Submitted:
26 March 2024
Posted:
26 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
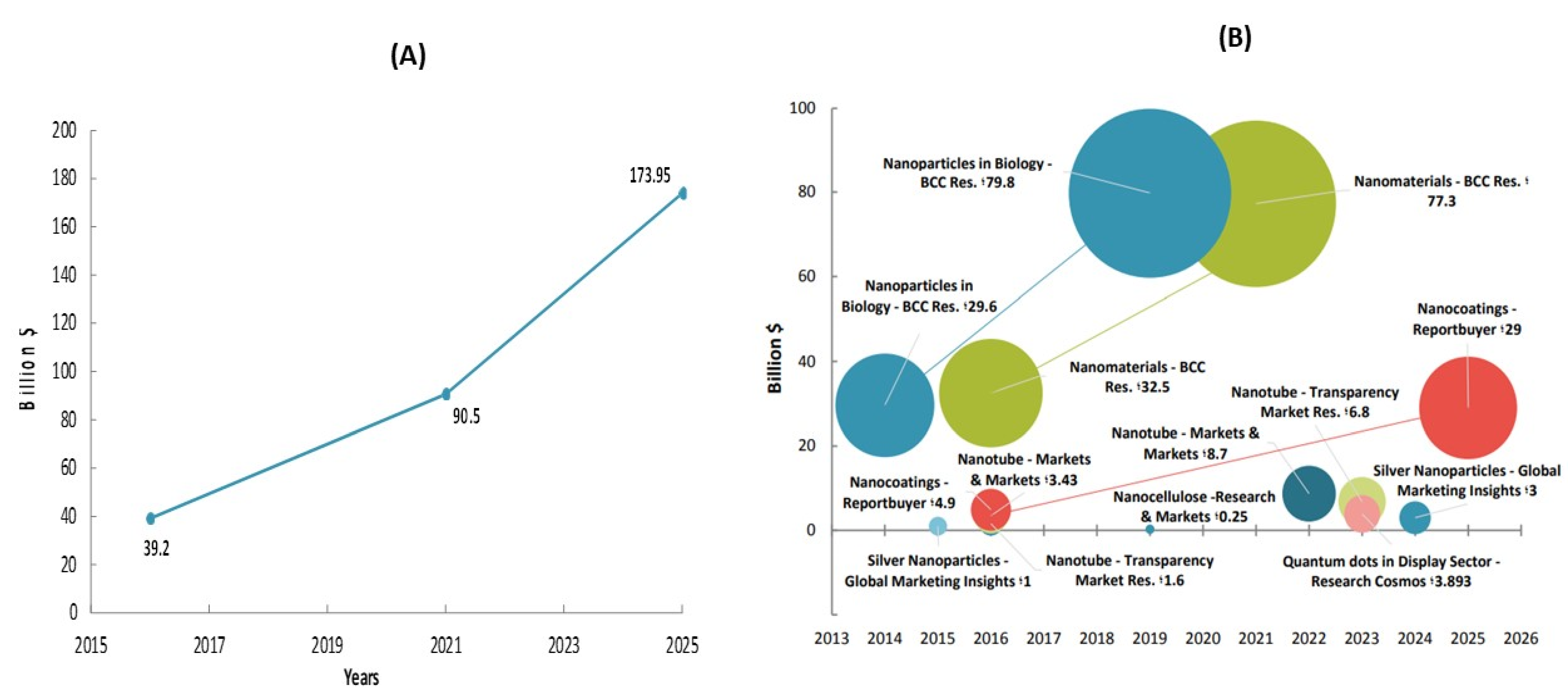
2. Materials and Methods
3. Results and Discussion
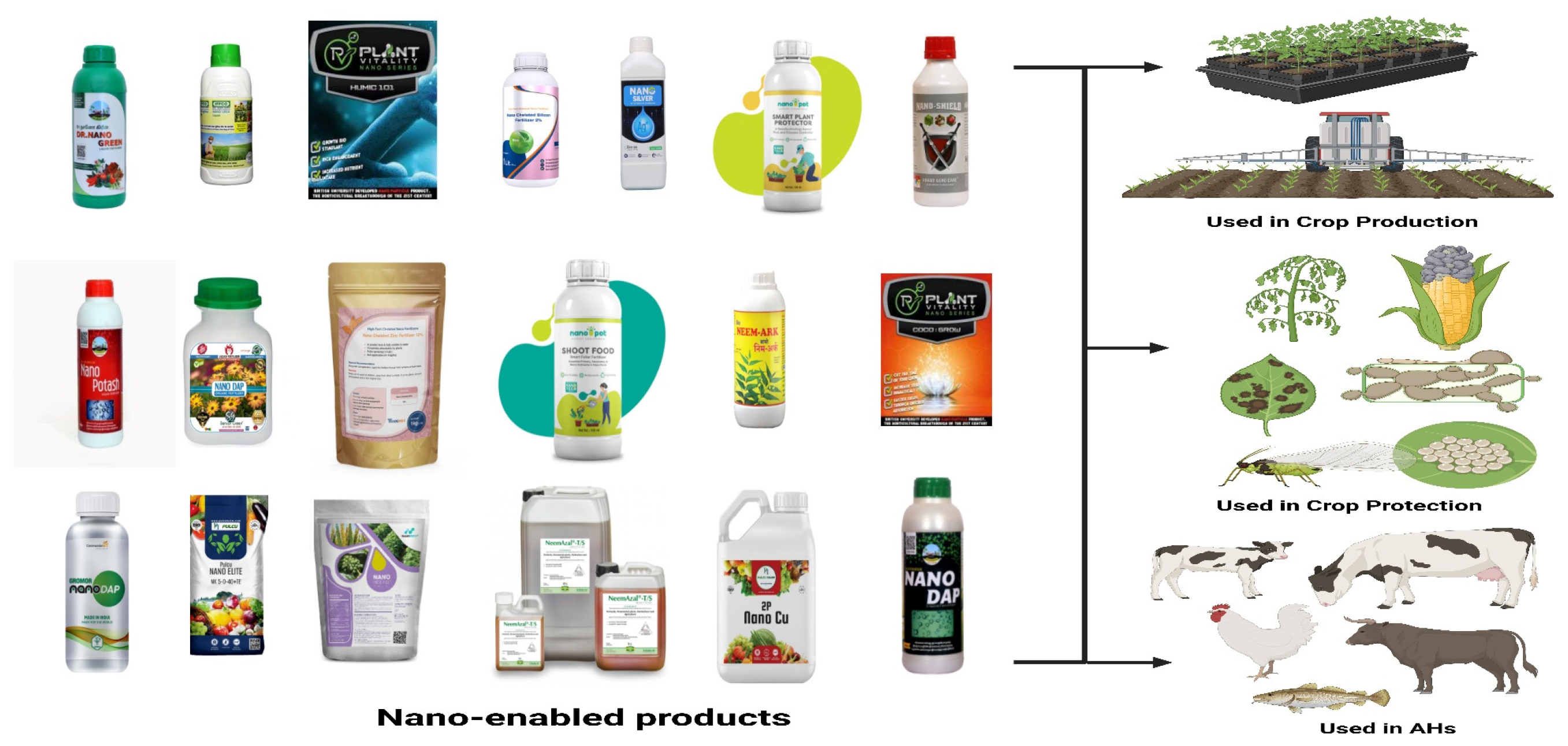
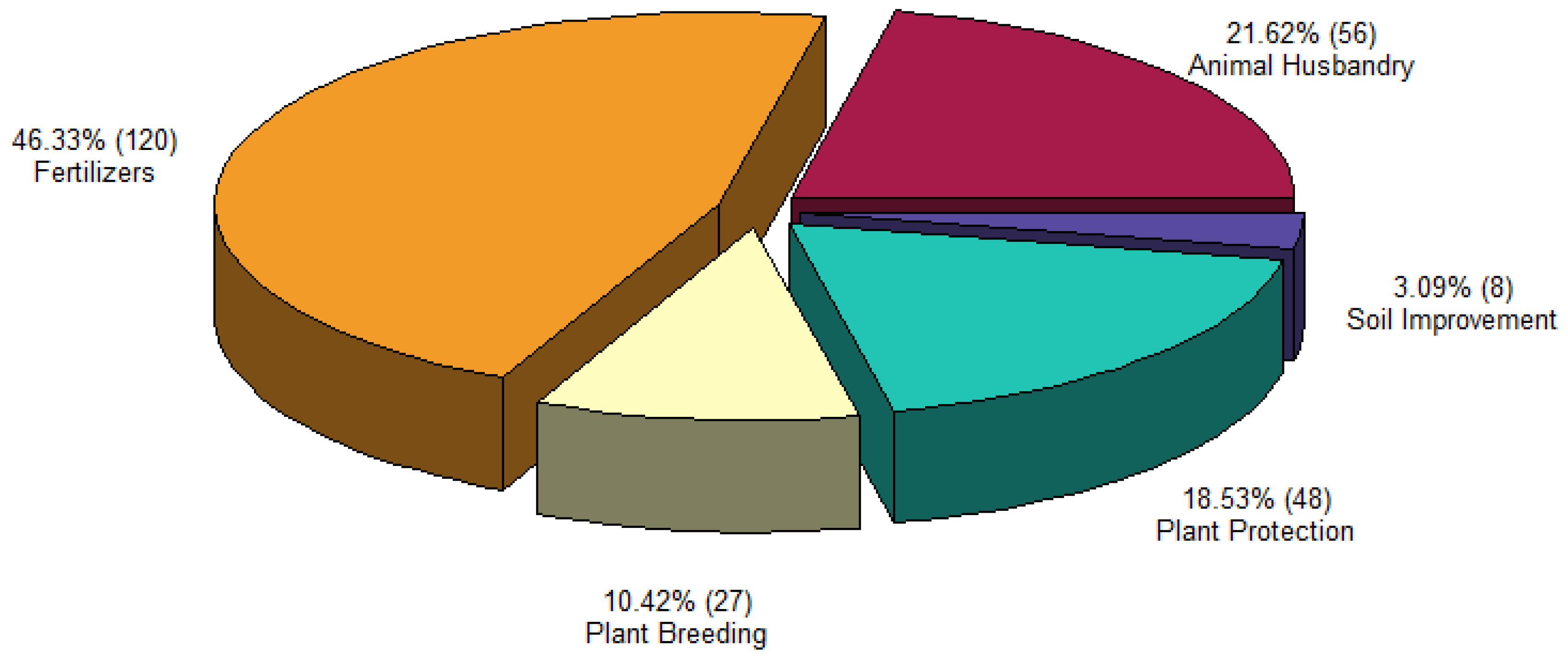
3.1. NPs in Agriculture and Animal Husbandry Products
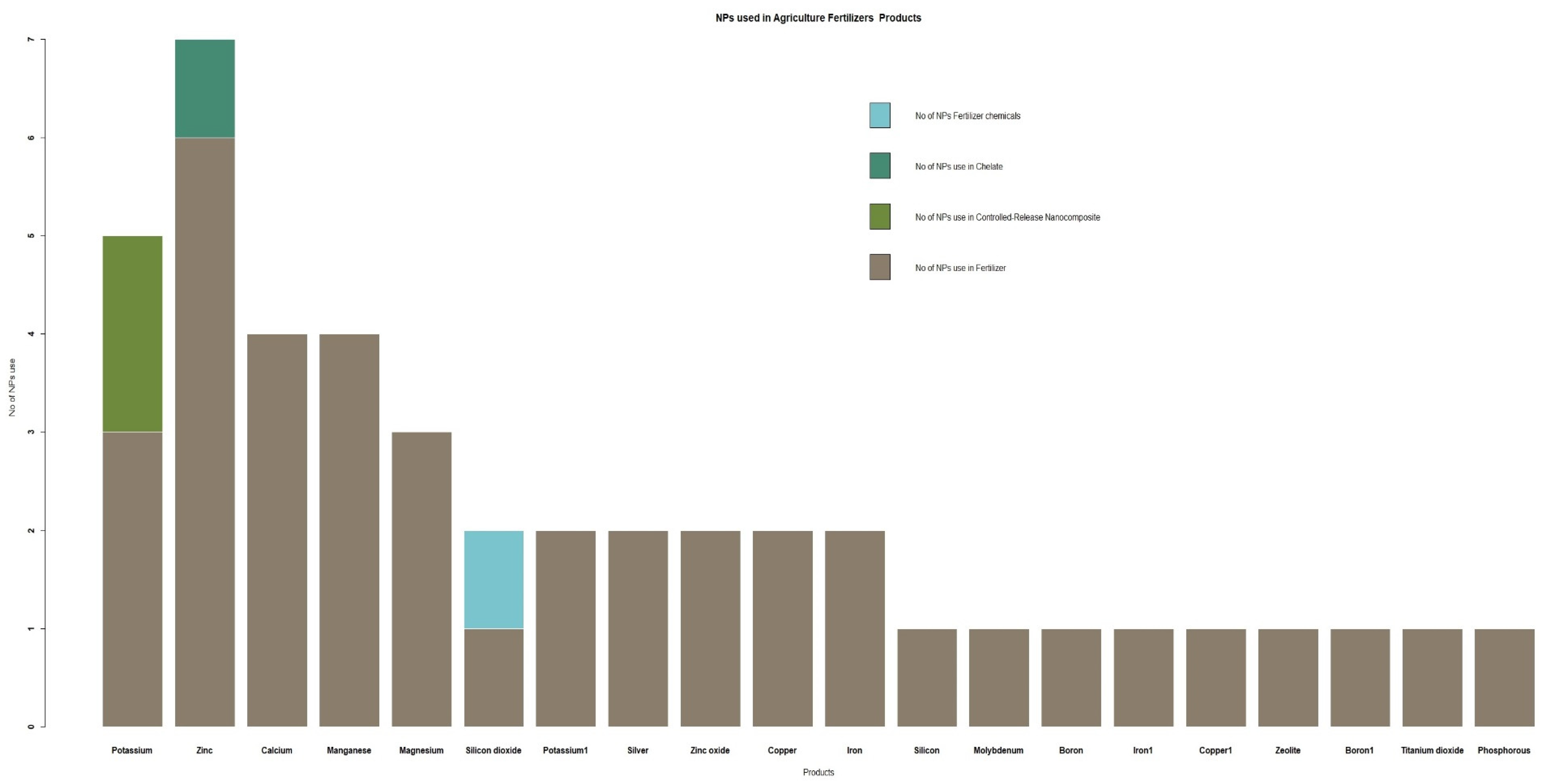
3.2. Type of NPs Utilized in Agriculture and AHs Products
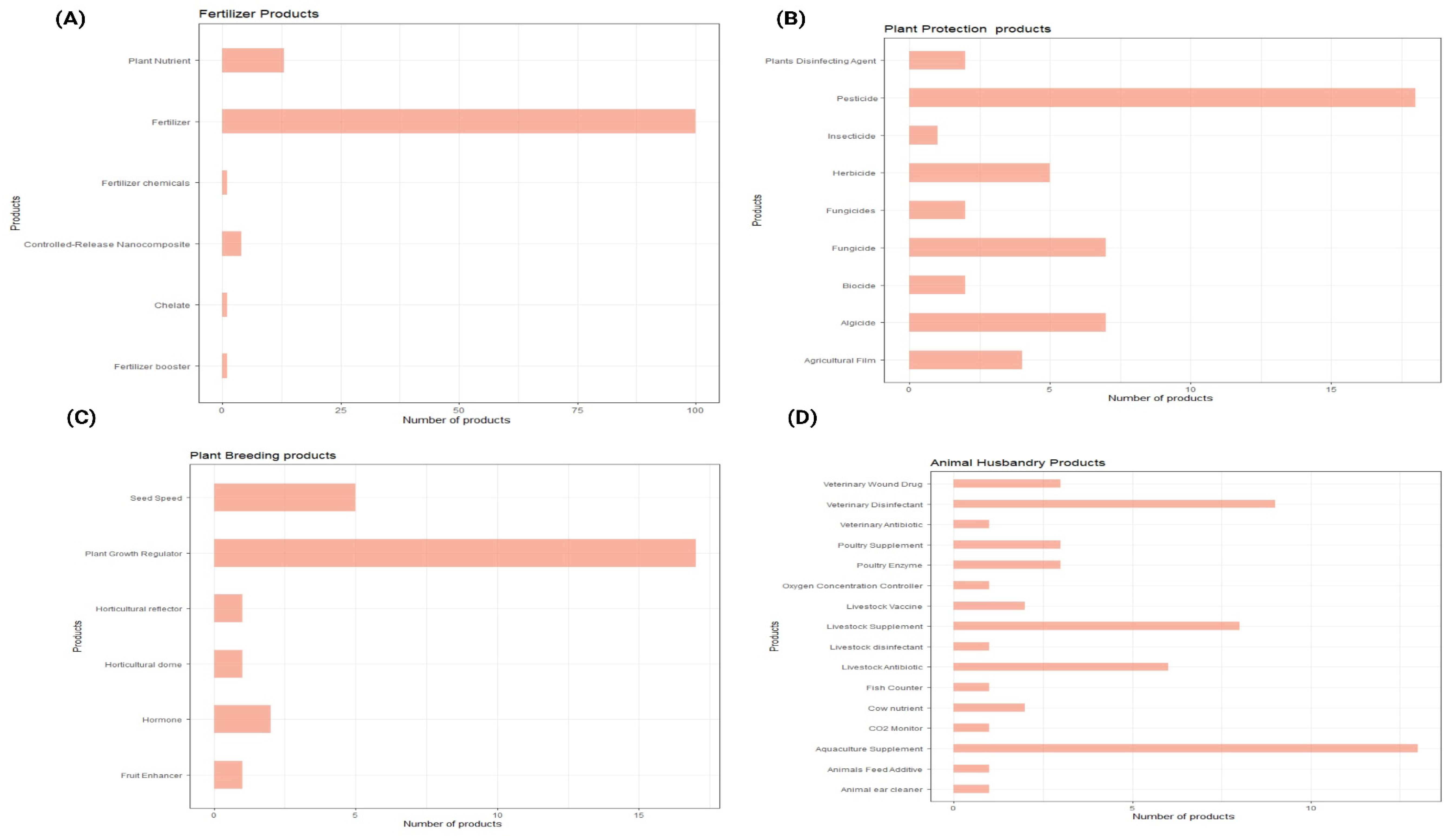
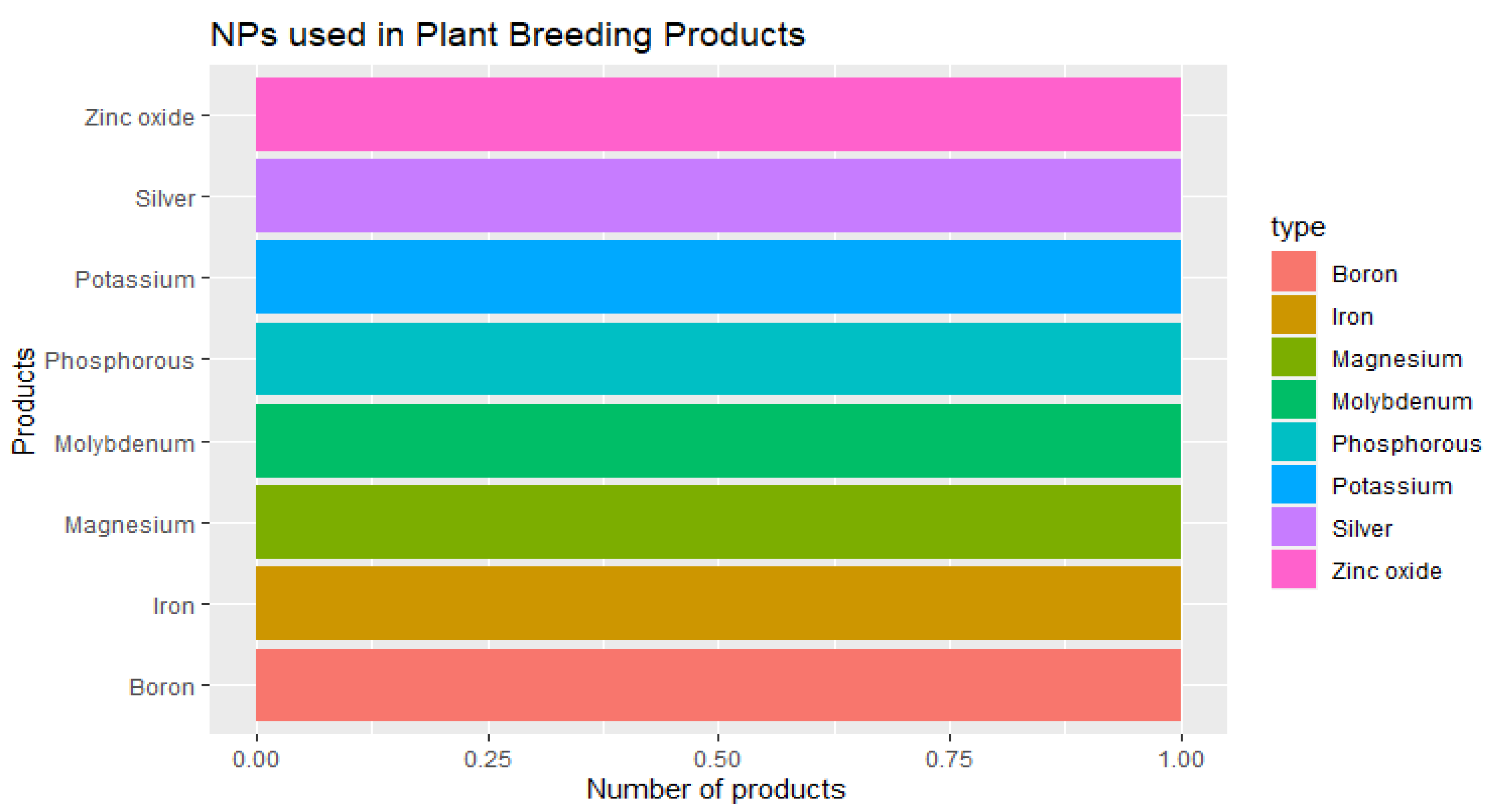
3.2.1. Crop Production
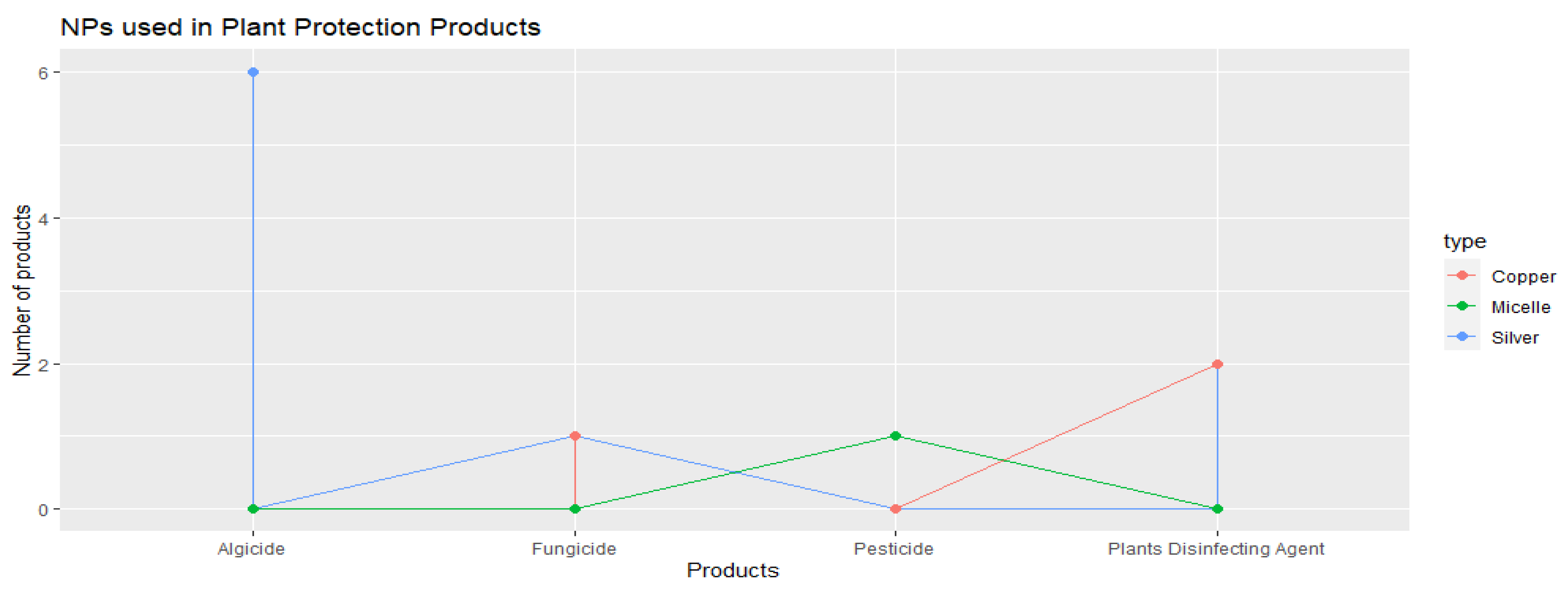
3.2.2. Crop Protection
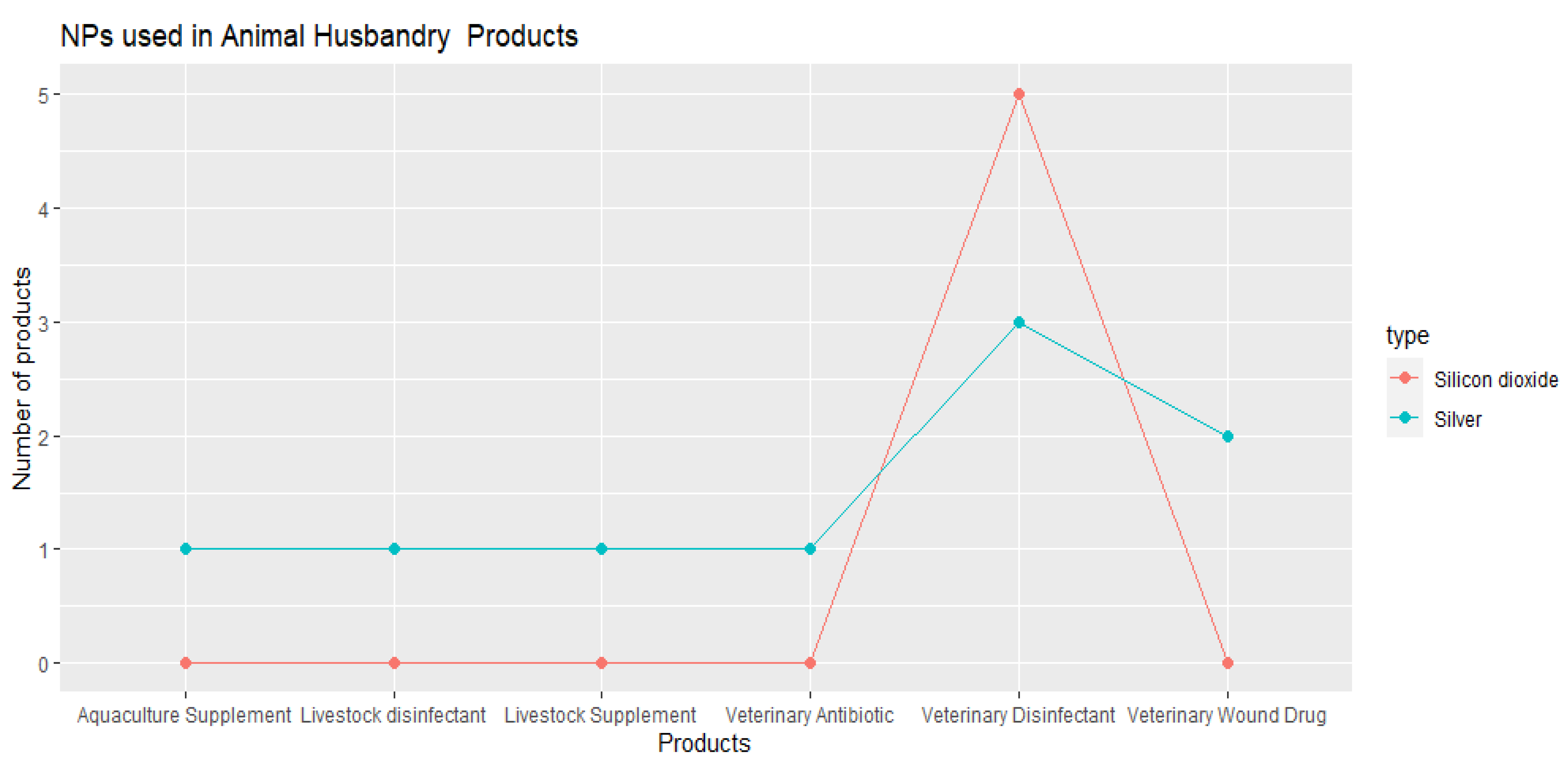
3.2.2. AHs
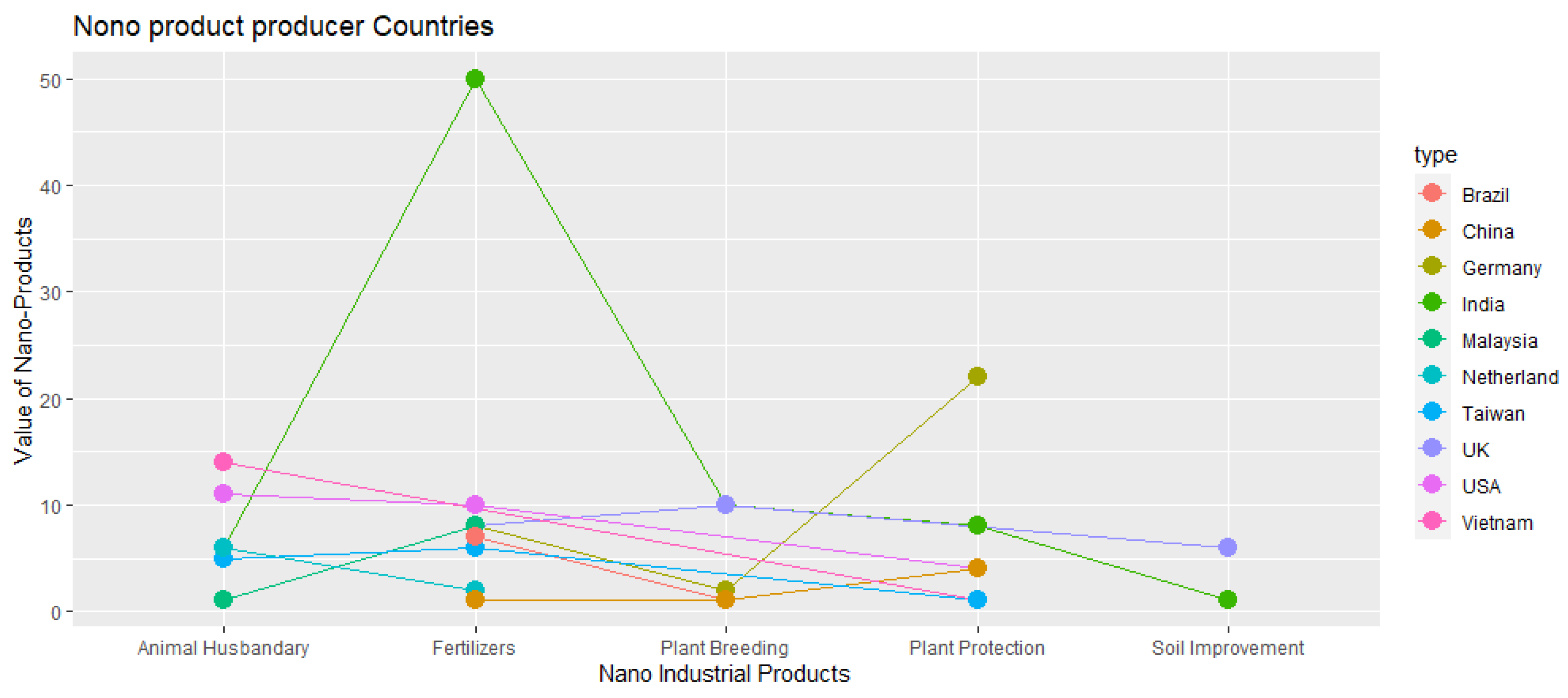
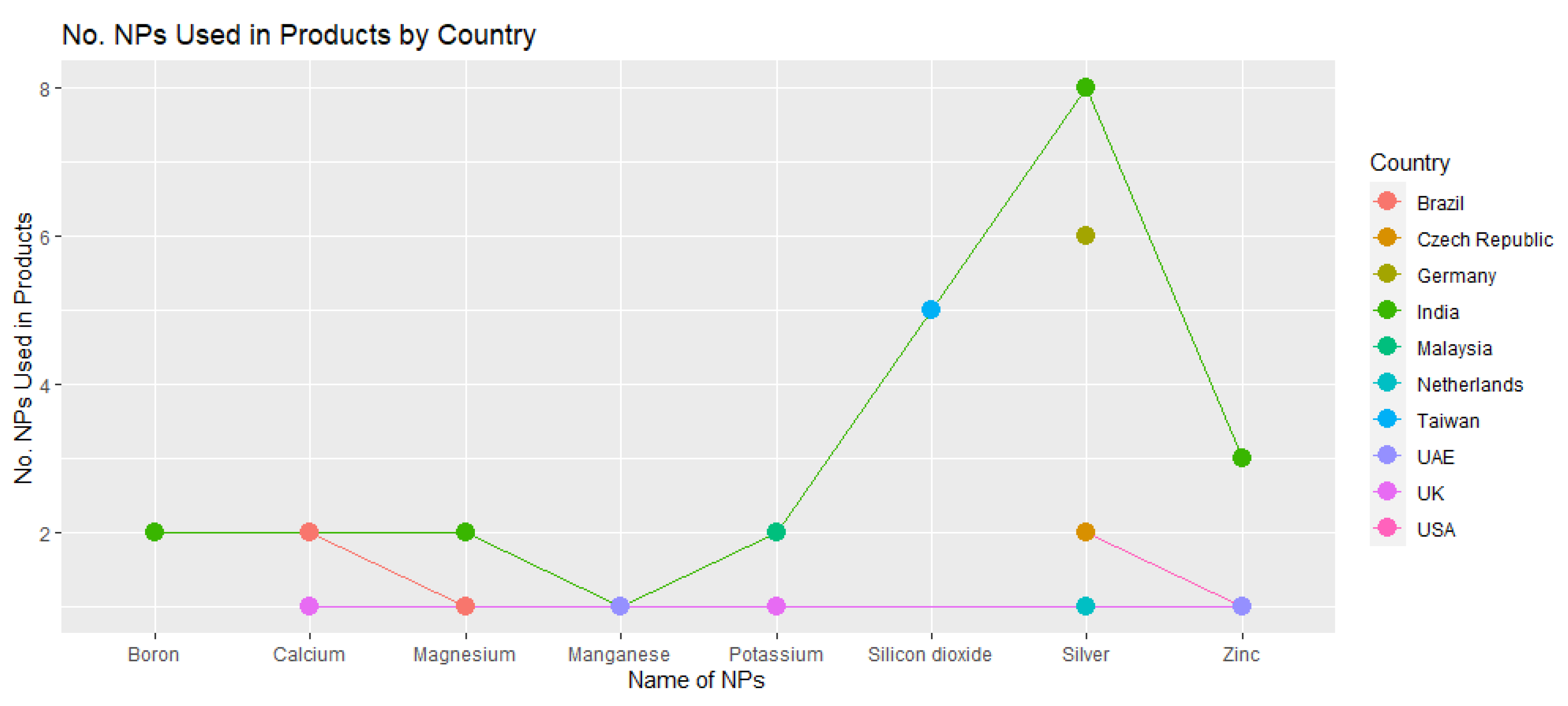
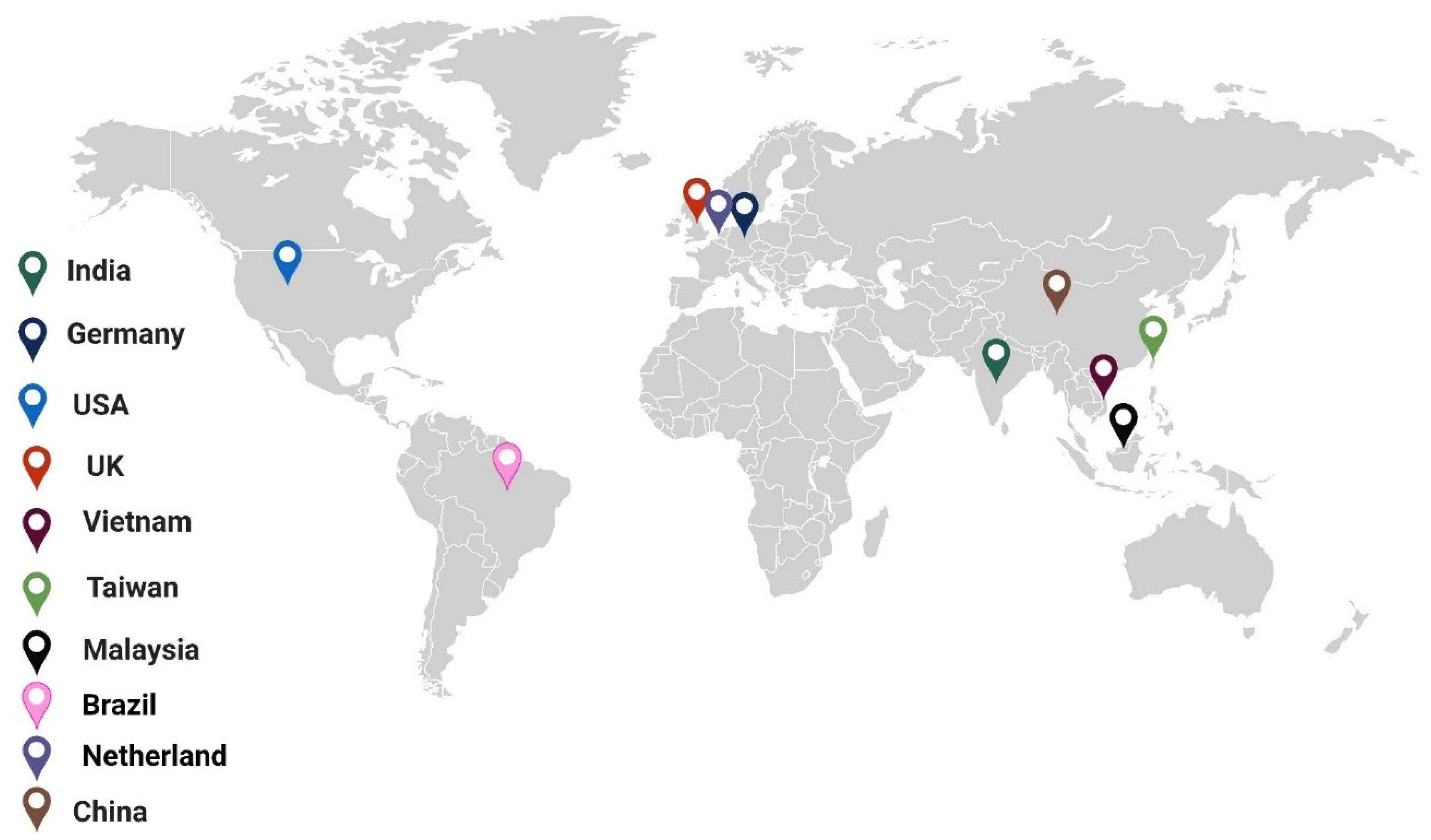
3.4. Global Diversity in the Utilization of Nanoproducts
4. Conclusion and Future Prospective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pouratashi, M.; Iravani, H. Farmers’ knowledge of integrated pest management and learning style preferences: Implications for information delivery. Int. J. Pest Manag. 2012, 58, 347–353. [Google Scholar] [CrossRef]
- Sun, X.; Lyu, J.; Ge, C. Knowledge and Farmers’ Adoption of Green Production Technologies: An Empirical Study on IPM Adoption Intention in Major Indica-Rice-Producing Areas in the Anhui Province of China. Int. J. Environ. Res. Public Health 2022, 19. [Google Scholar] [CrossRef] [PubMed]
- United Nations, D. of E. and S.A. Population Division, 2017. World Population Prospects: The 2017 Revision, Key Findings and Advance Tables. Working Paper No. ESA/P/WP/248. 2017.
