Submitted:
26 March 2024
Posted:
26 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Simultaneous Monitoring of Superoxide and Intracellular Calcium Ions in Neutrophils by Chemiluminescence and Fluorescence
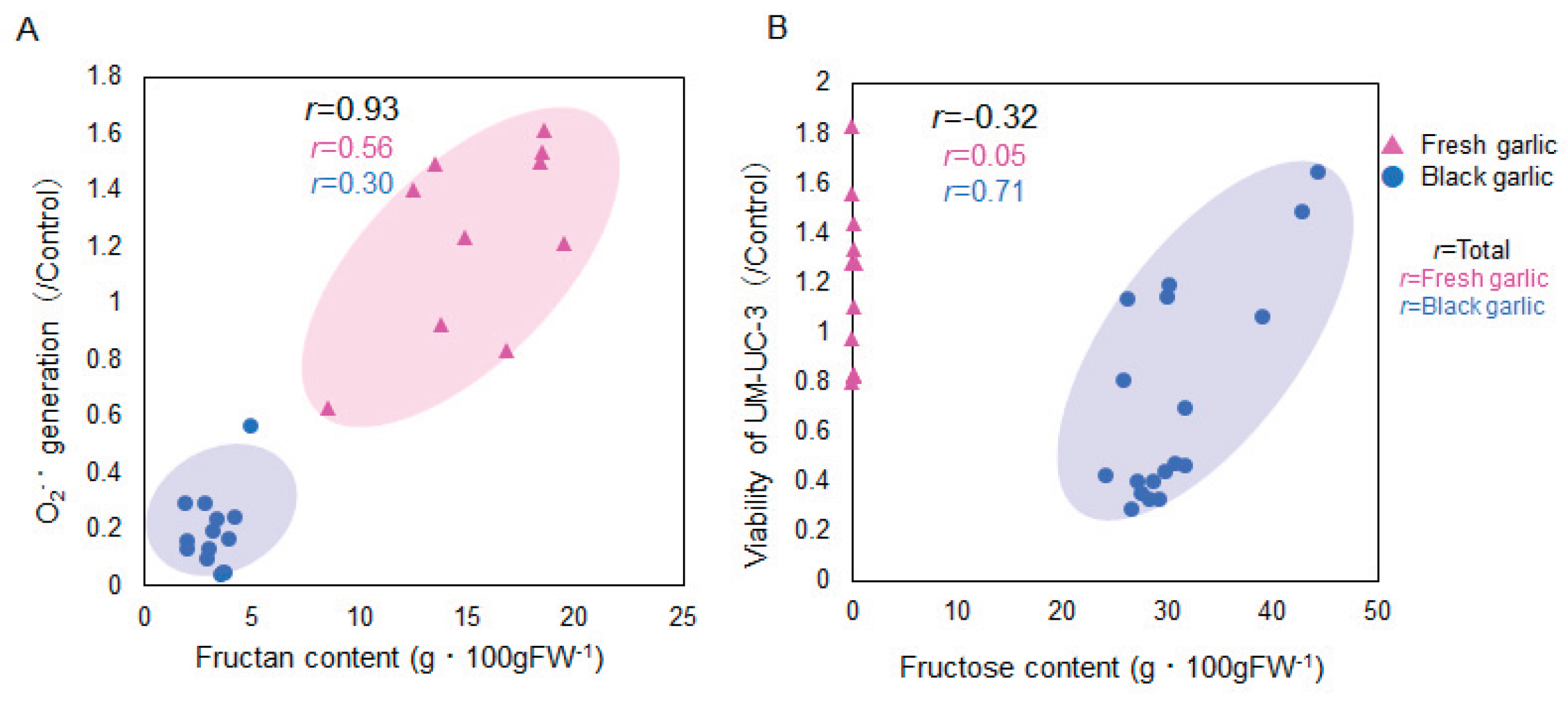
2.2. Evaluation of Antioxidant Activity
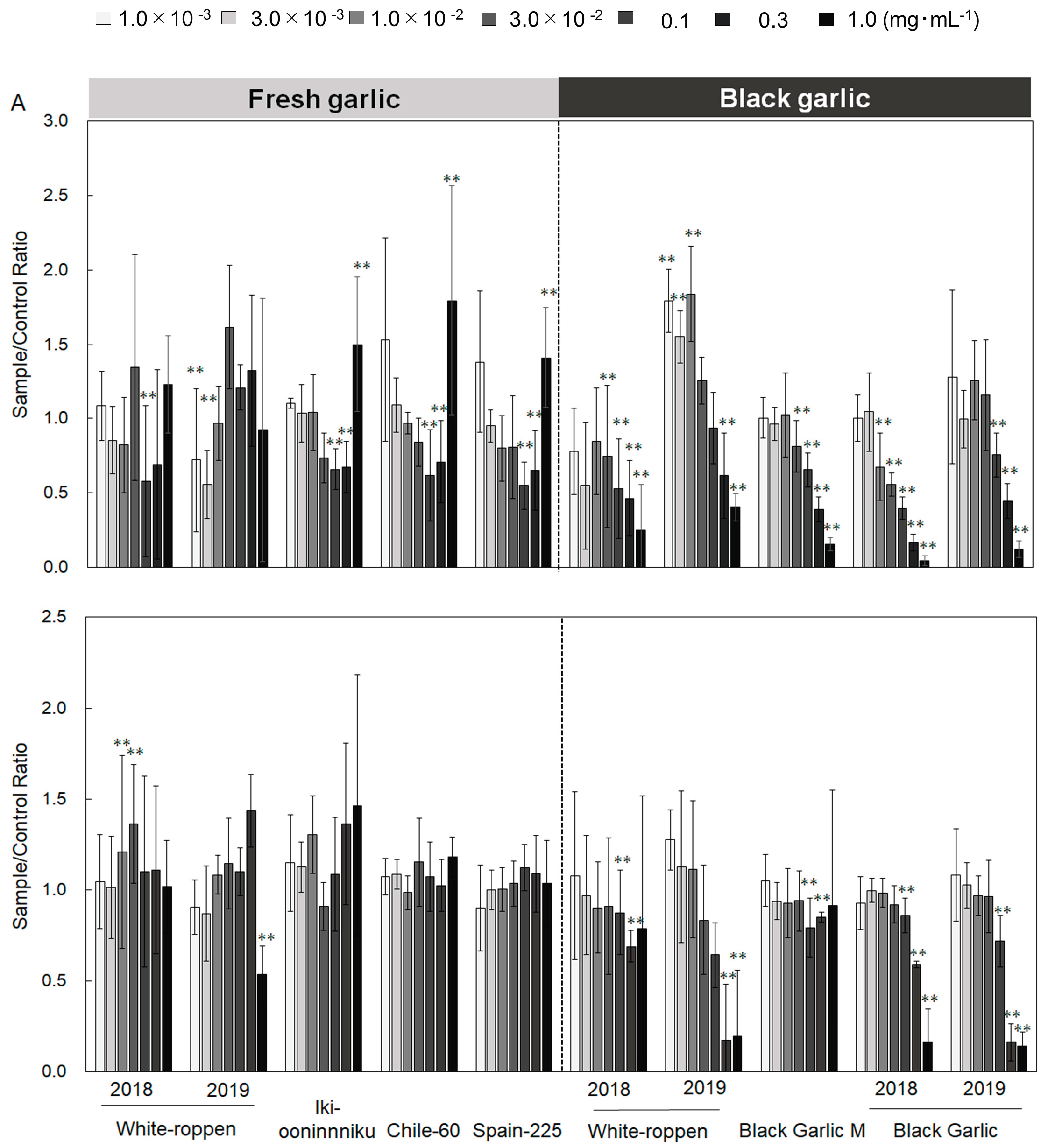
