Submitted:
30 July 2024
Posted:
31 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Background Inorganic Chemistry and Responses to Changing Water pH Values
1.2. Lifetime of Fossil Fuel Emissions in the Atmosphere
2. Experimental Methods
2.1. Modelling a Terrestrial Acidification Hypothesis
3. Results
3.1. The Possible Acidic Effect of Anthropogenic Combustion of Fossil Fuels since 1750
3.2. Modelling Acidification of Sea or Land Water
3.3. Titration of Seawater Alkalinity by Strong Acids and Atmospheric CO2
3.3. Titrate Modelled Stoichiometry of CO2 Formation from Addition of Strong Acid
3.4. Global Significance Possible for Rates of Acidification in Seawater
3.5. Modelling Acid Titrations in Fresh Water on Land Resulting from Farming
3.6. The Scale of Acidic Depositions on the Terrestrial Surface
| Acid | Source | Estimated total moles H+ and CO2 | Annual acid meq/m2 globally |
|---|---|---|---|
| Hydrogen ions (H+) [4,16] | Croplands, 15x1012 m2 sown alkalinity export | 10.0x1012 | 19.6 |
| “ | Forestry, 50x1012 m2 harvested alkalinity export | 5.0x1012 | 9.8 |
| “ | Rangelands, 100x1012 m2 alkalinity export | 5.0x1012 | 9.8 |
| Nitric, HNO3 [4,19] | Nitrification 1.5x108 t NH3 plus leached legume-N | 20x1012 | 39.2 |
| Agriculture total | 37.5 x1012 | 78.4 | |
| Sulphuric, H2SO4 [4] | Coal, oil, gas, wood combustion | 16x1012 | 64.0 |
| Sulphuric, H2SO4 or sulphurous [3,4] | Anaerobic sulphate respiration [4] from ca. 1013 moles C annually in sewage, discharged mainly to the ocean ; UV oxidation H2S and DMS [4]; oxidation of sulphides in aerated acid sulphate soils by drainage in urban habitation Stone & Ahern [23] | 25x1012 | 98.0 |
| Carbonic acid H2CO3 | Global respiration = assumed photosynthesis [3] | 15,300x1012 | 30,000.0 |
| Carbonic acid “[ | Coal, oil and gas fossil emissions [17] | 816x1012 | 1,599.7 |
| Carbonic acid “ | Increasing wildfires with population | 100x1012 | 392.1 |
| Nitric, sulphuric, acids | From increasing combustion in wildfires, ash countering acidification in forests | 16x1012 | 64.0 |
| Carbonic acid H2CO3 [3] | Cement, construction [3, Schulz] | 104.6 | |
| Total emissions | Approximate estimate only ±10% | 32,316.2 | |
| Weak acid | H2CO3 | pK 6.5, 8.9 | 31,991.8 |
| Total strong acids (H+) | C-fuels, agriculture, fire, smelting ores with sulphides (20 mequiv/m2) | pK ca. 0-1 | 324.4 |
| Wildfires 1.76 billion tonnes | |||
| Greening scenario [27] | 1% greater photosynthesis annually | +150x1012 | 300.0 |
| Photosynthesis | All sources + CO2 fertilisation | 15,600x1012 | -34,650.1 |
| Net CO2 | Weak acid—photosynthesis | 150x1012 | -1297.1 |
| CO2 to atmosphere | Current increase per annum 2.0 ppmv | 664.0 |
4. Discussion
4.1. Confirming Our Prima-Facie Case for Ongoing CO2 Emission from Acidification of Soil Inorganic Carbon
4.2. Intergovernmental Panel for Climate Change Reports
4.3. Integrating Rates of Acidifying Processes
4.4. Export of Produce as a Major Acidification Factor in CO2 Emission from Bicarbonate by Soils
4.5. Nitrification of Ammonia and Oxidation of Sulphur to Sulphuric Acid
4.6. Photosynthesis Reaction Kinetics and Increasing Temperature Affecting Greening
4.7. Unbalanced Acidification in Ecosystems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kennedy, I.R.; Runcie, J.W.; Zhang, S.; Ritchie, R.J. A New Look at physico-chemical causes of changing climate: Is the seasonal variation in seawater temperature a significant factor in establishing the partial pressure of carbon dioxide in the Earth’s atmosphere? Thermo 2022, 2, 401–434. [Google Scholar] [CrossRef]
- Keeling, C.D. ; TP, Wahlen M. ; van der Plicht, J. Interannual extremes in the rate of rise of atmospheric carbon dioxide since 1980. Nature 1995, 375, 666–670. [Google Scholar]
- Harvey, L.D. Global Warming 2000, The Hard Science. Prentice Hall, Harlow UK.
- Kennedy, I.R. Acid Soil and Acid Rain. 1992, Wiley, United Kingdom.
- Archer, D.; Eby, M.; Brovkin, V.; Ridgwell, A.; Cao, L.; Mikolajewicz, U.; Caldeira, K.; Matsumoto, K.; Munhoven, G.; Montenegro, A.; Tokos, K. Atmospheric lifetime of fossil fuel carbon dioxide. Annu. Rev. Earth Planet. Sci. 2009, 37, 117–134. [Google Scholar] [CrossRef]
- Ely, L.L.; Webb, R.H.; Enzel, R.H. Accuracy of post-bomb 137Cs and 14C in dating fluvial deposits. Quatern. Res. 1992, 38, 38,196–204. [Google Scholar] [CrossRef]
- Randerson, J.T.; Enting, I.G.; Schuur, A.G.; Calderia, K.; Fung, I.Y. Seasonal and latitudinal variability of troposphere Δ14CO2: Post bomb contributions from fossil fuels, oceans, the stratosphere, and the terrestrial biosphere. Glob. Biogeochem. Cyc. 2002, 16, 1112. [Google Scholar] [CrossRef]
- Bernard, S.; Frisén, J.; Spalding, K.L. A mathematical model for the interpretation of nuclear bomb test derived 14C incorporation in biological systems. Nucl. Instrum. Methods Phys. Res. Sect. B: Beam Interact. Mater. At. 2010, 268, 1295– 1298.
- Dickson, A.G.; Millero, F.J. x A comparison of the equilibrium constants for dissociation of carbonic acid in seawater media. Deep Sea Res. Contrib. 2019, 34, 1733–1743. [Google Scholar] [CrossRef]
- Millero, F.J.; Graham, T.B.; Huang, F.; Bustos-Serrano, H.; Pierrot, D. Dissociation constants of carbonic acid in seawater as a function of salinity and temperature. Mar. Chem. 2006, 100, 80–94. [Google Scholar] [CrossRef]
- Millero, F.; Huang, F.; Graham, T.; Pierrot, D. The dissociation of carbonic acid in NaCl solutions as a function of concentration and temperature. Geochim. 2007, 71, 46–55. [Google Scholar] [CrossRef]
- Millero, F.J. ; Thermodynamics of the carbon dioxide system in the oceans. Geochim. Cosmochim. Acta 1995, 59, 661–677. [Google Scholar] [CrossRef]
- Emerson, S.; Hedges, J. Carbonate Chemistry. In Chemical Oceanography and Marine Carbon Cycle, 2008, pp. 101–133, Cambridge University Press.
