1. Introduction
Musculoskeletal disorders affect one in two U.S. adults and are a leading cause of years lived with disability [
1]. Physical inactivity increases the risk for musculoskeletal disorders and early mortality [
1]. Conversely, there is a dose-response relationship between physical workloads and risk for musculoskeletal injuries (MSIs) [
2]. As such, MSI risk has been deemed proportional to the gap between an individual’s habitual physical activity (PA) level and their current PA level [
2]. Despite widespread interest, progress towards a comprehensive MSI risk prediction model has stagnated. Field-expedient biomechanical screens utilizing scoring systems, such as the Functional Movement Screen (FMS) and Y-balance test (YBT), have gained popularity due to early work showing promise in predicting injury. However, several investigations call into question the predictive validity of these tools, citing inconsistencies in the associations between scores and injury rates across heterogeneous samples [
3,
8]. Moreover, recent work has pointed to low test precision, tainting the practical utility of these tools [
5,
6].
Several studies have called for multifactorial models that consider the complex interplay between many injury risk factors, rather than just biomechanical determinants [
3,
12]. Popular movement screens do not consider other potentially risk-increasing factors such as age, sex, body mass index (BMI), exercise experience, overall fitness level, injury history, and other sociodemographic determinants such as military service, and socioeconomic status. Extant literature suggests that these factors may confound the association between injury rates and movement screen scores. For example, multiple studies involving the FMS and YBT in athlete populations show differences in the composite and component scores based on sex, injury history, and sport [
5,
6,
9]. Similar studies reported higher BMI and lower fitness levels in association with lower scores and higher MSI risk [
5,
6]. Factors such as age and sex, and performance-based characteristics such as strength, joint stability, and flexibility may be nested within injury history, which is arguably the strongest predictor of novel or recurrent injury [
10,
11]. This is exemplified by higher anterior cruciate ligament injury rates in females, whose susceptibility stems from sex-based differences in flexibility, joint stability, and biomechanics [
12].
Occupational exposures have also been shown to increase the risk of injury and pain warranting consideration in injury risk screens. Several studies indicate broad risk categories for MSIs related to occupation, including shift work, repetitive patterns or positions, high physical exertion, full-day sedentary work, computer work, and sleep problems [
13,
14,
15].
Multifactorial injury risk models incorporating modifiable and non-modifiable factors have demonstrated potential. In studies by Rhon et al. and Teyhen et al., significant injury risk factors were identified in military personnel [
7,
17,
18]. Rhon et al. found a combination of factors such as female sex, high BMI, pain during FMS tests or a score of ≤14, and poor fitness test scores linked to injuries [
17]. Teyhen and colleagues reported multiple self-reported factors associated with high injury risk, like smoking, prior surgery, musculoskeletal injury history, limited-duty days, and poor performance on fitness tests [
7]. A different study by Teyhen et al. showed that multiple individual factors created a sensitive predictive model for time-loss injuries in Army soldiers [
18].
In recent years, the identification of several clinical measures of musculoskeletal health has helped to build risk profiles through a better understanding of pain and injury mechanisms. C-reactive protein (CRP) is an inflammatory biomarker that has been linked to musculoskeletal pain in association with obesity, work-related stress, and rheumatism [
19]. Fibrinogen (FIB), a reactant marker of inflammation, is an established indicator of tissue injury that has been implicated as a deserving target for joint degeneration and chronic inflammation [
19]. Bone alkaline phosphatase (BAP) is a serum marker of bone formation and has been used to study the magnitude of musculoskeletal stress in response to training exertion [
20]. Urinary N-telopeptide (NTx) is a marker of bone resorption often used to monitor disease progression in osteoporosis [
21]. Although typically concerning inflammatory bowel diseases, H. pylori (HPY) is a bacterium that has been associated with osteoporosis, suggesting possible complex interactions between gastrointestinal health and bone loss [
22].
Owing to the complex interplay between human behaviors and adaptive physiological processes, ambiguity exists concerning biomarker dynamics in response to musculoskeletal disorders. For example, BAP and N-telopeptide responses were shown to be inconsistent despite similar sample characteristics and training regimens [
20]. Furthermore, bone turnover markers (BTM) have been shown to increase in proportion to fracture risk in some populations, but not others [
23]. Inflammation plays a complex role in pain perception, bone and muscle metabolism, and chronic disease. For example, in a fibromyalgia cohort, symptoms were worse in those with higher CRP, which was explained mostly by obesity and physical inactivity despite the prevailing theory that myalgia symptoms are not primarily caused by inflammation [
24].
Currently, no comprehensive injury risk model has been widely accepted. Due to the breadth of measures relevant to MSIs available in the National Health and Nutrition Examination Survey (NHANES), opportunities exist to explore multifactorial injury risk models underpinned by clinically relevant measures. Hence, the primary objective of this study is to investigate multifactorial models in association with injury, pain, physical functioning, pertinent biomarkers, and individual characteristics. Moreover, an endeavor is made to identify confounding variables that might exert an influence on these associations.
2. Materials and Methods
Data including sociodemographic variables, self-reported bone/joint injury, pain, difficulty performing activities of daily living (ADLs), physical activity, body composition, work hours, and markers of inflammation and bone turnover were collected from NHANES years 1999-2002. These years were chosen because they included joint pain, fitness measures, and other related variables that were not included in later data collection years. Descriptive population estimates (means and standard errors) were computed from complex design variables and stratified by sex, injury models, and case-control (CC) groups. Odds ratios for self-reported bone/joint injury were computed for sociodemographic factors, lifestyle factors, injury risk models, and case-control groups via logistic regression. Receiver operating characteristic (ROC) analysis was performed on the total sample and by sex to determine the predictive value of risk injury factors for pain and FD count.