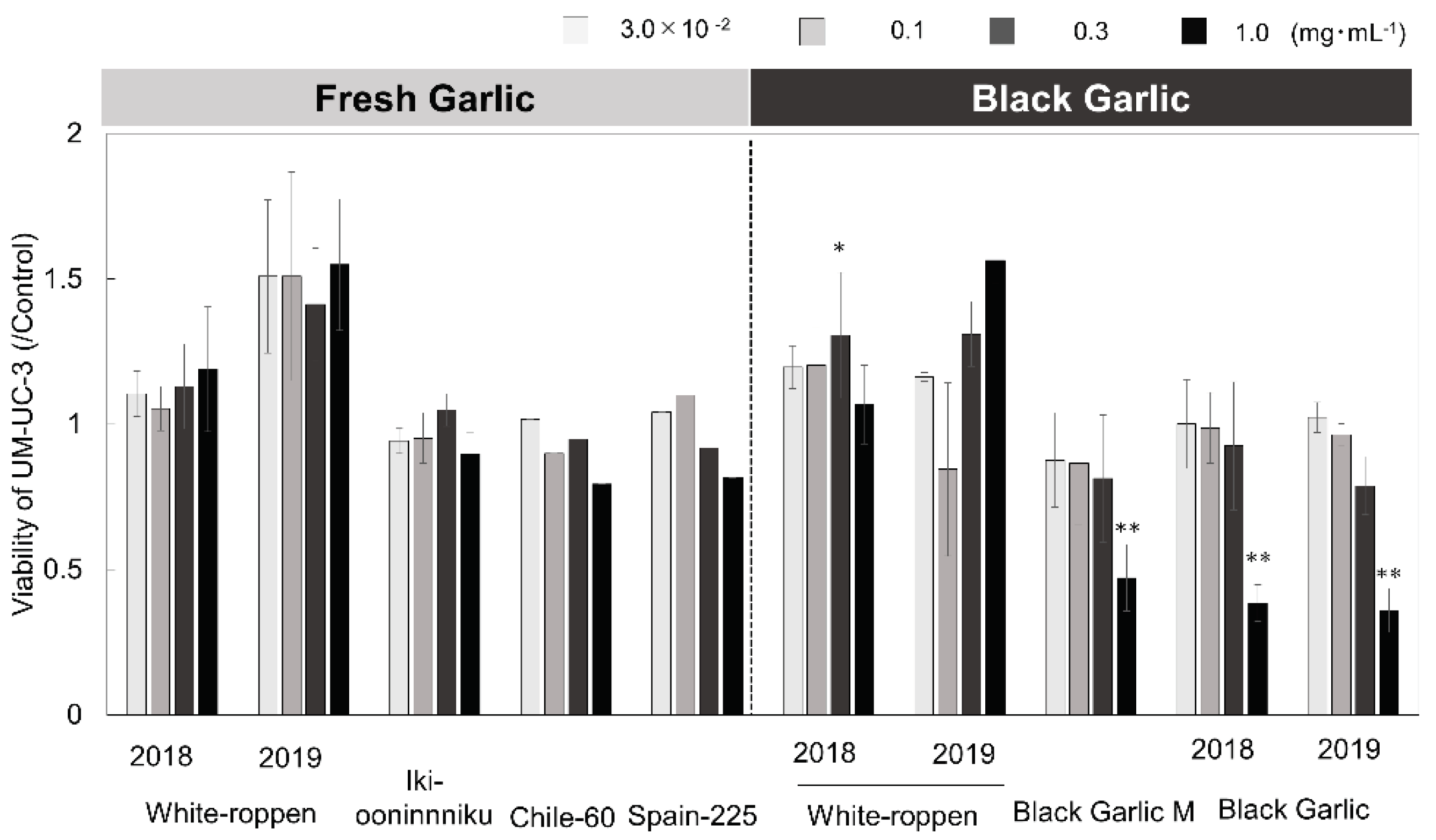
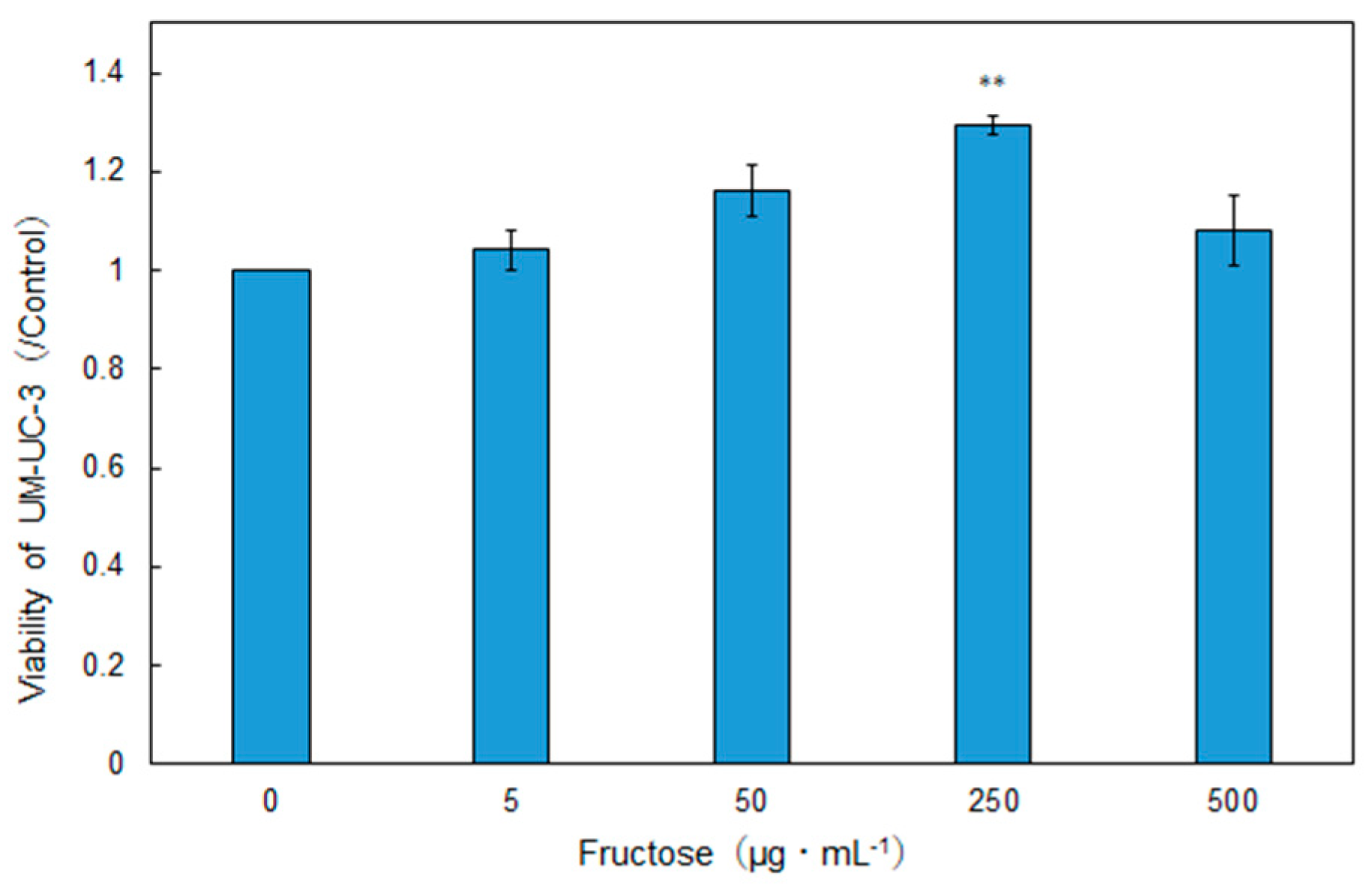
2.3. Evaluation of Anticancer Effect
2.4. Amino Acid and Sugar Contents
3. Materials and Methods
3.1. Plant Materials
3.2. Sample Preparation
3.2.1. Hot Ethanol Extraction
3.2.2. Dimethyl Sulfoxide (DMSO) Extraction
3.3. Simultaneous Evaluation for Antioxidant, Anti-Inflammatory, and Innate Immune Activation
3.4. DPPH Radical Scavenging Activity
3.5. Measurement of Cancer Cell Viability with CCK-8 Kit
3.6. Determination of Soluble Sugars (Glucose, Fructose, and Sucrose)
3.7. Determination of Fructan Content
3.8. Determination of Total Phenolic Compounds
3.9. Determination of Total Flavonoid Compounds
3.10. Determination of Amino Acid Contents
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
References
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H.B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Block, E. Garlic and Other Alliums The Lore and the Scinece; The Royal Society of Chemistry: 2010.