- World Population Prospects - Population Division - United Nations. Available online: https://population.un.org/wpp/ (accessed on 21 January 2024).
- Wu, Y.; Wang, X.; Zhang, L.; Zheng, Y.; Liu, X.; Zhang, Y. The critical role of biochar to mitigate the adverse impacts of drought and salinity stress in plants. Front. Plant Sci. 2023, 14, 1163451. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Khan, S.; Ma, X. Climate change impacts on crop yield, crop water productivity and food security – A review. Prog. Nat. Sci. 2009, 19, 1665–1674. [Google Scholar] [CrossRef]
- UN Report: Nature’s Dangerous Decline “Unprecedented”; Species Extinction Rates “Accelerating” - United Nations Sustainable Development. Available online: https://www.un.org/sustainabledevelopment/blog/2019/05/nature-decline-unprecedented-report/ (accessed on 21 January 2024).
- Rico, C.M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Chemistry, biochemistry of nanoparticles, and their role in antioxidant defense system in plants. Nanotechnol. Plant Sci. Nanoparticles Their Impact Plants 2015, 1–17. [Google Scholar] [CrossRef]
- Mughal, B.; Zaidi, S.Z.J.; Zhang, X.; Hassan, S.U. Biogenic Nanoparticles: Synthesis, Characterisation and Applications. Appl. Sci. 2021, Vol. 11, Page 2598 2021, 11, 2598. [Google Scholar] [CrossRef]
- Nanotechnology Market Size, Share & Growth Report, 2030. Available online: https://www.grandviewresearch.com/industry-analysis/nanotechnology-market-report (accessed on 21 January 2024).
