Submitted:
26 April 2024
Posted:
28 April 2024
You are already at the latest version
Abstract
Keywords:
1. Background
2. Halide Perovskites
2.1. HC(NH2)2PbI3 and Its Derivatives
2.2. (CH3NH3)x(HC(NH2)2)1-xPbI3 Perovskite
2.3. (HC(NH2)2)1−xCsxPbI3 Perovskites
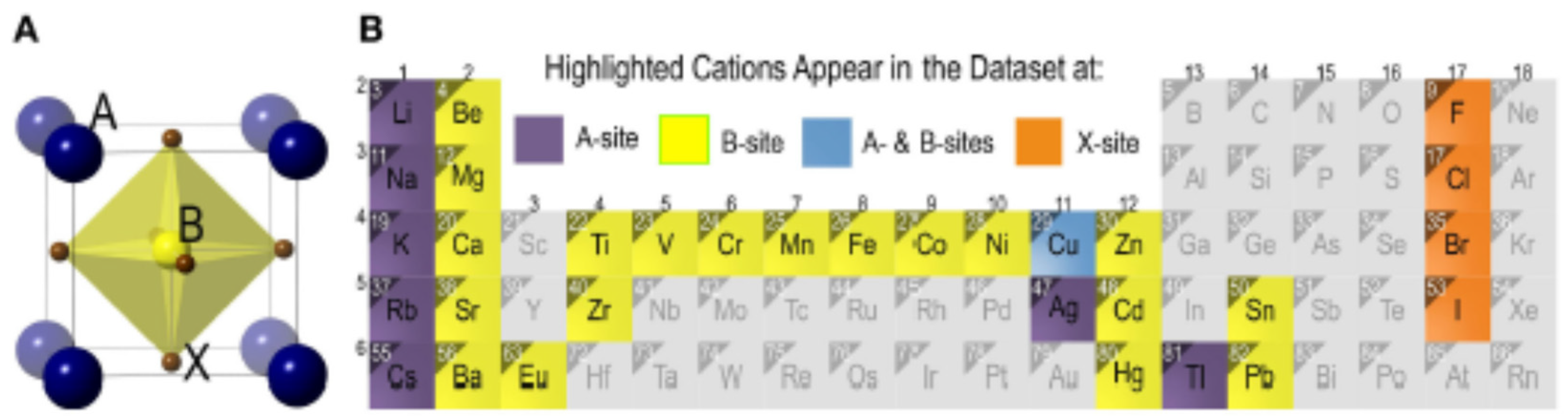
2.4. The Difficulty of Replacing Lead Atom by Other Metals
- a)
- Ionic radius: best outlined by the tolerance factor: Replacing the toxic Pb in perovskite crystals needs an atom with similar size. The excellent performance of Pb-based perovskites is mainly due to high structural symmetry and strong antibonding coupling between Pb and I.
- b)
- High polarizability: lead (II) is considered a softer or borderline hard/soft cation, has plarizable outer electrons, large size, low electronegative and should interact most strongly' with donor types. Typically a soft cation will covalently bond with a soft donor atom which has low electronegativity, highly polarizable low-lying empty orbitals and is easily oxidized, and a hard cation will form an ionic bond with a donor atom which has high electronegativity, low polarizability, and high energy empty orbitals and is hard to oxidize.
- c)
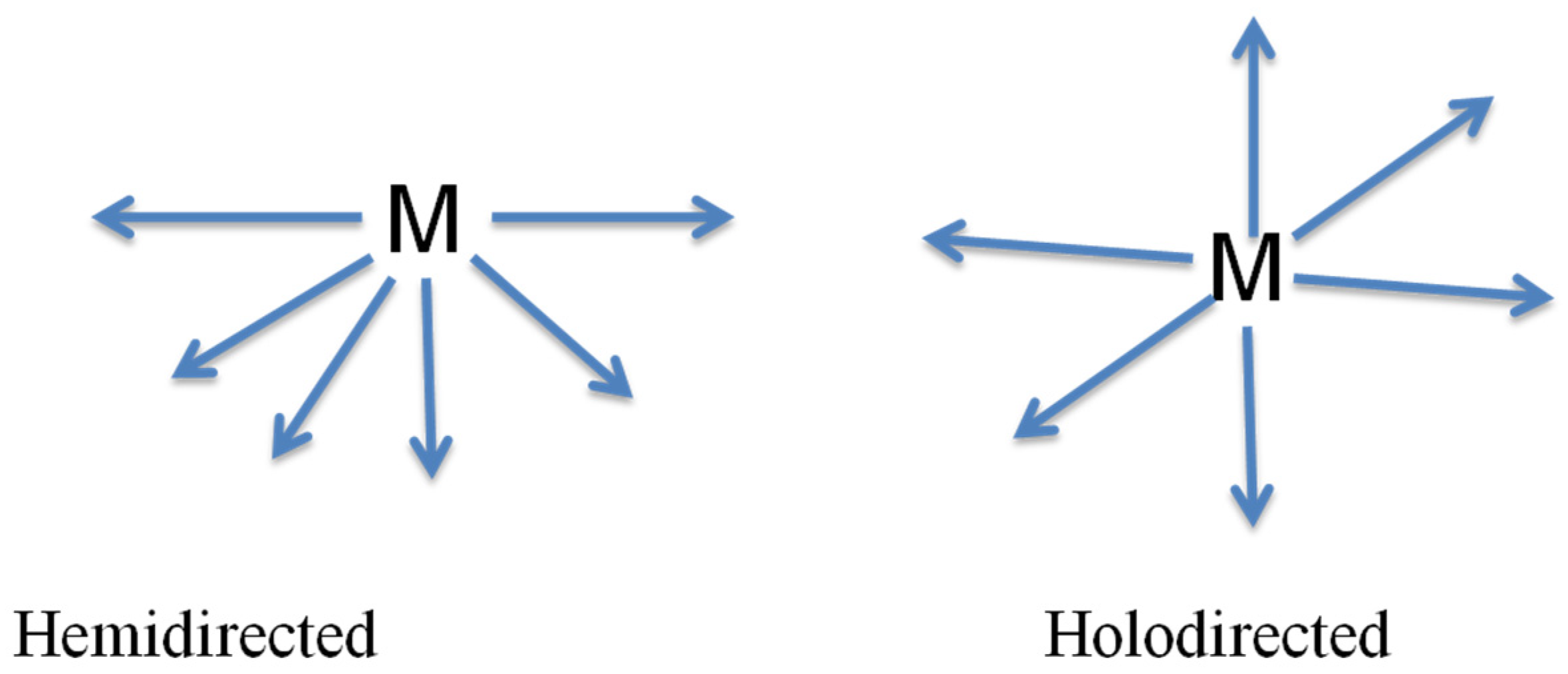
- Valence: a B site atom has in a perfect world a 2+ valence, different configurations are conceivable, and however, they require remuneration to accomplish charge neutrality. Lead (II) has stable oxidation state of +2 with coordination number of 6. All six PbII-X bonds of the halogen ligands, the holodirected structures in which the ligand atoms are connected to each other are clearly ionic, but the ionic character of the bonds decreases as the atomic number of the halogen ligand increases and greater transfer of electron density from the ligands to the lead occurs as the electronegativity of the ligand decreases and the bond become covalent bond. If the arrangement is holodirected geometry, the PbII-ligand bonds are all similar.
- d)
- Lone pairs: Ideally the B-site displays 6s2 lone pair. When considering every one of these elements, of each of the 120+ elements just lead has this alluring mix of properties.