- Orr, J.C.; Epitalon, J.M.; Gattuso, J.P. Comparison of the packages that compute ocean carbonate chemistry. Biogeosci. 2007, 12, 1483–1510. [Google Scholar] [CrossRef]
- Millero, F.J. Carbonate constants for estuarine waters. Mar. Freshwater Res. 2010, 61, 139–142. [Google Scholar] [CrossRef]
- Goulding, K.W.T. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Man. 2016, 32, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Le Quéré, C.; Peters, G.P.; Jones, M.W. Fossil CO2 emissions in the post-COVID-19 era. Nature Clim. Change 2020, 11, 197–199. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Smith, L. The mass of the atmosphere. J. Clim. 2005, 18, 864–875. [Google Scholar] [CrossRef]
- Dore, J.E.; Sadler, D.W.; Church, M.J.; Karl, D.M. ; Physical and biogeochemical modulation of ocean acidification in the central North Pacific. PNAS USA 2009, 106, 12235–12240. [Google Scholar] [CrossRef]
- Bromfield, S.M.; Cumming, R.W.; David, D.J.; Williams, C.H. Changes in soil pH, manganese and aluminium under subterranean pasture. Aust. J. Exp. Agric. Anim. Husb. 1988, 23, 181–191. [Google Scholar] [CrossRef]
- Prentice, I.C.; Farquhar, M.J.R.; Fasham, M.L.; Goulden, M.L.; Heimann, M.; Jarmilla, V.J.; Kheshgi, H.S.; Le Quere, C.; Scholes, R.J.; Wallace, D.W.R. The carbon cycle and atmospheric carbon dioxide. In Climate Change 2001, Chapter 3 The Scientific Basis, Contribution to the Third Assessment Report of the IPCC.
- Ciais, P.; Sabine, C.; Balu, G.; Bopp, L.; Brovkin, V.; Canadell, J.; Chabra, A. ; Defries. R.; Galloway J.; Heimann, M.; Jones, C.; Le Quere C.; Myneni, R.B.; Piao, S.; Thornton, P. Carbon and other biogeochemical cycles. 2013, In: Climate Change 2013: The Physical Basis Contribution of Working Group 1 to Fifth Assessment Report of the IPCC, Cambridge Press UK, USA.
- Stone, Y.; Ahern, C.R.; Blunden, B. Acid Sulfate Soil Manual. 1998, NSW Agriculture, Wollongbar, NSW 2477, Australia.https://www.epa.nsw.gov.au/~/media/EPA/Corporate%20Site/resources/epa/Acid-sulfate-Manual-1998.ashx.
- West, T.O.; McBride, A.C. The contribution of agricultural lime to carbon dioxide emissions in the United States: dissolution, transport and net emissions. Agric. Ecosys. Environ. 2005, 108, 145–154. [Google Scholar] [CrossRef]
- Zamanian, K.; Zarebanaadkouki, M.; Kuzyakov, Y. Nitrogen fertilization raises CO2 efflux from inorganic carbon: A global assessment. Glob. Change Biol. 2018, 24, 2810–2817. [Google Scholar] [CrossRef]
- Sharififar, A.; Minasny, B.; Arruays, D.; et al. Soil inorganic carbon, the other and equally important soil carbon pool: Distribution, controlling factors and the impact of climate change. Adv. Agron. 2023, 178, 165–231. [Google Scholar] [CrossRef]
- Sun, X.l.; Minasny, B.; Wu, Y.J.; Wang, H.L.; Fan, X.H.; Zhang, G.L. Soil organic carbon content increase in the east and south of China is accompanied by soil acidification. Sci. Total Env. 2022, 8587, 159253. [Google Scholar] [CrossRef]
- Püspök, J.F.; Zhao, S.; Calma, A.D.; Vourlitis, G.L.; Allison, S.D.; Aronson, E.L.; Schimel, J.P.; Hamam, E.J.; Homyak, P.M. Effects of experimental nitrogen deposition on soil organic carbon storage in Southern California drylands. Glob. Change Biol. 2022. [CrossRef] [PubMed]
- Raza, S.; Zamanian, K.; Ullah, S.; Kuzyakov, Y.; Virto, I.; Zhou, J. Inorganic carbon losses by soil acidification jeopardize global efforts on carbon sequestration and climate change mitigation. J. Clean. Prod. 2024, 315, 128036. [Google Scholar] [CrossRef]
- Huang, Y.; Song, X.; Wang, Y.P. : Canadell, J.G.; Luo, Y.; Ciais, P.: et al., Size, distribution, and vulnerability of the global soil inorganic carbon. Science 2024, 384, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Fan, L.; Zhou, J.; Banfield, C.C.; Kuzyakov, Y.; Zamanian, K. Nitrification-induced acidity controls CO2 emission from soil carbonates. Soil Biol. Biochem. 2024, 192, 10939. [Google Scholar] [CrossRef]
- Maes, S.L.; Dietrich, J.; Midolo, G.; Schwieger, S.; Kummu, M.; et al. Environmental drivers of increased ecosystem respiration in a warming tundra. Nature 2024, 629, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Scanes, E.; Scanes, P.R.; Ross, P.M. Climate change rapidly warms and acidifies Australian estuaries. Nature Commun. 2020, 11, 1803–1814. [Google Scholar] [CrossRef] [PubMed]
- Doney, S.C.; Mahowald, N.; Lima, I.; Feely, R.A.; Mackenzie, F.T.; Lamarque, J.F.; Rasch, P.J. Impact of anthropogenic atmospheric nitrogen and sulphur deposition on ocean acidification and the inorganic carbon system. PNAS 2007, 104, 14580–14585. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.R.; Edman, M.; Gallego-Urrea, J.A.; Claremor, B.; Hasselöv, I.M.; Omstedt, A.; Rutgersson, A. The potential future contribution of shipping to acidification of the Baltic Sea. Ambio 2018, 47, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Slattery, W.J.; Ridley, A.M.; Windsor, S.M. Ash alkalinity of animal and plant products. 1991, Austral. J. Exp. Agric. 31, No. 3.
- Chen, J.M.; Mo, G.; Deng, F. A joint global carbon inversion system using both CO2and 13CO2 atmospheric concentration data. Geosci. Model Develop. 2017, 10, 1131–1156. [Google Scholar] [CrossRef]
- Luoi, W.T.; Nelson, P.N.; Li, M.H.; Cai, J.P.; Zhang, Y.Y.; Zhang, Y.G.; Yang, S.; Wang, R.Z.; Wang, Z.W.; Wu, Y.N.; Han, X.G.; Jiang, Y. Contrasting pH buffering patterns in neutral-alkaline soils along a 3600 km transect in northern China. Biogeosci 2015, 12, 7047–7056. [Google Scholar] [CrossRef]
- Yoch, D. Dimethylsulfoniopropionate: Its sources, role in the marine food web, and biological degradation to dimethylsulfide. Appl. Environ. Micro. 2002, 68, 5804–5815. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.D. Dimethyl sulfide production and marine phytoplankton: The importance of species composition and cell size. Biol. Ocean. 1989, 6, 375–382. [Google Scholar]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Ann. Rev. Plant Biol. 1994, 40, 503–537. [Google Scholar] [CrossRef]
- Wright, G.C.; Rao, R.C.N.; Farquhar, G.D. Water-use efficiency and carbon isotope discrimination in peanut under water deficit conditions. Crop Sci. 1994, 34, 92–97. [Google Scholar] [CrossRef]
- Barbour, M.M.; Farquhar, G.D. (2001) Relative humidity-and ABA-induced variation in carbon and oxygen isotope ratios of cotton leaves. Plant Cell Environ. 2001, 23, 473–485. [Google Scholar] [CrossRef]
- Piao, S.; Wang, X.; Park, T.; Chen, C.; Lian, X.; He, Y.; Bjerke, J.W.; Chen, A.; Ciais, P.; Tømmervik, H.; Nemani, R.R.; Myneni, R.B. Characteristics, drivers and feedbacks of global greening. Nature Rev. 2020, 1, 14–27. [Google Scholar] [CrossRef]
- Mackay, D. Multimedia Environmental Models; The Fugacity Approach. 2001, 2nd Edition Lewis Publishers, Boca Raton.
