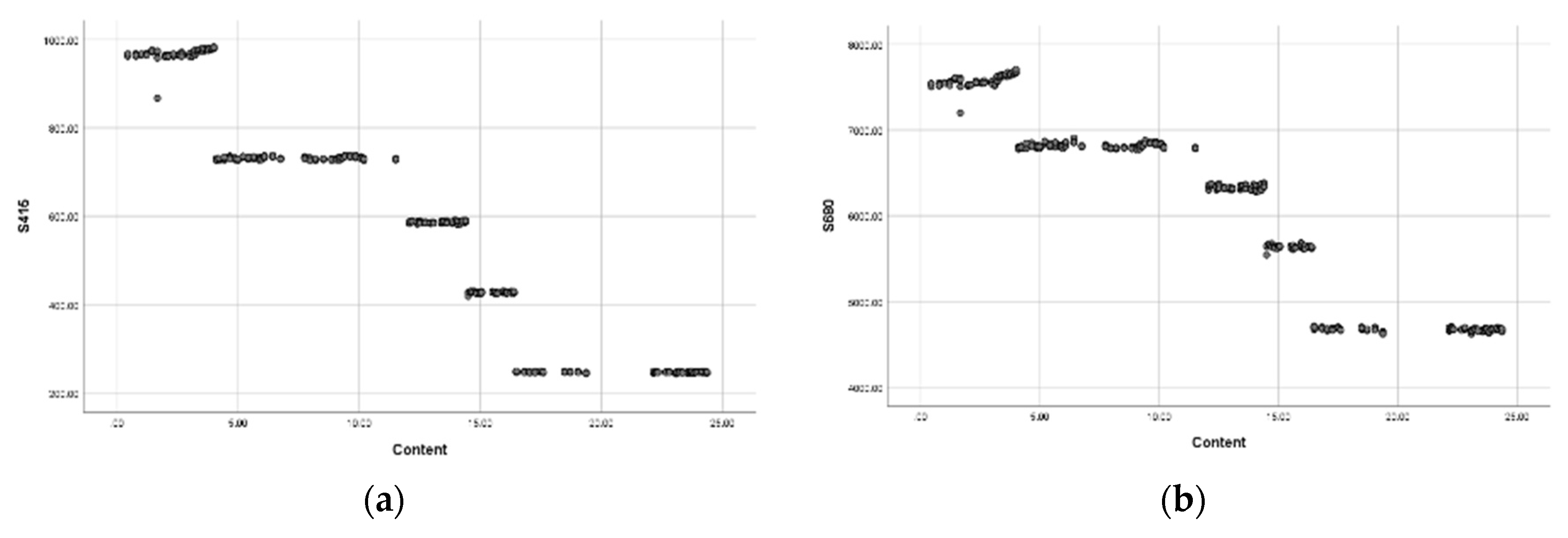
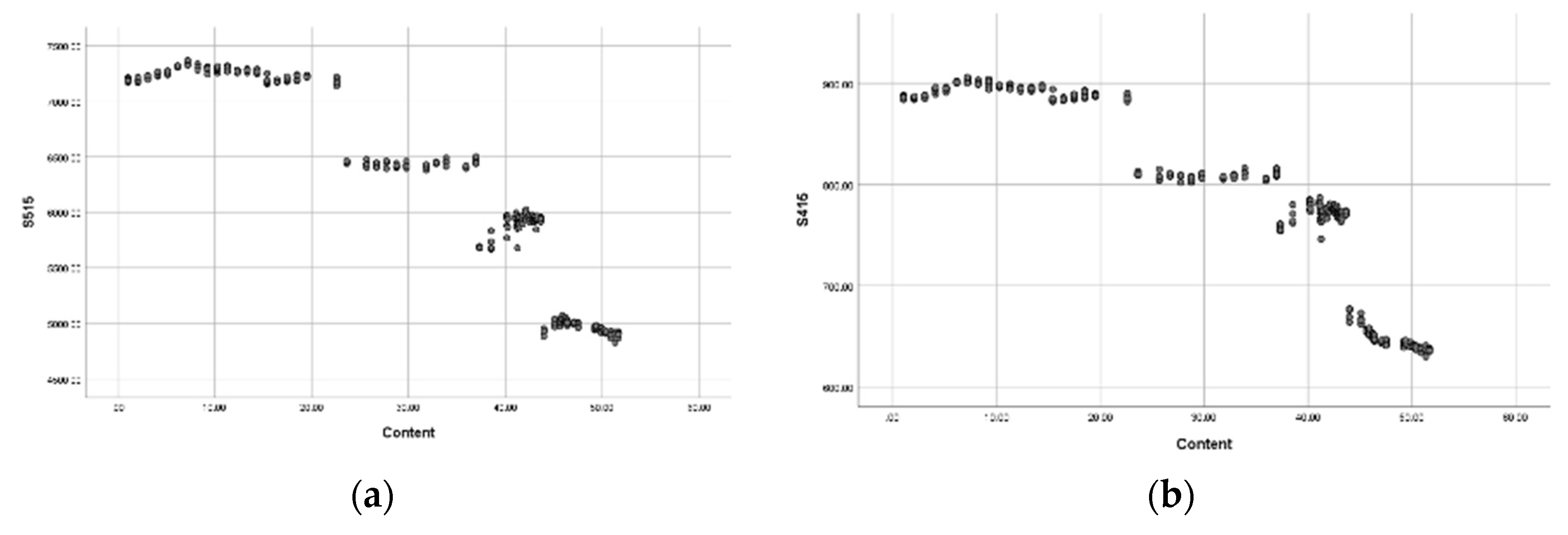
3.2. Establish Celibate Equation for Ammonium (NH4)
| Function | Eigenvalue | % of Variance | Cumulative % | Canonical Correlation |
|---|---|---|---|---|
| 1 | 53157.851a | 95.2 | 95.2 | 1.000 |
| 2 | 2417.922a | 4.3 | 99.5 | 1.000 |
| 3 | 219.119a | .4 | 99.9 | .998 |
| 4 | 54.845a | .1 | 100.0 | .991 |
| Function | ||||
| 1 | 2 | 3 | 4 | |
| S415 | -.048 | -.282 | -.243 | -.117 |
| S445 | -.059 | .020 | -.089 | .205 |
| S480 | -.026 | -.241 | .284 | .032 |
| S515 | .037 | .148 | -.116 | -.110 |
| S555 | -.051 | .202 | .078 | -.119 |
| S590 | .019 | -.059 | -.034 | .013 |
| S630 | .078 | -.063 | .002 | .076 |
| S680 | -.081 | -.018 | -.020 | -.007 |
| (Constant) | 1.507 | -36.104 | -19.740 | 15.526 |
3.3. Establish Predictive Equations for Nitrate (NO3-)
3.4. Cross-Validation for Group Classification of Ammonium (NH4+)
3.5. Accuracy and Errors of MJU Soil Tester from Soil Samples
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
References
- Abawi, G.S.; Gugino, B.K.; Idowu, O.J.; Moebius-Clune, B.N.; Schindelbeck, R.R.; Wolfe, D.W.; van Es, H.M. Use of an integrative soil health test for evaluation of soil management impacts. Renewable Agriculture and Food Systems 2009, 24, 214-224. [CrossRef]