- Ahmed, T.; Wang, C.K. Black Garlic and Its Bioactive Compounds on Human Health Diseases: A Review. Molecules 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Kang, D. Physicochemical Properties, Biological Activity, Health Benefits, and General Limitations of Aged Black Garlic: A Review. Molecules 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, M.; Yoshida, J.; Ide, N.; Sasaoka, T.; Yamaguchi, H.; Ono, K. Tetrahydro-b-Carboline Derivatives in Aged Garlic Extract Show Antioxidant Properties. The Journal of Nutrition 2006, 136, 726S–731S. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.G.; Kang, M.J.; Hong, S.S.; Choi, Y.H.; Shin, J.H. Antiinflammatory Effects of Functionally Active Compounds Isolated from Aged Black Garlic. Phytother Res 2017, 31, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Gweon, O.C.; Seo, Y.J.; Im, J.; Kang, M.J.; Kim, M.J.; Kim, J.I. Antioxidant effect of garlic and aged black garlic in animal model of type 2 diabetes mellitus. Nutr Res Pract 2009, 3, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Battin, E.E.; Brumaghim, J.L. Antioxidant activity of sulfur and selenium: a review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochem Biophys 2009, 55, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Horie, T.; Awazu, S.; Itakura, Y.; Fuwa, T. Identified Diallyl Polysulfides from an Aged Garlic Extract which Protects the Membranes from Lipid Peroxidation. Planta Medica 1992, 58, 468–469. [Google Scholar] [CrossRef]
- Imai, J.; Ide, N.; Nagae, S.; Moriguchi, T.; Matsuura, H.; Itakura, Y. Antioxidant and Radical Scavenging Effects of Aged Garlic Extract and its Constituents. Planta Medica 1994, 60, 417–420. [Google Scholar] [CrossRef]
- Beato, V.M.; Orgaz, F.; Mansilla, F.; Montaño, A. Changes in Phenolic Compounds in Garlic (Allium sativum L.) Owing to the Cultivar and Location of Growth. Plant Foods for Human Nutrition 2011, 66, 218–223. [Google Scholar] [CrossRef]
- Evans, C.A.R.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends in Plant Science 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Kazumura, K.; Sato, Y.; Satozono, H.; Koike, T.; Tsuchiya, H.; Hiramatsu, M.; Katsumata, M.; Okazaki, S. Simultaneous monitoring of superoxides and intracellular calcium ions in neutrophils by chemiluminescence and fluorescence: evaluation of action mechanisms of bioactive compounds in foods. J Pharm Biomed Anal 2013, 84, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yan, Y.X.; Yu, Q.T.; Deng, Y.; Wu, D.T.; Wang, Y.; Ge, Y.Z.; Li, S.P.; Zhao, J. Comparison of Immunomodulatory Effects of Fresh Garlic and Black Garlic Polysaccharides on RAW 264.7 Macrophages. J Food Sci 2017, 82, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Sunanta, P.; Chung, H.H.; Kunasakdakul, K.; Ruksiriwanich, W.; Jantrawut, P.; Hongsibsong, S.; Sommano, S.R. Genomic relationship and physiochemical properties among raw materials used for Thai black garlic processing. Food Sci Nutr 2020, 8, 4534–4545. [Google Scholar] [CrossRef] [PubMed]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. Journal of Agricultural and Food Chemistry 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Kazumura, K.; Sato, Y.; Satozono, H.; Mochizuki, M.; Wu, X.; Tsuchiya, H.; Koike, T.; Okazaki, S.; Osawa, T. Health progress for human on Anthocyan assessed by new evaluation method using the innate immune response of neutrophils In Proceedings (online) of the Annual Meeting 2013 Sendai of Japan Society for Bioscience, Biotechnology, and Agrochemistry, Japan Society for Bioscience, Biotechnology, and Agrochemistry: 2013b; p 1513.
