1. Introduction
Gestational diabetes (GDM), characterized by high blood sugar levels during pregnancy that affects both maternal and newborn outcomes, is a common complication of pregnancy [
1]. GDM is often classified into two categories, Class A1 and Class A2, with Class A1 being the milder form that can typically be managed with diet and lifestyle modifications, Despite being a milder form, GDM Class A1 cases can still present challenges in predicting and managing neonatal outcomes, particularly in identifying neonates who may require neonatal intensive care unit (NICU) admission [
2].
Fetal Doppler ultrasound, a non-invasive imaging technique, is a valuable tool in assessing fetal well-being and identifying potential complications [
3]. The fetal splenic artery PI, Doppler-derived parameter, has been used to evaluate fetal hemodynamics and perfusion [
4,
5]. Some studies have demonstrated a correlation between abnormal fetal splenic artery PI and adverse neonatal outcomes in various pregnancy complications, such as fetal growth restriction [6-10].
In this prospective study, it was investigated whether fetal splenic artery Doppler measured in cases followed for gestational diabetes class A1 predicts the need for neonatal intensive care. By identifying neonates at risk of requiring NICU care, healthcare professionals can better prepare and allocate resources, ensuring optimal care for both mothers and their babies.
2. Materials and Methods
In this study, 75 single pregnancy cases diagnosed with gestational diabetes mellitus class A1 who applied to our high-risk pregnancy outpatient clinic were evaluated. in one year period. Ethics committee approval was obtained.
The study group were aged 18-40 years, who presented at the polyclinic for routine antenatal follow-up at third trimester of pregnancy (>28 weeks) and had the diagnosis of gestational diabetes class A1. Only the pregnant women at 30-34 weeks of gestation (including the limit weeks) were included in the study. In the first examination of the pregnant cases, it was determined that they did not contain any high-risk pregnancy factors other than gestational diabetes, and liver, kidney, thyroid and hematological test results were within normal limits. Gestational diabetes mellitus (GDM) diagnosis was made with 75 g oral glucose tolerance test (OGTT). The 75 gram OGTT was used for diagnosing diabetes in pregnant individuals. The two-hour 75 gram OGTT was diagnostic of GDM when one glucose value was elevated. According to this test, fasting blood glucose was measured following an 8-hour fasting period, and then a solution containing 75 grams of glucose was drunk. Blood glucose was measured 1 hour and two hours after drinking the solution. GDM was diagnosed when fasting blood glucose was 92 or 1st hour blood glucose was 180 or second hour blood glucose was 153 mg/dL and above. Only the cases that were euglycemic with diet were accepted as GDM Class A1.
Cases with any systemic disease, use of drugs other than vitamins and iron, multiple pregnancy, smoking, biochemical/hormonal/hematological test disorders, and cases diagnosed with congenital fetal anomaly were not included in the studyCases with abnormal uterine, umbilical, middle cerebral artery Doppler findings were not included in the study. 15 cases not fullfiled the inclusion criteria were excluded from the study. 11 cases who did not come for pregnancy follow-ups and 4 cases who developed any pregnancy complications were excluded from the study.
In the study, all participating women provided informed consent. Key information collected included age, gravidity, parity, and body mass index (BMI), as well as sonographic measurements of fetal biometry, such as biparietal diameter (BPD), abdominal circumference (AC), femur length (FL), and estimated fetal weight (EFW). Gestational age was verified using first trimester ultrasound data.