2.1. Case-Control
To identify potential confounders, we extracted a CC sample using propensity score matching with a caliper of 0.5. Cases were matched with replacement by several covariates indicated in
Table 3. The Kolmogorov-Smirnov test was used to determine the normality of covariate distributions (p-value < 0.05). Covariate median differences between the CC groups were computed and then imputed into the logistic regression model to identify significant associations. Odds ratios were subsequently adjusted for identified confounders. Lastly, principal component analysis (PCA) was conducted to identify potential risk factor clusters predictive of injury.
3. Results
Data from over 21,033 NHANES respondents were included in the analysis. Descriptive population estimates are outlined in the Supplementary File. Approximately 3.2% of respondents reported a bone/joint injury causing difficulty or requiring assistance. Of those reporting joint pain in the past year, 32% reported pain symptoms due to injury, whereas 56% of those reporting bone/joint injury causing difficulty reported joint pain in the past year due to injury.
There was no association between sex and bone/joint injury (Male: OR 1.186 [95% C.I. 0.986 – 1.426]). There were strong associations for age group (40-49 years: OR 1.96 [95% C.I. 1.30 – 2.95]; 50-59 years: OR 2.09 [C.I. 1.37 – 3.17]; 60 and above: OR 4.82 [95% C.I. 3.32 – 7.01]), family PIR (Tercile 1: OR 1.88 [95% C.I. 1.23 – 2.89]), and veteran/military status (OR 1.52 [95% C.I. 1.14 – 2.01]).
There were significant associations between individual/lifestyle factors and self-reported bone/joint injury (
Table 1). Compared to normal BMI, those who were overweight (OR 1.85 [95% C.I. 1.25 – 2.72]) and obese (OR 2.87 [95% C.I. 2.09 – 3.96]) had close to double and triple the odds, respectively. Compared to heavy physical work, activity levels characterized by sitting most of the day were associated with over three times the odds (OR 3.053 [95% C.I. 1.23 – 7.58]). Muscle-strengthening activities were associated with a 44% (OR 0.563 [95% C.I. 0.40 – 0.80]) reduction in odds. For those reporting low back pain at the time of the survey, the odds were over 2.5 (OR 2.55 [95% C.I. 1.99 – 3.304]). Increases in pain count, functional difficulty (FD) count, and total factors increased the odds by 14.6% (OR 1.146 [95% C.I. 1.114 – 1.178]), 40.5% (OR 1.405 [95% C.I. 1.355 – 1.475]), and 2-fold (OR 2.024 [C.I. 95% 1.867 – 2.194]), respectively.
3.1. Sensitivity Analysis
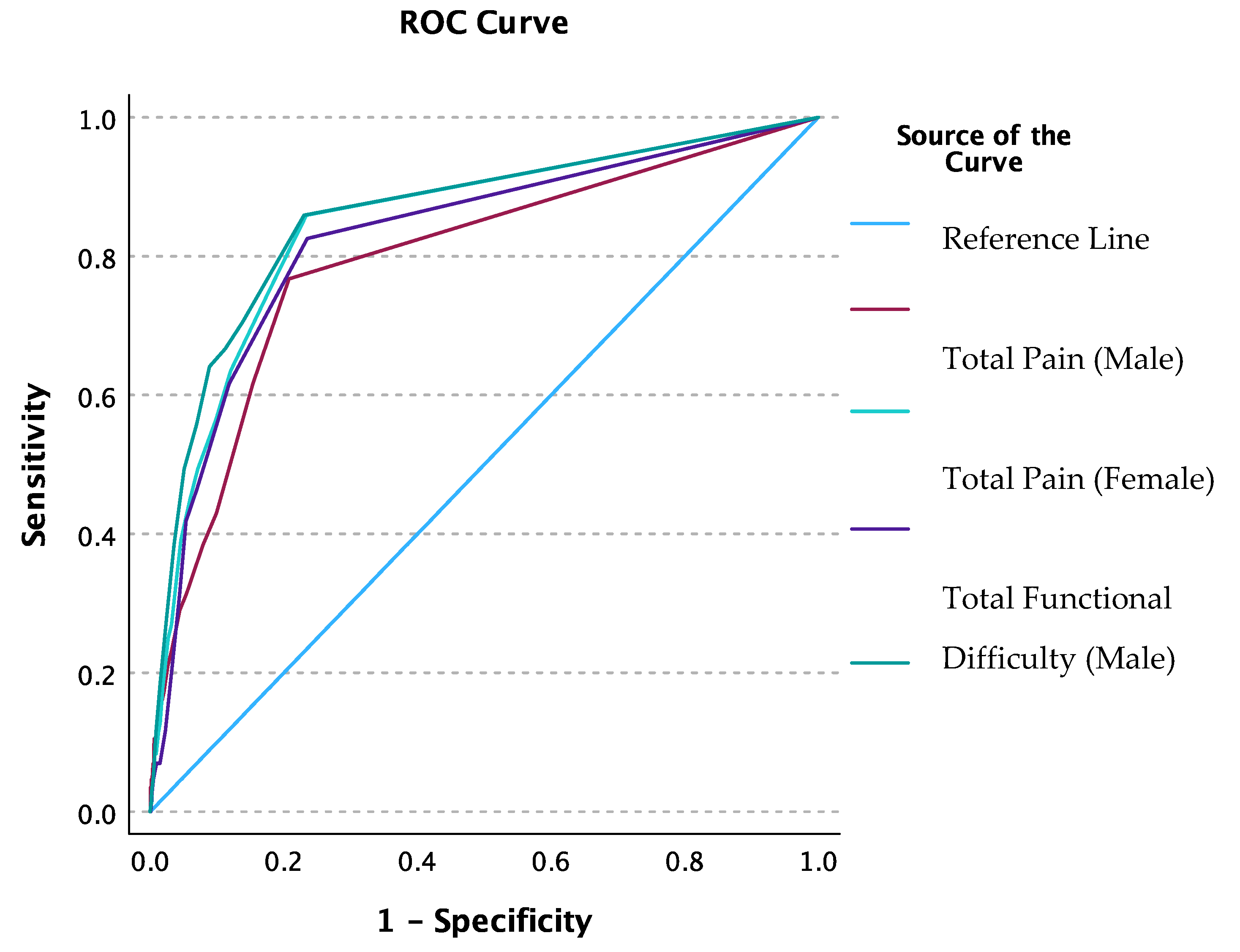
ROC analysis was conducted for injury factor group membership as a classifier for the total number of pain points and total FDs. Area under the ROC curve was 83% (C.I. 80% - 86%) for the total pain points and 85% (C.I. 82% - 87%) for the total FD. A total pain count of one or more optimized sensitivity at 70.2% and specificity at 83.4%. A total FD count of one or more optimized sensitivity at 67.4% and specificity at 87.2%. When ROC analysis was conducted by sex, the AUC for total pain points was higher for females at 85% (C.I. 81% - 88%) compared to males at 79% (C.I. 74% - 85%) (
Figure 1). For FDs, the AUC was again higher for females (86% [C.I. 82% - 89%]) compared to males (83% [C.I. 78% - 87%]). A total pain count of one or more optimized sensitivity and specificity for males at 61.6% and 84.6%, respectively; and for females at 75% and 82.2%, respectively. A total FD of one or more optimized sensitivity and specificity for males at 82.6% and 76.5%, respectively; and for females at 86% and 77%, respectively. The AUC difference between sex was not significant for pain (p-value = 0.11) or FDs (p-value = 0.314).
