Submitted:
26 March 2024
Posted:
26 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Bone Marrow Extraction and Product Preparation
2.4. BM-MSC Transplantation and Sampling
2.5. Immunological Factor Estimation
2.6. Biochemical Evaluation
2.7. Outcomes
2.8. Statistical Analysis
3. Results
3.1. Patients' Characterization and Safety Evaluation
3.2. Immunological Assessment
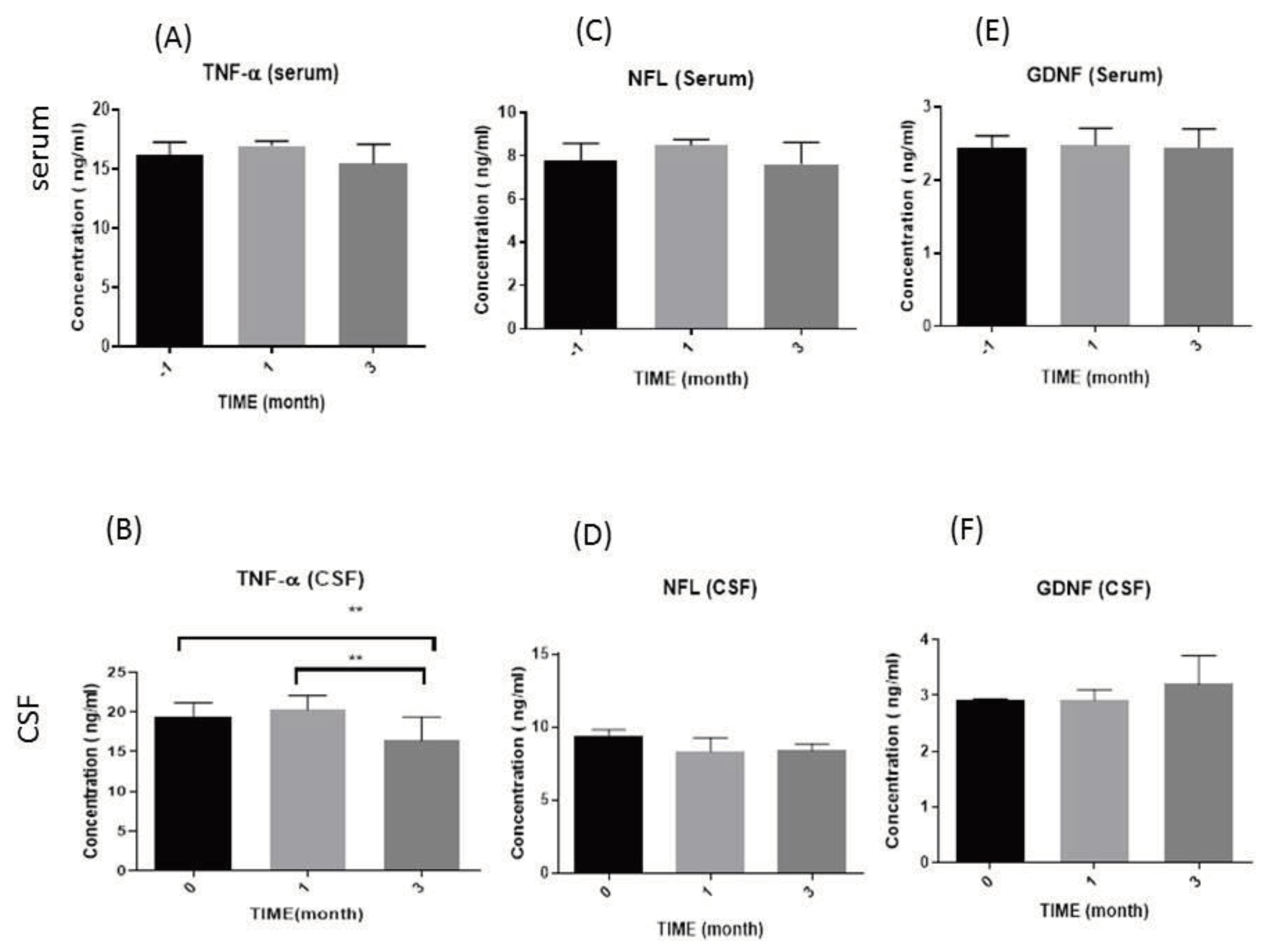
3.2.1. Tumor Necrosis Factor-Alpha (TNF-α) Levels
3.2.2. Neurofilament Light Chain (NFL) Levels
3.2.3. Glial cell-derived neurotrophic factor (GDNF) levels
3.3. Biochemical Evaluation
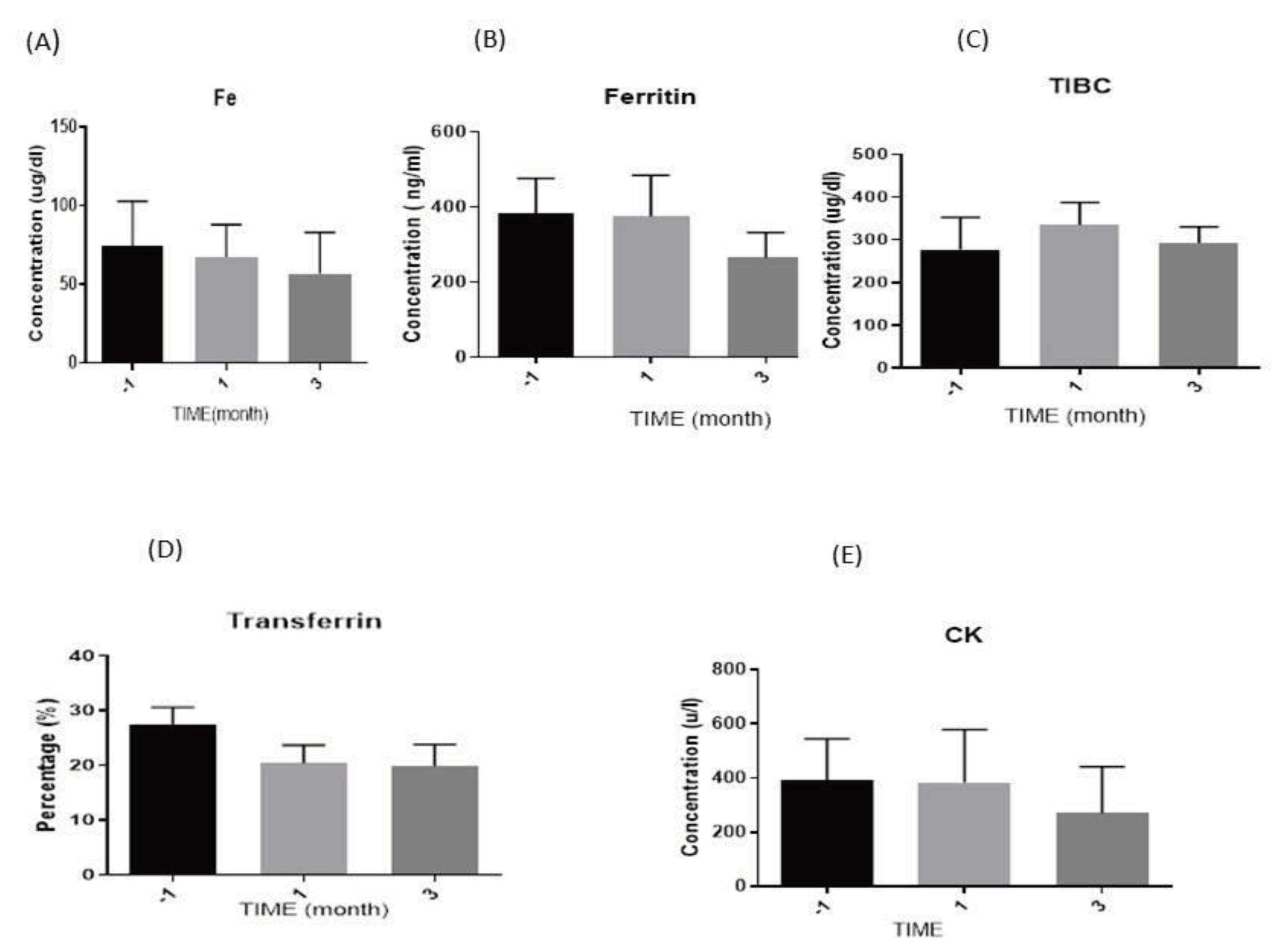
3.3.1. Iron (Fe) Levels
3.3.2. Ferritin Levels
3.3.3. Total Iron Binding Capacity (TIBC) Levels
3.3.4. Transferrin Levels
3.3.5. Creatine Kinase (CK) Levels
3.4. Correlation between Biochemical and Immunological Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, R.H.; Al-Chalabi, A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Goutman, S.A.; Petri, S.; Mazzini, L.; Savelieff, M.G.; Shaw, P.J.; Sobue, G. Amyotrophic