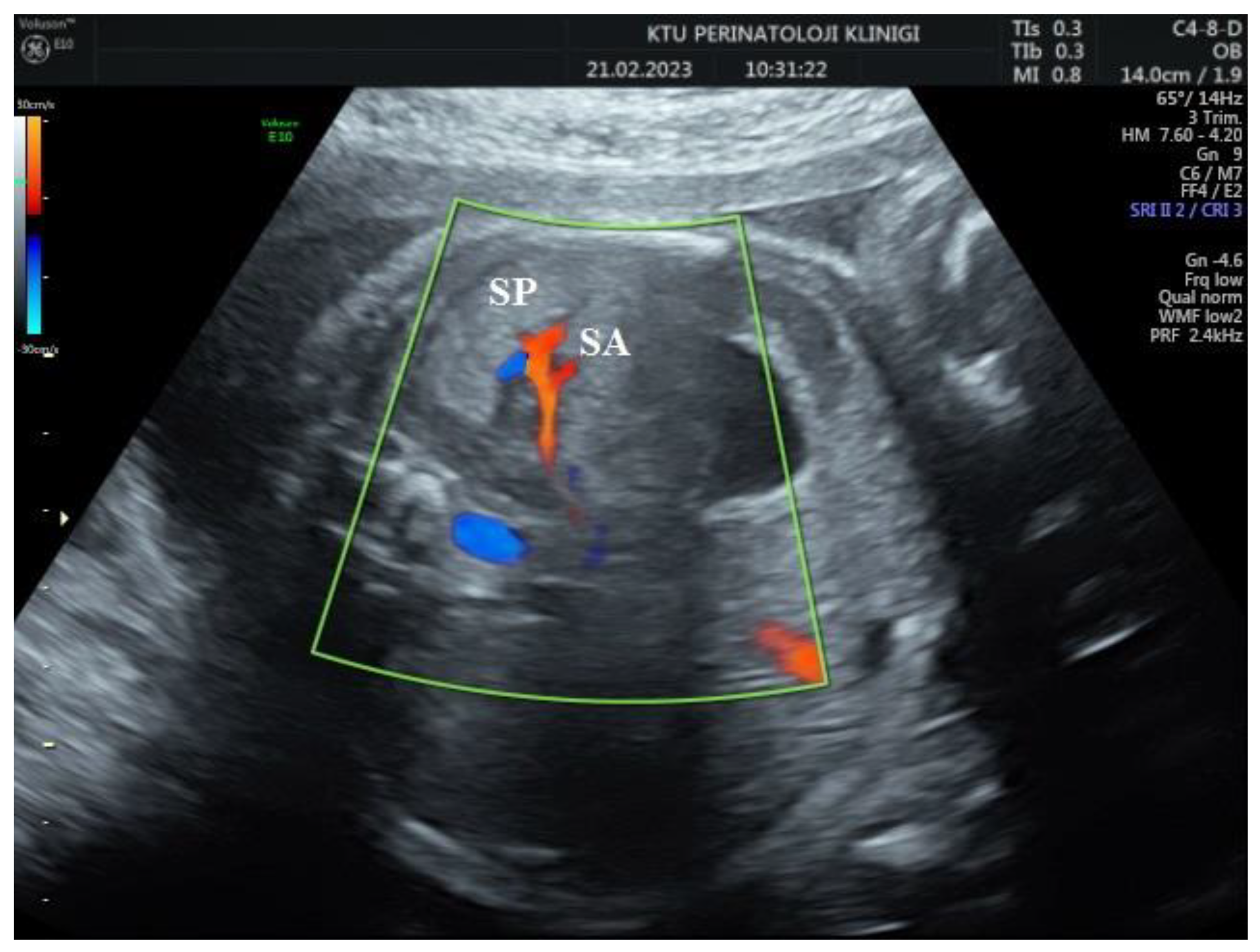
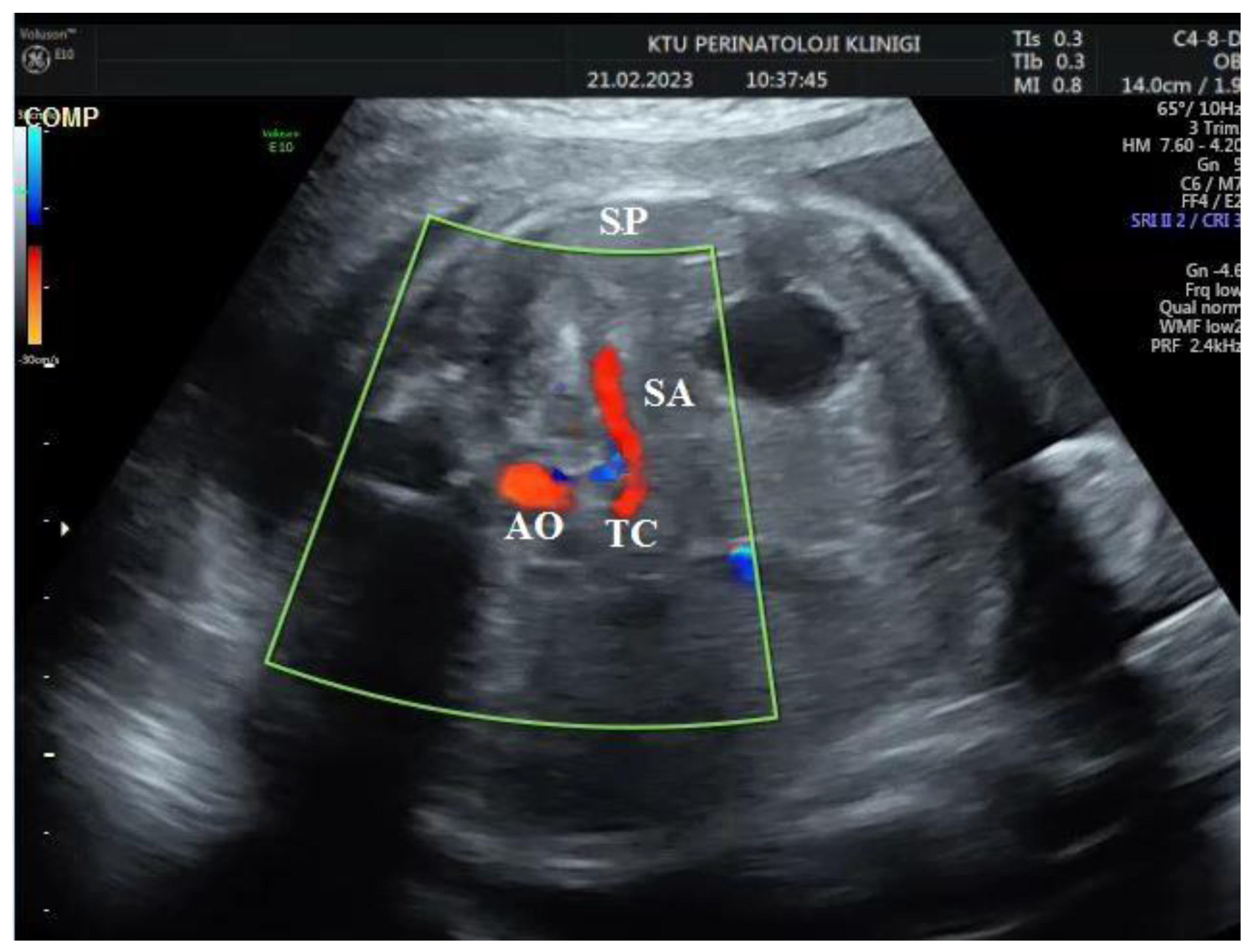
During the Doppler assessment, the patient was positioned on the examination table in a supine posture. It was measured when the fetus was inactive. A standard AC axial section view was obtained. Stomac pocket was visualized. Color Doppler was placed on the upper part of the stomach pocket and the splenic artery was visualized at the entrance to the spleen. Later, it was observed that the trace progressed towards the truncus celiacus and abdominal aorta. Color Doppler was placed at the entrance to the spleen with a 0-degree insomination angle, without the splenic artery entering the spleen. Fetal splenic artery Doppler parameters Peak systolic velocity (PSV), Pulsatility index (PI), Resistivity index (RI) and End diastolic velocity (EDV) were measured in all cases (
Figure 1 and
Figure 2).
All sonographic measurements was perfomed via the 2016 model VOLUSON GE E10 sonograph unit. All sonographic measurements were made at least twice by an experienced clinician. All cases were followed up until the end of pregnancy, and the birth data of the mother and the baby (fetal birthweight, fetal umbilical cord blood gas analysis) and the need for neonatal intensive care were recorded.
A total of 12 cases required neonatal intensive care due to respiratory distress, and their data were compared to those not needing intensive care.
Statistical Analysis
Data were analyzed using SPSS 13 software. The Levene test assessed variance homogeneity, while the Kolmogorov-Smirnov test evaluated normal distribution compatibility. Chi-square and Student's t-tests were employed for statistical analysis. To identify fetal splenic artery Doppler parameters for predicting neonatal intensive care requirements, ROC analysis was conducted. All data are presented as mean ± standard deviation or percentage. The ability of fetal doppler parameters to predict neonatal intensive care needs was assessed using binary logistic regression analysis.
The correlation between the parameters was evaluated with the help of Pearson correlation test. The situation where the p value was below 0.05 was considered significant.
Ethical Aproval
The study protocol was approved by current university ethic board (number 2020/37, date 28/01/2020).
3. Results
The data of 60 cases in total were evaluated . The demographic and sonographic factors of cases who need fetal intensive care and those not are shown in
Table 1.