3.2. Injury Risk Models
Risk models were constructed from the strength of associations from the univariate logistic regression analysis. Model 1 included those with three or more factors. Model 2 included risk factors in Model 1 plus those reporting low back pain during the past 3 months, and Model 3 included risk factors in the first two models plus those with more than one region-specific pain point within the past year. Those not selected to an elevated risk group will be referred to as the ‘low-risk group.’
All injury factor models were strongly associated with self-reported bone/joint injury. Model 1 was associated with odds of 2.24 (95% C.I. 1.71 – 2.92); Model 2 was associated with odds of 2.94 (95% C.I. 2.06 – 4.18); and Model 3 was associated with odds of 4.04 (95% C.I. 2.88 – 5.681). When adjusted for age, the association attenuated for Model 1 (OR 0.93 [95% C.I. 0.71 – 1.21]) but remained for Models 2 (OR 1.56 [95% C.I. 1.11 – 2.18]) and 3 (OR 2.15 [95% C.I. 1.54 – 2.99]). Males were more likely to be members of Model 1(OR 1.103 [95% C.I. 1.02 – 1.19]) and less likely to be members of Model 3 (OR 0.80 [95% C.I. 0.68 – 0.94]) compared to females (
Table 2).
There were strong associations for age group. Odds were highest in the 50–59-year-old group compared to the 20–39-year-old group across all models (Model 1: OR 31.08 [95% C.I. 23.86 – 40.48]; Model 2: OR 15.85 [C.I. 11.24 – 22.65] Model 3: OR 20.66 [C.I. 13.64 – 31.30]) (
Table 2). In those with veteran/military status, odds were highest for Model 1 and similar for Models 2 and 3 (Model 1: OR 2.14 [95% C.I. 1.805 – 2.526]) Model 2: OR 1.48 [95% C.I. 1.215 – 1.808]; Model 3: OR 1.58 (95% C.I. 1.253 – 2.00). A marginal trend increase in odds across risk models was observed for increases in FDs (Model 1: OR 1.28 [95% C.I. 1.25 – 1.31]; Model 2: OR 1.30 [95% C.I. 1.27 – 1.33]; Model 3: OR 1.35 [95% C.I. 1.31 – 1.39]). Compared to PIR Tercile 3, odds for Model 1 membership in Tercile 1 were significantly lower (OR 0.60 [95% C.I. 0.525 – 0.684]). Increases in CRP, FIB, and HPY significantly increased the odds of membership to all models (
Table 2). However, increases in BTM were significantly associated with decreased odds.
3.3. Case-Control Analysis
The control-matched sample included 467 cases of self-reported bone/joint injury. There were no differences in peak force-velocity, physical activity measures, and muscle strengthening frequency. Odds for injury group membership were significantly higher for age (24-year increase, OR 3.5 [95% C.I. 2.68 – 4.68]), BMI (4.5-unit increase, OR 1.53 [95% C.I. 1.29 – 1.81]), PBF (5.7% increase, OR 1.35 [95% C.I. 1.23 – 1.48]), total pain count (4-count increase, OR 2.4 [95% C.I. 1.37 – 4.21]), total FD (5-activity increase, OR 22.62 [95% C.I. 11.52 – 44.43]), and total factor count (2-factor increase, OR 5.81 [95% C.I. 4.29 – 7.88]) (
Table 3). However, odds were significantly decreased for BAP (1-unit increase, OR 0.971 [95% C.I. 0.956 – 0.986]) and NTx (63-unit increase, OR 0.963 [95% C.I. 0.941 – 0.985]). Odds of injury based on an increase in BMI within 1 year prior were equivocal (4.5-unit increase, OR 0.94 [95% C.I. 0.775 – 1.15]).
When adjusted for age, the associations attenuated for BAP (OR 0.986 [95% C.I. 0.986 – 1.003]) and NTx (OR 0.986 [95% C.I. 0.964 – 1.01]) (
Table 3). When adjusted for BMI, the odds of injury decreased with increases in BMD (OR 0.965 [C.I. 0.944 – 0.986]). When adjusted for age, BMI, BAP, NTx, TPF, total FD, total pain, and total factors, all associations attenuated except for total FD (OR 8.57 [95% C.I. 3.80 – 19.32]).
Table 3.