Lateral Sclerosis. Lancet 2022, 400, 1363–1380. [Google Scholar] [CrossRef] [PubMed]
- Mazzini, L.; Vescovi, A.; Cantello, R.; Gelati, M.; Vercelli, A. Stem Cells Therapy for ALS. Expert Opin. Biol. Ther. 2016, 16, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Wijesekera, L.C.; Nigel Leigh, P. Amyotrophic Lateral Sclerosis. Orphanet J. Rare Dis. 2009, 4, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Béland, L.-C.; Markovinovic, A.; Jakovac, H.; De Marchi, F.; Bilic, E.; Mazzini, L.; Kriz, J.; Munitic, I. Immunity in Amyotrophic Lateral Sclerosis: Blurred Lines between Excessive Inflammation and Inefficient Immune Responses. Brain Commun. 2020, 2, fcaa124. [Google Scholar] [CrossRef] [PubMed]
- Czaplinski, A.; Haverkamp, L.J.; Yen, A.A.; Simpson, E.P.; Lai, E.C.; Appel, S.H. The Value of Database Controls in Pilot or Futility Studies in ALS. Neurology 2006, 67, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System during Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441. [Google Scholar] [CrossRef] [PubMed]
- Olmos, G.; Lladó, J. Tumor Necrosis Factor Alpha: A Link between Neuroinflammation and Excitotoxicity. Mediators Inflamm. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Rao, M.V.; Nixon, R.A. Neurofilaments and Neurofilament Proteins in Health and Disease. Cold Spring Harb. Perspect. Biol. 2017, 9, a018309. [Google Scholar] [CrossRef]
- Sainio, M.T.; Rasila, T.; Molchanova, S.M.; Järvilehto, J.; Torregrosa-Muñumer, R.; Harjuhaahto, S.; Pennonen, J.; Huber, N.; Herukka, S.-K.; Haapasalo, A. Neurofilament Light Regulates Axon Caliber, Synaptic Activity, and Organelle Trafficking in Cultured Human Motor Neurons. Front. cell Dev. Biol. 2022, 9, 820105. [Google Scholar] [CrossRef]
- Barua, S.; Pathak, Y.V. Unilateral Ex Vivo Gene Therapy by GDNF in Neurodegenerative Diseases. In Gene Delivery Systems; CRC Press, 2022; pp. 155–161 ISBN 1003186068.