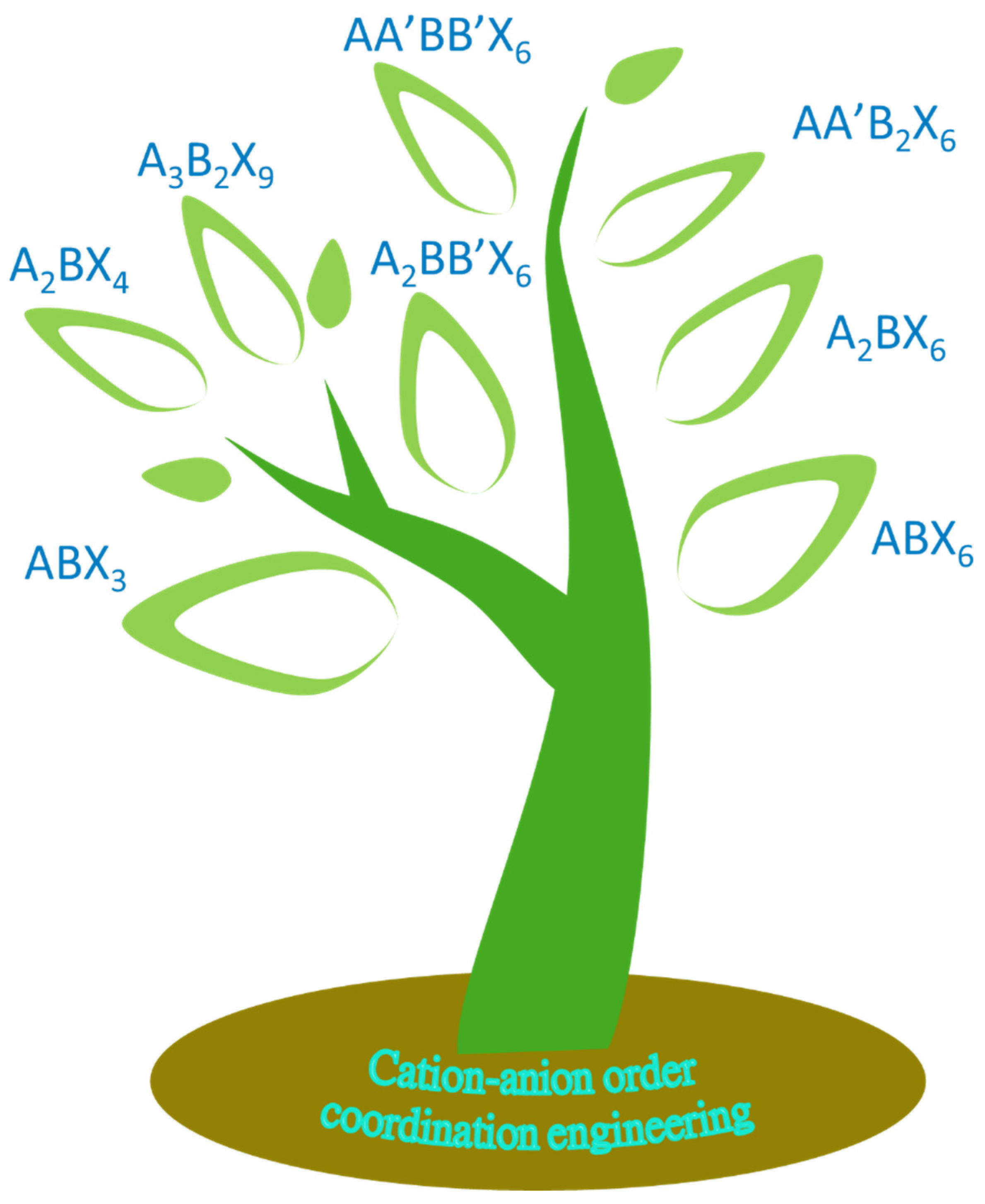
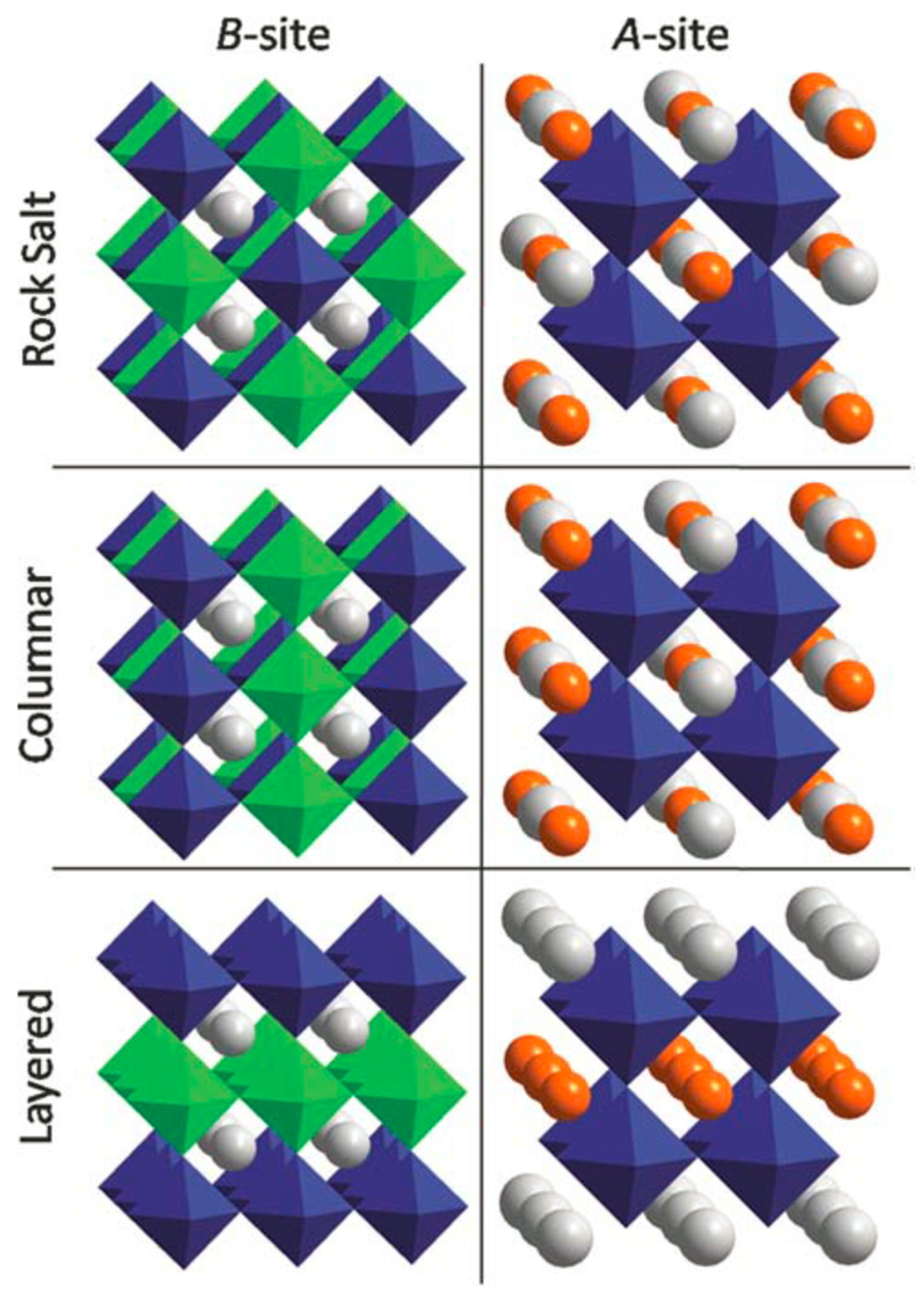
3. Coordination Engineering of Halide Perovskite Crystals
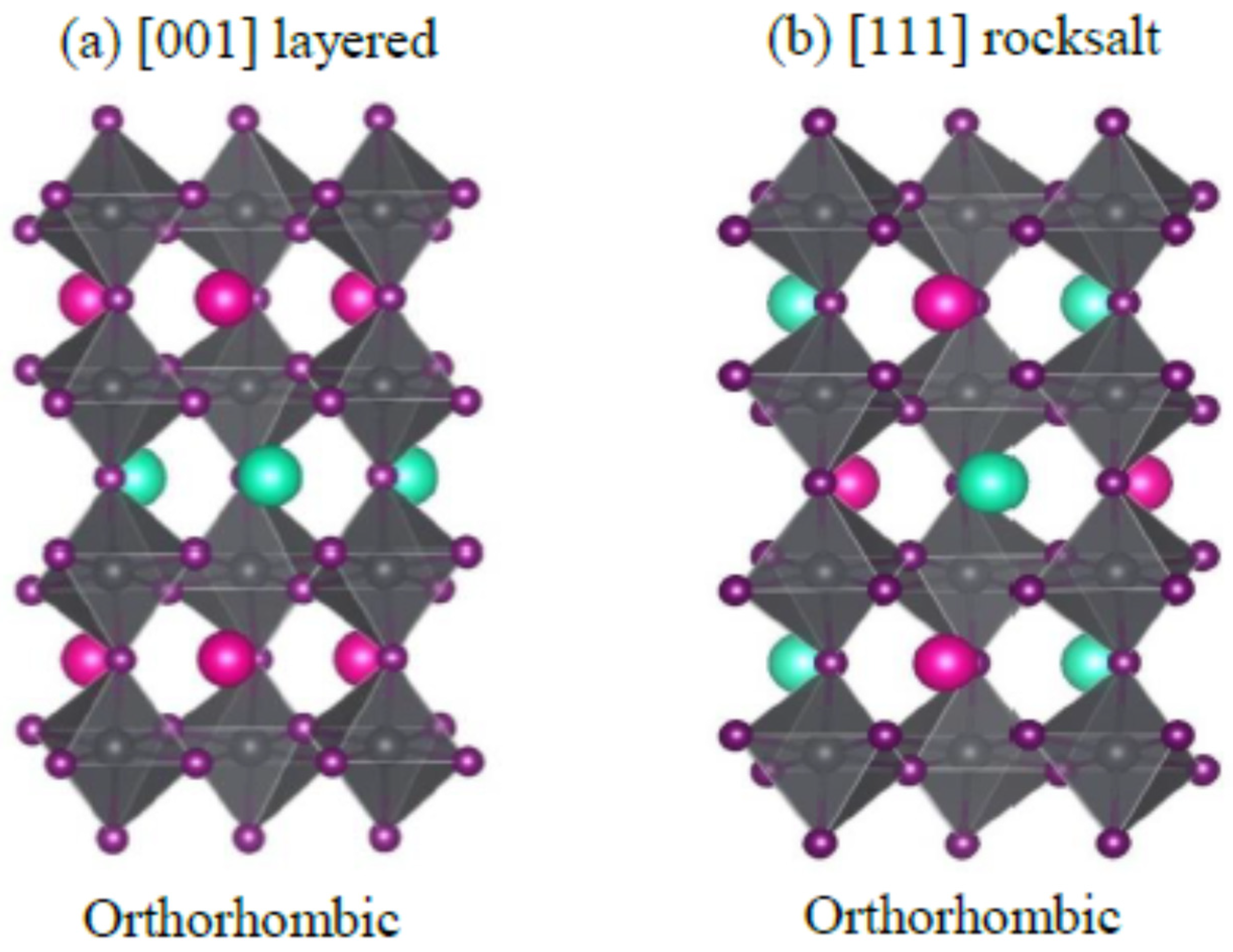
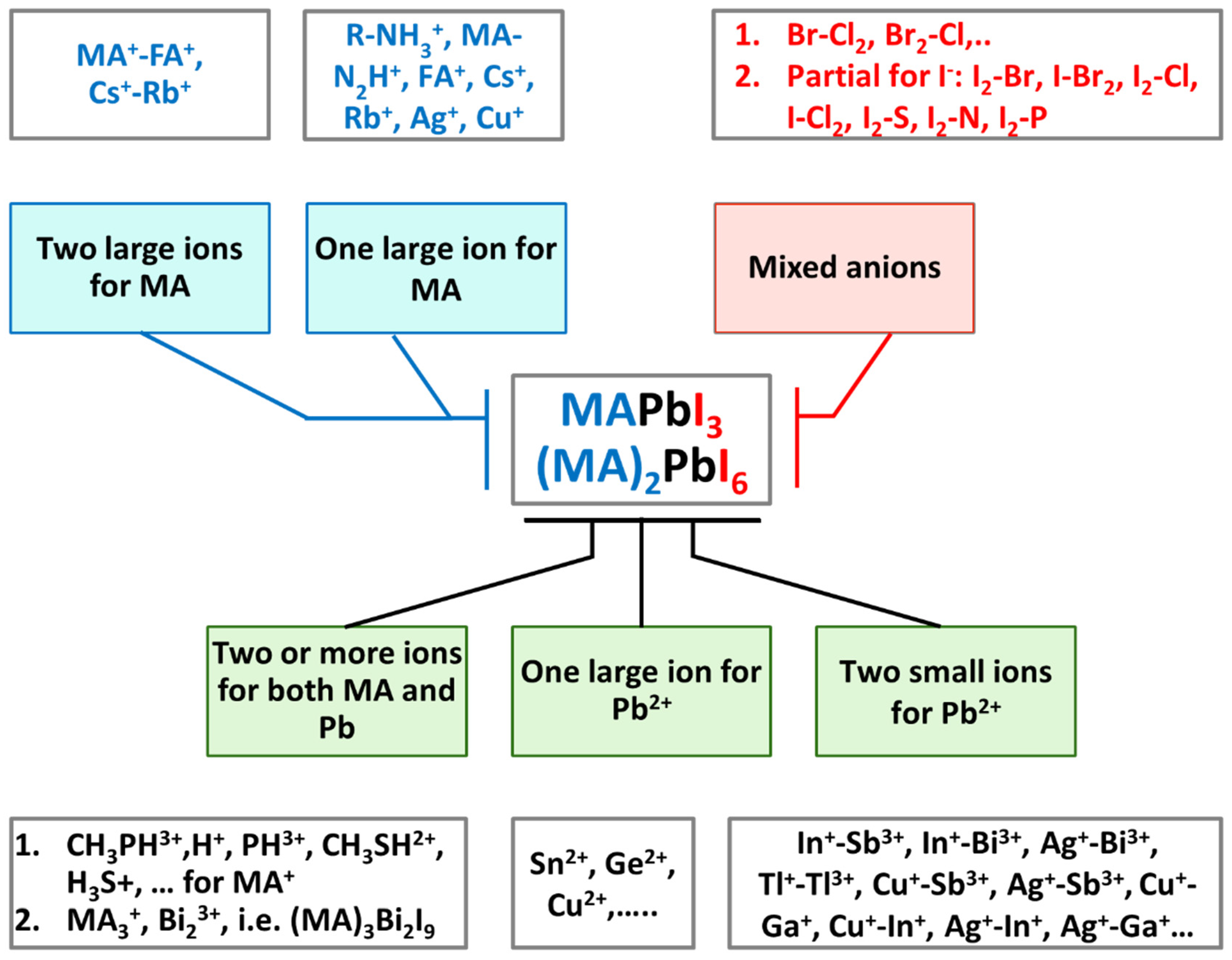
3.1. Cation and Anion Order Engineering of New Halide Perovskite
3.2. ABX3 Perovskite
3.3. ABX6 Perovskite
3.4. A2BX4 Perovskite
3.5. A2BX6 Perovskite
3.6. A3B2X9 Perovskite-Like 3D Framework
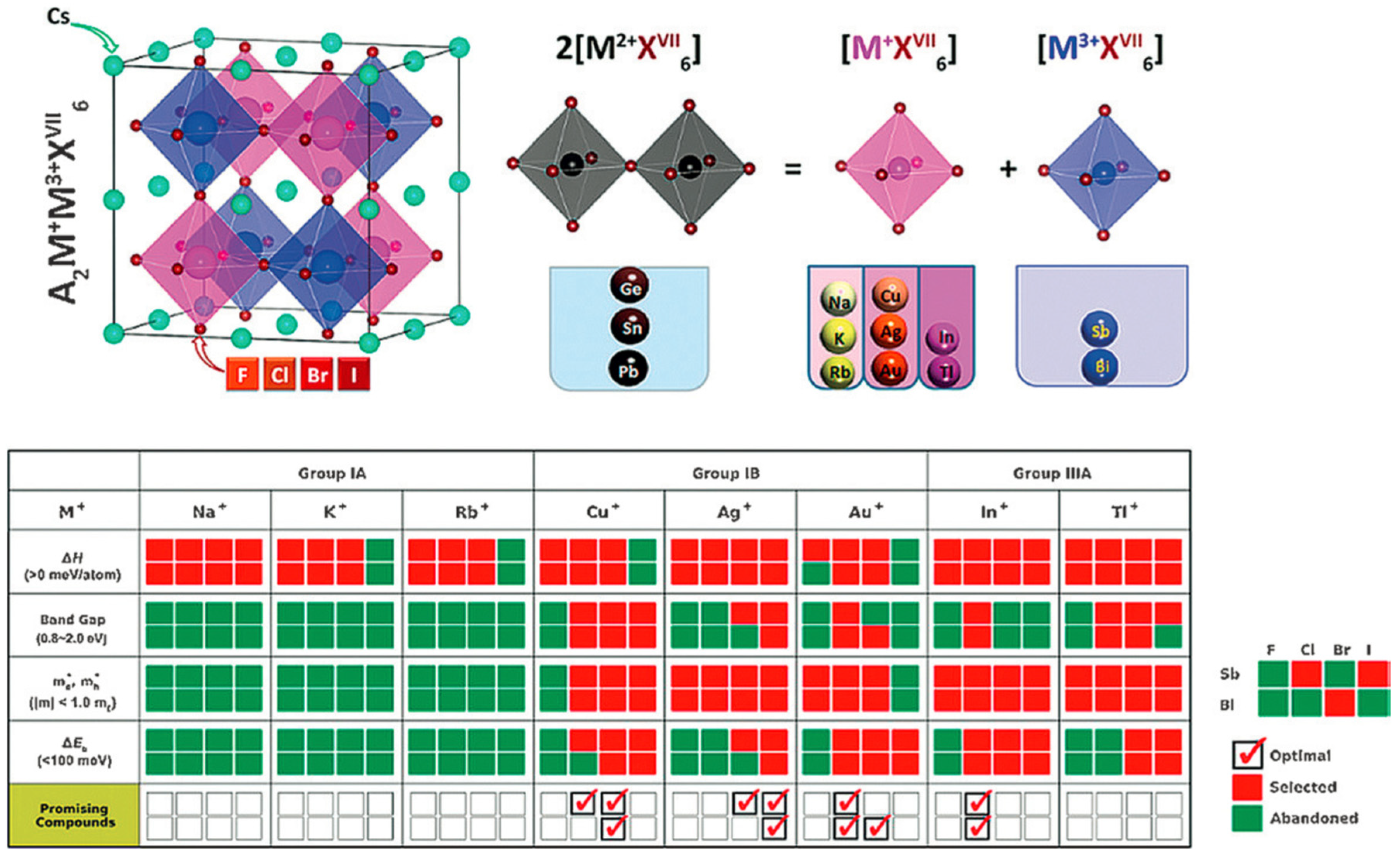
3.7. A2BB’X6 Double Perovskites
3.8. AA’B2X6 Double Perovskite
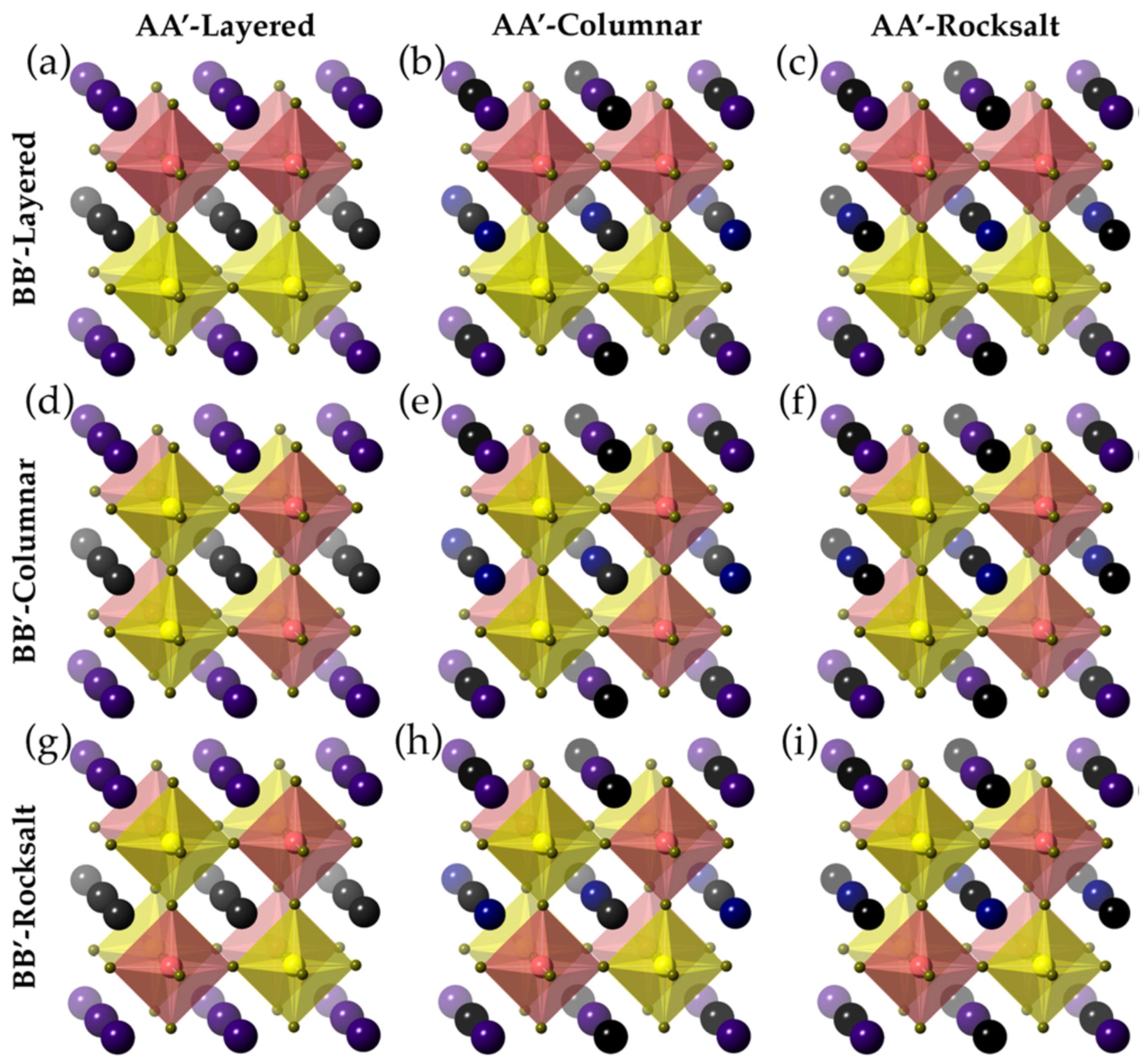
3.9. AA’BB’X6 Double Perovskite
4. Coordination Chemistry of Halide Perovskite Structures
4.1. Coordination Chemistry of Post Transition Metal Atoms
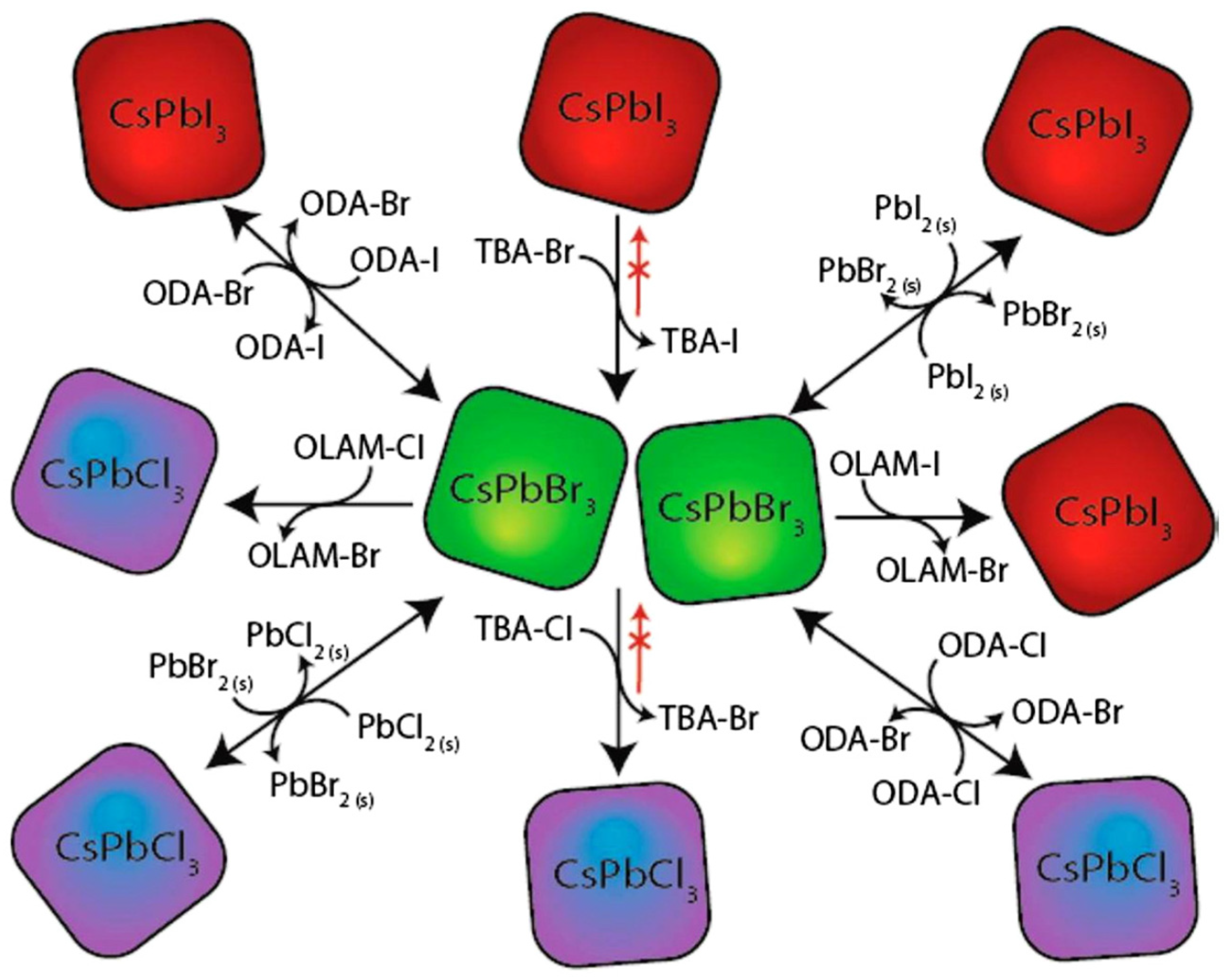
4.2. Proposed Ion Exchange and Ion Mixing Chemistry in Perovskites
4.3. Coordination Chemistry of Single Crystal Complexes Formation
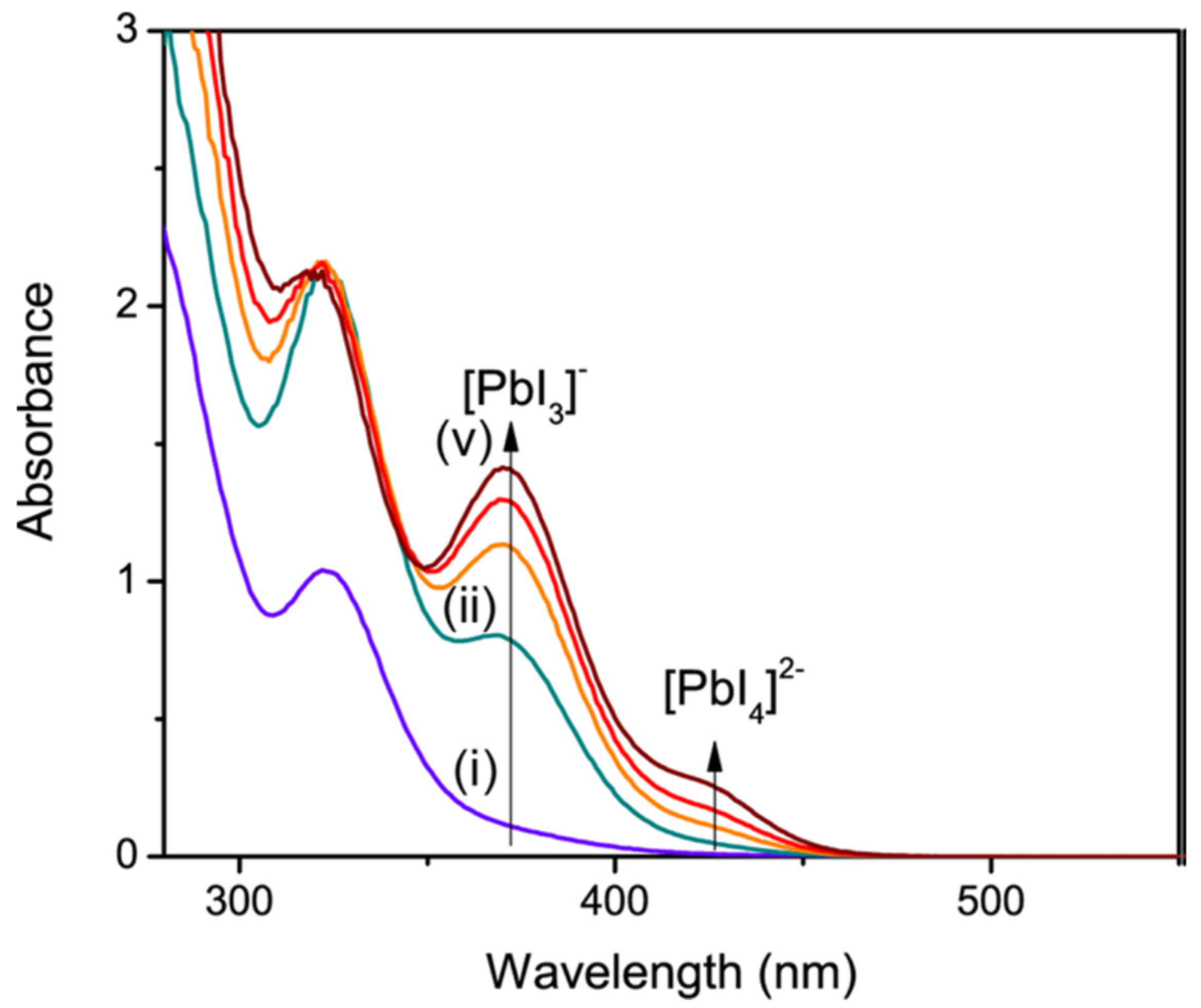
4.4. Coordination Chemistry Limits Crystallization of Halide Perovskites
5. Electronic Interaction during Coordination Chemistry
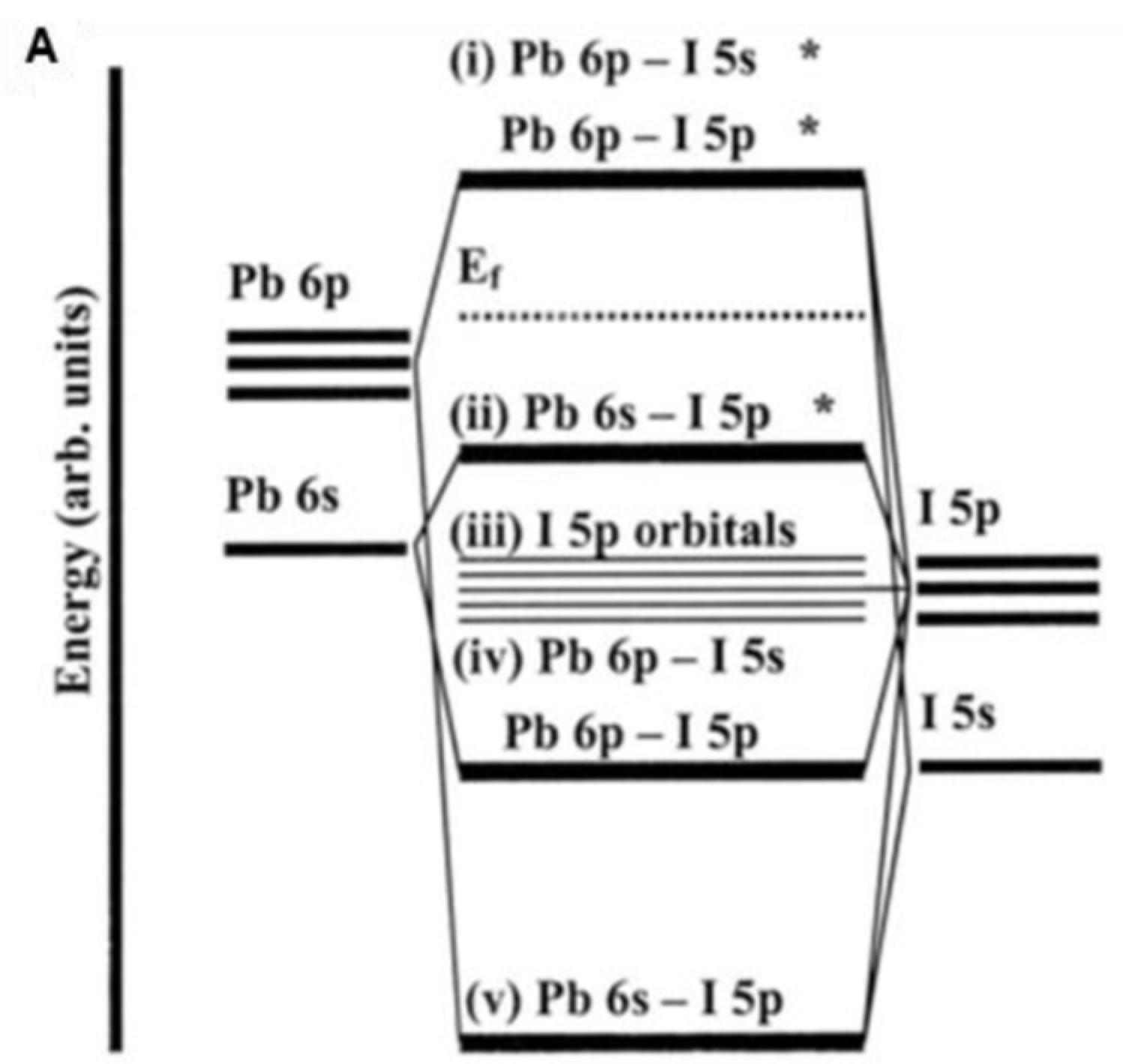
5.1. Bonding Idea in Lead Halide Perovskites
5.2. Complex Bonding Idea in Lead Halide Perovskites
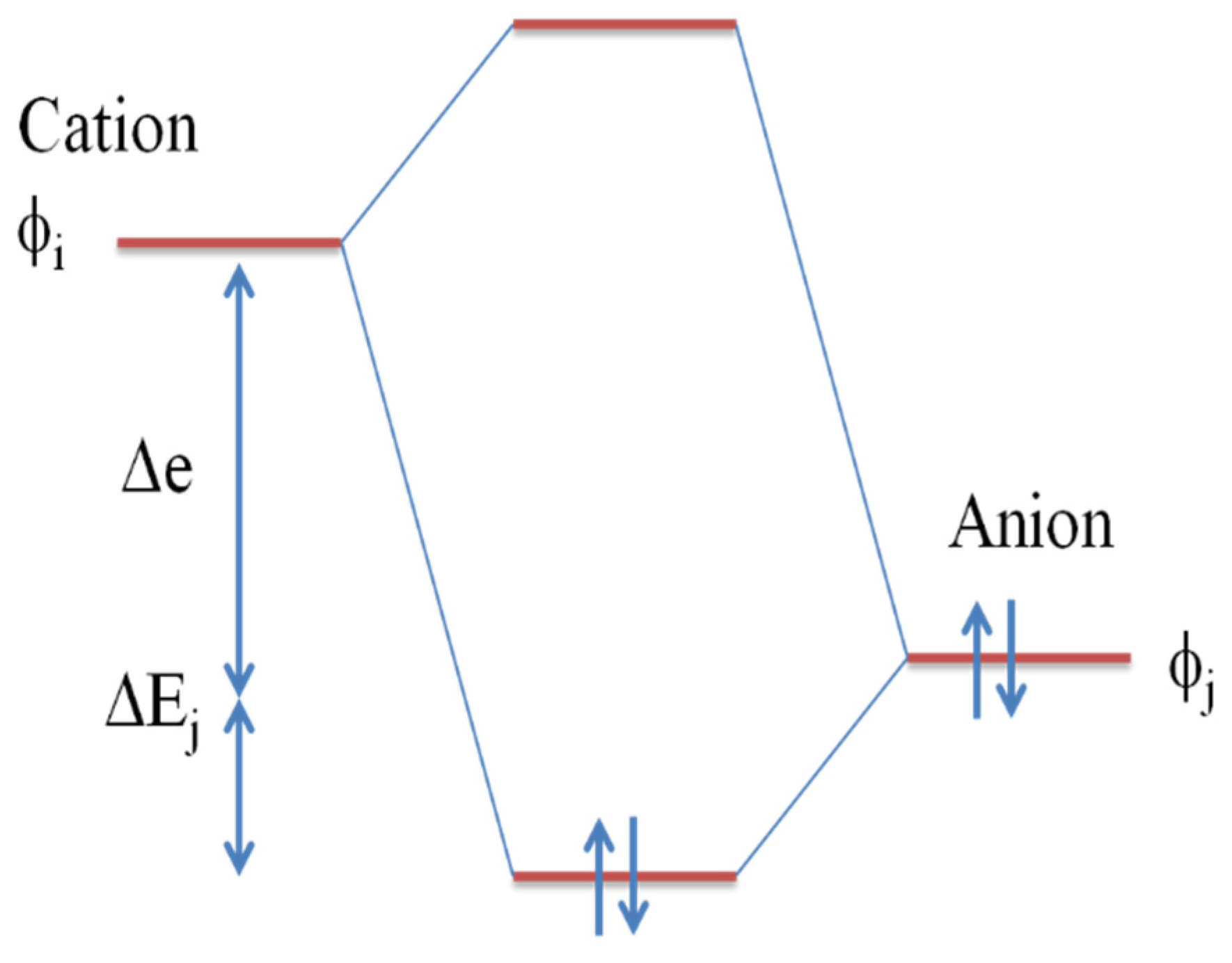
5.3. Electronegativity and Electronic Bandgap Tuning
5.4. Cation-Anion Orbital Interaction
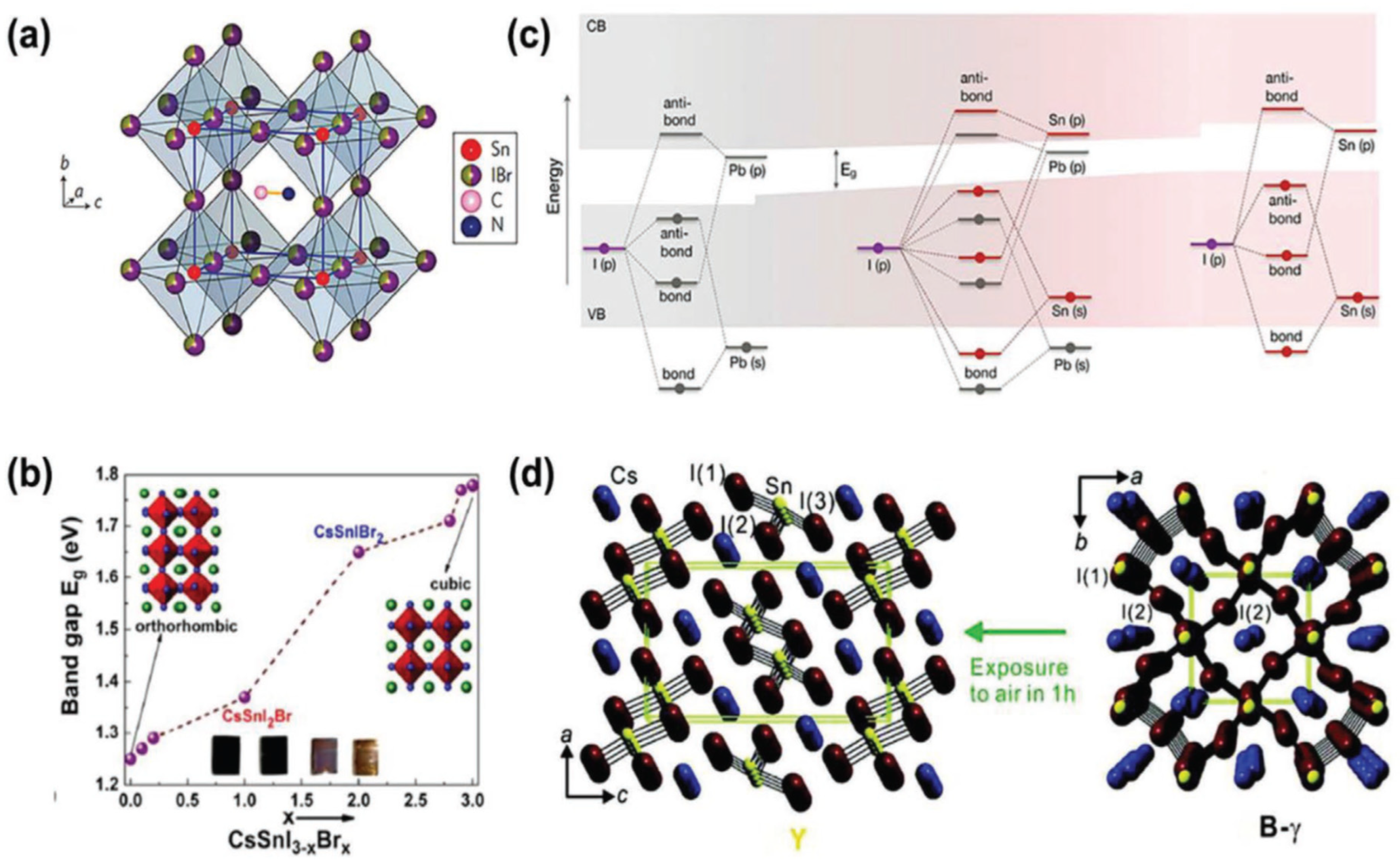
6. Properties of Different Halides Perovskite Structure
7. Energy Applications of Halide Perovskites beyond Photovoltaic
7.1. MAPbI3 as a Photocatalytic Material for HI Splitting
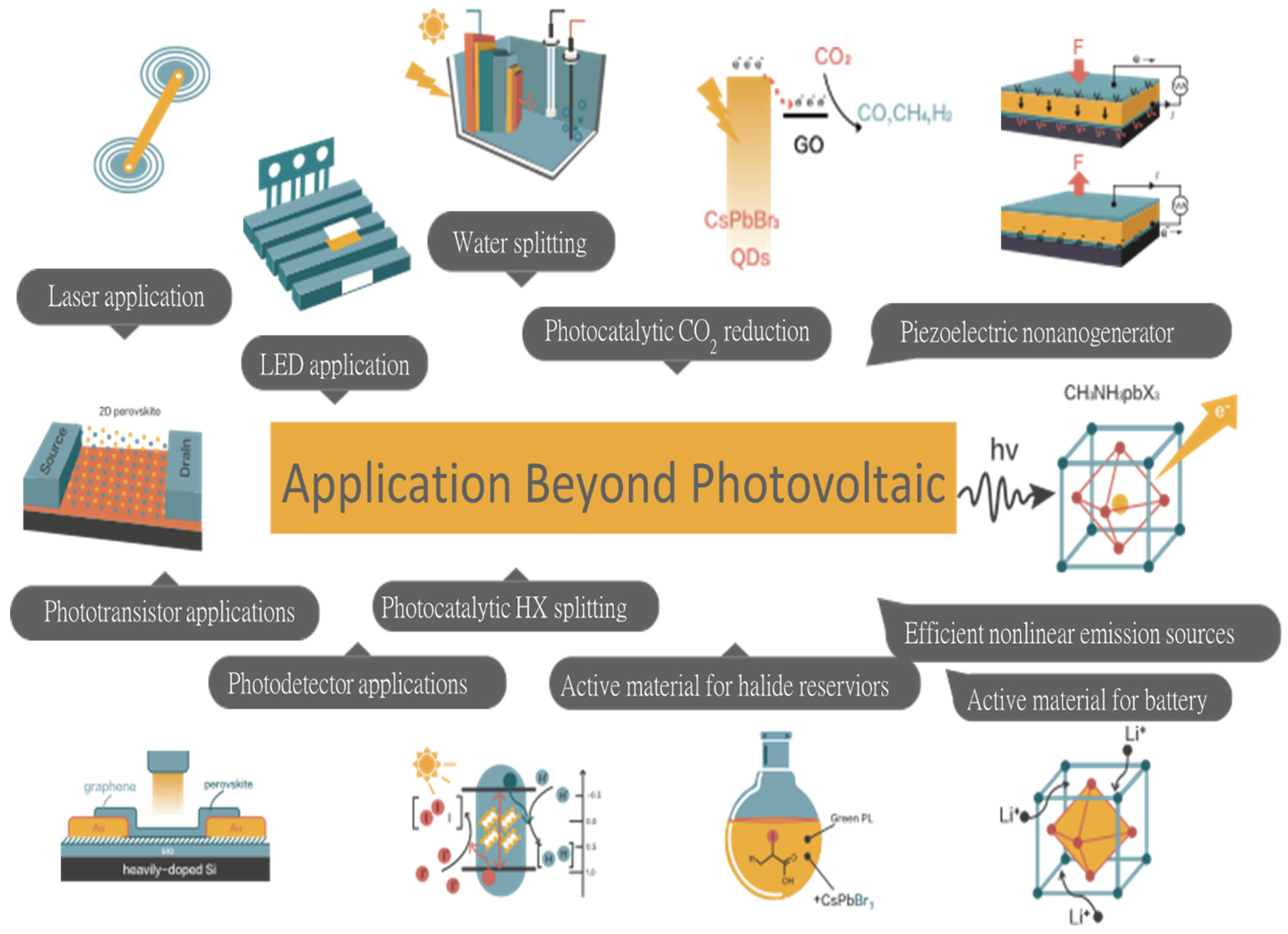
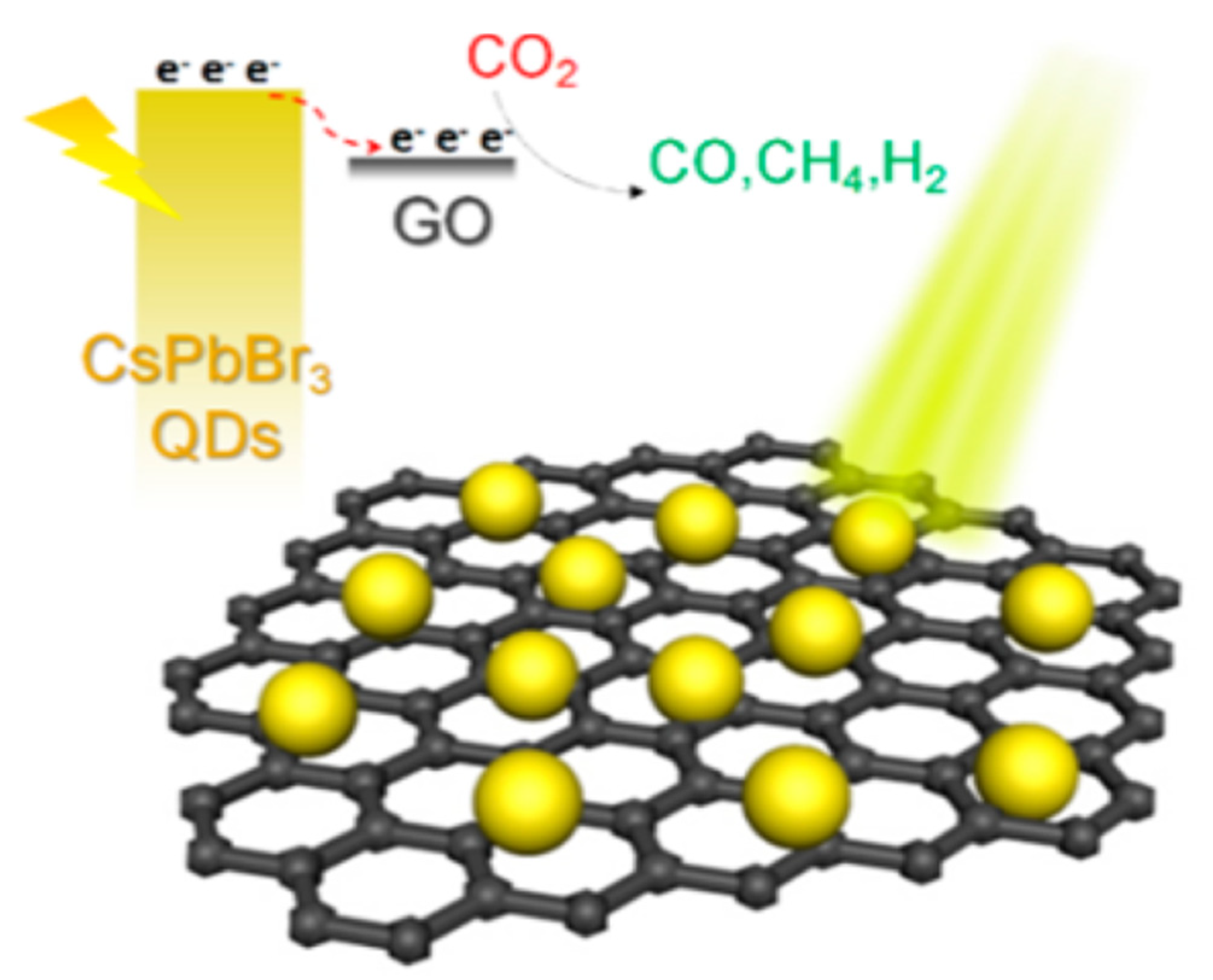
7.2. Perovskite QD-GO Nanocomposite for Photocatalytic Reduction of CO2
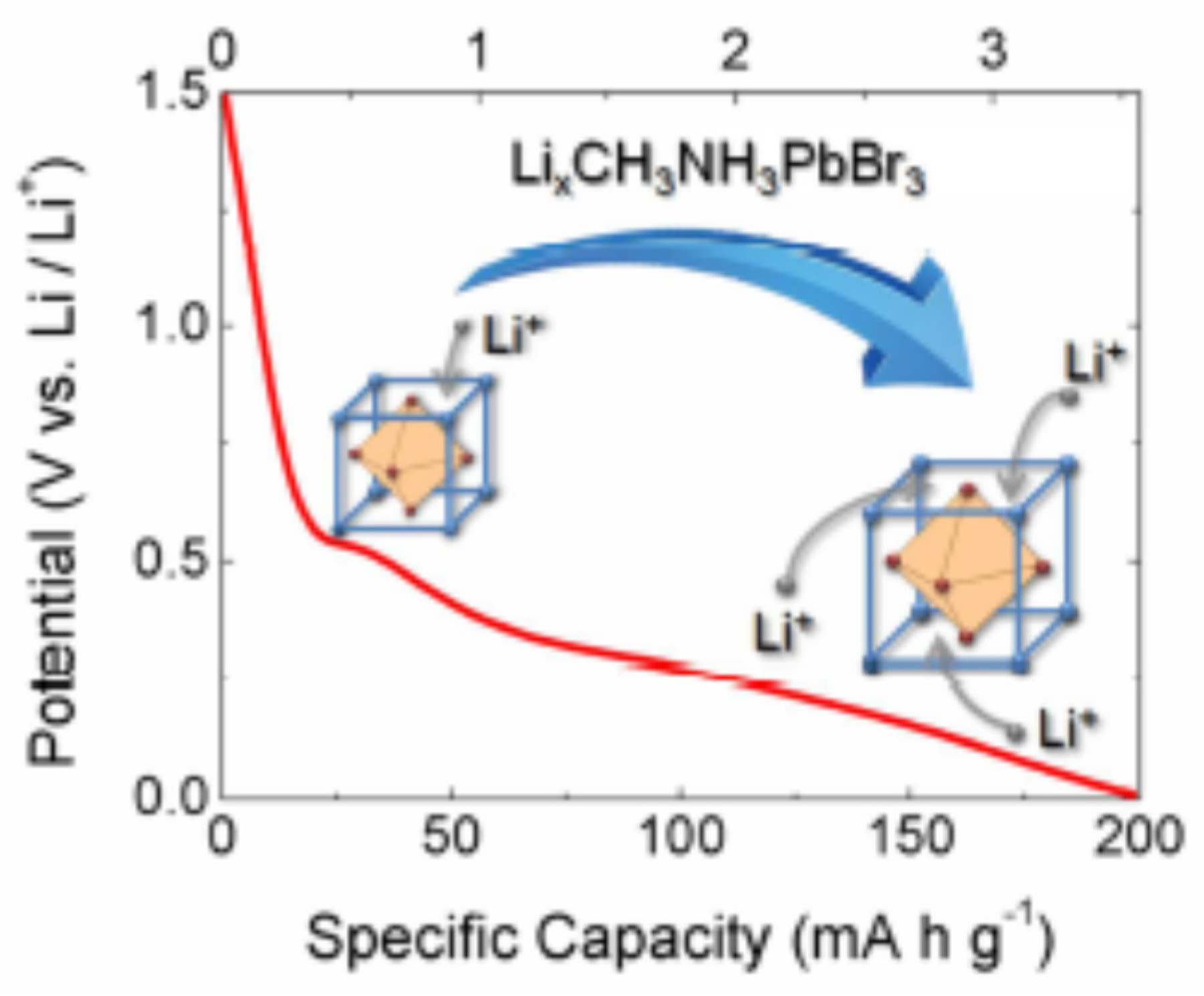
7.3. Halide Perovskite as Active Material for Battery
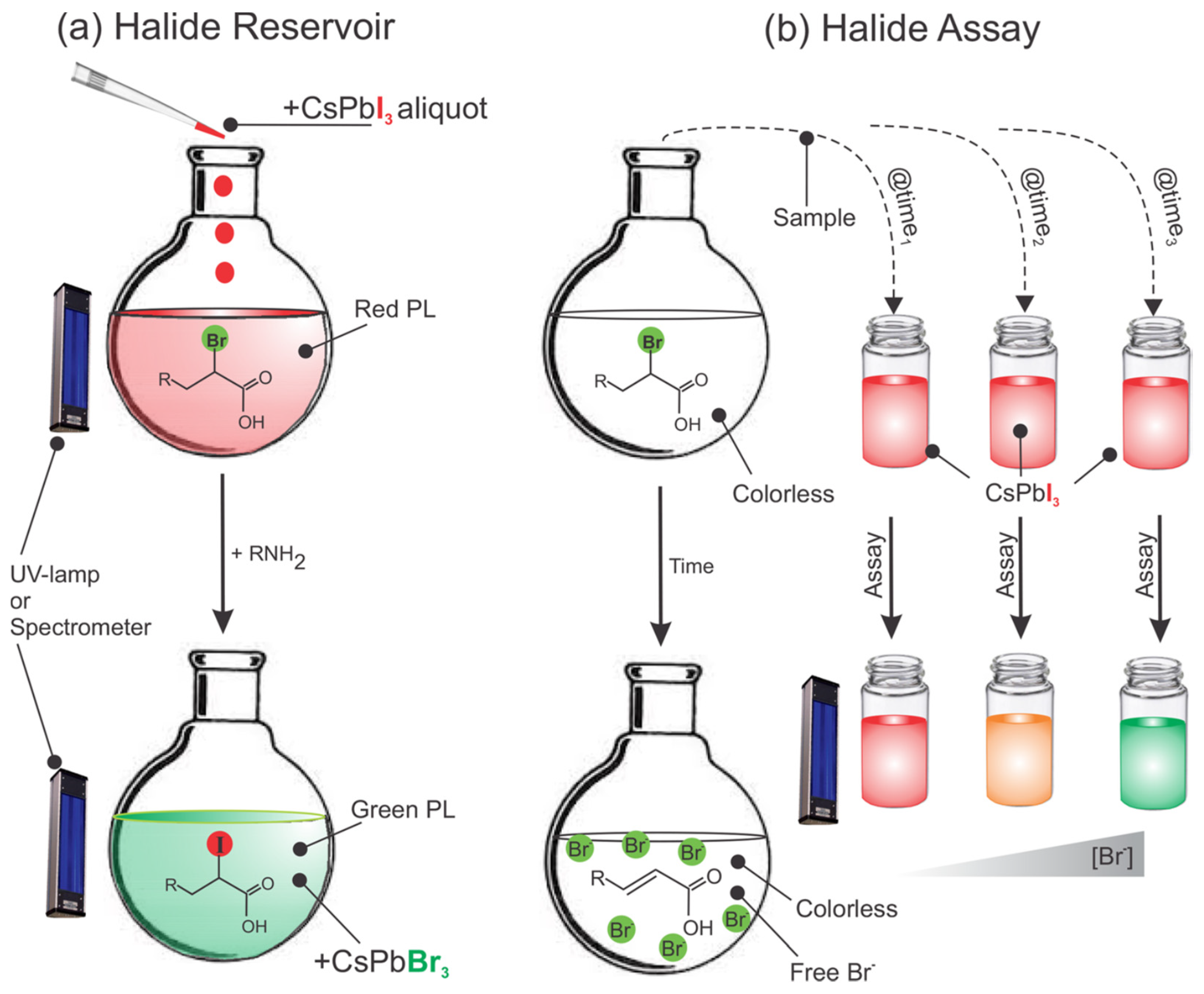
7.4. Halide Reservoir in Catalysis Applications
7.5. Piezoelectric Generators
7.6. What Could Happen in the Future of Halide Perovskites?
8. Concluding Remarks
- 1)
- Stability improvement as a way for flexible practical applications: currently, this is the first challenge that blocks practical applications of halide perovskites materials. This limitation is not only for device but also the material itself is easily prone to degrade. As a key parameter for any optoelectronic applications, materials environmental stability and durability determine the lifespan of the device. Hence, in depth sympathetic of the chemistry and engineering of halide perovskites is helpful to enhance stability in two ways: a) enhancing the hydrophobic character of halide perovskites to overcome the solubility and dissolution of these materials. This can be done by increasing the carbon chain in the organic tail to reduce its hydrophilic character or to increase inorganic character of the halide perovskites by completely replacing CH3NH3+ by water resistant in metal atoms such as Cs, Rb, etc. Using stoichiometric composition engineering of the organic tail with smaller amount of the organic tail could also enhance the hydrophobicity of these materials. b) Coordination engineering framework of halide perovskite structure that can overcome the stability issues in these materials.