The table displays odds ratios for injury group membership based on sex and imputed median differences. ‡ ISR = Immune Status Ratio, detected through IgG antibodies. †Reference category. Mean propensity scores for the injury and control groups were 0.167 and 0.037, respectively. *Significant at p <0.01.
Table 3.
The table displays odds ratios for injury group membership based on sex and imputed median differences. ‡ ISR = Immune Status Ratio, detected through IgG antibodies. †Reference category. Mean propensity scores for the injury and control groups were 0.167 and 0.037, respectively. *Significant at p <0.01.
| |
|
|
|
Age-Adjusted |
|
BMI-Adjusted |
| |
Units of Change |
Odds Ratio |
95% Confidence Interval |
Odds Ratio |
95% Confidence Interval |
Odds Ratio |
95% Confidence Interval |
| Lower |
Upper |
Lower |
Upper |
Lower |
Upper |
|
| Male |
Female †
|
0.869 |
0.639 |
1.183 |
|
|
|
|
|
|
|
| Age at screening |
24.00 |
3.545* |
2.683 |
4.684 |
|
|
|
3.095* |
2.26 |
4.24 |
|
Body mass index (kg/m2)
Change in BMI from 1 yr. ago |
4.50
4.50 |
1.528*
0.943 |
1.291
0.775 |
1.81
1.147 |
1.36* |
1.15 |
1.61 |
|
|
|
|
| Total percent fat (DXA) |
5.70 |
1.348* |
1.226 |
1.482 |
1.22* |
1.11 |
1.348 |
1.269* |
|
|
|
| Total pain count |
4.00 |
2.403* |
1.372 |
4.207 |
1.94* |
1.51 |
3.72 |
2.17* |
1.242 |
3.80 |
|
| Total functional difficulties |
5.00 |
22.621* |
11.517 |
44.431 |
13.16* |
6.66 |
25.99 |
17.81* |
9.05 |
35.04 |
|
| Bone mineral density (g/cm2) |
0.02 |
0.492 |
0.143 |
1.687 |
|
|
|
0.965* |
0.944 |
0.986 |
|
| Bone alkaline phosphatase (ug/L) |
1.00 |
0.971* |
0.956 |
0.986 |
0.986 |
0.969 |
1.003 |
0.973* |
0.955 |
0.991 |
|
| C-reactive protein (mg/dL) |
0.12 |
1.024 |
0.968 |
1.084 |
|
|
|
1.003 |
0.978 |
1.028 |
|
| Fibrinogen (mg/dL) |
13.00 |
1.051 |
0.993 |
1.113 |
|
|
|
|
|
|
|
| Helicobacter pylori (ISR)‡
|
0.15 |
1.043 |
1.00 |
1.088 |
|
|
|
|
|
|
|
| N-telopeptides (NTx) (nmol BCE) |
63.00 |
0.963* |
0.941 |
0.985 |
0.986 |
0.964 |
1.01 |
0.963* |
0.941 |
0.986 |
|
| Total factor count |
2.00 |
5.811* |
4.286 |
7.877 |
3.907* |
2.70 |
5.70 |
5.76* |
4.02 |
8.26 |
|
3.3.1. Predictive Validity
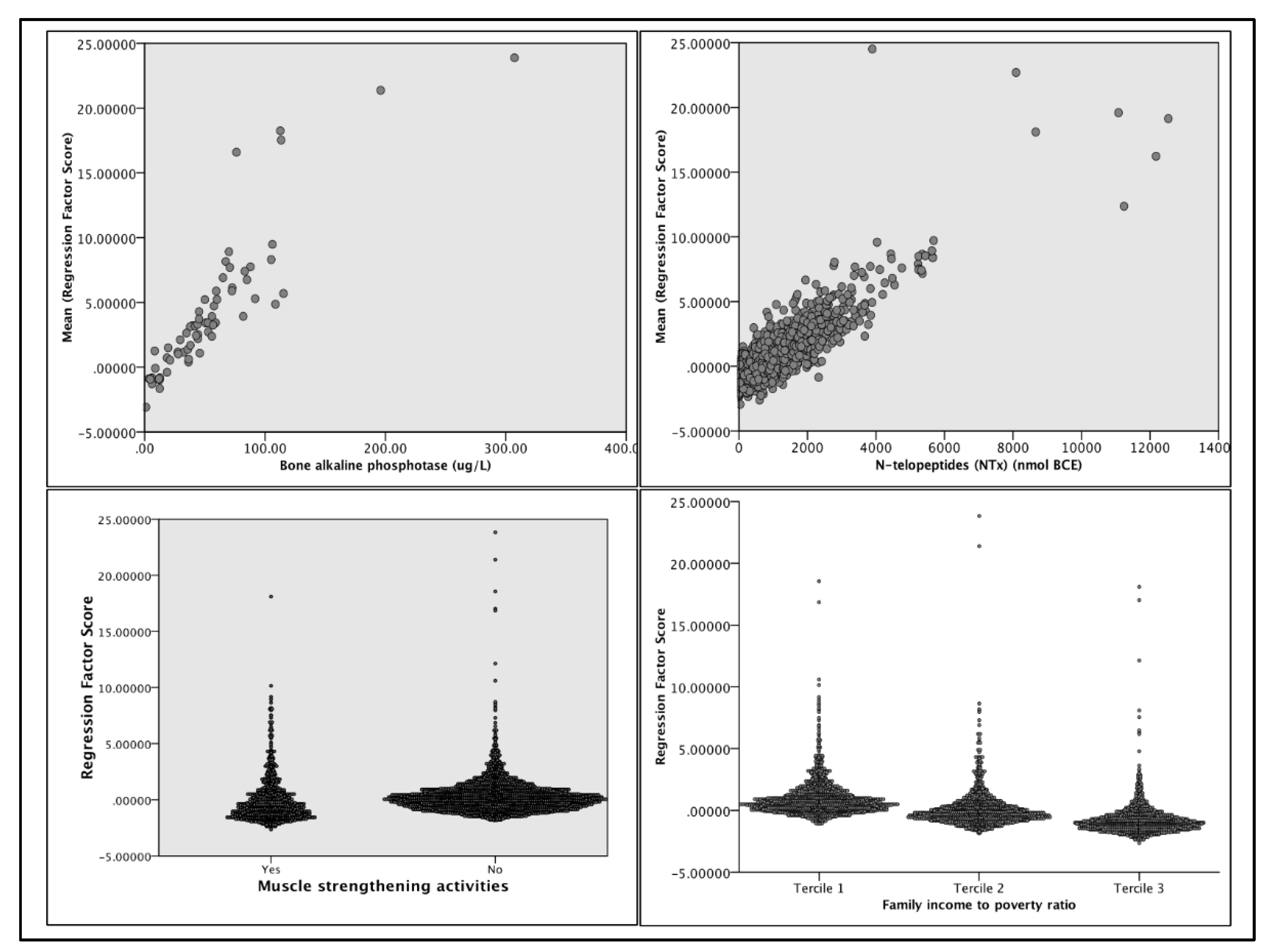