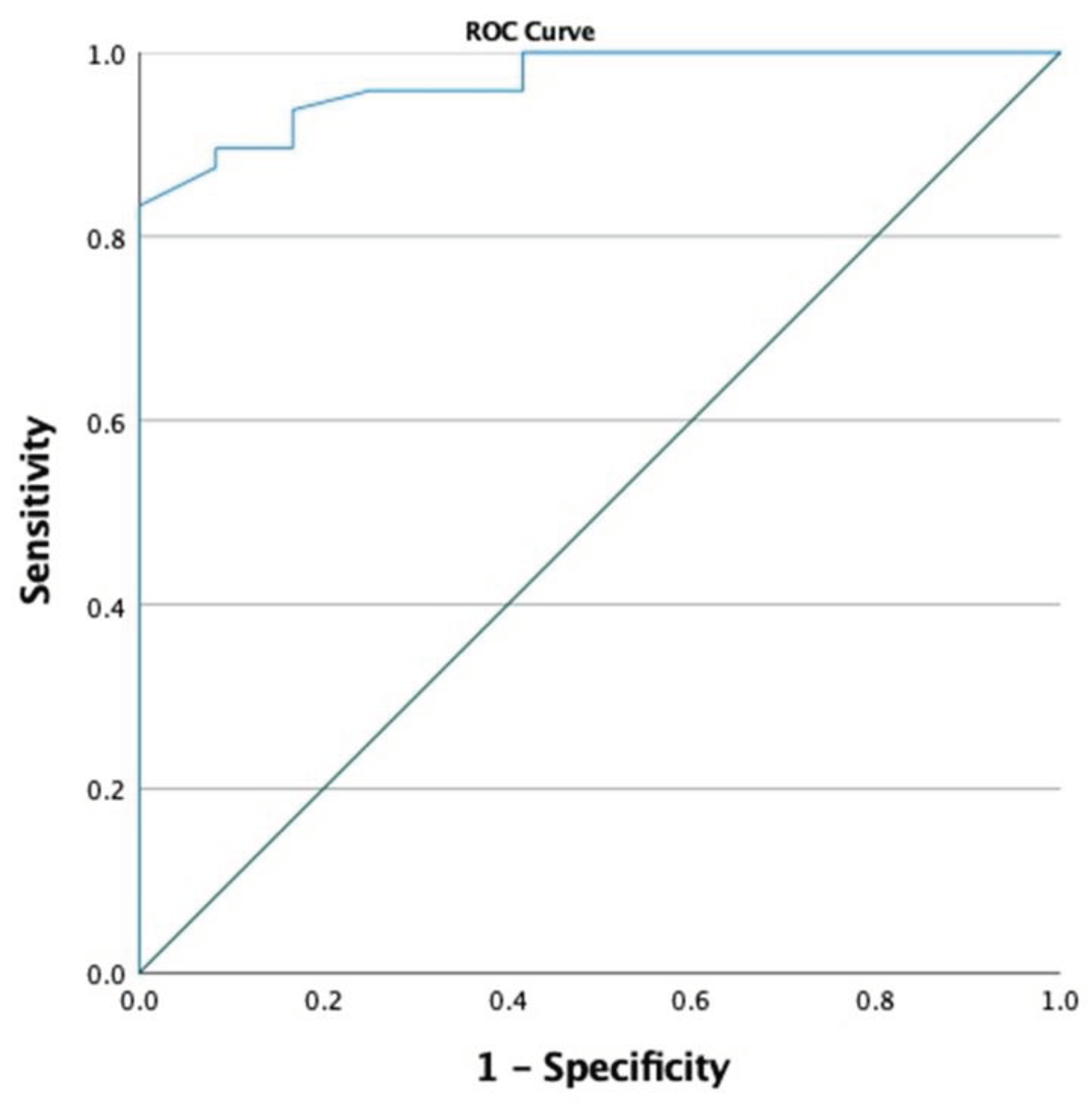
In cases with need fetal intensive care, fetal splenic PI index was found to be statistically significantly lower than in healthy cases without it (0.94 ± 0.29 vs. 1.70 ± 0.53, respectively, p<0.001, Student-t test,). When the fetal splenic PI cutoff value was selected as 1.105, the sensitivity was calculated as 97.9% and the specificity as 58.3% for predicting the neeed for fetal intensive care (AUC 0.968, p <0.001, 95% CI 0.929-0.998,
Figure 3). The use of low fetal splenic artery PI parameter is a significant and good indicator for predicting the need for fetal intensive care according to the binary logistic regression analysis result (p = 0.006).