- Im-Erb, R.; Bamroongrugsa, N.; Kawashima, K.; Amano, T.; Kato, S. Utilisation of coal ash to improve acid soil. J. Sci. Technol. 2004, 26, 697–708. [Google Scholar]
- Ukwattage, N.L.; Ranjith, P.G. Accelerated carbonation of coal combustion fly ash for atmospheric carbon dioxide sequestration and soil amendment. J. Poll. Effec. Contain. 2018, 6, 210–217. [Google Scholar]
- Back, S.K.; Mojannal, A.H.M.; Jo, H.H.; Kim, J.H.; Jeong, M.J.; Seo, Y.C.; Joung, H.T.; Kim, S.H. Increasing seawater alkalinity using fly ash to restore the pH and the effect of temperature on seawater flue gas desulphurization. J. Mater. Cyc. Waste Manag. 2019, 21, 962–973. [Google Scholar]
- Kim, A.G. (1999) The reaction of acid mine drainage with fly ash from coal combustion. 1999, Proc. Amer. Soc. Mining data Reclamation pp. 111–117.
- Ganguli, N.; Kennedy, I.R. (2013) Indigenous actinorhizal plants of Australia. J. Biosci. 2013, 38, 749–759. [Google Scholar] [CrossRef]
- Haruthaithanasan, M.; Pinyopusarerk, K.; Nicodemus, A.; Bush, D.; Thomson, L. Casuarinas for green economy and environmental sustainability. 2020, Proc.Sixth Inter. Casuarina Workshop, Krabi Thailand. Kasetsart Agricultural and Agro-Industrial Product Improvement Institute, Kasetsart University: Bangkok. 306 pp. ISBN 978-616-278-583-2.
- Bashan, Y.; Holguin, G.; de-Bashan, LE. Azospirillum-plant relationships: physiological, molecular, agricultural, and environmental advances (1997-2003) Can. J. Microbiol. 2004, 50, 521–577. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, I.R.; Choudhury, A.; Kecskés, M.L. Non-symbiotic bacterial diazotrophs in crop-farming systems: can their potential for plant growth promotion be better exploited? Soil Biol. Biochem. 2004, 36, 1229–1244. [Google Scholar] [CrossRef]
- Seneviratne, G.; Zavahir, J.S.; Bandara, W.R.S.M.; Weerasekara, M. Fungal-bacterial biofilms: their development for novel biotechnological applications. World J. Microbiol. Biotech. 2007, 24, 739–743. [Google Scholar] [CrossRef]
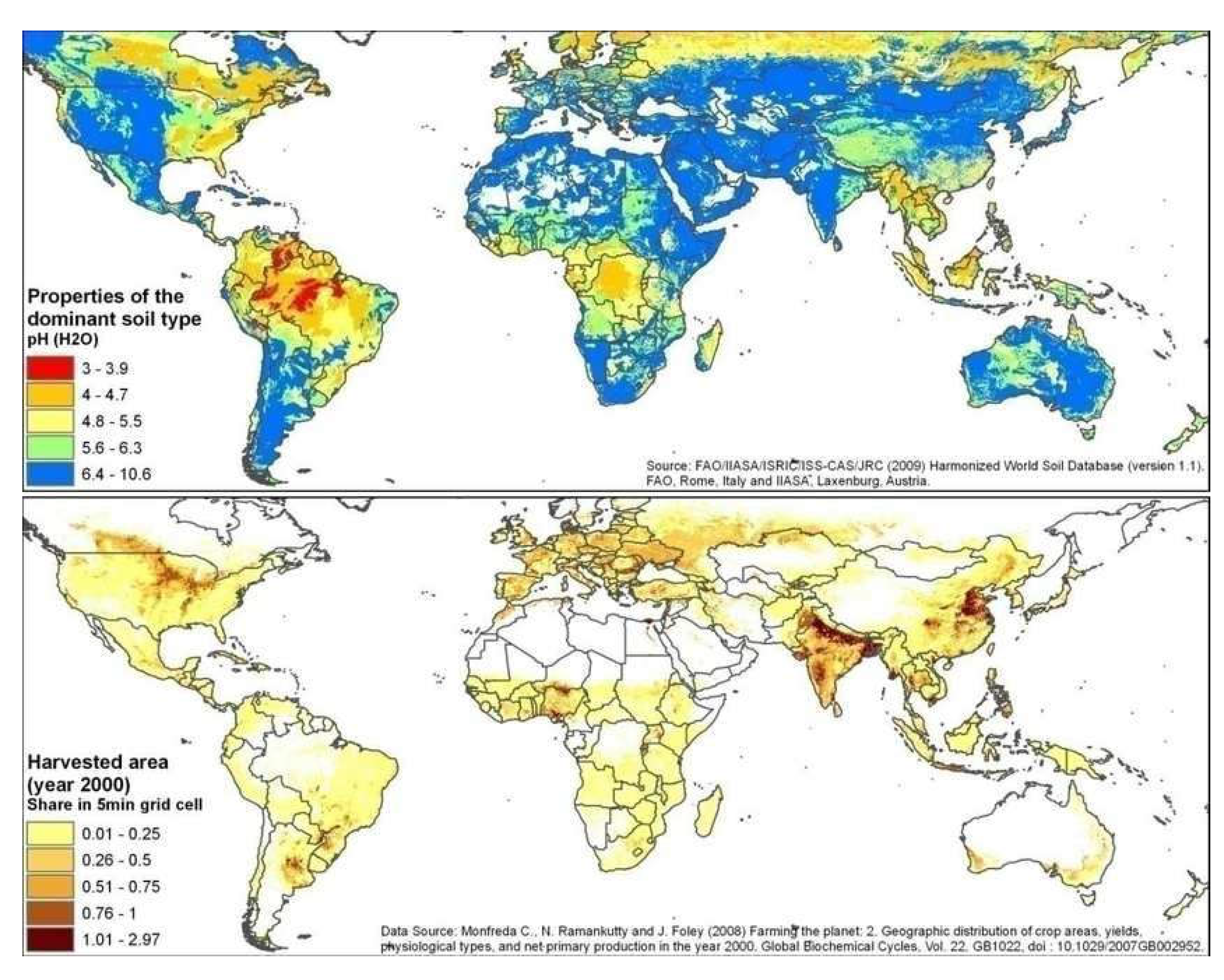
- Monfreda, C.; Ramankutty, N.; Foley, J. Farming the planet: 2. Geographic distribution of crop areas, yields, physiological types and net primary production in the year. Glob. Biochem. Cycl, 1022. [Google Scholar] [CrossRef]
- Kennedy, I.R.; Ganguli, N. N2-fixing trees for profitable farm-forestry. Agric. For. 2016, 62, 29–36. [Google Scholar] [CrossRef]
- Cael, B.B.; Bisson, K.; Boss, E.; Dutkiewicz, S.; Henson, S. Global climate-change trends detected in indicators of ocean ecology. 2023. [CrossRef]
- Canadell, J.G.; et al. , Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity and efficiency of natural sinks. Proc. Natl. Acad. Sci. USA 2007, 104, 18–866. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sutton, A.J.; Hu, C. Quantifying the atmospheric CO2 forcing effect on surface ocean pCO2 in the North Pacific subtropical gyre in the past two decades. Front. Mar. Sci. 2021, 8, 636861. [Google Scholar] [CrossRef]
- Hodzic, M.; Kennedy, I. R Kalman filter harmonic bank for Vostok ice core data analysis and climate predictions. 2021, Glaciers and the Polar Environment, Kanao, M., Godone, D., Dematteis, N. /: htpps. [CrossRef]
- Maddox, J. What remains to be discovered. p. 340, Macmillan 1998, London United Kingdom.