- Investor ideas, podcasts, stock news, investor awareness advertising for AI, Cannabis, Cleantech, Crypto, Defense, Fintech, Mining, Oil, Gaming Stocks. Available online: https://www.investorideas.com/news/2017/nanotech/02131Growth.asp (accessed on 21 January 2024).
- Nanotechnology Sees Big Growth in Products and Applications. Available online: https://www.bccresearch.com/pressroom/nan/nanotechnology-sees-big-growth-in-products-and-applications (accessed on 21 January 2024).
- Global Nanotechnology Market Will Grow by over $100 Billion. Available online: https://www.bccresearch.com/pressroom/nan/global-nanotechnology-market-will-grow-by-over-$100-billion (accessed on 21 January 2024).
- Global Nanotechnology Market Poised to Reach 183.7 Billion by 2028. Available online: https://www.bccresearch.com/pressroom/nan/global-nanotechnology-market-poised-to-reach-1837-billion-by-2028 (accessed on 21 January 2024).
- Kolenčík, M.; Ernst, D.; Komár, M.; Urík, M.; Šebesta, M.; Dobročka, E.; Černý, I.; Illa, R.; Kanike, R.; Qian, Y.; et al. Effect of Foliar Spray Application of Zinc Oxide Nanoparticles on Quantitative, Nutritional, and Physiological Parameters of Foxtail Millet (Setaria italica L.) under Field Conditions. Nanomater. 2019, Vol. 9, Page 1559 2019, 9, 1559. [Google Scholar] [CrossRef] [PubMed]