- 2)
- Toxicity reduction for mass production of halide perovskites: Toxicity is the second most challenging issue that hinders the commercialization and mass production of halide perovskites. Understanding the chemistry and engineering of halide perovskites is highly relevant to partially or completely avoid the toxicity in these materials. This can be done by a) complete removal of lead atom and replacing it with environmentally friendly metal atoms such as Ti, Sb, Bi, etc. b) completely replacing lead atom by at least less toxic metal atoms such as Sn and Ge, which could not affect the environment significantly. c) If both mechanisms may not be successful, mixing metal ions can be the least alternative to optimize the degree of toxicity in lead based halide perovskites. d) If all these modifications may not be successful, engineering other perovskite materials with new framework and new stoichoimetric composition as well as structure could be the least alternative to avoid toxicity.
- 3)
- Enhanced semiconducting properties such as optical and electrical properties as well as efficiency enhancement. This basic intention of coordination chemistry and coordination engineering of halide perovskite materials is to improve optical and electrical properties and to design new material with better semiconducting properties for better performance.
- 4)
- Another exciting behavior of halide perovskites is their wide range potential applications resulted due to enhanced semiconducting properties that may be benefited from and require the fundamental concepts of chemistry and engineering in addition to their photophysics properties: It is wondering that halide perovskites are highly applicable beyond photovoltaic applications, for instance, a) many optoelectronic devices such as laser, LED, photodetectors, transistors and nonlinear emission sources, b) photocatalytic activities such as efficient water, CO2 and HX splitting, c) storage devices such as active materials for LIB and Na ion battery as well as halide reservoirs for catalysis purpose. Moreover, discovery of new perovskite materials with multifunctional property and improved semiconducting properties: this point of view may be important to fabricate new device that fulfill the ‘Triple E’ rule: efficient, economical and environmental friendly solar cell device.
Acknowledgments
Conflict of Interest
Ethical Statement
References
- J. Berry, Buonassisi, T., Egger, D. A., Hodes, G., Kronik, L., Loo, Y. L., Lubomirsky, I., Marder, S. R., Mastai, Y., Miller, J. S., Mitzi, D. B., Paz, Y., Rappe, A. M., Riess, I., Rybtchinski, B., Stafsudd, O., Stevanovic, V., Toney, M. F., Zitoun, D.,... Cahen, D., Adv. Mater. , 2015, 27, 5102-5112.