- Seybold, C.A.; Hubbs, M.D.; Tyler, D.D. On-Farm Tests Indicate Effects of Long-Term Tillage Systems on Soil Quality. Journal of Sustainable Agriculture 2002, 19, 61-73. [CrossRef]
- Morton, J.; Baird, D.; Manning, M. A soil sampling protocol to minimise the spatial variability in soil test values in New Zealand hill country. New Zealand Journal of Agricultural Research 2000, 43, 367-375. [CrossRef]
- Lincy, C.T.; Lenin, F.A.; Jalbin, J. Deep residual network for soil nutrient assessment using optical sensors. Journal of Plant Nutrition and Soil Science 2023, n/a. [CrossRef]
- Kim, H.-J.; Sudduth, K.A.; Hummel, J.W. Soil macronutrient sensing for precision agriculture. Journal of Environmental Monitoring 2009, 11, 1810-1824. [CrossRef]
- Amoo, A.; Babalola, O. Ammonia-oxidizing microorganisms: Key players in the promotion of plant growth. Journal of soil science and plant nutrition 2017, 17, 935-947. [CrossRef]
- Singh, N.; Singh, P.; Pathak, P.K.; Gupta, K.J. Using Different Forms of Nitrogen to Study Hypersensitive Response Elicited by Avirulent Pseudomonas syringae. In Nitrogen Metabolism in Plants: Methods and Protocols, Gupta, K.J., Ed.; Springer New York: New York, NY, 2020; pp. 79-92.