- Sabir, S.; Arshad, M.; Chaudhari, S.K. Zinc oxide nanoparticles for revolutionizing agriculture: Synthesis and applications. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Zhang, Q.; Ying, Y.; Ping, J. Recent Advances in Plant Nanoscience. Adv. Sci. (Weinheim, Baden-Wurttemberg, Ger. 2022, 9. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, A.M.; Zuccari, G. Nanotechnological Manipulation of Nutraceuticals and Phytochemicals for Healthy Purposes: Established Advantages vs. Still Undefined Risks. Polym. 2021, 13, 2262. [Google Scholar] [CrossRef]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in sustainable agriculture: Recent developments, challenges, and perspectives. Front. Microbiol. 2017, 8, 1014. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Hassan, A.A.; Kim, K.H. Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J. Control. Release 2019, 294, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Nisha Raj, S.; Anooj, E.S.; Rajendran, K.; Vallinayagam, S. A comprehensive review on regulatory invention of nano pesticides in Agricultural nano formulation and food system. J. Mol. Struct. 2021, 1239, 130517. [Google Scholar] [CrossRef]
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of nanomaterials in agricultural production and crop protection: A review. Crop Prot. 2012, 35, 64–70. [Google Scholar] [CrossRef]
- Kaningini, A.G.; Nelwamondo, A.M.; Azizi, S.; Maaza, M.; Mohale, K.C. Metal Nanoparticles in Agriculture: A Review of Possible Use. Coatings 2022, 12, 1586. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Almutairi, K.F.; Alotaibi, M.; Shami, A.; Alhammad, B.A.; Battaglia, M.L. Nano-Fertilization as an Emerging Fertilization Technique: Why Can Modern Agriculture Benefit from Its Use? Plants 2021, Vol. 10, Page 2 2020, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.K.; Song, X.-P.; Joshi, A.; Rajput, V.D.; Singh, M.; Sharma, A.; Singh, R.K.; Li, D.-M.; Arora, J.; Minkina, T.; et al. Nanofertilizer Possibilities for Healthy Soil, Water, and Food in Future: An Overview. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Komarek, A.M.; De Pinto, A.; Smith, V.H. A review of types of risks in agriculture: What we know and what we need to know. Agric. Syst. 2020, 178, 102738. [Google Scholar] [CrossRef]
- Harwood, R.R. A history of sustainable agriculture. Sustain. Agric. Syst. 1990, 3–19. [Google Scholar] [CrossRef]
- Wildemeersch, J.C.J.; Garba, M.; Sabiou, M.; Fatondji, D.; Cornelis, W.M. Agricultural drought trends and mitigation in Tillaberí, Niger. Soil Sci. Plant Nutr. 2015, 61, 414–425. [Google Scholar] [CrossRef]
- Gondal, A.H.; Tayyiba, L. Prospects of Using Nanotechnology in Agricultural Growth, Environment and Industrial Food Products. Rev. Agric. Sci. 2022, 10, 68–81. [Google Scholar] [CrossRef]
- Romanovski, V.; Periakaruppan, R. Why metal oxide nanoparticles are superior to other nanomaterials for agricultural application? Nanometal Oxides Hortic. Agron. 2023, 7–18. [Google Scholar] [CrossRef]
- Nanotechnology in Agriculture Industry | NPD. Available online: https://product.statnano.com/industry/agriculture (accessed on 1 April 2023).