- M. Cai, Y. Wu, H. Chen, X. Yang, Y. Qiang and L. Han, Advanced science (Weinheim, Baden-Wurttemberg, Germany), 2017, 4, 1600269.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, Journal of American Chemical Society, 2009, 131, 6050-6051.
- A. Al-Ashouri, E. Köhnen, B. Li, A. Magomedov, H. Hempel, P. Caprioglio, J. A. Márquez, A. B. Morales Vilches, E. Kasparavicius, J. A. Smith, N. Phung, D. Menzel, M. Grischek, L. Kegelmann, D. Skroblin, C. Gollwitzer, T. Malinauskas, M. Jošt, G. Matič, B. Rech, R. Schlatmann, M. Topič, L. Korte, A. Abate, B. Stannowski, D. Neher, M. Stolterfoht, T. Unold, V. Getautis and S. Albrecht, Science, 2020, 370, 1300-1309.
- S. Collavini, S. F. Völker and J. L. Delgado, Angewandte Chemie International Edition, 2015, 54, 9757-9759.
- H. Zhou, Q. Chen, G. Li, S. Luo, T.-b. Song, H.-S. Duan, Z. Hong, J. You, Y. Liu and Y. Yang, Science, 2014, 345, 542-546.
- C. D. Bailie, M. G. Christoforo, J. P. Mailoa, A. R. Bowring, E. L. Unger, W. H. Nguyen, J. Burschka, N. Pellet, J. Z. Lee, M. Gratzel, R. Noufi, T. Buonassisi, A. Salleo and M. D. McGehee, Energy & Environmental Science, 2015, 8, 956-963.
- Y.-J. Kim, T.-V. Dang, H.-J. Choi, B.-J. Park, J.-H. Eom, H.-A. Song, D. Seol, Y. Kim, S.-H. Shin, J. Nah and S.-G. Yoon, Journal of Materials Chemistry A, 2016, 4, 756-763.
- T. A. Berhe, W.-N. Su, C.-H. Chen, C.-J. Pan, J.-H. Cheng, H.-M. Chen, M.-C. Tsai, L.-Y. Chen, A. A. Dubale and B.-J. Hwang, Energy & Environmental Science, 2016, 9, 323-356.
- J. Li, H.-L. Cao, W.-B. Jiao, Q. Wang, M. Wei, I. Cantone, J. Lü and A. Abate, Nature Communications, 2020, 11, 310.
- M. Ren, X. Qian, Y. Chen, T. Wang and Y. Zhao, Journal of Hazardous Materials, 2022, 426, 127848.
- X. Guo and C. Burda, Coordination Chemistry Reviews, 2016, 320-321, 53-65.
- Z. Li, A. Johnston, M. Wei, M. I. Saidaminov, J. Martins de Pina, X. Zheng, J. Liu, Y. Liu, O. M. Bakr and E. H. Sargent, Joule, 2020, 4, 631-643.
- K. Yan, M. Long, T. Zhang, Z. Wei, H. Chen, S. Yang and J. Xu, Journal of the American Chemical Society, 2015, 137, 4460-4468.
- J. Huang, D. Zhou, H. Yan, C. Meng, Y. Yang, J. Liu, M. Wang, P. Xu, Z. Peng, J. Chen and G. Li, Journal of Materials Chemistry C, 2024, 12, 4112-4122.
- S. Zuo, S. Chu, P. An, H. Hu, Z. Yin, L. Zheng and J. Zhang, Journal of Materials Science, 2021, 56, 9903-9913.
- Y. Qin, H. Zhong, J. Intemann, S. Leng, M. Cui, C. Qin, M. Xiong, F. Liu, A. Jen and K. Yao, Advanced Energy Materials, 2020, 10, 1904050.
- M. Mangrulkar and K. J. Stevenson, Journal, 2021, 11.
- M. Stumpp, R. Ruess, J. Müßener and D. Schlettwein, Materials Today Chemistry, 2017, 4, 97-105.
- S. Olga, E. S. Yudanova, N. A. Yeryukov, Y. A. Zhivodkov, T. Shamirzaev, E. A. Maximovskiy, S. Gromilov and I. Troitskaia, Journal of Crystal Growth, 2017, 462.