For the PCA, we imputed predictors that maximized both the Kaiser-Meyer-Olkin measure. The model explained over 57% of the total variance in injury group membership. Component 1 accounted for 17.5%; Component 2: 15.5%; Component 3: 12.34%; and Component 4: 11.83%. On Component 1, NTx (r=0.604) and BAP (r=0.603) loaded positively, while muscle-strengthening activities (MSA) (r=-0.404) and income tercile (r=-0.675) loaded negatively (
Figure 2). Low back pain (r=0.687) and physical activity (r=0.364) level loaded positively, and age (r=-0.716) loaded negatively on Component 2. For Component 3, BMI (r=-0.709) loaded negatively, and physical activity level (PAL)(r=0.668) loaded positively. Lastly, MSA (r=-0.618) and military/veteran status (r=-0.81) loaded negatively on Component 4.
3.4. Post Hoc Analyses
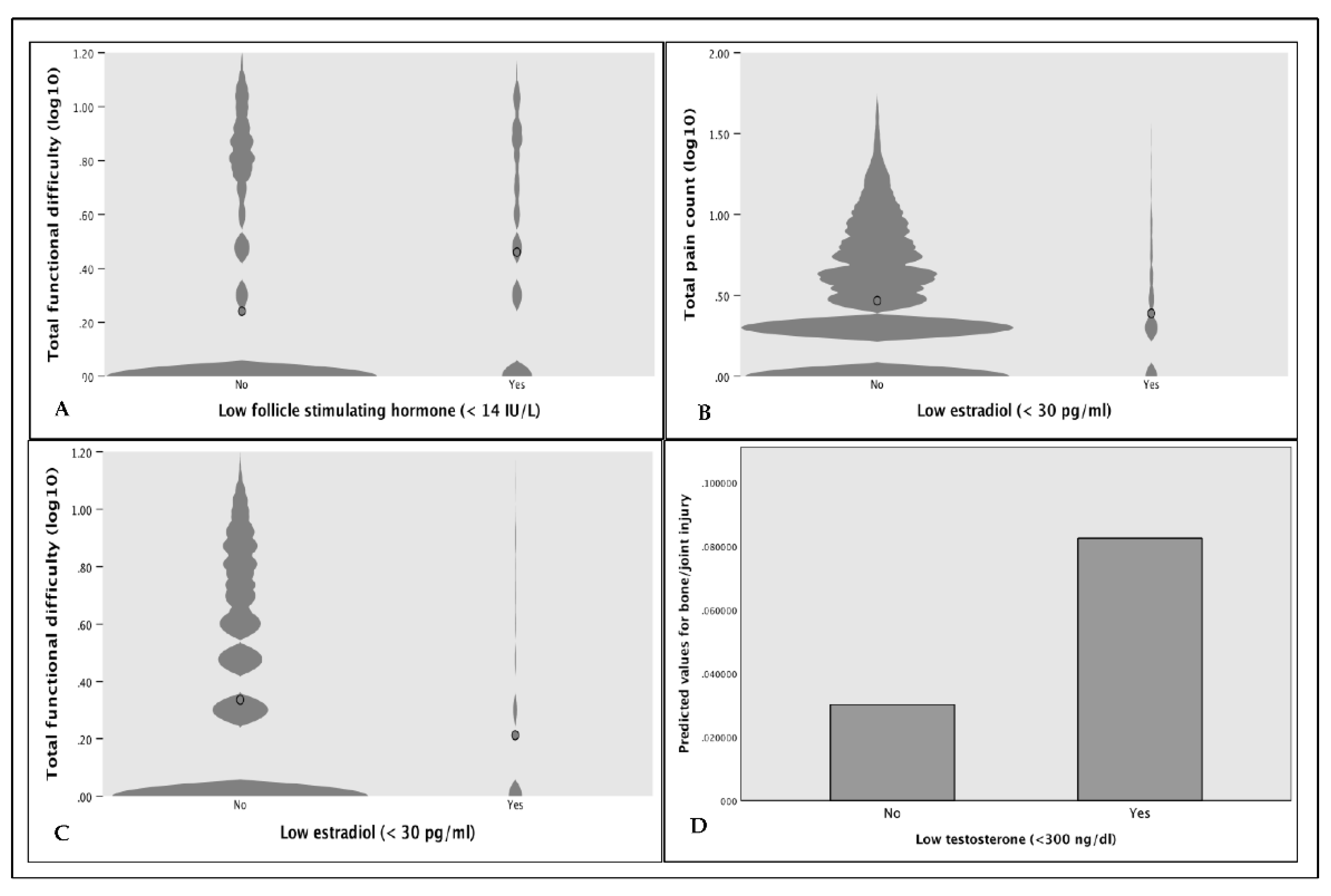
Due to the strong loadings of NTx and BAP on principal components 1 and 2, we performed post hoc analyses to explore differences in underlying conditions. Among those in the injury group, 16.2% [C.I. 12.9% - 20.2%] were told they had osteoporosis or brittle bones, compared to 4.4% in the control group (OR 4.19 [95% C.I. 1.32 – 13.33] p-value = 0.011). The proportion of respondents receiving treatment for osteoporosis was not significantly different between the groups (OR 0.351 [95% C.I. 0.079 – 1.552], p-value = 0.146). On average, those receiving treatment were older (= 4.94 years; p-value <0.001), and values for BAP (= -0.35; p-value <0.001) and NTx (= -111.57; p-value <0.001) were markedly lower except for higher BAP in those injured reporting treatment compared to injury and no treatment (p-value <0.001). Values for NTx and BAP were higher overall in the injury group regardless of treatment status, however, only significant for NTx (p-value = 0.012). BTM values were lowest in the control group receiving treatment. In the overall sample, men with total serum testosterone (T) below 300 ng/dl were over 2.8 times more likely to report bone/joint injury (p-value=0.038) (
Figure 3). Furthermore, serum estradiol (E2) below 30 pg/ml was associated with fewer pain points (OR 0.96 [95% C.I. 0.919 – 0.996], p-value = 0.03) and fewer FDs (OR 0.91 [95% C.I. 0.862 – 0.959], p-value <0.001). Similarly, follicle-stimulating hormone (FSH) below 14 IU/L (indicative of normal estrogen levels) was associated with fewer FDs (OR 0.798 [95% C.I. 0.71 – 0.90]).