- von Wirén, N.; Gojon, A.; Chaillou, S.; Raper, D. Mechanisms and Regulation of Ammonium Uptake in Higher Plants. In Plant Nitrogen, Lea, P.J., Morot-Gaudry, J.-F., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2001; pp. 61-77.
- George, J.; Holtham, L.; Sabermanesh, K.; Heuer, S.; Tester, M.; Plett, D.; Garnett, T. Small amounts of ammonium (NH$ _4^+ $) can increase growth of maize (Zea mays). Journal of Plant Nutrition and Soil Science 2016, 179, 717-725. [CrossRef]
- Britto, D.T.; Glass, A.D.M.; Kronzucker, H.J.; Siddiqi, M.Y. Cytosolic Concentrations and Transmembrane Fluxes of NH4 +/NH3. An Evaluation of Recent Proposals. Plant Physiology 2001, 125, 523-526. [CrossRef]
- Torres, M.J.; Simon, J.; Rowley, G.; Bedmar, E.J.; Richardson, D.J.; Gates, A.J.; Delgado, M.J. Chapter Seven - Nitrous Oxide Metabolism in Nitrate-Reducing Bacteria: Physiology and Regulatory Mechanisms. In Advances in Microbial Physiology, Poole, R.K., Ed.; Academic Press: 2016; Volume 68, pp. 353-432.
- Ambus, P.; Zechmeister-Boltenstern, S.; Butterbach-Bahl, K. Sources of nitrous oxide emitted from European forest soils. Biogeosciences 2006, 3, 135-145. [CrossRef]
- Liebig, M.A.; Doran, J.W.; Gardner, J.C. Evaluation of a Field Test Kit for Measuring selected Soil Quality Indicators. Agronomy Journal 1996, 88, 683-686. [CrossRef]
- Ditzler, C.; Tugel, A. Soil Quality Field Tools. Agronomy Journal - AGRON J 2002, 94. [CrossRef]
- Yeh, P.; Yeh, N.; Lee, C.-H.; Ding, T.-J. Applications of LEDs in optical sensors and chemical sensing device for detection of biochemicals, heavy metals, and environmental nutrients. Renewable and Sustainable Energy Reviews 2017, 75, 461-468. [CrossRef]
- Yeh, N.; Ding, T.J.; Yeh, P. Light-emitting diodes׳ light qualities and their corresponding scientific applications. Renewable and Sustainable Energy Reviews 2015, 51, 55-61. [CrossRef]
- Lapsiwala, P. Optical sensor for analysis of amonia, iron and manganese nutrients. International Journal of Engineering and Technology(UAE) 2018, 7, 288-291. [CrossRef]
- Mohd Yusof, K.; Isaak, S.; Che Abd Rashid, N.; Ngajikin, N.H. NPK DETECTION SPECTROSCOPY ON NON-AGRICULTURE SOIL. Jurnal Teknologi 2016, 78. [CrossRef]
- Yokota, M.; Okada, T.; Yamaguchi, I. An optical sensor for analysis of soil nutrients by using LED light sources. Measurement Science and Technology 2007, 18, 2197. [CrossRef]
- Zhang, C.; Kong, W.; Liu, F.; He, Y. Measurement of aspartic acid in oilseed rape leaves under herbicide stress using near infrared spectroscopy and chemometrics. Heliyon 2016, 2, e00064. [CrossRef]
- Keeney, D.R.; Nelson, D.W. Nitrogen—Inorganic Forms. In Methods of Soil Analysis; Agronomy Monographs; 1983; pp. 643-698.