- F. Shao, P. Qin, D. Wang, G. Zhang, B. Wu, J. He, W. Peng, T. C. Sum, D. Wang and F. Huang, ACS Appl Mater Interfaces, 2019, 11, 740-746.
- P. Gao, A. R. Bin Mohd Yusoff and M. K. Nazeeruddin, Nature Communications, 2018, 9, 5028.
- W.-J. Xu, S. Kopyl, A. Kholkin and J. Rocha, Coord. Chem. Rev., 2019, 387, 398-414.
- A. R. B. Mohd Yusoff, P. Gao and M. K. Nazeeruddin, Coord. Chem. Rev., 2018, 373, 258-294.
- R. Zhou, Z. Yang, J. Xu and G. Cao, Coord. Chem. Rev., 2018, 374, 279-313.
- M. I. Saidaminov, A. L. Abdelhady, B. Murali, E. Alarousu, V. M. Burlakov, W. Peng, I. Dursun, L. Wang, Y. He, G. Maculan, A. Goriely, T. Wu, O. F. Mohammed and O. M. Bakr, Nature Communications, 2015, 6, 7586.
- S. Govinda, B. P. Kore, M. Bokdam, P. Mahale, A. Kumar, S. Pal, B. Bhattacharyya, J. Lahnsteiner, G. Kresse, C. Franchini, A. Pandey and D. D. Sarma, The Journal of Physical Chemistry Letters, 2017, 8, 4113-4121.
- H. P C, H. K. Mulmudi, B. Ghosh, T. W. Goh, Y. Teng, T. Krishnamoorthy, M. Lockrey, K. Weber, T. M. Koh, S. Li, S. Mhaisalkar and N. Mathews, Chemistry of Materials, 2016, 28.
- S. Govinda, B. P. Kore, D. Swain, A. Hossain, C. De, T. N. Guru Row and D. D. Sarma, The Journal of Physical Chemistry C, 2018, 122, 13758-13766.
- T. Debnath, D. Sarker, H. Huang, Z.-K. Han, A. Dey, L. Polavarapu, S. V. Levchenko and J. Feldmann, Nature Communications, 2021, 12, 2629.
- B. Saparov, F. Hong, J.-P. Sun, H.-S. Duan, W. Meng, S. Cameron, I. G. Hill, Y. Yan and D. B. Mitzi, Chemistry of Materials, 2015, 27, 5622-5632.
- J. Feng and B. Xiao, The Journal of Physical Chemistry C, 2014, 118, 19655-19660.
- T. A. Berhe, Su, Wei-Nien, Hwang, Bing Joe, Journal, 2024, DOI: 10.20944/preprints202403.1615.v1.
- A. F. Akbulatov, S. A. Tsarev, M. Elshobaki, S. Y. Luchkin, I. S. Zhidkov, E. Z. Kurmaev, S. M. Aldoshin, K. J. Stevenson and P. A. Troshin, The Journal of Physical Chemistry C, 2019, 123, 26862-26869.
- W. Ke, C. C. Stoumpos, M. Zhu, L. Mao, I. Spanopoulos, J. Liu, O. Y. Kontsevoi, M. Chen, D. Sarma, Y. Zhang, M. R. Wasielewski and M. G. Kanatzidis, Science Advances, 2017, 3, e1701293.
- Z. Irshad, M. Adnan and J. K. Lee, Journal of Materials Science, 2022, 57, 1936-1946.
- J. Chen, J. Xu, C. Zhao, B. Zhang, X. Liu, S. Dai and J. Yao, ACS Applied Materials & Interfaces, 2019, 11, 4597-4606.
- E. López-Fraguas, S. Masi and I. Mora-Seró, ACS Applied Energy Materials, 2019, 2, 8381-8387.
- S. Yue, S. C. McGuire, H. Yan, Y. S. Chu, M. Cotlet, X. Tong and S. S. Wong, ACS Omega, 2019, 4, 18219-18233.
- S. M. Liga and G. Konstantatos, Journal of Materials Chemistry C, 2021, 9, 11098-11103.
- X. Lu, Z. Zhao, K. Li, Z. Han, S. Wei, C. Guo, S. Zhou, Z. Wu, W. Guo and C.-m. L. Wu, RSC Advances, 2016, 6, 86976-86981.
- S. Alnujaim, A. Bouhemadou, M. Chegaar, A. Guechi, S. Bin-Omran, R. Khenata, Y. Al-Douri, W. Yang and H. Lu, The European Physical Journal B, 2022, 95, 114.