- Lopez, G.; Ahmadi, S.H.; Amelung, W.; Athmann, M.; Ewert, F.; Gaiser, T.; Gocke, M.I.; Kautz, T.; Postma, J.; Rachmilevitch, S.; et al. Nutrient deficiency effects on root architecture and root-to-shoot ratio in arable crops. Front. Plant Sci. 2023, 13, 1067498. [Google Scholar] [CrossRef]
- Bethanis, J.; Golia, E.E. Micro- and nano-plastics in agricultural soils: A critical meta-analysis of their impact on plant growth, nutrition, metal accumulation in plant tissues and crop yield. Appl. Soil Ecol. 2024, 194, 105202. [Google Scholar] [CrossRef]
- Herrera, W.; Vera, J.; Aponte, H.; Hermosilla, E.; Fincheira, P.; Parada, J.; Tortella, G.; Seabra, A.B.; Diez, M.C.; Rubilar, O. Meta-analysis of metal nanoparticles degrading pesticides: what parameters are relevant? Environ. Sci. Pollut. Res. 2023, 30, 60168–60179. [Google Scholar] [CrossRef]
- Pirzadah, B.; Pirzadah, T.B.; Jan, A.; Hakeem, K.R. Nanofertilizers: A Way Forward for Green Economy. Nanotechnol. Life Sci. 2020, 99–112. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; ALmoshadak, A.S.; Shafi, M.E.; Albaqami, N.M.; Saad, A.M.; El-Tahan, A.M.; Desoky, E.S.M.; Elnahal, A.S.M.; Almakas, A.; Abd El-Mageed, T.A.; et al. Vital roles of sustainable nano-fertilizers in improving plant quality and quantity-an updated review. Saudi J. Biol. Sci. 2021, 28, 7349–7359. [Google Scholar] [CrossRef] [PubMed]
- Upadhayay, V.K.; Chitara, M.K.; Mishra, D.; Jha, M.N.; Jaiswal, A.; Kumari, G.; Ghosh, S.; Patel, V.K.; Naitam, M.G.; Singh, A.K.; et al. Synergistic impact of nanomaterials and plant probiotics in agriculture: A tale of two-way strategy for long-term sustainability. Front. Microbiol. 2023, 14, 1133968. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Rajput, V.D.; Varshney, A.; Ghazaryan, K.; Minkina, T. Small Tech, Big Impact: Agri-nanotechnology Journey to Optimize Crop Protection and Production for Sustainable Agriculture. Plant Stress 2023, 10, 100253. [Google Scholar] [CrossRef]
- Shelar, A.; Nile, S.H.; Singh, A.V.; Rothenstein, D.; Bill, J.; Xiao, J.; Chaskar, M.; Kai, G.; Patil, R. Recent Advances in Nano-Enabled Seed Treatment Strategies for Sustainable Agriculture: Challenges, Risk Assessment, and Future Perspectives. Nano-Micro Lett. 2023, 15, 1–37. [Google Scholar] [CrossRef]
- Kumari, S.; Sehrawat, K.D.; Phogat, D.; Sehrawat, A.R.; Chaudhary, R.; Sushkova, S.N.; Voloshina, M.S.; Rajput, V.D.; Shmaraeva, A.N.; Marc, R.A.; et al. Ascophyllum nodosum (L.) Le Jolis, a Pivotal Biostimulant toward Sustainable Agriculture: A Comprehensive Review. Agric. 2023, 13, 1179. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, K.; Abd-Elsalam, K.A. Nanofertilizers: Types, Delivery and Advantages in Agricultural Sustainability. Agrochem. 2023, 2, 296–336. [Google Scholar] [CrossRef]
- An, C.; Sun, C.; Li, N.; Huang, B.; Jiang, J.; Shen, Y.; Wang, C.; Zhao, X.; Cui, B.; Wang, C.; et al. Nanomaterials and nanotechnology for the delivery of agrochemicals: strategies towards sustainable agriculture. J. Nanobiotechnology 2021 201 2022, 20, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, J.; Matysiak, K. Advances in Crop Protection in Organic Farming System. Agric. 2023, Vol. 13, Page 1947 2023, 13, 1947. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, Z.; Sun, D.W. Bioinspired Nanomodification Strategies: Moving from Chemical-Based Agrosystems to Sustainable Agriculture. ACS Nano 2021, 15, 12655–12686. [Google Scholar] [CrossRef]
- Goyal, V.; Rani, D.; Ritika; Mehrotra, S.; Deng, C.; Wang, Y. Unlocking the Potential of Nano-Enabled Precision Agriculture for Efficient and Sustainable Farming. Plants 2023, 12, 3744. [Google Scholar] [CrossRef]
- Yadav, N.; Garg, V.K.; Chhillar, A.K.; Rana, J.S. Recent advances in nanotechnology for the improvement of conventional agricultural systems: A review. Plant Nano Biol. 2023, 4, 100032. [Google Scholar] [CrossRef]
- Ur Rahim, H.; Qaswar, M.; Uddin, M.; Giannini, C.; Herrera, M.L.; Rea, G. Nano-Enable Materials Promoting Sustainability and Resilience in Modern Agriculture. Nanomaterials 2021, 11. [Google Scholar] [CrossRef]
- Ha, N.M.C.; Nguyen, T.H.; Wang, S.L.; Nguyen, A.D. Preparation of NPK nanofertilizer based on chitosan nanoparticles and its effect on biophysical characteristics and growth of coffee in green house. Res. Chem. Intermed. 2019, 45, 51–63. [Google Scholar] [CrossRef]
- Farooq, M.A.; Hannan, F.; Islam, F.; Ayyaz, A.; Zhang, N.; Chen, W.; Zhang, K.; Huang, Q.; Xu, L.; Zhou, W. The potential of nanomaterials for sustainable modern agriculture: present findings and future perspectives. Environ. Sci. Nano 2022, 9, 1926–1951. [Google Scholar] [CrossRef]
- Azam, M.; Bhatti, H.N.; Khan, A.; Zafar, L.; Iqbal, M. Zinc oxide nano-fertilizer application (foliar and soil) effect on the growth, photosynthetic pigments and antioxidant system of maize cultivar. Biocatal. Agric. Biotechnol. 2022, 42, 102343. [Google Scholar] [CrossRef]
- Dapkekar, A.; Deshpande, P.; Oak, M.D.; Paknikar, K.M.; Rajwade, J.M. Zinc use efficiency is enhanced in wheat through nanofertilization. Sci. Reports 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Fedenia, L.N.; Sharifan, H.; Ma, X.; Lombardini, L. Effects of foliar application of zinc sulfate and zinc nanoparticles in coffee (Coffea arabica L.) plants. Plant Physiol. Biochem. PPB 2019, 135, 160–166. [Google Scholar] [CrossRef] [PubMed]
- García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vázquez-Alvarado, R.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar Application of Zinc Oxide Nanoparticles and Zinc Sulfate Boosts the Content of Bioactive Compounds in Habanero Peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Kolenčík, M.; Ernst, D.; Urík, M.; Ďurišová, Ľ.; Bujdoš, M.; Šebesta, M.; Dobročka, E.; Kšiňan, S.; Illa, R.; Qian, Y.; et al. Foliar Application of Low Concentrations of Titanium Dioxide and Zinc Oxide Nanoparticles to the Common Sunflower under Field Conditions. Nanomater. 2020, 10, 1619. [Google Scholar] [CrossRef] [PubMed]
- Davarpanah, S.; Tehranifar, A.; Davarynejad, G.; Abadía, J.; Khorasani, R. Effects of foliar applications of zinc and boron nano-fertilizers on pomegranate (Punica granatum cv. Ardestani) fruit yield and quality. Sci. Hortic. (Amsterdam). 2016, 210, 57–64. [Google Scholar] [CrossRef]
- Khattab, S.; Alkuwayti, M.A.; Yap, Y.K.; Meligy, A.M.A.; Bani Ismail, M.; El Sherif, F. Foliar Spraying of ZnO Nanoparticals on Curcuma longa Had Increased Growth, Yield, Expression of Curcuminoid Synthesis Genes, and Curcuminoid Accumulation. Horticulturae 2023, 9, 355. [Google Scholar] [CrossRef]
- Gao, M.; Chen, Y.; Wu, L.; Wang, Y. Changes in the Profiles of Yield, Yield Component, Oil Content, and Citral Content in Litsea cubeba (Lour.) Persoon Following Foliar Fertilization with Zinc and Boron. For. 2019, 10, 59. [Google Scholar] [CrossRef]
- Nekoukhou, M.; Fallah, S.; Abbasi-Surki, A.; Pokhrel, L.R.; Rostamnejadi, A. Improved efficacy of foliar application of zinc oxide nanoparticles on zinc biofortification, primary productivity and secondary metabolite production in dragonhead. J. Clean. Prod. 2022, 379, 134803. [Google Scholar] [CrossRef]
- Shebl, A.; Hassan, A.A.; Salama, D.M.; Abd El-Aziz, M.E.; Abd Elwahed, M.S.A. Green synthesis of nanofertilizers and their application as a foliar for cucurbita pepo l. J. Nanomater. 2019, 2019. [Google Scholar] [CrossRef]
- Garza-Alonso, C.A.; Juárez-Maldonado, A.; González-Morales, S.; Cabrera-De la Fuente, M.; Cadenas-Pliego, G.; Morales-Díaz, A.B.; Trejo-Téllez, L.I.; Tortella, G.; Benavides-Mendoza, A. ZnO nanoparticles as potential fertilizer and biostimulant for lettuce. Heliyon 2023, 9, 12787. [Google Scholar] [CrossRef] [PubMed]
- Yusefi-Tanha, E.; Fallah, S.; Rostamnejadi, A.; Pokhrel, L.R. Zinc Oxide Nanoparticles (ZnONPs) as Nanofertilizer: Improvement on Seed Yield and Antioxidant Defense System in Soil Grown Soybean (Glycine max cv. Kowsar). bioRxiv, 0396. [Google Scholar] [CrossRef]