4. Discussion
In this exploratory analysis, important insights were revealed into the complex nature of musculoskeletal injury prediction in the U.S. population. To our knowledge, this is the first comprehensive epidemiological investigation on MSI risk in the U.S. population establishing various factor cluster models supported by clinical markers. From a sociodemographic standpoint, increasing age, and lower socioeconomic level significantly increased the odds of self-reported injury. We also found an over 50% increase in MSI risk for those with a veteran/military background compared to the nonmilitary population. Recent work supports sociodemographic risk factors for MSIs; however, less is known about the causes of increased risk from military service [
25,
26]. Although active-duty service members tend to be fitter than age-matched civilian counterparts, veterans have comparatively higher chronic disease rates including musculoskeletal disorders [
26]. Exposures common to career military service are certainly culpable, such as regular, repetitive, and sustained physical work and combat-related hazards requiring rapid and intensive physical responses. It is plausible that the combination of discriminant factors discussed heretofore in addition to service-related exposures could explain the increased MSI risk.
Supporting previous studies, our findings showed that lifestyle factors including increasing BMI, sedentary lifestyle, and self-reported low back pain substantially increased the odds for self-reported injury while muscle strengthening decreased the odds [
25,
27]. Low back pain was highly prevalent in the U.S. population at 40% and added 70% increased risk in Model 2 compared to Model 1. We did not find an association between bone/joint injury and weekly work hours. Instead, overall physical activity level was a significant factor, aligning with previous work [
15]. In the risk model analysis, we found factor clusters of three or more greatly increased the odds of bone/joint injury and were associated with increased inflammatory markers. When adjusted for age, Model 1 associations attenuated while Models 2 and 3 remained significant, highlighting the effects of non-age-related factors on injury risk. Furthermore, there was an average of over 1.5 injury factors in the low-risk group, emphasizing the importance of using factor clusters to differentiate MSI risk.
A strength of this study was the inclusion of a CC analysis to explore potential confounders. Compared to controls matched on multiple variables, the injury group was older and had elevated BMI, higher TPF, more risk factors, and higher BTM. Addressing reverse causality, we found no significant association between injury and an increase in BMI in the previous year, suggesting that BMI did not increase because of injury but was a potential causal factor. Only after adjustment for BMI did increases in BMD significantly decrease the odds of injury, indicating that BMI confounded the relationship between BMD and injury. Multiple studies have demonstrated a positive relationship between BMI and BMD [
35]. However, our findings support recent work showing a saturation effect of BMI on BMD [
35].
Interestingly, we found conflicting results regarding the associations between BTM and bone/joint injury risk. While BAP and NTx were inversely associated with membership to the injury group in the logistic regression, the PCA and post hoc analyses showed a positive relationship. Going beyond basic correlation or regression analysis, PCA attempts to capture maximum variance by creating new uncorrelated components from linear combinations of predictor variables. Instead of eliminating less-predictive collinear variables, such as in stepwise regression, PCA loads combinations of correlated predictors onto components from the most to the least predictive of the dependent variable. In the aggregate sample, age, and BTM were predictive of injury but inversely correlated. The PCA accurately combined positively loaded NTx and BAP with other strongly correlated variables onto Component 1 that were more predictive of injury compared to the combination of variables correlated with age. This explains the strong loading of age on Component 2 and attenuated odds ratios for BAP and NTx after adjustment for age in the logistic regression. These findings are supported by the observed pattern of BTM values that decline with age but are higher in children and in those at risk for fractures [
28].
Another strength of this study was the use of ROC curve analysis to clarify the precision of a multifactorial model to predict pain and FDs. Test sensitivity and specificity were high, albeit optimized for 1 count of each. It is important to note that joint pain and FDs were not exclusively associated with injury. Moreover, the association between pain and injury attenuated after adjustment for other significant predictors, supporting work showing pain to be a suboptimal predictor of injury or movement performance [
29,
30]. Consequently, total joint pain was an unremarkable predictor of bone/joint injury.