- C. C. Stoumpos, L. Frazer, D. J. Clark, Y. S. Kim, S. H. Rhim, A. J. Freeman, J. B. Ketterson, J. I. Jang and M. G. Kanatzidis, Journal of the American Chemical Society, 2015, 137, 6804-6819.
- A. Ashfaq, S. Tahir, S. Mushtaq, R. S. Alqurashi, M. Haneef, N. Almousa, U. u. Rehman and R. S. Bonilla, Materials Today Communications, 2023, 35, 106016.
- C. Zou, Z. Zhu, C.-Y. Huang and L. Lin, The intrinsic properties of MAPbxSn1-xBr3 perovskite single crystals (Withdrawal Notice), SPIE, 2018.
- X.-G. Zhao, D. Yang, Y. Sun, T. Li, L. Zhang, L. Yu and A. Zunger, Journal of the American Chemical Society, 2017, 139, 6718-6725.
- Q. Zhang, F. Hao, J. Li, Y. Zhou, Y. Wei and H. Lin, Sci Technol Adv Mater, 2018, 19, 425-442.
- L. Hnuna and Z. Pachuau, Physica Scripta, 2023, 98.
- M. Wang, W. Wang, B. Ma, W. Shen, L. Liu, K. Cao, S. Chen and W. Huang, Nano-Micro Letters, 2021, 13, 62.
- M. R. Filip, S. Hillman, A. A. Haghighirad, H. J. Snaith and F. Giustino, The Journal of Physical Chemistry Letters, 2016, 7, 2579-2585.
- H. Yao, F. Zhou, Z. Li, Z. Ci, L. Ding and Z. Jin, Advanced Science, 2020, DOI: 10.1002/advs.201903540, 1903540.
- Z. Zhang, Q. Sun, Y. Lu, F. Lu, X. Mu, S.-H. Wei and M. Sui, Nature Communications, 2022, 13, 3397.
- T. A. Berhe, E. K. Ashebir and B. T. Abay, Journal, 2024, DOI: 10.20944/preprints202403.1340.v1.
- N. Zibouche and M. S. Islam, ACS Applied Materials & Interfaces, 2020, 12, 15328-15337.
- Y. Liu, I. J. Cleveland, M. N. Tran and E. S. Aydil, The Journal of Physical Chemistry Letters, 2023, 14, 3000-3006.
- W. Ke, C. Stoumpos and M. Kanatzidis, Advanced Materials, 2018, 31, 1803230.
- X.-H. Zhao, Y.-L. Tang, T.-Y. Tang, X.-F. Diao, L.-K. Gao, Q. Xie, B. Shi, L. Yuan and L.-M. Lu, Materials Today Communications, 2021, 26, 102180.
- T. Ghrib, A. Rached, E. Algrafy, I. A. Al-nauim, H. Albalawi, M. G. B. Ashiq, B. U. Haq and Q. Mahmood, Materials Chemistry and Physics, 2021, 264, 124435.
- H. Zhang, Y. Xu, Q. Sun, J. Dong, Y. Lu, B. Zhang and W. Jie, CrystEngComm, 2018, 20, 4935-4941.
- T. Li, Y. Hu, C. A. Morrison, W. Wu, H. Han and N. Robertson, Sustainable Energy & Fuels, 2017, 1, 308-316.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Letters, 2015, 15, 3692-3696.
- D. B. Mitzi and P. Brock, Inorganic chemistry, 2001, 40, 2096-2104.
- I. M. Alsalamah, A. Shaari, N. A. M. Alsaif, S. A. Yamusa, G. Lakshminarayana and N. Rekik, Chemical Physics, 2023, 573, 111978.
- D. Ju, X. Jiang, H. Xiao, X. Chen, X. Hu and X. Tao, Journal of Materials Chemistry A, 2018, 6, 20753-20759.
- D. Amgar, T. Binyamin, V. Uvarov and L. Etgar, Nanoscale, 2018, 10, 6060-6068.
- D. Jayan K, Optical Materials, 2021, 122, 111671.
- B. Kshirsagar, N. Jaykhedkar, K. Jain, S. Kishor, V. Shah, L. M. Ramaniah and S. Tiwari, The Journal of Physical Chemistry C, 2021, 125, 2592-2606.
- F. El-Mellouhi, E. T. Bentria, A. Marzouk, S. N. Rashkeev, S. Kais and F. H. Alharbi, npj Computational Materials, 2016, 2, 16035.
- S. Farshad Akhtarianfar, S. Shojaei and S. Khameneh Asl, Solar Energy, 2021, 220, 70-79.
- R. Chen, C. Liu, Y. Chen, C. Ye, S. Chen, J. Cheng, S. Cao, S. Wang, A. Cui, Z. Hu, H. Lin, J. Wu, X. Y. Kong and W. Ren, The Journal of Physical Chemistry C, 2023, 127, 635-641.
- M.-H. Jung, RSC Advances, 2021, 11, 32590-32603.
- Q. Mahmood, G. Nazir, S. Bouzgarrou, A. I. Aljameel, A. Rehman, H. Albalawi, B. Ul Haq, T. Ghrib and A. Mera, Journal of Solid State Chemistry, 2022, 308, 122887.
- R. Ishikawa, K. Ueno and H. Shirai, Organic Electronics, 2019, 78, 105596.
- M.-C. Tsai, w.-n. su and B. Hwang, CuPbX3 and AgPbX3 Inorganic Perovskites for Solar cell Applications, 2024.
- H. Zheng, J. Dai, J. Duan, F. Chen, G. Zhu, F. Wang and C. Xu, Journal of Materials Chemistry C, 2017, 5, 12057-12061.
- Robert F. Service, science, 2016, 354.
- T. M. Koh, K. Fu, Y. Fang, S. Chen, T. C. Sum, N. Mathews, S. G. Mhaisalkar, P. P. Boix and T. Baikie, The Journal of Physical Chemistry C, 2014, 118, 16458-16462.
- G. E. Eperon, S. D. Stranks, C. Menelaou, M. B. Johnston, L. M. Herz and H. J. Snaith, Energy & Environmental Science, 2014, 7, 982-988.
- S. Pang, H. Hu, J. Zhang, S. Lv, Y. Yu, F. Wei, T. Qin, H. Xu, Z. Liu and G. Cui, Chemistry of Materials, 2014, 26, 1485-1491.
- N. Pellet, P. Gao, G. Gregori, T. Y. Yang, M. K. Nazeeruddin, J. Maier and M. Grätzel, Angewandte Chemie (International ed. in English), 2014, 53, 3151-3157.
- J. W. Lee, D. J. Seol, A. N. Cho and N. G. Park, Adv Mater, 2014, 26, 4991-4998.
- M. Hu, L. Liu, A. Mei, Y. Yang, T. Liu and H. Han, Journal of Materials Chemistry A, 2014, 2, 17115-17121.
- A. Binek, F. C. Hanusch, P. Docampo and T. Bein, The Journal of Physical Chemistry Letters, 2015, 6, 1249-1253.
- C. C. Stoumpos, C. D. Malliakas and M. G. Kanatzidis, Inorganic chemistry, 2013, 52, 9019-9038.
- P. G. N. Pellet, G. Gregori, T. Y. Yang, M. K. Nazeeruddin, J. Maier and M. Gr¨atzel, Angew. Chem., Int. Ed. Engl., 2014, 53, 3151-3157.
- D.-H. K. Jin-Wook Lee, Hui-Seon Kim, Seung-Woo Seo, Sung Min Cho, and Nam-Gyu Park *, Adv. Energy Mater. , 2015, 5, 1501310.
- N. Ahn, D. Y. Son, I. H. Jang, S. M. Kang, M. Choi and N. G. Park, J Am Chem Soc, 2015, 137, 8696-8699.
- D.-H. K. J.-W. Lee, H.-S. Kim, S.-W. Seo, S. M. Cho, N.-G. Park,, Adv. Energy Mater., 2015, 5, 1501310.
- M. Y. Z. Li, J.-S. Park, S.-H. Wei, J. J. Berry, K. Zhu,, Chem. Mater. , 2016, 28, 284.
- P. W. B. a. P. M. W. M. W. Lufaso, Acta Crystallogr., Sect. B: Struct. Sci., 2006, 62, 397-410.
- B. J. K. a. P. M. W. C. J. Howard, Acta Crystallogr., Sect. B: Struct. Sci., 2003, 59, 463-471.
- D. Iwanaga, Y. Inaguma and M. Itoh, Materials Research Bulletin, 2000, 35, 449-457.
- S. Chakraverty, A. Ohtomo, D. Okuyama, M. Saito, M. Okude, R. Kumai, T. Arima, Y. Tokura, S. Tsukimoto, Y. Ikuhara and M. Kawasaki, Physical Review B, 2011, 84, 064436.
- P. D. Battle, T. C. Gibb, C. W. Jones and F. Studer, Journal of Solid State Chemistry, 1989, 78, 281-293.
- K. B. G. M. T. Anderson, G. A.. Taylor and K. R. Poeppelmeier, Prog. Solid State Chem., 1993, 22, 197-233.
- M. W. L. a. P. M. W. P. W. Barnes, Acta Crystallogr., Sect. B: Struct. Sci., 2006, 62, 384-396.
- R. D. H. a. A. W. S. P. Woodward, J. Mater. Res., 1994, 9, 2118-2127.
- H. W. P. K. Davies, A. Y. Borisevich, I. E. Molodetsky and L. Farber,, Annu. Rev. Mater. Res.,, 2008, 38, 369-401.
- G. K. a. P. M. Woodward*, J. Mater. Chem., 2010, 20, 5785-5796.
- N. Selivanov, A. Samsonova, R. Kevorkyants, I. Krauklis, B. Stroganov, M. Triantafyllou Rundell, D. Bahnemann, C. Stoumpos, A. Emeline and Y. Kapitonov, Advanced Functional Materials, 2021, 31.
- A. Alaei, A. Circelli, Y. Yuan, Y. Yang and S. Lee, Materials Advances, 2020, DOI: 10.1039/D0MA00643B.
- S. H. Pengfei FU, Jiang TANG, Zewen XIAO, Front. Optoelectron., 2021, 14, 252-259.
- O. Muller, andRoy,R., NewYork: Springer., 1974.
- J. Bisquert, F. Fabregat-Santiago, I. Mora-Seró, G. Garcia-Belmonte, E. M. Barea and E. Palomares, Inorganica Chimica Acta, 2008, 361, 684-698.
- O. Muller, andRoy,R., NewYork: Springer., 1974.
- P. V. B. Ghanshyam Pilania1*, Chiho Kim3 and Turab Lookman2, Frontiers in Materials 2016.
- L. A. F. Sergey A. Adonin, Maxim N. Sokolov, Gennady V. Shilov, Denis V. Korchagin, Vladimir P. Fedin, Sergey M. Aldoshin, Keith J. Stevenson, and Pavel A. Troshin*, Adv. Energy Mater., 2017, 1701140.
- R. A. J. S. L. Lawton, J. Am. Chem. Soc., 1966, 88, 616.
- R. A. J. S. L. Lawton, Inorg. Chem., 1971, 10, 709.
- R. A. J. S. L. Lawton, Inorg. Chem., 1968, 7, 2124.
- R. A. J. S. L. Lawton, Inorg. Chem., 1966, 5, 743.
- R. J. S. Lawton, Inorg. Chem., 1971, 10, 2813.
- Z. L. Cheng, J., CrystEngComm., 2010, 12, 2646−2662.
- E. R. H. Dohner, E. T.; Karunadasa, H. I., J. Am. Chem. Soc. , 2014, 136, 1718−1721.
- E. R. J. Dohner, A.; Bradshaw, L. R.; Karunadasa, H. I.,, J. Am. Chem. Soc., 2014, 136, 13154−13157.
- B. S. Lee, C. C.; Zhou, N.; Hao, F.; Malliakas, C.; Yeh, C.-Y.; Marks, T. J.; Kanatzidis, M. G.; Chang, R. P. H., J. Am. Chem. Soc. , 2014, 136, 15379− 15385.
- I. C. H. Smith, E. T.; Solis-Ibarra, D.; McGehee, M. D.; Karunadasa, H. I. A Angew. Chem., 2014, 126, 11414−11417.
- R. C. K. R. E. Brandt, R. L. Z. Hoye, J. R. Poindexter, M. W. B. Wilson, S. Sulekar, F. Lenahan, P. X. T. Yen, V. Stevanovic´, J. C. Nino, M. G. Bawendi, T. Bounassisi,, J. Phys. Chem. Lett. , 2015, 6, 4297.
- A. J. E. R. N. T. Hahn, S. K. Beal, R. R. Fullon, C. B. Mullins,, J. Phys. Chem. C, 2012, 116, 24878.
- D. R. S. Sfaelou, V. Dracopoulos, P. Lianos,, RSC Adv., 2015, 5, 95813.
- M. R. B. E. T. McClure, W. Windl, P. M. Woodward,, Chem. Mater., 2016, 28, 1348.
- Z. Y. Y. Kim, A. Jain, O. Voznyy, G.-H. Kim, M. Liu, L. N. Quan, F. P. G. de Arquer, R. Comin, J. Z. Fan, E. H. Sargent,, Angew. Chem., Int. Ed., 2016, 55, 9586.
- F. H. B. Saparov, J.-P. Sun, H.-S. Duan, W. Meng, S. Cameron, I. G. Hill, Y. Yan, D. B. Mitzi,, Chem. Mater., 2015, 27, 5622.
- H. K. M. P. C. Harikesh, B. Ghosh, T. W. Goh, Y. T. Teng, K. Thirumal, M. Lockrey, K. Weber, T. M. Koh, S. Li, S. Mhaisalkar, N. Mathews,, Chem. Mater., 2016, 28, 7496.
- I. K. C. Hebig, J. Flohre, T. Kirchartz,, ACS Energy Lett., 2016, 1, 309.
- H. S. Huang X, Biswas P, et al., J Phys Chem C., 2016, 120, 28924-28932.
- Y. J. Lyu MQ, Cai ML, et al., Nano Research., 2016, 9(3, 692-702.
- J.-H. Y. X.-G. Zhao, Y. Fu, D. Yang, Q. Xu, L. Yu, S.-H. Wei, L. Zhang,, J. Am. Chem. Soc., 2017, 139, 2630-2638.
- S. H. M. R. Filip, A. A. Haghighirad, H. J. Snaith, F. Giustino,, J. Phys. Chem. Lett., 2016, 7, 2579-2585.
- T. H. A. H. Slavney, A. M. Lindenberg, H. I. Karunadasa,, J. Am. Chem. Soc., 2016, 138, 2138-2141.
- K. Z. D. Z. Xiao, T. Hu, W. Meng, J. Wang, D. B. Mitzi, Y. Yan,, J. Am. Chem. Soc., 2017, 139, 6054-6057.
- W. M. Z. Xiao, J. Wang, D. B. Mitzi, Y. Yan,, Mater. Horiz., 2017, 4, 206-216.
- A. B. a. T. N. Z. Amirbekova Gulzhanat1, Physics department, al-Farabi Kazakh National University, Kazakhstan, 2017.
- Abdykadyrov B., ANM abstracts, 2015.
- G. P. a. B. P. Uberuaga, JOURNAL OF APPLIED PHYSICS, , 2015, 117, 114103.
- K. G. M. Stamplecoskie, J. S.; Kamat, P. V., Energy Environ. Sci., 2015, 8, 208−215.