- Manjunatha, S.B.; Biradar, D.P.; Aladakatti, Y.R. Nanotechnology and its applications in agriculture: a review. J. Farm Sci. 2016, 29, 1–13. [Google Scholar]
- Wang, G.; Wang, J.; Han, X.; Chen, R.; Xue, X. Effects of Spraying Calcium Fertilizer on Photosynthesis, Mineral Content, Sugar–Acid Metabolism and Fruit Quality of Fuji Apples. Agron. 2022, 12, 2563. [Google Scholar] [CrossRef]
- Lan-lan, Y.; Kai-zheng, L.; Guo-hui, Q.; Xue-mei, Z.; Han, L.; Su-ping, G.; Lan-lan, Y.; Kai-zheng, L.; Guo-hui, Q.; Xue-mei, Z.; et al. Optimum application amount and times of calcium nitrate for better fruit quality and lower incidence of apple bitter pit. J. Plant Nutr. Fertil. 2020, 26, 765–772. [Google Scholar] [CrossRef]
- Atzrodt, J.; Derdau, V.; Kerr, W.; Reid, M. Applications of hydrogen isotopes in the life sciences. Angew. Chemie Int. Ed. 2017, 1–26. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.B.; Dal Sasso, G.; Carmona, F.J.; Miguel-Rojas, C.; Pérez-De-Luque, A.; Masciocchi, N.; Guagliardi, A.; Delgado-López, J.M. Engineering Biomimetic Calcium Phosphate Nanoparticles: A Green Synthesis of Slow-Release Multinutrient (NPK) Nanofertilizers. ACS Appl. Bio Mater. 2020, 3, 1344–1353. [Google Scholar] [CrossRef]
- Carmona, F.J.; Dal Sasso, G.; Ramírez-Rodríguez, G.B.; Pii, Y.; Delgado-López, J.M.; Guagliardi, A.; Masciocchi, N. Urea-functionalized amorphous calcium phosphate nanofertilizers: optimizing the synthetic strategy towards environmental sustainability and manufacturing costs. Sci. Reports 2021, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Álvarez, E.P.; Ramírez-Rodríguez, G.B.; Carmona, F.J.; Martínez-Vidaurre, J.M.; Masciocchi, N.; Guagliardi, A.; Garde-Cerdán, T.; Delgado-López, J.M. Towards a more sustainable viticulture: foliar application of N-doped calcium phosphate nanoparticles on Tempranillo grapes. J. Sci. Food Agric. 2021, 101, 1307–1313. [Google Scholar] [CrossRef]
- Gaiotti, F.; Lucchetta, M.; Rodegher, G.; Lorenzoni, D.; Longo, E.; Boselli, E.; Cesco, S.; Belfiore, N.; Lovat, L.; Delgado-López, J.M.; et al. Urea-doped calcium phosphate nanoparticles as sustainable nitrogen nanofertilizers for viticulture: Implications on yield and quality of pinot gris grapevines. Agronomy 2021, 11, 1026. [Google Scholar] [CrossRef]
- Diedrick, K.; Agronomist, A. PIONEER AGRONOMY SCIENCES CROP INSIGHTS Manganese Fertility in Soybean Production.
- Yang, S.X.; Deng, H.; Li, M.S. Manganese uptake and accumulation in a woody hyperaccumulator, Schima superba. https://pse.agriculturejournals.cz/doi/10.17221/401-PSE.html 2008, 54, 441–446. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Galavi, M.; Ahmadvand, G. Effect of zinc and manganese foliar application on yield, quality and enrichment on potato (Solanum tuberosum L.). Asian J. Plant Sci. 2007, 6, 1256–1260. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, H.; Lal, R. Effects of Stabilized Nanoparticles of Copper, Zinc, Manganese, and Iron Oxides in Low Concentrations on Lettuce (Lactuca sativa) Seed Germination: Nanotoxicants or Nanonutrients? Water. Air. Soil Pollut. 2016, 227, 1–14. [Google Scholar] [CrossRef]
- Salama, D.M.; Abd El-Aziz, M.E.; Shaaban, E.A.; Osman, S.A.; Abd El-Wahed, M.S. The impact of nanofertilizer on agro-morphological criteria, yield, and genomic stability of common bean (Phaseolus vulgaris L.). Sci. Reports 2022, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Salama, D.M.; Abd El-Aziz, M.E.; Osman, S.A.; Abd Elwahed, M.S.A.; Shaaban, E.A. Foliar spraying of MnO2-NPs and its effect on vegetative growth, production, genomic stability, and chemical quality of the common dry bean. Arab J. Basic Appl. Sci. 2022, 29, 26–39. [Google Scholar] [CrossRef]
- Cakmak, I. Magnesium in crop production, food quality and human health. Plant Soil 2013, 368, 1–4. [Google Scholar] [CrossRef]
- Peng, W.T.; Zhang, L.D.; Zhou, Z.; Fu, C.; Chen, Z.C.; Liao, H. Magnesium promotes root nodulation through facilitation of carbohydrate allocation in soybean. Physiol. Plant. 2018, 163, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Hailes, K.J.; Aitken, R.L.; Menzies, N.W. Magnesium in tropical and subtropical soils from north-eastern Australia. II. Response by glasshouse-grown maize to applied magnesium. Soil Res. 1997, 35, 629–642. [Google Scholar] [CrossRef]
- Yang, G.H.; Yang, L.T.; Jiang, H.X.; Li, Y.; Wang, P.; Chen, L.S. Physiological impacts of magnesium-deficiency in Citrus seedlings: Photosynthesis, antioxidant system and carbohydrates. Trees - Struct. Funct. 2012, 26, 1237–1250. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil 2013, 368, 87–99. [Google Scholar] [CrossRef]
- Leusbrock, I.; Metz, S.J.; Rexwinkel, G.; Versteeg, G.F. The solubility of magnesium chloride and calcium chloride in near-critical and supercritical water. J. Supercrit. Fluids 2010, 53, 17–24. [Google Scholar] [CrossRef]
- Wolf, F.I.; Cittadini, A. Chemistry and biochemistry of magnesium. Mol. Aspects Med. 2003, 24, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, J.; Liu, L.; Rosanoff, A.; Xiong, X.; Zhang, Y.; Pei, T. Effects of Magnesium Fertilizer on the Forage Crude Protein Content Depend upon Available Soil Nitrogen. J. Agric. Food Chem. 2018, 66, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Grzebisz, W. Magnesium - Food and human health. J. Elem. 2011, 16, 299–323. [Google Scholar] [CrossRef]
- Shang, Y.; Kamrul Hasan, M.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Mol. 2019, 24, 2558. [Google Scholar] [CrossRef] [PubMed]
- Mattiello, E.M.; Ruiz, H.A.; Neves, J.C.L.; Ventrella, M.C.; Araújo, W.L. Zinc deficiency affects physiological and anatomical characteristics in maize leaves. J. Plant Physiol. 2015, 183, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Monreal, C.M.; Derosa, M.; Mallubhotla, S.C.; Bindraban, P.S.; Dimkpa, C. Nanotechnologies for increasing the crop use efficiency of fertilizer-micronutrients. Biol. Fertil. Soils 2015, 52, 423–437. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Raliya, R.; Mahawar, H.; Rathore, I. Development of Zinc Nanofertilizer to Enhance Crop Production in Pearl Millet (Pennisetum americanum). Agric. Res. 2014, 3, 257–262. [Google Scholar] [CrossRef]
- Palmqvist, N.G.M.; Seisenbaeva, G.A.; Svedlindh, P.; Kessler, V.G. Maghemite Nanoparticles Acts as Nanozymes, Improving Growth and Abiotic Stress Tolerance in Brassica napus. Nanoscale Res. Lett. 2017, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Yang, F.; Liu, C.; Gao, Q.; Wan, Z.; Gu, F.; Wu, C.; Ma, Z.; Zhou, J.; Yang, P. Influences of nano-TiO2 on the chloroplast aging of spinach under light. Biol. Trace Elem. Res. 2005, 104, 249–260. [Google Scholar] [CrossRef]
- Wang, W.; Tarafdar, J.; research, P.B.-J. of nanoparticle; 2013, undefined Nanoparticle synthesis and delivery by an aerosol route for watermelon plant foliar uptake. Springer 2013, 15. [Google Scholar] [CrossRef]
- Zahra, Z.; Arshad, M.; Rafique, R.; Mahmood, A.; Habib, A.; Qazi, I.A.; Khan, S.A. Metallic Nanoparticle (TiO2 and Fe3O4) Application Modifies Rhizosphere Phosphorus Availability and Uptake by Lactuca sativa. J. Agric. Food Chem. 2015, 63, 6876–6882. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Biswas, P. Environmentally benign bio-inspired synthesis of Au nanoparticles, their self-assembly and agglomeration. RSC Adv. 2015, 5, 42081–42087. [Google Scholar] [CrossRef]
- Jiang, M.; Song, Y.; Kanwar, M.K.; Ahammed, G.J.; Shao, S.; Zhou, J. Phytonanotechnology applications in modern agriculture. J. Nanobiotechnology 2021, 19, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Ashraf, M. Nanoparticles potentially mediate salt stress tolerance in plants. Plant Physiol. Biochem. 2021, 160, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Chhipa, H. Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 2017, 15, 15–22. [Google Scholar] [CrossRef]