- Hirano; Kasai. Oxidative stress and cancer. Bio Clinica 1998, 13, 51–54. [Google Scholar]
- Sigounas, G.; Hooker, J.L.; Wei Li, A.; Anagnostou; Steiner, M. S-Allylmercaptocysteine, a stable thioallyl compound, induces apoptosis in erythroleukemia cell lines. Nutrition and Cancer 1997, 28, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Dirsch, V.M.; Gerbes, A.L.; Vollmar, A.M. Ajoene, a Compound of Garlic, Induces Apoptosis in Human Promyeloleukemic Cells, Accompanied by Generation of Reactive Oxygen Species and Activation of Nuclear Factor κB. Molecular Pharmacology 1998, 53, 402–407. [Google Scholar] [CrossRef]
- Liu, H.; Huang, D.; McArthur, D.L.; Boros, L.G.; Nissen, N.; Heaney, A.P. Fructose induces transketolase flux to promote pancreatic cancer growth. Cancer Res 2010, 70, 6368–6376. [Google Scholar] [CrossRef]
- Ryu, K.; Ide, N.; Matsuura, H.; Itakura, Y. Nα-(1-Deoxy-D-fructos-1-yl)-L-Arginine, an Antioxidant Compound Identified in Aged Garlic Extract The Journal of Nutrition 2001, 131, 972S–976S. [CrossRef]
- Saito, H. ニンニクの化学 (Garlic science); Asakura Publishing Co., Ltd: 2008.