- Joseph S. Manser, ‡ Makhsud I. Saidaminov,∥ Jeffrey A. Christians,†,‡ Osman M. Bakr,∥ and Prashant V. Kamat*,†,‡,§, Acc. Chem. Res., 2016, 49, 330−338.
- i. P. i. C. C. R. D. Hancock, ed. A. F. Williams, C. Floriani and A. E. Merbach,, VCHA:VCH, Basel,, 1992, 129.
- M. S. S. R. D. Hancock, S. M. Dobson and J. C. A. Boeyens,, Inorg. Chim. Acta,, 1988, 154, 229.
- P. Pyykkö, Chem. Rev., 1988, 88, 563.
- G. A. H. P. Schwerdtfeger, M. Dolg and M. A. Bennett,, J. Am. Chem. Soc., 1992, 114, 7518.
- A. B. A. Andrés, A. Carachalios, A. Bianchi, P. Dapporto, E. Garcia-España, P. Paoletti and P. Paoli,, J. Chem. Soc., Dalton Trans., , 1993, 3507.
- J. Parr, Po(vhedron, 1997, 16, 551-566.
- B.-K. M. J.-H. Chung, Y.K. Kim, K.-H. Kim and T.-Y. Kwon,, J Mol Struct, 2014, 1076, 698-703.
- D. E.-G. C. Platas-Iglesias, T. Enriquez-Perez, F. Avecilla, A. deBlas and T. Rodriguez-Blas,, Inorg Chem, 2005, 44, 2224-2233.
- H. L. J.W. Nugent, J.H. Reibenspies and R.D. Hancock,, 2015, 91, 120-127.
- J. P. G. a. C. W. B. L. Shimonni-Livny, Inorg Chem, , 1998, 17, 1853.
- A. Walsh and G.W. Watson, J Solid State Chem, 2005, 178, 1422.
- J. P. G. a. C. W. B. L. Shimoni-Livny, Inorg Chem,, 1998, 37, 1853-1867.
- A. L. S. J. B. Hoffman, and P. V. Kamat,, J. Am. Chem. Soc., 2016, 138(27), 8603-8611.
- G. Li et al., J. Phys. Chem. C 2015, 119(48), 26883-26888.
- V. D. I. Quinten A. Akkerman, ‡ Sara Accornero,† Alice Scarpellini,† Annamaria Petrozza,‡ Mirko Prato,*,† and Liberato Manna*,†, J. Am. Chem. Soc., 2015, 137, 10276−10281.
- C. G. f. B. I. V. Markov World Scientifi c, Singapore 1995.
- Y. C. Z. S. Yang, Y. Hou, X. Chen, Y. Chen, Y. Wang, H. Zhao,H. G. Yang,, Chem. Mater., 2014, 26, 6705.
- I. M. O. Horváth J. Photochem. Photobiol. A, 1998 95, 114.
- G. e. al, APL,, 2014, 133902.
- S. e. al., Nat. Commun., 2015, 6.
- F. B. Brivio, K. T.; Walsh, A.; van Schilfgaarde, M., Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 155204.
- M. R. Kepenekian, R.; Katan, C.; Sapori, D.; Pedesseau, L.; Even, J., ACS Nano 2015, 9, 11557−11567.
- M. I. Kim, J.; Freeman, A. J.; Ihm, J.; Jin, H., Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 6900−6904.
- J. P. Even, J. Phys. Chem. Lett., 2015, 6, 2238−2242.
- A. M. Amat, E.; Ronca, E.; Quarti, C.; Umari, P.;Nazeeruddin, M. K.; Grätzel, M.; De Angelis, F., Nano Lett., 2014, 14, 3608−3616.
- A. D. S. Stroppa, D.; Barone, P.; Bokdam, M.; Kresse, G.; Franchini, C.; Whangbo, M.-H.; Picozzi, S., Nat. Commun., 2014, 5, 5900.
- I. B. D. Koutselas, L.; Papavassiliou, G. C., J. Phys.: Condens. Matter., 1996, 8, 1217−1227.
- F. Z. Chiarella, A.; Licci, F.; Borriello, I.; Cantele, G.; Ninno, D.; Cassinese, A.; Vaglio, R., Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 045129.
- T. A. Umebayashi, K.; Kondo, T.; Nakao, A., Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 155405.
- F. W. Brivio, A. B.; Walsh, A., APL Mater., 2013, 1, 042111.
- Y. H. P. Chang, C. H.; Matsuishi, K., J. Korean Phys. Soc., 2004, 44, 889−893.
- I. C. Borriello, G.; Ninno, D., Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 235214.
- B. R. R. Vincent, K. N.; Cameron, T. S.; Knop, O., Can. J. Chem., 1987, 65, 1042−1046.
- T. A. Umebayashi, K.; Kondo, T.; Nakao, A., Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 155405.
- F. B. Brivio, K. T.; Walsh, A.; van Schilfgaarde, M., Matter Mater. Phys., 2014, 89, 155204.
- E. U.-G. S. García-Martína, M.C. Knapp, G. King, P.M. Woodward,, 2009, 1148.
- T. K. K. Kobayashi, H. Sawada, K. Terakura, Y. Tokura,, Nature 1998, 395(6703), 677-680.
- G. S. R. Ubic, M.T. Sebastian,, Ceramic Transactions, 2009, 204, 177-185.
- I. D. Brown*, Chem. Rev., 2009, 109,, 6858-6919.
- J. M. B. Frost, K. T.; Brivio, F.; Hendon, C. H.; van Schilfgaarde, M.; Walsh, A., Nano Lett., 2014, 14, 2584−2590.
- T. A. Dittrich, C.; Prajongtat, P.; Rech, B.; Lux-Steiner, M. C., J. Phys. Chem. C, 2015, 119, 23968−23972.
- L. Pauling, Cornell University Press: Ithaca, NY, 1960.
- F. S. Hao, C. C.; Chang, R. P. H.; Kanatzidis, M. G., J. Am. Chem. Soc.,, 2014, 136, 8094−8099.
- J. K. B. T. A. Albright, M.-H. Whangbo,, 2nd ed., Wiley, New York, , 2013.
- F. Z. Huanhuan Yao, Zhizai Li, Zhipeng Ci,* Liming Ding,* and Zhiwen Jin*, Adv. Sci. , 2020, 7.
- C. Bernal and K. Yang, The Journal of Physical Chemistry C, 2014, 118, 24383–24388.
- L.-y. Huang and W. R. L. Lambrecht, Physical Review B, 2013, 88, 165203.
- A. Goyal, S. McKechnie, D. Pashov, W. Tumas, M. van Schilfgaarde and V. Stevanović, Chemistry of Materials, 2018, 30, 3920-3928.
- F. J. Iftikhar, Q. Wali, S. Yang, Y. Iqbal, R. Jose, S. Munir, I. A. Gondal and M. E. Khan, Organic Electronics, 2021, 91, 106077.
- T. Kim and B. Park, Chemistry of Materials, 2024, 36, 675-681.
- G. Giorgi and K. Yamashita, Journal of Materials Chemistry A, 2015, 3, 8981-8991.
- C. He and X. Liu, Light: Science & Applications, 2023, 12, 15.
- J. S. Manser, J. A. Christians and P. V. Kamat, Chemical Reviews, 2016, 116, 12956-13008.
- L. Chouhan, S. Ghimire, C. Subrahmanyam, T. Miyasaka and V. Biju, Chemical Society Reviews, 2020, 49, 2869-2885.
- H. C. Weerasinghe, N. Macadam, J.-E. Kim, L. J. Sutherland, D. Angmo, L. W. T. Ng, A. D. Scully, F. Glenn, R. Chantler, N. L. Chang, M. Dehghanimadvar, L. Shi, A. W. Y. Ho-Baillie, R. Egan, A. S. R. Chesman, M. Gao, J. J. Jasieniak, T. Hasan and D. Vak, Nature Communications, 2024, 15, 1656.
- Y.-S. Jung, K. Hwang, Y.-J. Heo, J.-E. Kim, D. Vak and D.-Y. Kim, Advanced Optical Materials, 2018, 6, 1701182.
- C. Gong, S. Tong, K. Huang, H. Li, H. Huang, J. Zhang and J. Yang, Solar RRL, 2020, 4, 1900204.
- Y. Y. Kim, T.-Y. Yang, R. Suhonen, M. Välimäki, T. Maaninen, A. Kemppainen, N. J. Jeon and J. Seo, Advanced Science, 2019, 6, 1802094.
- J. Ma, X. Zheng, H. Lei, W. Ke, C. Chen, Z. Chen, G. Yang and G. Fang, Solar RRL, 2017, 1, 1700118.
- Z. Li, P. Li, G. Chen, Y. Cheng, X. Pi, X. Yu, D. Yang, L. Han, Y. Zhang and Y. Song, ACS Applied Materials & Interfaces, 2020, 12, 39082-39091.
- A. Verma, D. Martineau, S. Abdolhosseinzadeh, J. Heier and F. Nüesch, Materials Advances, 2020, 1, 153-160.
- C. Shan, Z. Wang, Z. Wang, T. Wang, D. Luo, K. Wang, X. W. Sun and A. K. K. Kyaw, Flexible and Printed Electronics, 2022, 7, 015010.
- G. Vescio, J. Sanchez-Diaz, J. L. Frieiro, R. S. Sánchez, S. Hernández, A. Cirera, I. Mora-Seró and B. Garrido, ACS Energy Lett, 2022, 7, 3653-3655.
- D. Richmond, M. McCormick, T. K. Ekanayaka, J. D. Teeter, B. L. Swanson, N. Benker, G. Hao, S. Sikich, A. Enders, A. Sinitskii, C. C. Ilie, P. A. Dowben and A. J. Yost, Journal of visualized experiments : JoVE, 2019, DOI: 10.3791/58760.
- C. Chen, C. Ran, Q. Yao, J. Wang, C. Guo, L. Gu, H. Han, X. Wang, L. Chao, Y. Xia and Y. Chen, Advanced Science, 2023, 10, 2303992.
- C. C. Stoumpos and M. G. Kanatzidis, Accounts of Chemical Research, 2015, 48, 2791-2802.
- M. Laska, Z. Krzemińska, K. Kluczyk-Korch, D. Schaadt, E. Popko, W. A. Jacak and J. E. Jacak, Nano Energy, 2020, 75, 104751.
- J. E. Jacak and W. A. Jacak, Materials, 2022, 15, 2254.
- M. Ren, L. Fang, Y. Zhang, F. T. Eickemeyer, Y. Yuan, S. M. Zakeeruddin, M. Grätzel and P. Wang, Adv Mater, 2024, DOI: 10.1002/adma.202403403, e2403403.
- S. D. S. Stranks, H. J., Nat. Nanotechnol., 2015, 10, 391−402.
- G. M. Xing, N.; Lim, S.; Yantara, N.; Liu, X.; Sabba, D.; Graetzel, M.; Mhaisalkar, S.; Sum, T., Nat. Mater., 2014, 13, 476−480.
- Z.-K. M. Tan, R. S.; Lai, M. L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.; Sadhanala, A.; Pazos, L. M.; Credgington, D.; Hanusch, F.; Bein, T.; Snaith, H. J.; Friend, R. H., Nat. Nanotechnol., 2014, 9, 687−692.
- X. Y. C. Chin, D.; Yin, J.; Bruno, A.; Soci, C., Nat. Commun., 2015, 6, 7383.
- B. Huskinson, Rugolo, J., Mondal, S. K. & Aziz, M. J., Energy Environ. Sci. , 2012, 5, 8690 8698.
- G. R. B. Taylor, M., J. Hyg., 1982, 89, 321 328.
- R. S. C. Yeo, D.-T., J. Electrochem. Soc., 1980, 127, 549 555.
- J. A. e. a. Baglio, J. Electrochem. Soc., 1982, 129, 1461 1472.
- N. e. a. Singh, Energy Environ. Sci., 2014, 7, 978 981.
- D. C. Powers, Hwang, S. J., Zheng, S.-L. & Nocera, D. G., Inorg. Chem., 2014, 53, 9122 9128.
- J. R. McKone, Potash, R. A., DiSalvo, F. J. & Abruña, H. D., Phys. Chem. Chem. Phys., 2015, 17, 13984 13991.
- S. Ardo, Park, S. H.,Warren, E. L. & Lewis, N. S., Energy Environ. Sci. , 2015, 8, 1484 1492.
- W. J. C. Sunghak Park1†, ChanWoo Lee1, Sangbaek Park1, Hyo-Yong Ahn1 and Ki Tae Nam1,2*, NATURE ENERGY www.nature.com/natureenergy 1© 2016.
- S. N. Kazim, M. K.; Grätzel, M.; Ahmad, S., Angew. Chem., Int. Ed., 2014, 53, 2812−2824.
- M. Z. He, D.; Wang, M.; Lin, C.; Lin, Z., J. Mater. Chem. A, 2014, 2, 5994−6003.
- M. P. He, X.; Liu, X.; Jiang, B.; He, Y.; Snaith, H.; Lin, Z., Angew. Chem., Int. Ed., 2016, 55, 4280−4284.
- Y. Y. Kim, E.; Voznyy, O.; Comin, R.; Walters, G.; Gong, X.; Kanjanaboos, P.; Nogueira, A. F.; Sargent, E. H., ACS Appl. Mater. Interfaces, 2015, 7, 25007−25013.
- L. C. P. s. Schmidt, A.; González-Carrero, S.; Malinkiewicz, O.; Agouram, S.; Mínguez Espallargas, G.; Bolink, H. J.; Galian, R. E.; Pérez-Prieto, J., J. Am. Chem. Soc., 2014, 136, 850−853.
- M.-Z. Y. Yang-Fan Xu, † Bai-Xue Chen, Xu-Dong Wang, Hong-Yan Chen, Dai-Bin Kuang,* and Cheng-Yong Su, J. Am. Chem. Soc. , 2017, , 139, 5660−5663.
- a. G. G.-B. Nuria Vicente, J. Phys. Chem. Lett., 2017.
- Jiantie Xu1, Yonghua Chen1,* & Liming Dai1, nature comm., 2015, 6, 8103.
- L. Y. Protesescu, S.; Bodnarchuk, M. I.; Krieg, F.; Caputo, R.; Hendon, C. H.; Yang, R. X.; Walsh, A.; Kovalenko, M. V., Nano Lett.,, , 2015, 15, 3692−3696.
- A. B. L. Wong, M.; Eaton, S. W.; Yu, Y.; Lin, E.; Dou, L.; Fu, A.; Yang, P., Nano Lett., 2015, 15, 5519−5524.
- N. T. Pellet, J.; Maier, J.; Grätzel, M., Chem. Mater., 2015, 27, 2181−2188.
- D. M. P. Jang, K.; Kim, D. H.; Park, J.; Shojaei, F.; Kang, H. S.; Ahn, J.-P.; Lee, J. W.; Song, J. K., Nano Lett., 2015, 15, 5191−5199.
- G. P. Nedelcu, L.; Yakunin, S.; Bodnarchuk, M. I.; Grotevent, M. J.; Kovalenko, M. V., Nano Lett., 2015, 15, 5635−5640.
- Q. A. D. I. Akkerman, V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L., J. Am. Chem. Soc., 2015, 137, 10276−10281.
- Tennyson L. Doane, † Kayla L. Ryan,† Laxmikant Pathade,† Kevin J. Cruz,† Huidong Zang,‡ Mircea Cotlet,‡ and Mathew M. Maye*,†, ACS Nano, 2016, 10, 5864−5872.
- C. M. L. Starks, C. L., Academic Press: New York,, 1978, pp 13−56.
- G. W. Gribble, Chem. Soc. Rev., 1999, 28, 335−346.