- Luchansky, M.S.; Bailey, R.C. High-Q Optical Sensors for Chemical and Biological Analysis. Analytical Chemistry 2012, 84, 793-821. [CrossRef]
- Chen, W.; Kaya Özdemir, Ş.; Zhao, G.; Wiersig, J.; Yang, L. Exceptional points enhance sensing in an optical microcavity. Nature 2017, 548, 192-196. [CrossRef]
- Cavedo, F.; Esmaili, P.; Norgia, M. Remote Reflectivity Sensor for Industrial Applications. Sensors 2021, 21. [CrossRef]
- Portone, A.; Borrego-Varillas, R.; Ganzer, L.; Di Corato, R.; Qualtieri, A.; Persano, L.; Camposeo, A.; Cerullo, G.; Pisignano, D. Conformable Nanowire-in-Nanofiber Hybrids for Low-Threshold Optical Gain in the Ultraviolet. ACS Nano 2020, 14, 8093-8102. [CrossRef]
- Prosa, M.; Benvenuti, E.; Kallweit, D.; Pellacani, P.; Toerker, M.; Bolognesi, M.; Lopez-Sanchez, L.; Ragona, V.; Marabelli, F.; Toffanin, S. Organic Light-Emitting Transistors in a Smart-Integrated System for Plasmonic-Based Sensing. Advanced Functional Materials 2021, 31, 2104927. [CrossRef]
- Liu, X.; Ma, Y. Sensitive carbon monoxide detection based on light-induced thermoelastic spectroscopy with a fiber-coupled multipass cell [Invited]. Chinese Optics Letters 2022, 20, 031201. [CrossRef]
- Jiang, X.; Qavi, A.J.; Huang, S.H.; Yang, L. Whispering-Gallery Sensors. Matter 2020, 3, 371-392. [CrossRef]
- Shen, Z.; Du, M. High-performance refractive index sensing system based on multiple Fano resonances in polarization-insensitive metasurface with nanorings. Opt. Express 2021, 29, 28287-28296. [CrossRef]
- Fay, C.D.; Nattestad, A. LED PEDD Discharge Photometry: Effects of Software Driven Measurements for Sensing Applications. Sensors 2022, 22. [CrossRef]
- Bhandari, A.K. A logarithmic law based histogram modification scheme for naturalness image contrast enhancement. Journal of Ambient Intelligence and Humanized Computing 2020, 11, 1605-1627. [CrossRef]
- Priyadarshini, N.; Sarkar, M. A High Dynamic Range CMOS Image Sensor using Programmable Linear-Logarithmic Counter for Low Light Imaging Applications. In Proceedings of the 2020 IEEE International Symposium on Circuits and Systems (ISCAS), 12-14 Oct 2020, 2020; pp. 1-5. [CrossRef]
- Brunetti, A.M.; Choubey, B. A Low Dark Current 160 dB Logarithmic Pixel with Low Voltage Photodiode Biasing. Electronics 2021, 10. [CrossRef]
- Zhang, G.; Gu, Z.; Zhao, Q.; Ren, J.; Han, S.; Lu, W. A Multi-Node Detection Algorithm Based on Serial and Threshold in Intelligent Sensor Networks. Sensors 2020, 20. [CrossRef]
- Mennel, L.; Symonowicz, J.; Wachter, S.; Polyushkin, D.K.; Molina-Mendoza, A.J.; Mueller, T. Ultrafast machine vision with 2D material neural network image sensors. Nature 2020, 579, 62-66. [CrossRef]
- Croffie, M.E.T.; Williams, P.N.; Fenton, O.; Fenelon, A.; Metzger, K.; Daly, K. Optimising Sample Preparation and Calibrations in EDXRF for Quantitative Soil Analysis. Agronomy 2020, 10. [CrossRef]
- Moldoveanu, S.; David, V. Chapter 12 - Comments on Sample Preparation in Chromatography for Different Types of Materials. In Modern Sample Preparation for Chromatography, Moldoveanu, S., David, V., Eds.; Elsevier: Amsterdam, 2015; pp. 411-446.