- Yuan, H.; Sun, L.; Chen, M.; Wang, J. The Comparison of the Contents of Sugar, Amadori, and Heyns Compounds in Fresh and Black Garlic. J Food Sci 2016, 81, C1662–C1668. [Google Scholar] [CrossRef] [PubMed]
- Etoh, T. Studies on the sterility in garlic, Allium sativum L. Memoirs of the Faculty of Agriculture, Kagoshima University 1985, 21, 77–132. [Google Scholar]
- Hirata, S.; Abdelrahman, M.; Yamauchi, N.; Shigyo, M. Diversity evaluation based on morphological, physiological and isozyme variation in genetic resources of garlic (Allium sativum L.) collected worldwide. Genes and Genetic Systems, 2016; 91, 161–173. [Google Scholar]
- Kondo, S.; Tsuda, K.; Muto, N.; Nakatani, S. Changes in Antioxidant Activitu during Fruit Development in Citrus Fruit. Hort.Res.(Japan) 2002, 1, 63–66. [Google Scholar] [CrossRef]
- Percheron, F. Dosage colorimetrique du fructose et des fructo-franosides par l` acide thiobarbiturique. C.R.Acad.Sci 1962, 255, 2521–2522. [Google Scholar]
- Folin, O.; Denis, W. A Colorimetric Method for the Determination of Phenols (and Phenol Derivatives) in Urine. Journal of Biological Chemistry 1915, 22, 305–308. [Google Scholar] [CrossRef]