A moderate positive correlation was found in terms of fetal umbilical cord pH and BE at delivery and prenatal fetal splenic doppler PI in all cases (Pearson correlation, r=0.334, p=0.009, Pearson Correlation test).
4. Discussion
This study evaluated the relationship between fetal splenic artery Doppler parameters and the need for NICU in pregnant women with GDM class A1. The results showed that a lower fetal splenic artery PI was significantly associated with an increased need for NICU, with a sensitivity of 97.9% and specificity of 58.3% for predicting the need for neonatal intensive care when the fetal splenic PI was taken as 1.105. The results also showed a moderate positive correlation between prenatal fetal splenic Doppler PI and fetal umbilical cord pH and BE at delivery.
The importance of fetal Doppler parameters in predicting adverse neonatal outcomes has been well-documented in the literature [
7,
11]. Previous studies have focused on umbilical and middle cerebral artery Doppler measurements in high-risk pregnancies, including those with GDM [
12]. Limited research has been conducted on the relationship between fetal splenic artery Doppler parameters and neonatal outcomes, particularly in pregnancies complicated by GDM class A1. In the past decade, abnormal Doppler velocity patterns in various fetal vessels and their correlation with fetal outcomes have been extensively studied [13-15]. Recently, the pathophysiology of blood redistribution in relation to fetal growth deprivation has been evaluated [
16,
17]. Epidemiological evidence suggests that changes in blood distribution due to fetal growth deprivation may increase the risk of myocardial damage and abnormal liver function in newborns [
18,
19]. Decreased resistance in the splenic artery has been suggested as an indicator of fetal distress, and a decreased fetal liver circulation has recently been proposed as a predictor for the prognosis of fetal growth restriction [
7,
20].
The assessment of fetal well-being is crucial in prenatal care, particularly for high-risk pregnancies. Fetal splenic artery Doppler ultrasonography is a non-invasive technique that has been increasingly utilized for evaluating fetal well-being and predicting adverse perinatal outcomes. The technique measures blood flow velocities in various vessels, including the splenic artery, which supplies blood to the spleen, an organ essential for fetal immune system development The Doppler indices, such as resistance index (RI), pulsatility index (PI), and systolic/diastolic ratio (S/D), can help assess blood flow and detect abnormalities in fetal circulation [
21].
Abuhamad et al. (1992) were the first to report the use of Doppler velocimetry in evaluating the main splenic artery of fetuses [
6]. They noted a reduction in the splenic artery RI in a subset of fetuses classified as small for gestational age (SGA). The authors theorized that the associated chronic hypoxia led to the release of erythropoietin, which increased splenic flow to support increased erythropoiesis. This reduction in RI in some SGA fetuses was later confirmed by Capponi et al. in a subsequent study [
22].
Several theories attempt to explain the reduced splenic artery pulsatility index (PI) observed in cases of fetal growth restriction. Recent research has shown that compensatory vasodilation in the splenic artery helps maintain low venous perfusion to the fetal liver [
20,
23]. The exact connection between a decreased splenic artery PI and fetal growth restriction remains unclear. However, one study discovered that a lower splenic artery PI correlated with higher perinatal mortality rates, lower Apgar scores, and metabolic acidosis. This suggests that severe fetal deprivation may lead to more significant hemodynamic changes in the spleen, potentially identifying fetuses at an increased risk of perinatal death [
24].
In a study, it was determined that fetal splenic artery PI values were lower in fetuses small for gestational age compared to normal fetuses. This supports the argument that vascular resistance is lower in SGA fetuses [
25].
In another study, fetal splenic artery Doppler measurement was performed in pregnancies with late-onset fetal growth restriction and similar results were reported. The researchers found that decreased splenic artery PI was significantly and positively correlated with a higher probability of experiencing adverse obstetric outcomes [
3].
Neonatal outcomes of splenic artery Dopplers in gestational diabetic patients have not yet been studied. As a result, our study is the first of its kind in the literature. The current study demonstrates that fetal splenic artery PI could serve as a valuable indicator for predicting the need for neonatal intensive care in GDM class A1 pregnancies. A possible explanation for this finding could be that a lower PI reflects increased placental resistance and, consequently, impaired fetal blood flow, which could negatively impact fetal growth and wellbeing. This notion is supported by the observed moderate positive correlation between prenatal fetal splenic Doppler PI and fetal umbilical cord pH and BE at delivery, suggesting a relationship between splenic artery PI and fetal acid-base status.