Our findings align with work supporting the connection between inflammatory responses and musculoskeletal disorders brought on by metabolic disease processes, which implicates MSIs as sequelae of chronic disease. For instance, elevated markers of inflammation and muscle and bone degradation are often concomitant in metabolic conditions such as menopause, osteoporosis, diabetes, obesity, and cardiovascular disease [
21,
22,
31]. As a direct driver of inflammation and bone loss, fibrinogen may potentiate the differentiation of monocytes into osteoclasts via the receptor activator of the NF-kB ligand (RANKL) pathway [
31]. Coincidentally, impaired fibrinolysis has been shown to play a role in the pathology of chronic metabolic diseases including those mentioned previously [
31]. In a susceptible host, HPY may induce local and systemic inflammatory responses, releasing pro-inflammatory factors, which further activate osteoclasts resulting in bone resorption [
22].
Our investigation corroborated other studies reporting associations between hormonal dysregulation, higher BTM, and injury variables in both males and females [
32,
33]. Stressors such as aging, sleep deprivation, lack of recovery time, excessive workloads, and changes in hormone homeostasis are associated with proinflammatory processes and disruptions in bone metabolism through pathways shared by the immune and bone systems [
34]. However, the resulting pathological cascade has been shown to vary in different populations for different reasons. Several studies in active participants have shown short-term (hours/days), dose-dependent increases in BTM in response to novel physical activity [56]. The return to baseline is prolonged with higher relative increases in both intensity and duration. Similar studies have also shown long-term (weeks) decreases in BTM, implicating excessive workloads and inadequate recovery as causal factors [
20]. Meanwhile, increased BTM and low E2 in post-menopausal women were associated with a 3-fold increase in fracture risk after adjustment for BMD [
20,
34]. In elderly men, mounting evidence suggests declining testosterone levels promote bone resorption and decreased BMD contributing to increased osteoporotic fracture risk [
33].
Characterizing the human milieu, our PCA results may have captured clustered risk factors in those with bone/joint injury. Because increases in BTM appear to be acute in response to PA and decreases are resultant of age or chronic stressors, our cross-sectional observations of BTM appear to reflect habitual behavior such as PA or strength training, non-modifiable factors such as age and socioeconomic level, and underlying chronic disease. This is supported by factor loadings that are consistent with studies showing similar influences of age, BMI, musculoskeletal disorders, mechanical loading, and activity levels on BTM [
21]. Component 1 indicates a pattern related to lack of exercise, socioeconomic disparities, and BTM dysregulation. Component 2 suggests a pattern of higher activity levels and lower back pain in younger individuals. Similarly, Component 3 exhibits a pattern related to higher activity levels and lower BMI, possibly reflecting inadequate recovery. Finally, Component 4 displays a pattern possibly related to lack of exercise in civilians compared to those with military/veteran status.
4.1. Limitations
The most apparent limitations of this study are self-reported injury and disease status. Moreover, no clinical measures of injury severity were available. Studies have shown that region-specific pain and MSIs differ based on sex, occupation, or previous injury [
14,
15,
25]. Our investigation focused solely on broad injury risk, and therefore, we did not differentiate risk based on a plurality of injury locales or examine differential risk profiles based on sex. Due to retrospective analysis, it is unclear whether ADL difficulty could be used as an injury predictor or whether it is exclusively the result of injury, despite the prevalence of FDs in the control group and association with risk factor models. Future studies should seek to clarify the associations between common region-specific injuries and ADL exposures in recreationally active adults.
5. Conclusions
Findings from this study support a combination of modifiable and non-modifiable factors in assessing injury risk in the U.S. population. Moreover, these risk factors were validated by clinical measures of inflammation and chronic disease. Together with other biomarkers, BTM may offer valuable insights regarding musculoskeletal injury risk as they reflect age-related physiological processes, overall lifestyle, and underlying disease. In practice, injury risk could be systematically assessed by capturing accessible information through intake questionnaires, which are commonly used within clinical and non-clinical settings. The strong independent effect of ADL difficulty ratings on bone/joint injury calls into question the validity of movement screens based on supposed fundamental movement pattern ideals. Presumably, functional difficulty ratings vary according to subjective norms for physical functioning. Therefore, idealized movement criteria would be rendered invalid for movement contexts in which the ideal performance departs from movement strategies deemed functional by the individual. Ratings of ADL difficulty may be useful in identifying high-risk groups; however, more research is needed. Practitioners concerned with MSI risk should consider a multifactorial pre-screening tool to foster safe and effective physical activity participation and reduce the burden of musculoskeletal disorders.
Supplementary Materials
The following supporting information can be downloaded at: Preprints.org, Figure S1: title; Table S1: title; Video S1: title.
Author Contributions
AE: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data Curation, Writing – Original Draft, Visualization. PSG: Conceptualization, Methodology, Validation, Writing – Review & Editing. JS: Conceptualization, Validation, Writing – Review & Editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
The data used throughout this study were derived from the following resources available in the public domain:
https://www.cdc.gov/nchs/nhanes/index.htm. Accordingly, this research is considered exempt under 45 CFR 46.104.
Informed Consent Statement
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. These data were derived from the following resources available in the public domain:
https://www.cdc.gov/nchs/nhanes/index.htm.
Conflicts of Interest
None
References
- Collaborators U. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591-606. [CrossRef]
- Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: U.S. Department of Health and Human Services, 2008.