- Vu, Q.H.; Hang, T.T.M.; Yaguchi, S.; Ono, Y.; Pham, T.M.P.; Yamauchi, N.; Shigyo, M. Assessment of biochemical and antioxidant diversities in a shallot germplasm collection from Vietnam and its surrounding countries. Genetic Resources and Crop Evolution 2013, 60, 1297–1312. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013, 48, 452–458. [Google Scholar] [CrossRef]

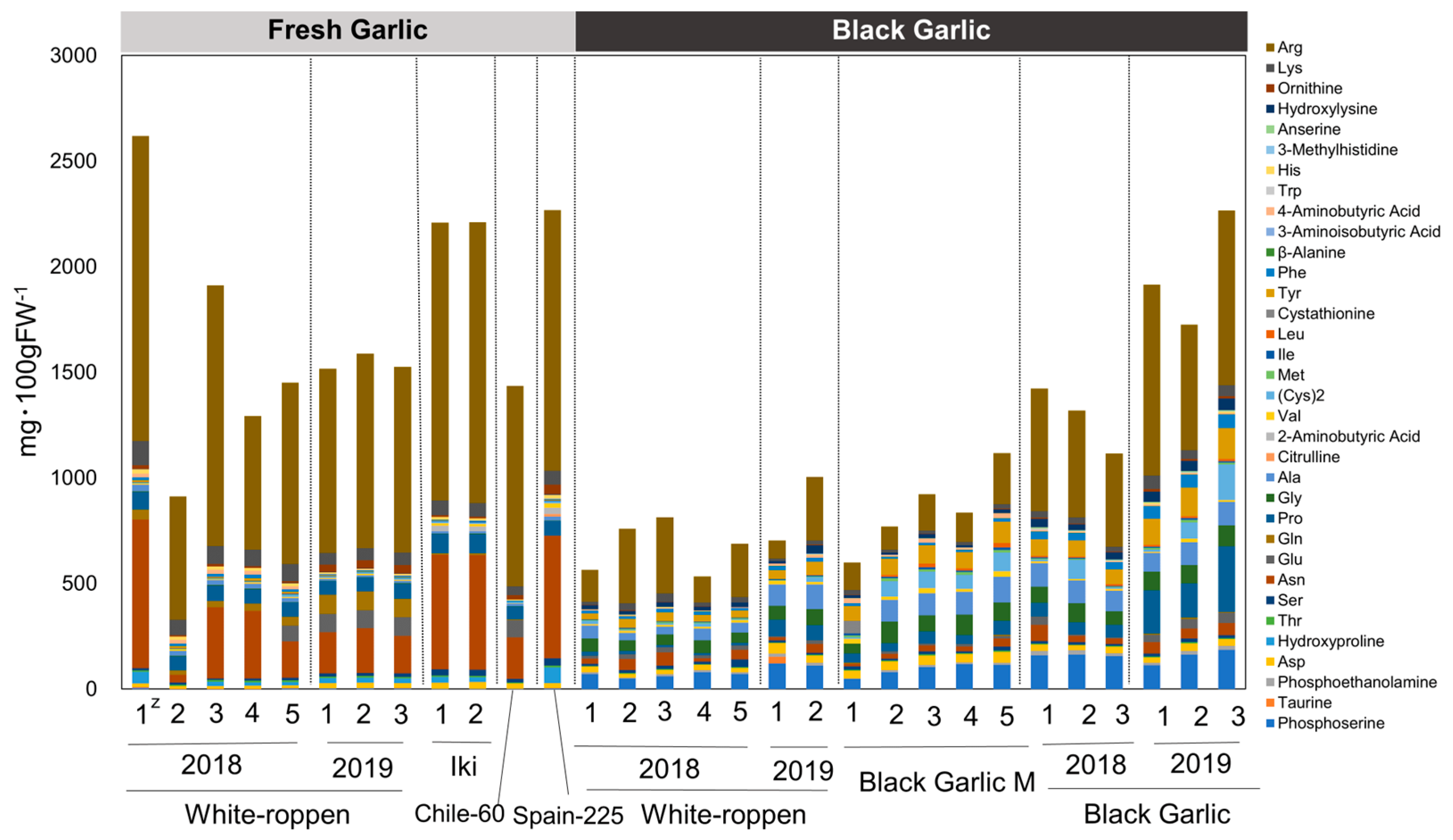
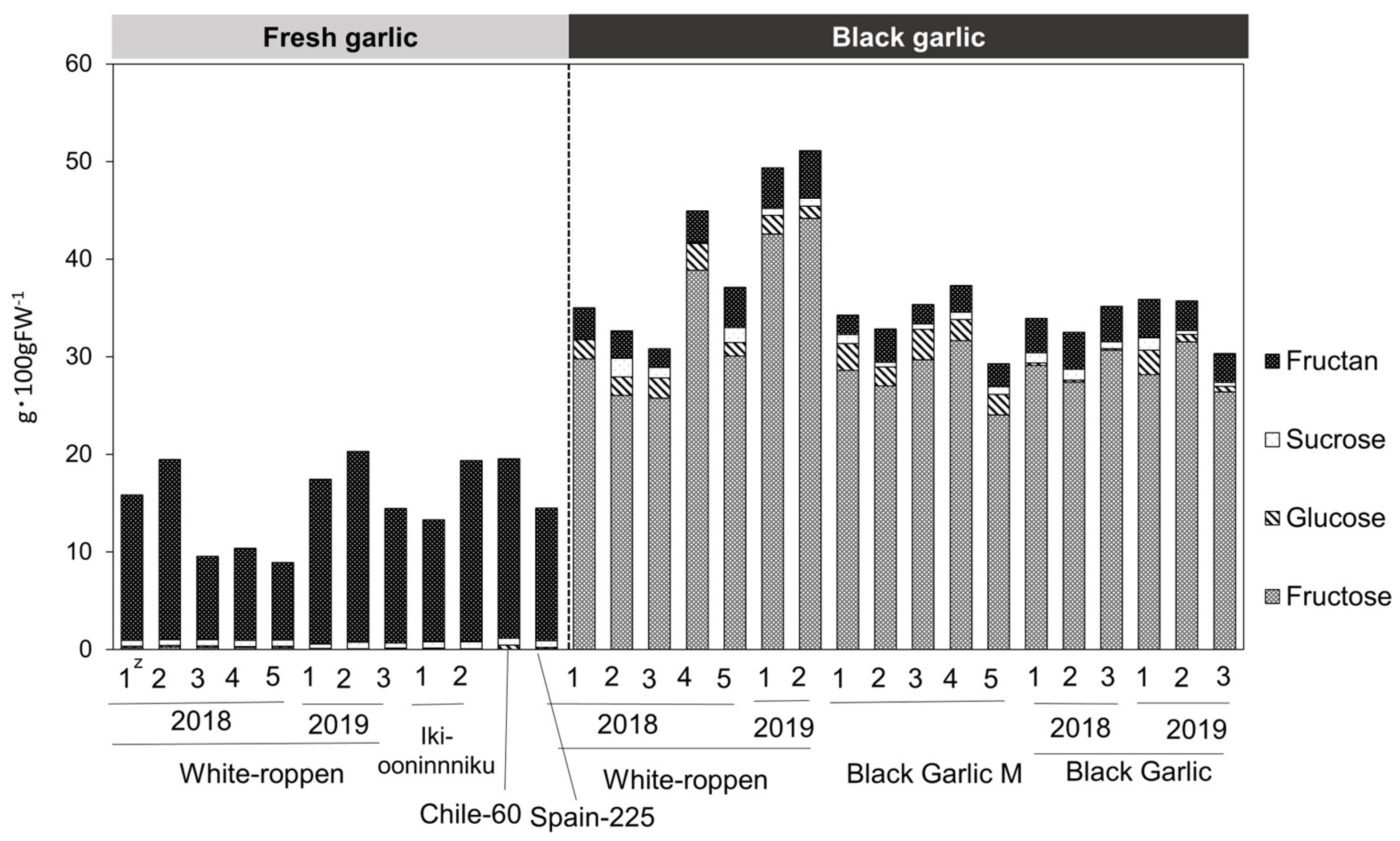
| Materials | Number of bulbs | Collected site |
Accession information | |
|---|---|---|---|---|
| Fresh garlic | ‘White-roppen’ 2018 | 5 | Japanese local market | - |
| ‘White-roppen’ 2019 | 3 | Japanese local market | - | |
| ‘Iki-ooninnniku’ | 2 | Saga University, Japan | Hirata et al. (2016b) | |
| ‘Chile-60’ | 1 | Chile | Etoh (1985) | |
| ‘Spain-225’ | 1 | Spain | Hirata et al. (2016b) | |
| Black garlic | ‘White-roppen’ 2018 | 5 | Processed White-roppen 2018 | - |
| ‘White-roppen’ 2019 | 2 | Processed White-roppen 2019 | - | |
| Black Garlic M | 5 | Japanese local market | - | |
| Black Garlic 2018 | 3 | Indonesian local market | - | |
| Black Garlic 2019 | 3 | Indonesian local market | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