The results of this study are consistent with previous findings that have demonstrated the utility of fetal Doppler measurements in predicting neonatal outcomes in high-risk pregnancies. However, the current study adds to the existing body of knowledge by specifically focusing on GDM class A1 pregnancies and examining the association between fetal splenic artery Doppler parameters and neonatal outcomes.
The most important limitation of our study is the small number of cases. Additionally, the study did not examine the long-term neonatal outcomes beyond the need for neonatal intensive care. Future research with larger sample sizes and follow-up of neonatal outcomes is warranted to further validate and expand upon these findings.
5. Conclusions
We believe that using fetal splenic artery Doppler measurements in mothers with gestational diabetes mellitus (GDM) can help predict which neonates may require NICU. We aimed to determine the best method for predicting NICU needs for infants born to mothers with GDM. As our study suggests, the evaluation of fetal splenic artery Doppler could be a brief and valuable answer to this question.
We kindly recommend this simple, rapid, and valuable evaluation of fetal splenic artery Doppler to obstetricians. By doing so, it may be a safe approach to alert the neonatologist that NICU might be required, allowing for better preparedness and resource allocation.
The results of this study suggest that fetal splenic artery PI could be a valuable indicator for predicting the need for neonatal intensive care in pregnant women with GDM class A1. This finding could have potential clinical implications, as it may help guide prenatal care and counseling for women with GDM, thereby potentially improving neonatal outcomes. Further research is needed to confirm and extend these findings in larger populations and to explore the long-term neonatal outcomes associated with fetal splenic artery Doppler parameters.
Authors contribution
All authors (MA, HA, ESGG, and SG) participated and contributed o data collection, literature review, and editing the manuscript.
Institutional Review Board statement
The study protocol was approved by current university ethic board (number 2020/37, date 28/01/2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Any data set is available on request to the Corresponding author.
Acknowledgments
The authors thank Koc EA for her contributions at the beginning of the study.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- American Diabetes, A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes care 2018, 41, S13–S27. [Google Scholar] [CrossRef] [PubMed]
- Landon, M.B. , Spong, C.Y., Thom, E., Carpenter, M.W., Ramin, S.M., Casey, B., Wapner, R.J., Varner, M.W., Rouse, D.J., Thorp Jr, J.M. A multicenter, randomized trial of treatment for mild gestational diabetes. New England Journal of Medicine 2009, 361, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Alfirevic, Z. , Stampalija, T., Dowswell, T. Fetal and umbilical Doppler ultrasound in high-risk pregnancies. The Cochrane database of systematic reviews 2017, 6, CD007529. [Google Scholar] [PubMed]
- Cohen D., A. , R. . The fetal splenic artery:The fetal splenic artery: Doppler assessment and its application for the evaluation of fetal well-being.. Ultrasound in Obstetrics & Gynecology 2001, 17, 105–109. [Google Scholar]
- Vedmedovska, N. , Rezeberga, D., Teibe, U., Zodzika, J., Donders, G.G. Adaptive changes in the splenic artery and left portal vein in fetal growth restriction. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine 2012, 31, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Abuhamad, A.Z. , Mari, G. , Bogdan, D., Evans III, A.T. Doppler flow velocimetry of the splenic artery in the human fetus: is it a marker of chronic hypoxia? American journal of obstetrics and gynecology 1995, 172, 820–825. [Google Scholar]
- Capponi, A. , Rizzo, G., Arduini, D., Romanini, C. Splenic artery velocity waveforms in small-for-gestational-age fetuses: relationship with pH and blood gases measured in umbilical blood at cordocentesis. American journal of obstetrics and gynecology 1997, 176, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Ozalp, M. , Demir, O., Ozbay, G., Akbas, M., Aran, T., Osmanagaoglu, M.A. The utility of foetal splenic artery Doppler measurement in the diagnosis of late-onset foetal growth restriction. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology 2022, 42, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Oz, U. , Kovanci, E., Jeffress, A., Mendilicioglu, I., Mari, G., Bahado-Singh, R.O. Splenic artery Doppler in the prediction of the small-for-gestational age infant. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2002, 20, 346–350. [Google Scholar]