- Kennedy, I.R.; Hodzic, M. Applying the action principle of classical mechanics to the thermodynamics of the troposphere. Appl. Mech. 2023, 4, 729–775. [Google Scholar] [CrossRef]
- Mothapo, N.; Chen, H.; Cubeta, M.A.; Grossman, J.M.; Fuller, F.; Shi, W. Phylogenetic, taxonomic and functional diversity of fungal denitrifiers and associated N2O production efficacy. Soil Biol. Biochem. 2015, 83, 160–175. [Google Scholar] [CrossRef]
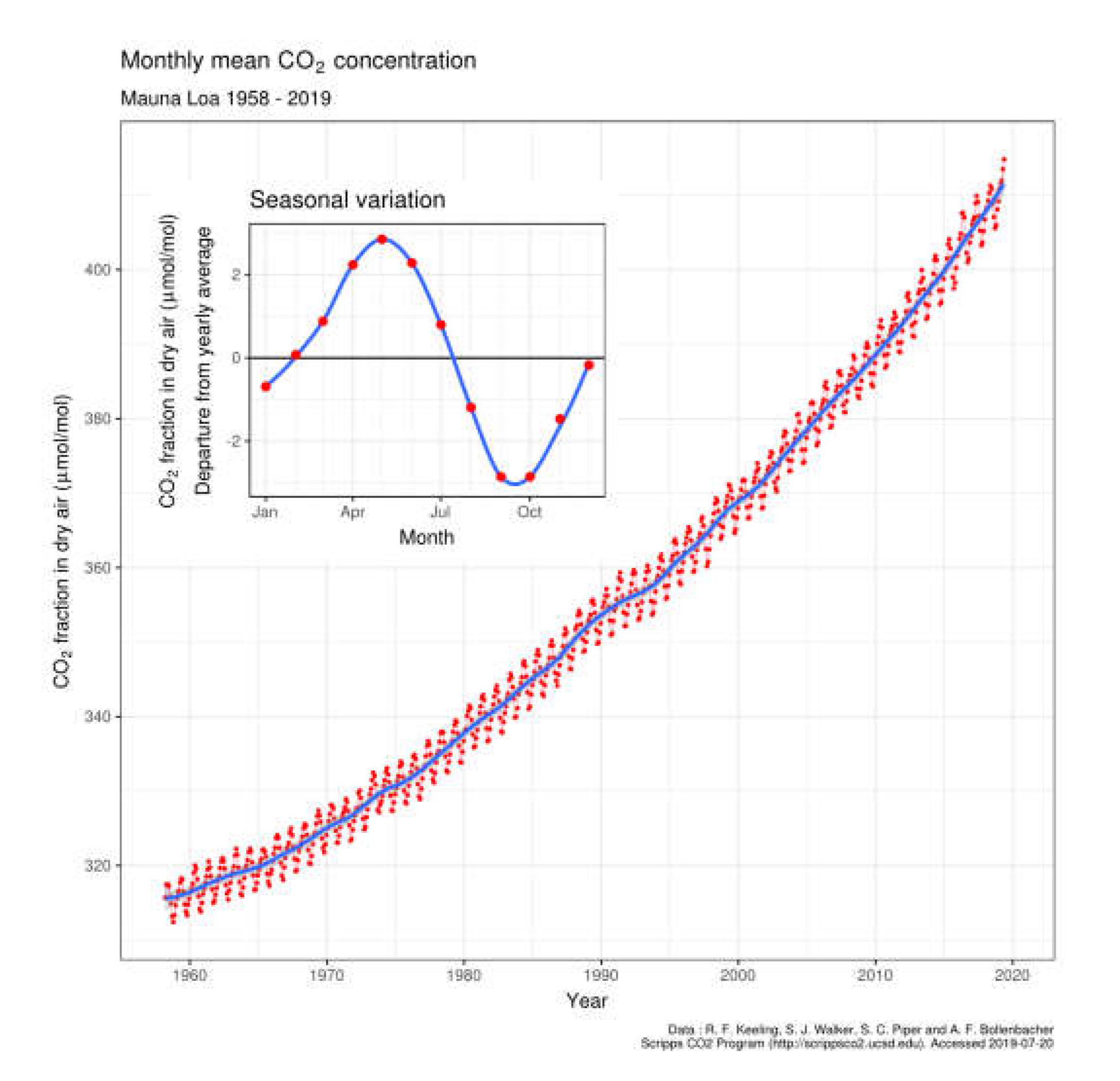
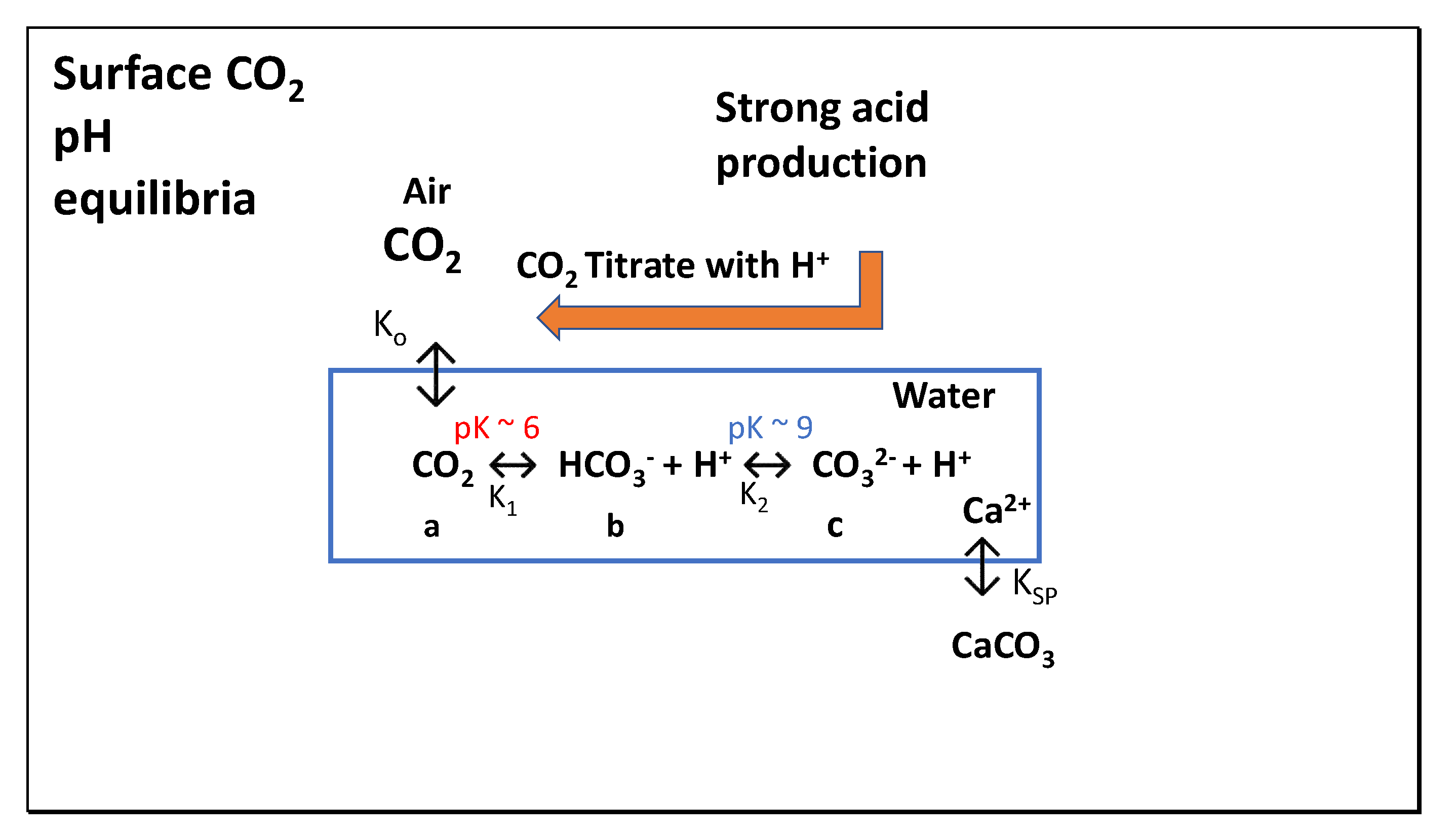
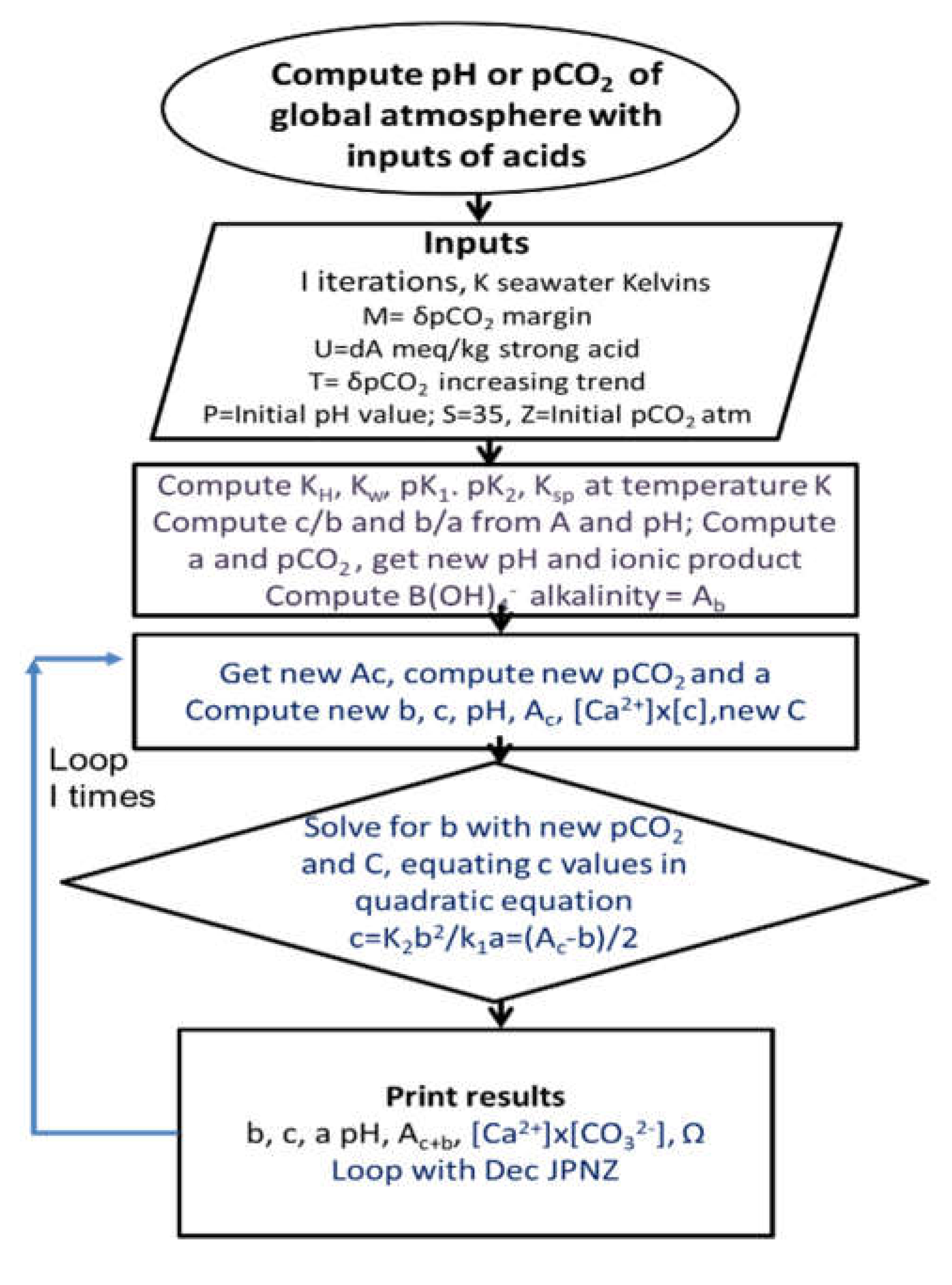
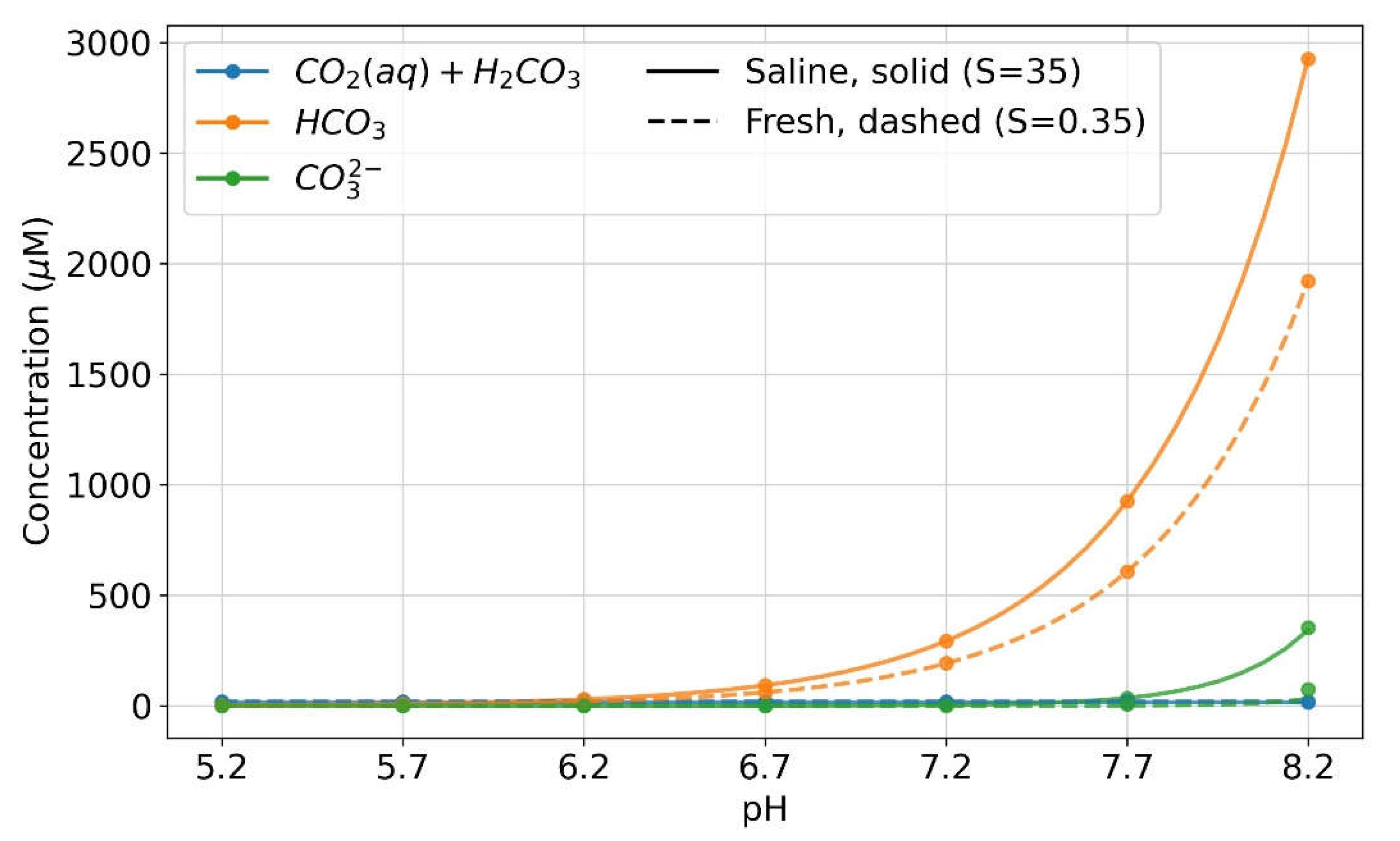