- Hellmich, H.L.; Kos, L.; Cho, E.S.; Mahon, K.A.; Zimmer, A. Embryonic Expression of Glial Cell-Line Derived Neurotrophic Factor (GDNF) Suggests Multiple Developmental Roles in Neural Differentiation and Epithelial-Mesenchymal Interactions. Mech. Dev. 1996, 54, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Haney, M.J.; Jin, Y.S.; Uvarov, O.; Vinod, N.; Lee, Y.Z.; Langworthy, B.; Fine, J.P.; Rodriguez, M.; El-Hage, N. GDNF-Expressing Macrophages Restore Motor Functions at a Severe Late-Stage, and Produce Long-Term Neuroprotective Effects at an Early-Stage of Parkinson’s Disease in Transgenic Parkin Q311X (A) Mice. J. Control. Release 2019, 315, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Kokić, A.N.; Stević, Z.; Stojanović, S.; Blagojević, D.P.; Jones, D.R.; Pavlović, S.; Niketić, V.; Apostolski, S.; Spasić, M.B. Biotransformation of Nitric Oxide in the Cerebrospinal Fluid of Amyotrophic Lateral Sclerosis Patients. Redox Rep. 2005, 10, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Halon, M.; Kaczor, J.J.; Ziolkowski, W.; Flis, D.J.; Borkowska, A.; Popowska, U.; Nyka, W.; Wozniak, M.; Antosiewicz, J. Changes in Skeletal Muscle Iron Metabolism Outpace Amyotrophic Lateral Sclerosis Onset in Transgenic Rats Bearing the G93A HmSOD1 Gene Mutation. Free Radic. Res. 2014, 48, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-P.; Wei, Q.-Q.; Ou, R.-W.; Hou, Y.-B.; Zhang, L.-Y.; Yuan, X.-Q.; Yao, Y.-Q.; Jia, D.-S.; Zhang, Q.; Li, W.-X. Creatine Kinase in the Diagnosis and Prognostic Prediction of Amyotrophic Lateral Sclerosis: A Retrospective Case-Control Study. Neural Regen. Res. 2021, 16, 591. [Google Scholar] [CrossRef] [PubMed]
- Tavakol-Afshari, J.; Boroumand, A.R.; Farkhad, N.K.; Moghadam, A.A.; Sahab-Negah, S.; Gorji, A. Safety and Efficacy of Bone Marrow Derived-Mesenchymal Stem Cells Transplantation in Patients with Amyotrophic Lateral Sclerosis. Regen. Ther. 2021, 18, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. El Escorial Revisited: Revised Criteria for the Diagnosis of Amyotrophic Lateral Sclerosis. Amyotroph. lateral Scler. other Mot. neuron Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Faruqi, A.; Mukkamalla, S.K.R. Iron Binding Capacity. 2020.
- Koerper, M.A.; Dallman, P.R. Serum Iron Concentration and Transferrin Saturation in the Diagnosis of Iron Deficiency in Children: Normal Developmental Changes. J. Pediatr. 1977, 91, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Colpo, G.D.; Ascoli, B.M.; Wollenhaupt-Aguiar, B.; Pfaffenseller, B.; Silva, E.G.; Cirne-Lima, E.O.; Quevedo, J.; Kapczinski, F.; Rosa, A.R. Mesenchymal Stem Cells for the Treatment of Neurodegenerative and Psychiatric Disorders. An. Acad. Bras. Cienc. 2015, 87, 1435–1449. [Google Scholar] [CrossRef]
- Rufino, R.A.; Pereira-Rufino, L.D.S.; Vissoto, T.C.S.; Kerkis, I.; Neves, A.D.C.; da Silva, M.C.P. The Immunomodulatory Potential Role of Mesenchymal Stem Cells in Diseases of the Central Nervous System. Neurodegener. Dis. 2022. [Google Scholar] [CrossRef]