- Palmqvist, N.G.M.; Seisenbaeva, G.A.; Svedlindh, P.; Kessler, V.G. Maghemite Nanoparticles Acts as Nanozymes, Improving Growth and Abiotic Stress Tolerance in Brassica napus. Nanoscale Res. Lett. 2017, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, T.R.; Salleh, M.A.M.; Sijam, K.; Rahim, R.A.; Mohsenifar, A.; Safarnejad, R.; Tabatabaei, M. Fluorometric immunoassay for detecting the plant virus Citrus tristeza using carbon nanoparticles acting as quenchers and antibodies labeled with CdTe quantum dots. Microchim. Acta 2016, 183, 2277–2287. [Google Scholar] [CrossRef]
- Hayles, J.; Johnson, L.; Worthley, C.; Losic, D. Nanopesticides: a review of current research and perspectives. New Pestic. Soil Sensors 2017, 193–225. [Google Scholar] [CrossRef]
- Shukla, P.; Chaurasia, P.; Younis, K.; Qadri, O.S.; Faridi, S.A.; Srivastava, G. Nanotechnology in sustainable agriculture: studies from seed priming to post-harvest management. Nanotechnol. Environ. Eng. 2019, 4, 1–15. [Google Scholar] [CrossRef]
- Ocsoy, I.; Paret, M.L.; Ocsoy, M.A.; Kunwar, S.; Chen, T.; You, M.; Tan, W. Nanotechnology in plant disease management: DNA-directed silver nanoparticles on graphene oxide as an antibacterial against Xanthomonas perforans. ACS Nano 2013, 7, 8972–8980. [Google Scholar] [CrossRef]
- Fernando, S.; Gunasekara, T.; Holton, J. Antimicrobial Nanoparticles: applications and mechanisms of action. Sri Lankan J. Infect. Dis. 2018, 8, 2. [Google Scholar] [CrossRef]
- Hoseinnejad, M.; Jafari, S.M.; Katouzian, I. Inorganic and metal nanoparticles and their antimicrobial activity in food packaging applications. Crit. Rev. Microbiol. 2018, 44, 161–181. [Google Scholar] [CrossRef]
- Karimi, E. Antimicrobial activities of nanoparticles. Nanotechnol. Agric. Crop Prod. Prot. 2019, 171–206. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Firoz, M.; Al-Khaishany, M.Y. Role of nanoparticles in plants. Nanotechnol. Plant Sci. Nanoparticles Their Impact Plants 2015, 19–35. [Google Scholar] [CrossRef]
- Wang, Z.; Yue, L.; Dhankher, O.P.; Xing, B. Nano-enabled improvements of growth and nutritional quality in food plants driven by rhizosphere processes. Environ. Int. 2020, 142, 105831. [Google Scholar] [CrossRef]
- Ahmad, N.; Sharma, S.; Alam, M.K.; Singh, V.N.; Shamsi, S.F.; Mehta, B.R.; Fatma, A. Rapid synthesis of silver nanoparticles using dried medicinal plant of basil. Colloids Surfaces B Biointerfaces 2010, 81, 81–86. [Google Scholar] [CrossRef]
- Ahmad, N.; Sharma, S.; Ahmad, N.; Sharma, S. Green Synthesis of Silver Nanoparticles Using Extracts of Ananas comosus. Green Sustain. Chem. 2012, 2, 141–147. [Google Scholar] [CrossRef]
- Kotakadi, V.S.; Gaddam, S.A.; Subba Rao, Y.; Prasad, T.N.V.K.V.; Varada Reddy, A.; Sai Gopal, D.V.R. Biofabrication of silver nanoparticles using Andrographis paniculata. Eur. J. Med. Chem. 2014, 73, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Baliyarsingh, B.; Mishra, A.; Rath, S. Evaluation of insecticidal and repellency activity of leaf extracts of Andrographis paniculata against Tribolium castaneum (red flour beetle). Int. J. Trop. Insect Sci. 2021, 41, 765–773. [Google Scholar] [CrossRef]
- Rajan, R.; Chandran, K.; Harper, S.L.; Yun, S. Il; Kalaichelvan, P.T. Plant extract synthesized silver nanoparticles: An ongoing source of novel biocompatible materials. Ind. Crops Prod. 2015, 70, 356–373. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, Y.; Pan, J.; Liu, X. Formulation and evaluation of norcanthridin nanoemulsions against the Plutella xylostella (Lepidotera: Plutellidae). BMC Biotechnol. 2019, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Arvind Bharani, R.; Karthick Raja Namasivayam, S. Biogenic silver nanoparticles mediated stress on developmental period and gut physiology of major lepidopteran pest Spodoptera litura (Fab.) (Lepidoptera: Noctuidae)—An eco-friendly approach of insect pest control. J. Environ. Chem. Eng. 2017, 5, 453–467. [Google Scholar] [CrossRef]
- Santhoshkumar, T.; Rahuman, A.A.; Bagavan, A.; Marimuthu, S.; Jayaseelan, C.; Kirthi, A.V.; Kamaraj, C.; Rajakumar, G.; Zahir, A.A.; Elango, G.; et al. Evaluation of stem aqueous extract and synthesized silver nanoparticles using Cissus quadrangularis against Hippobosca maculata and Rhipicephalus (Boophilus) microplus. Exp. Parasitol. 2012, 132, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Buteler, M.; Sofie, S.W.; Weaver, D.K.; Driscoll, D.; Muretta, J.; Stadler, T. Development of nanoalumina dust as insecticide against Sitophilus oryzae and Rhyzopertha dominica. Int. J. Pest Manag. 2015, 61, 80–89. [Google Scholar] [CrossRef]
- Pavela, R.; Murugan, K.; Canale, A.; Benelli, G. Saponaria officinalis-synthesized silver nanocrystals as effective biopesticides and oviposition inhibitors against Tetranychus urticae Koch. Ind. Crops Prod. 2017, 97, 338–344. [Google Scholar] [CrossRef]
- Ghidan, A.Y.; Al-Antary, T.M.; Awwad, A.M. Green synthesis of copper oxide nanoparticles using Punica granatum peels extract: Effect on green peach Aphid. Environ. Nanotechnology, Monit. Manag. 2016, 6, 95–98. [Google Scholar] [CrossRef]
- Ibarra-Laclette, E.; Blaz, J.; Pérez-Torres, C.A.; Villafán, E.; Lamelas, A.; Rosas-Saito, G.; Ibarra-Juárez, L.A.; García-ávila, C. de J.; Martínez-Enriquez, A.I.; Pariona, N. Antifungal Effect of Copper Nanoparticles against Fusarium kuroshium, an Obligate Symbiont of Euwallacea kuroshio Ambrosia Beetle. J. Fungi 2022, 8, 347. [Google Scholar] [CrossRef]
- Lopez-Lima, D.; Mtz-Enriquez, A.I.; Carrión, G.; Basurto-Cereceda, S.; Pariona, N. The bifunctional role of copper nanoparticles in tomato: Effective treatment for Fusarium wilt and plant growth promoter. Sci. Hortic. (Amsterdam). 2021, 277, 109810. [Google Scholar] [CrossRef]
- Viet, P. Van; Nguyen, H.T.; Cao, T.M.; Hieu, L. Van Fusarium Antifungal Activities of Copper Nanoparticles Synthesized by a Chemical Reduction Method. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef]
- Pariona, N.; Mtz-Enriquez, A.I.; Sánchez-Rangel, D.; Carrión, G.; Paraguay-Delgado, F.; Rosas-Saito, G. Green-synthesized copper nanoparticles as a potential antifungal against plant pathogens. RSC Adv. 2019, 9, 18835–18843. [Google Scholar] [CrossRef]
- Gaba, S.; Rai, A.K.; Varma, A.; Prasad, R.; Goel, A. Biocontrol potential of mycogenic copper oxide nanoparticles against Alternaria brassicae. Front. Chem. 2022, 10, 966396. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Falkeborg, M.; Zheng, Y.; Yang, T.; Xu, X. Formulation and characterization of nanostructured lipid carriers containing a mixed lipids core. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 430, 76–84. [Google Scholar] [CrossRef]
- Youssef, F.S.; El-Banna, H.A.; Elzorba, H.Y.; Galal, A.M. Application of some nanoparticles in the field of veterinary medicine. Int. J. Vet. Sci. Med. 2019, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Preet, S.; Tomar, R.S. Anthelmintic effect of biofabricated silver nanoparticles using Ziziphus jujuba leaf extract on nutritional status of Haemonchus contortus. Small Rumin. Res. 2017, 154, 45–51. [Google Scholar] [CrossRef]
- B. , A. Evaluation of the Anthelmintic Activity (in- vitro) of Neem Leaf Extract-Mediated Silver Nanoparticles against Haemonchus contortus. Int. J. Pure Appl. Biosci. 2017, 5, 118–128. [Google Scholar] [CrossRef]
- Diaz, R.; Mackey, B.; Chadalavada, S.; kainthola, J.; Heck, P.; Goel, R. Enhanced Bio-P removal: Past, present, and future – A comprehensive review. Chemosphere 2022, 309, 136518. [Google Scholar] [CrossRef]
- Lürling, M.; Mackay, E.; Reitzel, K.; Spears, B.M. Editorial – A critical perspective on geo-engineering for eutrophication management in lakes. Water Res. 2016, 97, 1–10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