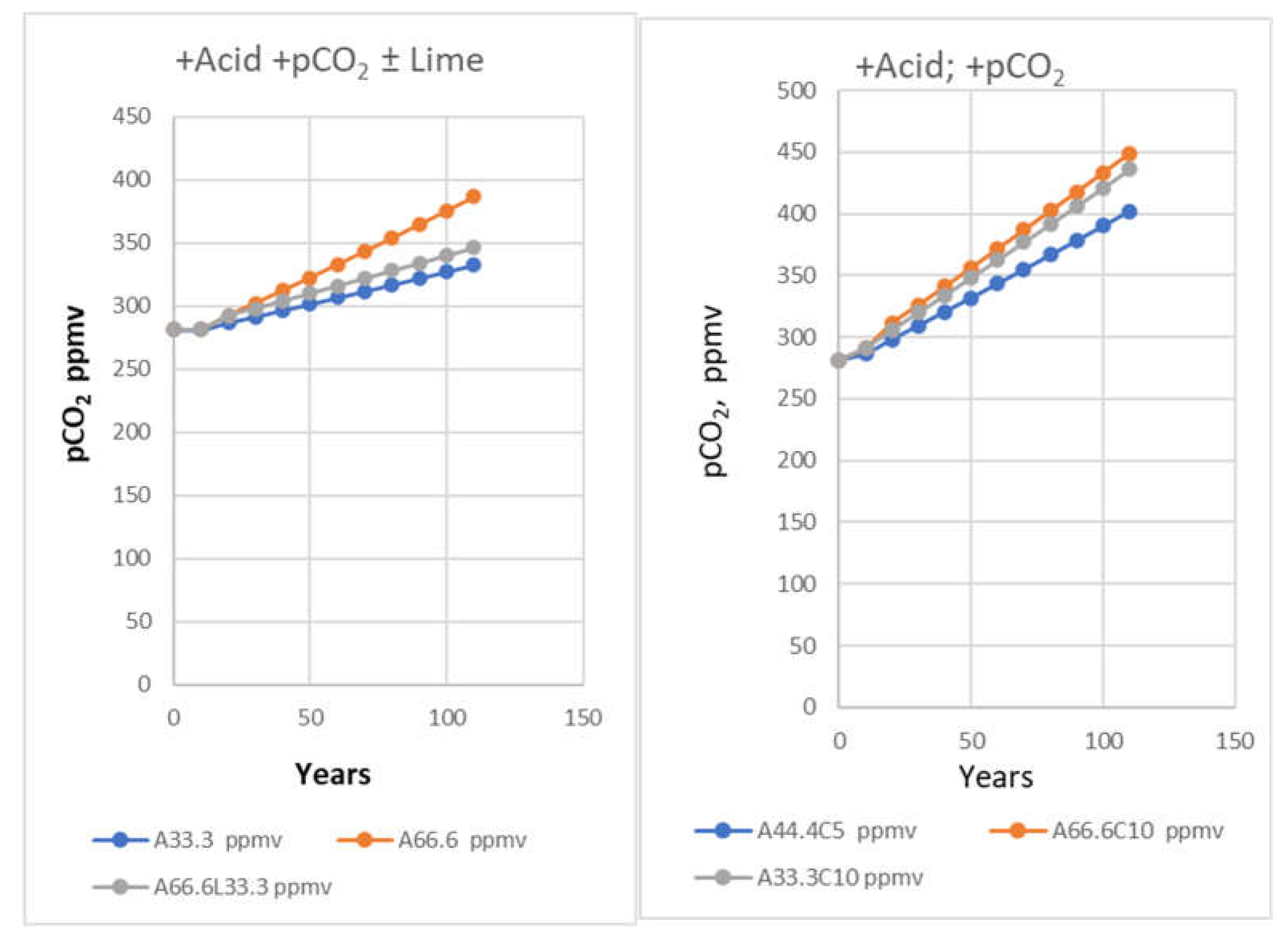
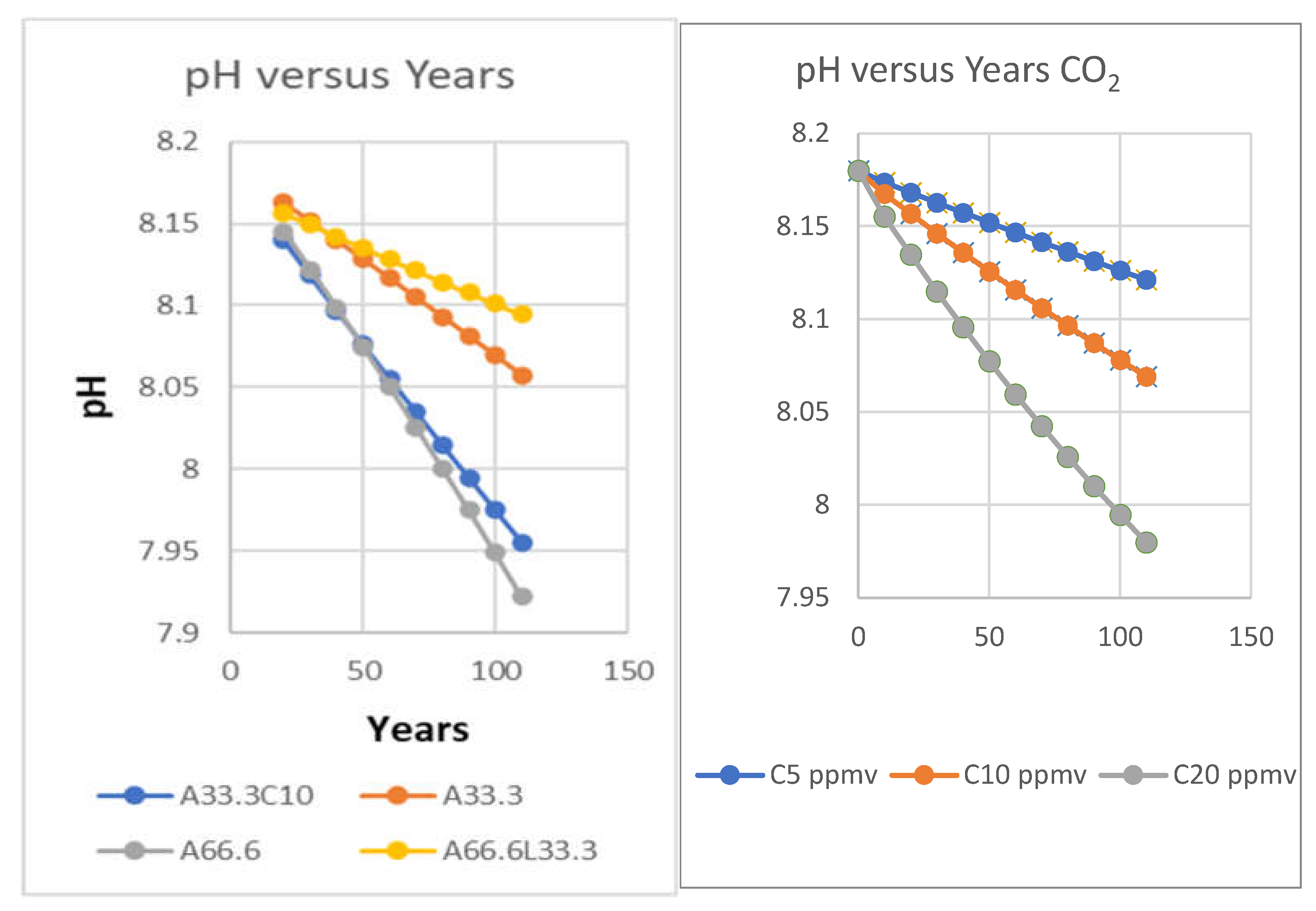
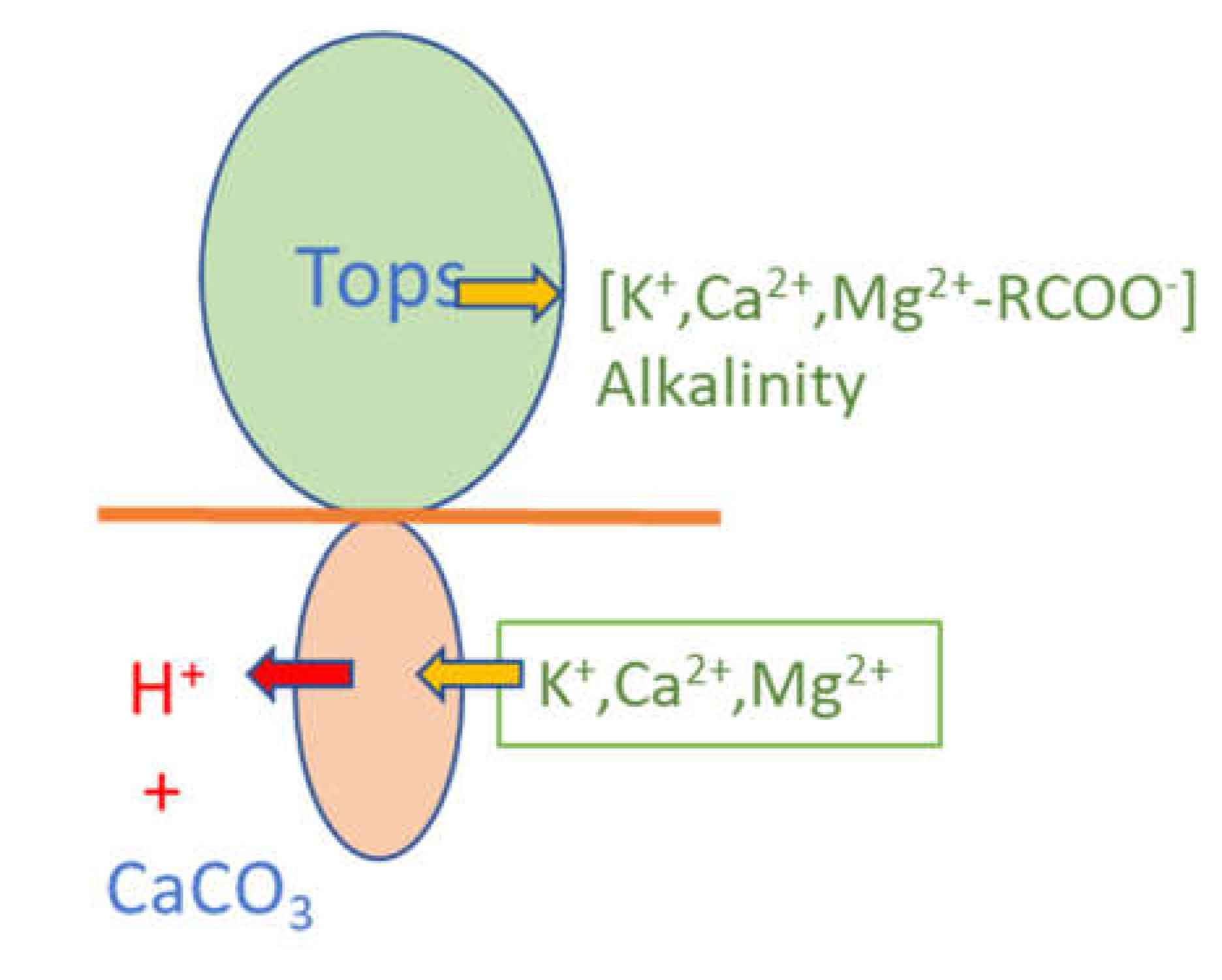
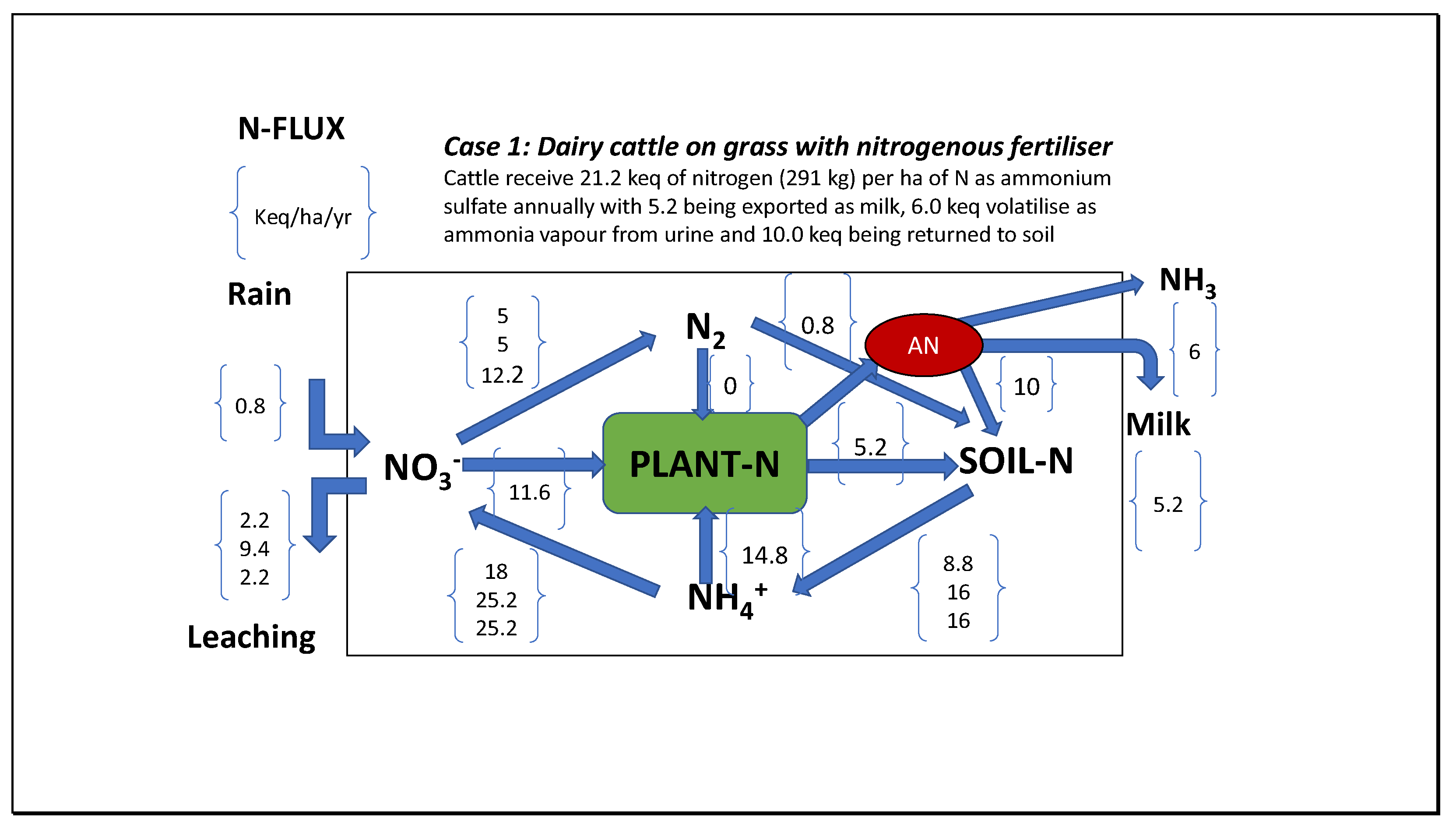
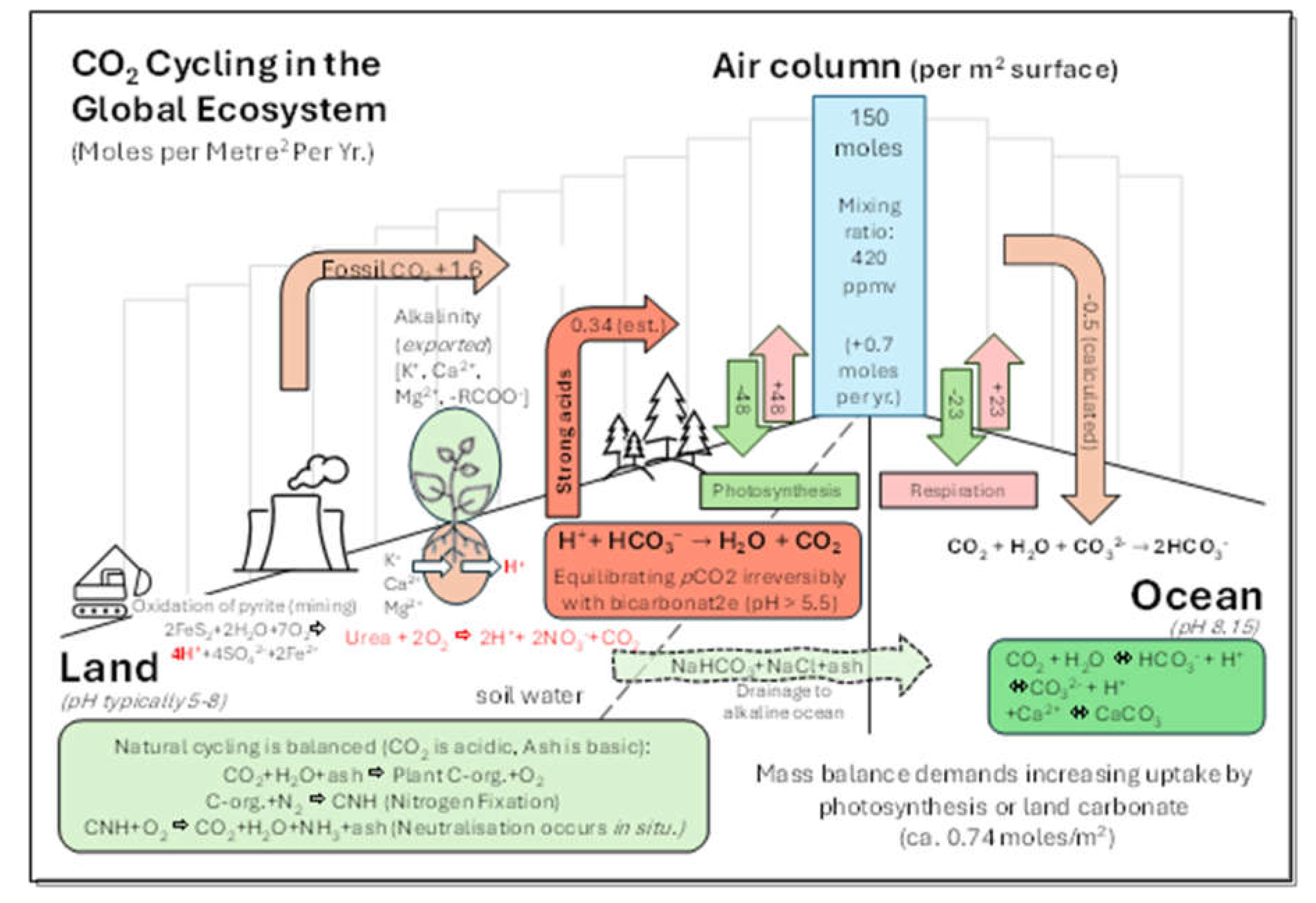
| Year | ppmv. | Moles atmospheric CO2/m2 | Increase moles/m2 | Total global atmospheric CO2 Teratonnes (1012) |
|---|---|---|---|---|
| 1750 | 280 | 95.61 | 0 | 2.1459 |
| 2015 | 400 | 140.00 | 44.390 | 3.1422 |
| Fuel | Tonnes | Gram CO2 | Moles C | Moles S (5%) | Moles SO2/m2 | Total acidic moles CO2/m2 |
|---|---|---|---|---|---|---|
| Total global CO2 emissions since 1750 | 2.5x1012 | 2.5x1018 | 5.682x1016 | 1.065x1015 | 2.130 | 4.26 |