- J. I. n. e. De Roo, M.; Geiregat, P.; Nedelcu, G.; Walravens, W.; Maes, J.; Martins, J. C.; Van Driessche, I.; Kovalenko, M. V.; Hens, Z., ACS Nano, 2016, 10, 2071−2081.
- H. L. Ran Ding, Xiaoli Zhang,* Juanxiu Xiao, Rahul Kishor, Huaxi Sun, Bowen Zhu, Geng Chen, Fei Gao, Xiaohua Feng, Jingsheng Chen, Xiaodong Chen, Xiaowei Sun,* and Yuanjin Zheng*, Adv. Funct. Mater., 2016.
- T. D. Siegler, A. Dawson, P. Lobaccaro, D. Ung, M. E. Beck, G. Nilsen and L. L. Tinker, ACS Energy Lett, 2022, 7, 1728-1734.
- L. Qiu, L. K. Ono and Y. Qi, Materials Today Energy, 2018, 7, 169-189.
- N. Soleimanioun, M. Rani, B. Singh, G. S. S. Saini and S. K. Tripathi, Journal of Alloys and Compounds, 2021, 861, 158207.
- S. Murugan and E. C. Lee, Materials (Basel, Switzerland), 2023, 16.
- Q. Zhang, F. Hao, J. Li, Y. Zhou, Y. Wei and H. Lin, Science and Technology of Advanced Materials, 2018, 19, 425-442.
- V. Chauhan, D. Tripathi, P. Singh, A. Sharma, M. K. Khanna, R. Kumar, R. Bhatnagar and T. Kumar, Inorganic Chemistry Communications, 2023, 157, 111421.
- W. Zhang, X. Zheng, X. Chen, X. Jiang, H. Wang and G. Zhang, Frontiers in Nutrition, 2023, 10.
- E. Hamed, A. Meki and N. Abd El-Mottaleb, Journal of physiology and biochemistry, 2010, 66, 143-151.
- Q. Zhai, A. Narbad and W. Chen, Nutrients, 2015, 7, 552-571.
- M. E. Sears, TheScientificWorldJournal, 2013, 2013, 219840.
- B. Pourrut, M. Shahid, C. Dumat, P. Winterton and E. Pinelli, Reviews of environmental contamination and toxicology, 2011, 213, 113-136.
- H.-C. Kim, T.-W. Jang, H.-J. Chae, W.-J. Choi, M.-N. Ha, B.-J. Ye, B.-G. Kim, M.-J. Jeon, S.-Y. Kim and Y.-S. Hong, Annals of Occupational and Environmental Medicine, 2015, 27, 30.
- Z. Yi, N. Ladi, X. Shai, H. Li, Y. Shen and M. Wang, Nanoscale Advances, 2019, 1.
- M. Cheng, J. Jiang, C. Yan, Y. Lin, M. Mortazavi, A. B. Kaul and Q. Jiang, Journal, 2024, 14.
- H. Chen, Advanced Functional Materials, 2017, 27, 1605654.
- A. K. Jena, A. Kulkarni and T. Miyasaka, Chemical Reviews, 2019, 119, 3036-3103.
- X. Tian, S. D. Stranks and F. You, Science Advances, 2020, 6, eabb0055.
- R. Pandey, S. Bhattarai, K. Sharma, J. Madan, A. K. Al-Mousoi, M. K. A. Mohammed and M. K. Hossain, ACS Applied Electronic Materials, 2023, 5, 5303-5315.
- Q. Emery, M. Remec, G. Paramasivam, S. Janke, J. Dagar, C. Ulbrich, R. Schlatmann, B. Stannowski, E. Unger and M. Khenkin, ACS Applied Materials & Interfaces, 2022, 14, 5159-5167.
- Y. Wang, I. Ahmad, T. Leung, J. Lin, W. Chen, F. Liu, A. M. C. Ng, Y. Zhang and A. B. Djurišić, ACS Materials Au, 2022, 2, 215-236.
- R. K. Raman, S. A. Gurusamy Thangavelu, S. Venkataraj and A. Krishnamoorthy, Renewable and Sustainable Energy Reviews, 2021, 151, 111608.
- Y. Xu, R. Xia, J. Gao, S. Wang, J. Zhu, W. Xiong, N. Yuan and J. Ding, Journal, 2023, 16.
- Q. Zhong, M. Cao and Q. Zhang, Nanoscale, 2021, 13, 19341-19351.
- M. Lyu, J.-H. Yun, P. Chen, M. Hao and L. Wang, Advanced Energy Materials, 2017, 7, 1602512.
- A. H. Slavney, R. W. Smaha, I. C. Smith, A. Jaffe, D. Umeyama and H. I. Karunadasa, Inorganic chemistry, 2017, 56, 46-55.
- H. Hu, B. Dong and W. Zhang, Journal of Materials Chemistry A, 2017, 5, 11436-11449.
- J. K. Rony, M. Islam, M. Saiduzzaman, K. M. Hossain, S. Alam, A. Biswas, M. H. Mia, S. Ahmad and S. K. Mitro, Journal of Materials Research and Technology, 2024, 29, 897-909.
- E. Magliano, P. Mariani, A. Agresti, S. Pescetelli, F. Matteocci, B. Taheri, A. Cricenti, M. Luce and A. Di Carlo, ACS Applied Energy Materials, 2023, 6, 10340-10353.
- A. Fangnon, M. Dvorak, V. Havu, M. Todorović, J. Li and P. Rinke, ACS Applied Materials & Interfaces, 2022, 14, 12758-12765.
- D. Koushik, W. J. H. Verhees, Y. Kuang, S. Veenstra, D. Zhang, M. A. Verheijen, M. Creatore and R. E. I. Schropp, Energy & Environmental Science, 2017, 10, 91-100.
- S. S. Azad, R. Keshavarzi, V. Mirkhani, M. Moghadam, S. Tangestaninejad and I. Mohammadpoor-Baltork, Scientific Reports, 2024, 14, 6466.
- R. Hosseinian Ahangharnejhad, Z. Song, T. Mariam, J. J. Gardner, G. K. Liyanage, Z. S. Almutawah, B. M. M. Anwar, M. Junda, N. J. Podraza, A. B. Phillips, Y. Yan and M. J. Heben, ACS Applied Energy Materials, 2021, 4, Medium: X; Size: p. 7571-7578.
- Y. Li, X. Lu, Y. Mei, C. Dong, D. T. Gangadharan, K. Liu, Z. Wang, S. Qu, M. I. Saidaminov, W. Zhang and F. Tan, Advanced Functional Materials, 2023, 33, 2301920.
- Y. Wang, H. Ran, Y. Zhao, Y. Lu, X. Chen and Y. Tang, Solar RRL, 2024, DOI: 10.1002/solr.202301075.
- Q.-Q. Chu, Z. Sun, D. Wang, B. Cheng, H. Wang, C.-P. Wong and B. Fang, Matter, 2023, 6, 3838-3863.
- P. Zhu, C. Chen, J. Dai, Y. Zhang, R. Mao, S. Chen, J. Huang and J. Zhu, Advanced Materials, 2024, 36, 2307357.
- R. Wang, T. Huang, J. Xue, J. Tong, K. Zhu and Y. Yang, Nature Photonics, 2021, 15.
- H. Bi, J. Liu, Z. Zhang, L. Wang, R. Beresneviciute, D. Tavgeniene, G. Kapil, C. Ding, A. K. Baranwal, S. R. Sahamir, Y. Sanehira, H. Segawa, S. Grigalevicius, Q. Shen and S. Hayase, ACS Energy Lett, 2023, 8, 3852-3859.
- T. Leijtens, K. A. Bush, R. Prasanna and M. D. McGehee, Nature Energy, 2018, 3, 828-838.
- R. Lin, Y. Wang, Q. Lu, B. Tang, J. Li, H. Gao, Y. Gao, H. Li, C. Ding, J. Wen, P. Wu, C. Liu, S. Zhao, K. Xiao, Z. Liu, C. Ma, Y. Deng, L. Li, F. Fan and H. Tan, Nature, 2023, 620, 994-1000.
- N. E. Boukortt, C. Triolo, S. Santangelo and S. Patanè, Journal, 2023, 16.
- B. Chen, Z. Yu, A. Onno, Z. Yu, S. Chen, J. Wang, Z. C. Holman and J. Huang, Science Advances, 2022, 8, eadd0377.
- L. Li, Y. Wang, X. Wang, R. Lin, X. Luo, Z. Liu, K. Zhou, S. Xiong, Q. Bao, G. Chen, Y. Tian, Y. Deng, K. Xiao, J. Wu, M. Saidaminov, H. Lin, C.-Q. Ma, Z. Zhao, W. Yingju and H. Tan, Nature Energy, 2022, 7, 1-10.
- Z. Wang, Z. Song, Y. Yan, S. Liu and D. Yang, Advanced Science, 2019, 6, 1801704.
- J. Yuan, A. Hazarika, Q. Zhao, X. Ling, T. Moot, W. Ma and J. M. Luther, Joule, 2020, 4, 1160-1185.
- C.-Y. Huang, H. Li, Y. Wu, C.-H. Lin, X. Guan, L. Hu, J. Kim, X. Zhu, H. Zeng and T. Wu, Nano-Micro Letters, 2022, 15, 16.
- Q. Zhao, A. Hazarika, X. Chen, S. P. Harvey, B. W. Larson, G. R. Teeter, J. Liu, T. Song, C. Xiao, L. Shaw, M. Zhang, G. Li, M. C. Beard and J. M. Luther, Nature Communications, 2019, 10, 2842.
- X. Ling, J. Yuan and W. Ma, Accounts of Materials Research, 2022, 3, 866-878.
- L. Hu, Q. Zhao, S. Huang, J. Zheng, X. Guan, R. Patterson, J. Kim, L. Shi, C.-H. Lin, Q. Lei, D. Chu, W. Tao, S. Cheong, R. D. Tilley, A. W. Y. Ho-Baillie, J. M. Luther, J. Yuan and T. Wu, Nature Communications, 2021, 12, 466.
- J. Chen, D. Jia, E. M. J. Johansson, A. Hagfeldt and X. Zhang, Energy & Environmental Science, 2021, 14, 224-261.
- A. u. Rehman, E. P. Van Kerschaver, E. Aydin, W. Raja, T. G. Allen and S. De Wolf, Progress in Photovoltaics: Research and Applications, 2023, 31, 429-442.
- A. De Rose, D. Erath, V. Nikitina, J. Schube, D. Güldali, Ä. Minat, T. Rößler, A. Richter, S. Kirner, A. Kraft and A. Lorenz, Solar Energy Materials and Solar Cells, 2023, 261, 112515.
- A. Al-Ashouri, A. Magomedov, M. Roß, M. Jošt, M. Talaikis, G. Chistiakova, T. Bertram, J. A. Márquez, E. Köhnen, E. Kasparavičius, S. Levcenco, L. Gil-Escrig, C. J. Hages, R. Schlatmann, B. Rech, T. Malinauskas, T. Unold, C. A. Kaufmann, L. Korte, G. Niaura, V. Getautis and S. Albrecht, Energy & Environmental Science, 2019, 12, 3356-3369.
- M. Jošt, L. Kegelmann, L. Korte and S. Albrecht, Advanced Energy Materials, 2020, 10, 1904102.
- Z. Li, Y. Zhao, X. Wang, Y. Sun, Z. Zhao, Y. Li, H. Zhou and Q. Chen, Joule, 2018, 2, 1559-1572.
- S. Albrecht and B. Rech, Nature Energy, 2017, 2, 16196.
- P. Wu, J. Wen, Y. Wang, Z. Liu, R. Lin, H. li, H. Luo and H. Tan, Advanced Energy Materials, 2022, 12.
- J. Liu, E. Aydin, J. Yin, M. De Bastiani, F. H. Isikgor, A. U. Rehman, E. Yengel, E. Ugur, G. T. Harrison, M. Wang, Y. Gao, J. I. Khan, M. Babics, T. G. Allen, A. S. Subbiah, K. Zhu, X. Zheng, W. Yan, F. Xu, M. F. Salvador, O. M. Bakr, T. D. Anthopoulos, M. Lanza, O. F. Mohammed, F. Laquai and S. De Wolf, Joule, 2021, 5, 3169-3186.
- J. Liu, M. De Bastiani, E. Aydin, G. T. Harrison, Y. Gao, R. R. Pradhan, M. K. Eswaran, M. Mandal, W. Yan, A. Seitkhan, M. Babics, A. S. Subbiah, E. Ugur, F. Xu, L. Xu, M. Wang, A. u. Rehman, A. Razzaq, J. Kang, R. Azmi, A. A. Said, F. H. Isikgor, T. G. Allen, D. Andrienko, U. Schwingenschlögl, F. Laquai and S. De Wolf, Science, 2022, 377, 302-306.
- P. Wu, D. Thrithamarassery Gangadharan, M. I. Saidaminov and H. Tan, ACS Central Science, 2023, 9, 14-26.
- C. C. Boyd, J. Xu, K. A. Bush, J. A. Raiford, R. Cheacharoen and M. D. McGehee, Burlingame, California, 2019.

| No. | Hybrid organic inorganic halide perovskite | Inorganic halide perovskites |
|---|---|---|
| 1 | MAPbX3[26,27] | Rb3Sb2I9[28] |
| 2 | FAPbX3[29,30] | Cs3Sb2I9[31] |
| 3 | MASnX3[32] | Rb6Pb5Br16[33] |
| 4 | FASnX3[34,35] | Rb6Pb5I16[33] |
| 5 | MAxFA1_xPbI3[36] | CsRbPb2I6 |
| 6 | FA1−xCsxPbI3[37] | Cs2SnI6[38] |
| 7 | MA1–xFAxGeI3[39] | Cs2TiIxBr6−x[40] |
| 8 | MAGeX3[41] | Cs2InBiCl6[42] |
| 9 | FAGeI3[43] | Cs2InSbCl6[42] |
| 10 | C(NH2)3GeI3[43] | Cs2TiX6[44] |
| 11 | MAPbxSn1-xBr3[45] | Rb2CuInCl6[46] |
| 12 | MAPb1-xInxI3Clx[47] | Rb2AgInBr6[48] |
| 13 | FA0.8Cs0.2SnI3[49] | Cs2BiAgCl6[50] |
| 14 | (PEA)2(FA)8Sn9I28[51] | Cs2AgBiBr6[52,53] |
| 15 | (BA)2(MA)3Sn4I13[54] | Cs2AgInBr6[55] |
| 16 | (FA)x(MA)1−xSnX3[56] | In2TiX6[57] |
| 17 | (CH3)3NHGeI3[43] | K2TiX6[58] |
| 18 | CH3C(NH2)2GeI3[43] | Cs3Bi2I9[59] |
| 19 | C5H6NBiI4[60] | CsPbX3[61] |
| 20 | (H3NC6H12NH3)BiI5[62] | RbPbX3[63] |
| 21 | MA3Sb2I9[64] | Cs1−xRbxPbX3[65] |
| 22 | (FA)2BiCuI6[66] | CsSnX3[67] |
| 23 | (NH4)3Sb2I9[49] | H3SPbX3[68] |
| 24 | HC(NH2)2PbI3[69] | CsGeI3[70] |
| 25 | (CH3NH3)1-x(HC(NH2)2)xPbI3[71] | Tl2TiX6[72] |
| 26 | (HC(NH2)2)0.9Cs0.1PbI3[73] | CuPbX3[74] |
| 27 | [HC(NH2)2]x[CH3NH3]1−xPbI3[75] | AgPbX3[74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