- Cui, G.; Guo, H.; Li, H.; Zhai, Y.; Gong, Z.; Wu, J.; Liu, J.; Dong, Y.; Hou, S.; Liu, J. RVG-Modified Exosomes Derived from Mesenchymal Stem Cells Rescue Memory Deficits by Regulating Inflammatory Responses in a Mouse Model of Alzheimer’s Disease. Immun. Ageing 2019, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Homem, C.C.F.; Repic, M.; Knoblich, J.A. Proliferation Control in Neural Stem and Progenitor Cells. Nat. Rev. Neurosci. 2015, 16, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, H.; Wang, Y.; Gu, G.; Zhang, W.; Xia, R. Neural Stem Cell Transplantation Decreases Neuroinflammation in a Transgenic Mouse Model of Alzheimer’s Disease. J. Neurochem. 2016, 136, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-H.; Allen, K.; Oei, F.; Leoni, E.; Kuhle, J.; Tree, T.; Fratta, P.; Sharma, N.; Sidle, K.; Howard, R. Systemic Inflammatory Response and Neuromuscular Involvement in Amyotrophic Lateral Sclerosis. Neurol. Neuroinflammation 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Cudkowicz, M.E.; Lindborg, S.R.; Goyal, N.A.; Miller, R.G.; Burford, M.J.; Berry, J.D.; Nicholson, K.A.; Mozaffar, T.; Katz, J.S.; Jenkins, L.J. A Randomized Placebo-controlled Phase 3 Study of Mesenchymal Stem Cells Induced to Secrete High Levels of Neurotrophic Factors in Amyotrophic Lateral Sclerosis. Muscle Nerve 2022, 65, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.D.; Cudkowicz, M.E.; Windebank, A.J.; Staff, N.P.; Owegi, M.; Nicholson, K.; McKenna-Yasek, D.; Levy, Y.S.; Abramov, N.; Kaspi, H. NurOwn, Phase 2, Randomized, Clinical Trial in Patients with ALS: Safety, Clinical, and Biomarker Results. Neurology 2019, 93, e2294–e2305. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, Y.; Dong, S.; Yang, W.; Qian, T.; Liu, X.; Cheng, Q.; Wang, J.; Chen, X. Role of Blood Neurofilaments in the Prognosis of Amyotrophic Lateral Sclerosis: A Meta-Analysis. Front. Neurol. 2021, 1731. [Google Scholar] [CrossRef] [PubMed]
- Feneberg, E.; Oeckl, P.; Steinacker, P.; Verde, F.; Barro, C.; Van Damme, P.; Gray, E.; Grosskreutz, J.; Jardel, C.; Kuhle, J. Multicenter Evaluation of Neurofilaments in Early Symptom Onset Amyotrophic Lateral Sclerosis. Neurology 2018, 90, e22–e30. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, X.; Li, S.; Yang, F.; Wang, H.; Cui, F.; Huang, X. CSF Neurofilament Light Chain Elevation Predicts ALS Severity and Progression. Front. Neurol. 2020, 11, 919. [Google Scholar] [CrossRef]
- Cohen, J.A.; Lublin, F.D.; Lock, C.; Pelletier, D.; Chitnis, T.; Mehra, M.; Gothelf, Y.; Aricha, R.; Lindborg, S.; Lebovits, C. Evaluation of Neurotrophic Factor Secreting Mesenchymal Stem Cells in Progressive Multiple Sclerosis. Mult. Scler. J. 2023, 29, 92–106. [Google Scholar] [CrossRef]
- Petrou, P.; Kassis, I.; Levin, N.; Paul, F.; Backner, Y.; Benoliel, T.; Oertel, F.C.; Scheel, M.; Hallimi, M.; Yaghmour, N. Beneficial Effects of Autologous Mesenchymal Stem Cell Transplantation in Active Progressive Multiple Sclerosis. Brain 2020, 143, 3574–3588. [Google Scholar] [CrossRef] [PubMed]