| Group | Function | |||
| 1 | 2 | 3 | 4 | |
| 1.00 | 376.221 | -51.416 | 7.433 | -3.190 |
| 2.00 | 105.310 | 40.947 | -10.562 | 8.717 |
| 3.00 | -46.107 | 57.960 | -7.391 | -14.077 |
| 4.00 | -182.240 | 32.644 | 34.424 | 3.001 |
| 5.00 | -263.740 | -54.924 | -7.556 | .615 |
| Group | Predicted Group Membership | Total | ||||||
| 1.00 | 2.00 | 3.00 | 4.00 | 5.00 | ||||
| Cross-validated | Count | 1.00 | 103 | 0 | 0 | 0 | 0 | 103 |
| 2.00 | 0 | 141 | 0 | 0 | 0 | 141 | ||
| 3.00 | 0 | 0 | 85 | 0 | 0 | 85 | ||
| 4.00 | 0 | 0 | 0 | 70 | 0 | 70 | ||
| 5.00 | 0 | 0 | 0 | 0 | 140 | 140 | ||
| % | 1.00 | 100.0 | .0 | .0 | .0 | .0 | 100.0 | |
| 2.00 | .0 | 100.0 | .0 | .0 | .0 | 100.0 | ||
| 3.00 | .0 | .0 | 100.0 | .0 | .0 | 100.0 | ||
| 4.00 | .0 | .0 | .0 | 100.0 | .0 | 100.0 | ||
| 5.00 | .0 | .0 | .0 | .0 | 100.0 | 100.0 | ||
| Function | Eigenvalue | % of Variance | Cumulative % | Canonical Correlation |
| 1 | 5686.972a | 99.6 | 99.6 | 1.000 |
| 2 | 17.653a | .3 | 99.9 | .973 |
| 3 | 4.491a | .1 | 100.0 | .904 |
| Function | |||
| 1 | 2 | 3 | |
| S415 | .198 | .015 | .026 |
| S480 | -.120 | -.021 | .013 |
| S515 | .015 | .021 | -.020 |
| S555 | .013 | .002 | .013 |
| (Constant) | -29.245 | -101.595 | -62.687 |
| Group | Function | ||
| 1 | 2 | 3 | |
| 1.00 | -88.279 | 1.881 | 1.775 |
| 2.00 | 8.222 | 5.715 | -3.718 |
| 3.00 | -8.922 | -6.097 | -1.206 |
| 4.00 | 115.291 | .960 | 1.770 |
| Group | Predicted Group Membership | Total | |||||
| 1.00 | 2.00 | 3.00 | 4.00 | ||||
| Cross-validated | Count | 1.00 | 100 | 0 | 0 | 0 | 100 |
| 2.00 | 0 | 55 | 0 | 0 | 55 | ||
| 3.00 | 0 | 0 | 95 | 0 | 95 | ||
| 4.00 | 0 | 0 | 0 | 80 | 80 | ||
| % | 1.00 | 100.0 | .0 | .0 | .0 | 100.0 | |
| 2.00 | .0 | 100.0 | .0 | .0 | 100.0 | ||
| 3.00 | .0 | .0 | 100.0 | .0 | 100.0 | ||
| 4.00 | .0 | .0 | .0 | 100.0 | 100.0 | ||
| Group | Predicted Group Membership | Total | ||||||
| 1.00 | 2.00 | 3.00 | 4.00 | 5.00 | ||||
| Cross-validated | Count | 1.00 | 37 | 0 | 0 | 0 | 0 | 330/499 |
| 2.00 | 97 | 249 | 61 | 11 | 0 | |||
| 3.00 | 0 | 0 | 34 | 0 | 0 | |||
| 4.00 | 0 | 0 | 0 | 10 | 0 | |||
| 5.00 | 0 | 0 | 0 | 0 | 0 | |||
| % | 1.00 | 27.61 | 0.00 | 0.00 | 0.00 | 0.00 | 66.13/100 | |
| 2.00 | 72.39 | 100.00 | 64.21 | 52.38 | 0.00 | |||
| 3.00 | 0.00 | 0.00 | 35.79 | 0.00 | 0.00 | |||
| 4.00 | 0.00 | 0.00 | 0.00 | 47.62 | 0.00 | |||
| 5.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| Group | Predicted Group Membership | Total | |||||
| 1.00 | 2.00 | 3.00 | 4.00 | ||||
| Cross-validated | Count | 1.00 | 340 | 5 | 25 | 20 | 405/500 |
| 2.00 | 0 | 15 | 20 | 10 | |||
| 3.00 | 5 | 10 | 30 | 0 | |||
| 4.00 | 0 | 0 | 0 | 20 | |||
| % | 1.00 | 98.55 | 16.67 | 33.33 | 40.00 | 81/100 | |
| 2.00 | 0.00 | 50.00 | 26.67 | 20.00 | |||
| 3.00 | 1.45 | 33.33 | 40.00 | 0.00 | |||
| 4.00 | 0.00 | 0.00 | 0.00 | 40.00 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