- Tongsong, T. , Tongprasert, F., Srisupundit, K., Luewan, S. Splenic artery: peak systolic velocity of normal fetuses. Archives of gynecology and obstetrics 2010, 281, 829–832. [Google Scholar] [CrossRef]
- Baschat, A.A. Fetal growth restriction - from observation to intervention. Journal of perinatal medicine 2010, 38, 239–246. [Google Scholar] [CrossRef]
- Ebrashy, A. , Azmy, O., Ibrahim, M., Waly, M., Edris, A. Middle cerebral/umbilical artery resistance index ratio as sensitive parameter for fetal well-being and neonatal outcome in patients with preeclampsia: case-control study. Croatian medical journal 2005, 46, 821–825. [Google Scholar] [PubMed]
- Verburg, B.O. , Jaddoe, V.W., Wladimiroff, J.W., Hofman, A., Witteman, J.C., Steegers, E.A. Fetal hemodynamic adaptive changes related to intrauterine growth: the Generation R Study. circulation 2008, 117, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Baschat, A.A. , Cosmi, E., Bilardo, C.M., Wolf, H., Berg, C., Rigano, S., Germer, U., Moyano, D., Turan, S., Hartung, J. Predictors of neonatal outcome in early-onset placental dysfunction. Obstetrics & Gynecology 2007, 109, 253–261. [Google Scholar]
- Cheema, R. , Dubiel, M., Breborowicz, G., Gudmundsson, S. Fetal cerebral venous Doppler velocimetry in normal and highâ€risk pregnancy. Ultrasound in Obstetrics and Gynecology: The Official Journal of the International Society of Ultrasound in Obstetrics and Gynecology 2004, 24, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Bellotti, M. , Pennati, G., De Gasperi, C., Bozzo, M., Battaglia, F.C., Ferrazzi, E. Simultaneous measurements of umbilical venous, fetal hepatic, and ductus venosus blood flow in growth-restricted human fetuses. American journal of obstetrics and gynecology 2004, 190, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Kiserud, T. , Ebbing, C., Kessler, J., Rasmussen, S. Fetal cardiac output, distribution to the placenta and impact of placental compromise. Ultrasound in Obstetrics and Gynecology 2006, 28, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Mäkikallio, K. , Vuolteenaho, O., Jouppila, P., Räsänen, J. Ultrasonographic and biochemical markers of human fetal cardiac dysfunction in placental insufficiency. circulation 2002, 105, 2058–2063. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V. , Marcellini, M., Marchesini, G., Vanni, E., Manco, M., Villani, A., Bugianesi, E. Intrauterine growth retardation, insulin resistance, and nonalcoholic fatty liver disease in children. Diabetes care 2007, 30, 2638–2640. [Google Scholar] [CrossRef]
- Ebbing, C. , Rasmussen, S., Godfrey, K.M., Hanson, M.A., Kiserud, T. Redistribution pattern of fetal liver circulation in intrauterine growth restriction. Acta Obstetricia et Gynecologica Scandinavica 2009, 88, 1118–1123. [Google Scholar] [CrossRef]
- Baschat, A.A. Doppler application in the delivery timing of the preterm growth-restricted fetus: another step in the right direction. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2004, 23, 111–118. [Google Scholar]
- Rizzo, G. , Capponi, A., Arduini, D., Romanini, C. The value of fetal arterial, cardiac and venous flows in predicting pH and blood gases measured in umbilical blood at cordocentesis in growth retarded fetuses. BJOG: An International Journal of Obstetrics & Gynaecology 1995, 102, 963–969. [Google Scholar]
- Dubiel, M. , Korszun, P., Breborowicz, G., Gudmundsson, S. Fetal hepatic artery blood flow velocimetry in normal and high-risk pregnancies. Prenatal and Neonatal Medicine 2001, 6, 151–156. [Google Scholar]
- Vedmedovska, N. , Rezeberga, D., Teibe, U., Zodzika, J., Donders, G.G. Adaptive changes in the splenic artery and left portal vein in fetal growth restriction. Journal of Ultrasound in Medicine 31, 223-229.
- Mari, G. , Abuhamad, A.Z., Uerpairojkit, B., Martinez, E., Copel, J.A. Blood flow velocity waveforms of the abdominal arteries in appropriate- and small-for-gestational-age fetuses. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 1995, 6, 15–18. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).