- Bonazza NA, Smuin D, Onks CA, Silvis ML, Dhawan A. Reliability, validity, and injury predictive value of the functional movement screen: a systematic review and meta-analysis. The American Journal of Sports Medicine. 2017;45(3):725-32. [CrossRef]
- Teyhen D et al. Consortium for health and military performance and American College of Sports Medicine Summit: Utility of functional movement assessment in identifying musculoskeletal injury risk. Current Sports Medicine Reports. 2014;13(1):52-63. [CrossRef]
- Wang D, Chen J, Lai W, Vail J, Rugg CM, Hame SL. Predictive value of the functional movement screen for sports-related injury in NCAA division I athletes. Orthopaedic Journal of Sports Medicine. 2017;5(3_suppl3):2325967117S00132. [CrossRef]
- O’connor FG, Deuster PA, Davis J, Pappas CG, Knapik JJ. Functional movement screening: predicting injuries in officer candidates. Medicine And Science in Sports and Exercise. 2011;43(12):2224-30. [CrossRef]
- Teyhen DS et al. What risk factors are associated with musculoskeletal injury in US Army Rangers? A prospective prognostic study. Clinical Orthopaedics and Related Research®. 2015;473(9):2948-58. [CrossRef]
- Moore E, Chalmers S, Milanese S, Fuller JT. Factors influencing the relationship between the functional movement screen and injury risk in sporting populations: a systematic review and meta-analysis. Sports Medicine. 2019;49(9):1449-63. [CrossRef]
- Chimera NJ, Smith CA, Warren M. Injury history, sex, and performance on the functional movement screen and Y balance test. Journal of Athletic Training. 2015;50(5):475-85. [CrossRef]
- Fulton J et al. Injury risk is altered by previous injury: a systematic review of the literature and presentation of causative neuromuscular factors. International Journal of Sports Physical Therapy. 2014;9(5):583: PMID: 25328821.
- Toohey LA, Drew MK, Cook JL, Finch CF, Gaida JE. Is subsequent lower limb injury associated with previous injury? A systematic review and meta-analysis. British Journal of Sports Medicine. 2017;51(23):1670-8. [CrossRef]
- Matzkin E, Garvey K. Sex differences in common sports-related injuries. NASN School Nurse. 2019;34(5):266-9. [CrossRef]
- Uehli K et al. Sleep problems and work injuries: a systematic review and meta-analysis. Sleep Medicine Reviews. 2014;18(1):61-73. [CrossRef]
- Fischer D, Lombardi DA, Folkard S, Willetts J, Christiani DC. Updating the “Risk Index”: A systematic review and meta-analysis of occupational injuries and work schedule characteristics. Chronobiology International. 2017;34(10):1423-38. [CrossRef]
- Dzakpasu FQ et al. Musculoskeletal pain and sedentary behaviour in occupational and non-occupational settings: a systematic review with meta-analysis. International Journal of Behavioral Nutrition and Physical Activity. 2021;18(159). [CrossRef]
- Macedo LG, Battié MC. The association between occupational loading and spine degeneration on imaging–a systematic review and meta-analysis. BMC Musculoskeletal Disorders. 2019;20:1-5. [CrossRef]
- Rhon DI et al. Much work remains to reach consensus on musculoskeletal injury risk in military service members: a systematic review with meta-analysis. European Journal of Sport Science. 2022;22(1):16-34. [CrossRef]
- Teyhen DS et al. Identification of risk factors prospectively associated with musculoskeletal injury in a warrior athlete population. Sports Health. 2020;12(6):564-72. [CrossRef]
- Afifi N et al. Value of albumin-fibrinogen ratio and CRP-albumin ratio as predictor marker of disease activity in Egyptian RA patients, correlated with musculoskeletal sonography. Open Access Rheumatology: Research and Reviews. 2020:241-8. [CrossRef]
- Hughes JM et al. Bone formation is suppressed with multi-stressor military training. European Journal of Applied Physiology. 2014;114:2251-9. [CrossRef]
- Roberts HM, Law RJ, Thom JM. The time course and mechanisms of change in biomarkers of joint metabolism in response to acute exercise and chronic training in physiologic and pathological conditions. European Journal of Applied Physiology. 2019;119(11-12):2401-2420. [CrossRef]
- Wang T et al. Relationship between Helicobacter pylori infection and osteoporosis: a systematic review and meta-analysis. BMJ Open. 2019;9(6). [CrossRef]
- Napoli N et al. Bone turnover markers do not predict fracture risk in type 2 diabetes. Journal of Bone and Mineral Research. 2020;35(12):2363-71. [CrossRef]
- Zetterman T, Markkula R, Kalso E. Elevated highly sensitive C-reactive protein in fibromyalgia associates with symptom severity. Rheumatology Advances in Practice. 2022;6(2). [CrossRef]
- Alkassabi O et al. Risk factors to persistent pain following musculoskeletal injuries: a systematic literature review. International Journal of Environmental Research and Public Health. 2022; 29;19(15):9318. [CrossRef]
- Hinojosa R, Hinojosa MS. Activity-limiting musculoskeletal conditions in US veterans compared to non-veterans: results from the 2013 National Health Interview Survey. PLoS One. 2016;11(12). [CrossRef]
- Briggs MS et al. Relations of C-reactive protein and obesity to the prevalence and the odds of reporting low back pain. Archives of Physical Medicine and Rehabilitation. 2013;94(4):745-52. [CrossRef]
- Zhang Yet al. Variation of Bone Turnover Markers in Childhood and Adolescence. International Journal of Clinical Practice. 2023;2023. [CrossRef]
- Rhon DI et al. Much work remains to reach consensus on musculoskeletal injury risk in military service members: a systematic review with meta-analysis. European Journal of Sport Science. 2022;22(1):16-34. [CrossRef]
- Kazman JB et al. Factor structure of the functional movement screen in marine officer candidates. Journal of Strength Conditioning Research. 2014;28(3):672–8. [CrossRef]
- Luyendyk JP, Schoenecker JG, Flick MJ. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood. 2019;133(6):511-520. [CrossRef]
- Chlebowski RT et al. Estrogen alone and joint symptoms in the Women’s Health Initiative randomized trial. Menopause. 2013;20(6):600-8. [CrossRef]
- Shigehara K et al. Testosterone and bone health in men: a narrative review. Journal of Clinical Medicine. 2021;10(3):530. [CrossRef]
- Garnero P et al. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. Journal of Bone and Mineral Research. 2000;15(8):1526-36. [CrossRef]
- Ma M, Feng Z, Liu X, Jia G, Geng B, Xia Y. The saturation effect of body mass index on bone mineral density for people over 50 years old: a cross-sectional study of the US population. Frontiers in Nutrition. 2021;8. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).