- Cabanes, C.; Bonilla, S.; Tabares, L.; Martínez, S. Neuroprotective Effect of Adult Hematopoietic Stem Cells in a Mouse Model of Motoneuron Degeneration. Neurobiol. Dis. 2007, 26, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Monsour, M.; Garbuzova-Davis, S.; Borlongan, C. V Patching Up the Permeability: The Role of Stem Cells in Lessening Neurovascular Damage in Amyotrophic Lateral Sclerosis. Stem Cells Transl. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, Y.; Shang, H. Aberrations of Biochemical Indicators in Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. Transl. Neurodegener. 2021, 10, 1–12. [Google Scholar] [CrossRef]
- Mitchell, R.M.; Simmons, Z.; Beard, J.L.; Stephens, H.E.; Connor, J.R. Plasma Biomarkers Associated with ALS and Their Relationship to Iron Homeostasis. Muscle Nerve 2010, 42, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, I.; Hasegawa, T.; Honda, A.; Ozawa, K.; Hayashi, Y.; Hashimoto, K.; Yamada, M.; Koumura, A.; Sakurai, T.; Kimura, A. Patterns of Levels of Biological Metals in CSF Differ among Neurodegenerative Diseases. J. Neurol. Sci. 2011, 303, 95–99. [Google Scholar] [CrossRef]
- Moghadam, M.S.; Azimian, H.; Afshari, J.T.; Toossi, M.T.B.; Farkhad, N.K.; Aghaee-Bakhtiari, S.H. Chromosomal Instability in Various Generations of Human Mesenchymal Stem Cells Following the Therapeutic Radiation Doses. 2023. [Google Scholar] [CrossRef]
- Devos, D.; Moreau, C.; Kyheng, M.; Garçon, G.; Rolland, A.S.; Blasco, H.; Gelé, P.; Timothée Lenglet, T.; Veyrat-Durebex, C.; Corcia, P. A Ferroptosis–Based Panel of Prognostic Biomarkers for Amyotrophic Lateral Sclerosis. Sci. Rep. 2019, 9, 2918. [Google Scholar] [CrossRef]
- Goodall, E.F.; Haque, M.S.; Morrison, K.E. Increased Serum Ferritin Levels in Amyotrophic Lateral Sclerosis (ALS) Patients. J. Neurol. 2008, 255, 1652–1656. [Google Scholar] [CrossRef]
- Hertel, N.; Kuzma-Kozakiewicz, M.; Gromicho, M.; Grosskreutz, J.; de Carvalho, M.; Uysal, H.; Dengler, R.; Petri, S.; Körner, S. Analysis of Routine Blood Parameters in Patients with Amyotrophic Lateral Sclerosis and Evaluation of a Possible Correlation with Disease Progression—a Multicenter Study. Front. Neurol. 2022, 13. [Google Scholar] [CrossRef]
- Sheykhansari, S.; Kozielski, K.; Bill, J.; Sitti, M.; Gemmati, D.; Zamboni, P.; Singh, A.V. Redox Metals Homeostasis in Multiple Sclerosis and Amyotrophic Lateral Sclerosis: A Review. Cell Death Dis. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rathore, K.I.; Redensek, A.; David, S. Iron Homeostasis in Astrocytes and Microglia Is Differentially Regulated by TNF-α and TGF-β1. Glia 2012, 60, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, T.; Cheng, Y.; Wu, Y.; Zhu, L.; Gu, Z.; Wu, Y.; Cai, L.; Wu, Y.; Zhang, Y. Melatonin Ameliorates Neurological Deficits through MT2/IL-33/Ferritin H Signaling-Mediated Inhibition of Neuroinflammation and Ferroptosis after Traumatic Brain Injury. Free Radic. Biol. Med. 2023, 199, 97–112. [Google Scholar] [CrossRef] [PubMed]
| Biochemical parameters | Immunological parameters | ||
|---|---|---|---|
| NFL(ng/ml) | TNF-α (ng/ml) | GDNF(ng/ml) | |
| Ferritin (ng/ml) | ...... | -(0.52*) | -(0.48*) |
| Fe (µg/dl) | 0.48* | ...... | ...... |
| CK (u/l) | 0.44* | ...... | ...... |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