| Annual CO2 emissions StatisticaTM 2020 | 3.7x1010 | 3.68x1016 | 8.364x1014 | 1.55x1013 | 0.032* | 0.06 |
| Salt (NaCl ‰) | 35 | 35 | 35 | 35 | 0.35 | 0.35 | 0.35 | 0.35 |
| pH | 8.2 | 7.2 | 6.2 | 5.2 | 8.2 | 7.2 | 6.2 | 5.2 |
| [CO2] μM = a | 15.8 | 15.8 | 15.8 | 15.8 | 19.2 | 19.2 | 19.2 | 19.2 |
| [HCO3-] μM = b | 2930.2 | 293.0 | 29.3 | 0.93 | 1924.7 | 192.5 | 19.2 | 1.92 |
| [CO32-] μM = c | 352.1 | 0.35 | 0.0004 | 0.000 | 74.3 | 0.07 | 0.001 | 0.000 |
| [HCO3-]/[CO32-] | 8.27 | 82.7 | 827.4 | 8274.4 | 25.7 | 257.5 | 2574.9 | 25749.1 |
| C=a+b+c μM | 3298.1 | 312.3 | 45.1 | 16.7 | 2018.2 | 217.4 | 38.4 | 21.12 |
| Acidifying process | Moles of global strong acid production | Moles of CO2 increase (meq/m2) |
Statistical confidence |
|---|---|---|---|
| Increase in atmospheric CO2 (2021) | 664.0 | Strong | |
| Sources of strong acid production | Sulphuric, nitric | , protons | |
| Fossil fuels (2% fossil CO2 moles untrapped SO2) | 32.8x1012 | 64.0±10 | Moderate |
| Acidification from agriculture and forestry | 37.5x1012 | 78.4±10 | Moderate |
| Increasing wildfire acidification S and N | 44x1012 | 64.0±20 | Low |
| Anaerobic sulphate respiration, H2S, DMS | 25x1012 | 98.0±20 | Low |
| Mining exposure, refining metal, sulfides, pyrites | 10x1012 | 20.0±50 | Low |
| Total decrease in alkalinity from strong acids | 197x1012 | 324.4±110 | Approximate |
| Product exported | Ash alkalinity mmoles H+/kg |
CaCO3 equivalent kg/tonne |
Estimated limestone kg/ha | H+ per m2 per crop |
|---|---|---|---|---|
| Lamb | 340 | 17 | 1700 (1 t/ha) | 0.34 |
| Milk | 80 | 4 | 400 (1 t/ha | 0.08 |
| Clover | 822 | 41 | 41,100 (5t/ha) | |
| Lucerne | 1203 | 60 | 60.000 (10t/ha) | 6.00 |
| Wheat | 184 | 9 | 9,000 (5t/ha) | 0.90 |
| Lupin | 404 | 20 | 20,000 (5 t/ha) | 2.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





