Submitted:
26 March 2024
Posted:
28 March 2024
You are already at the latest version
Abstract
Keywords:
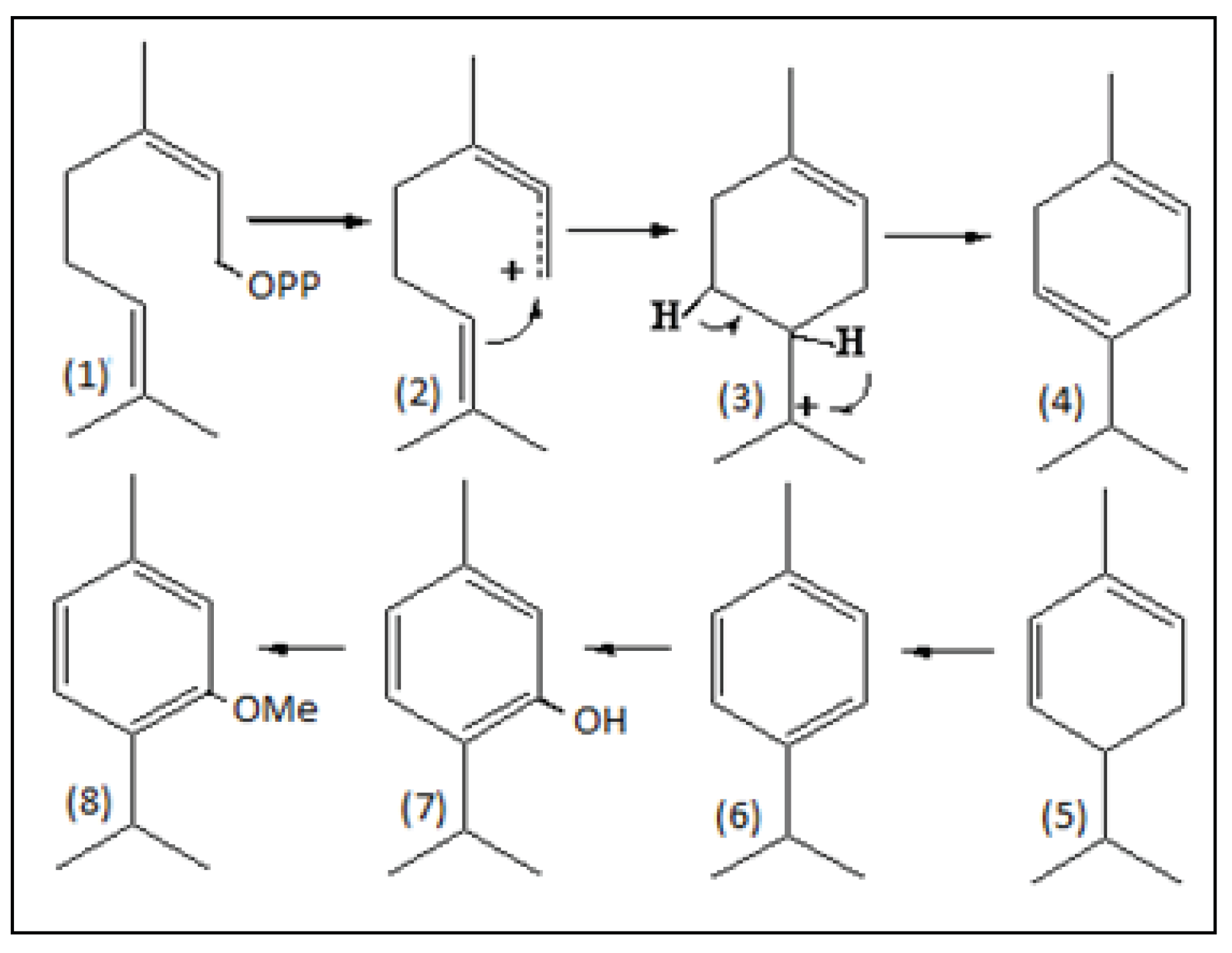
1. Introduction
2. Results and Discussion
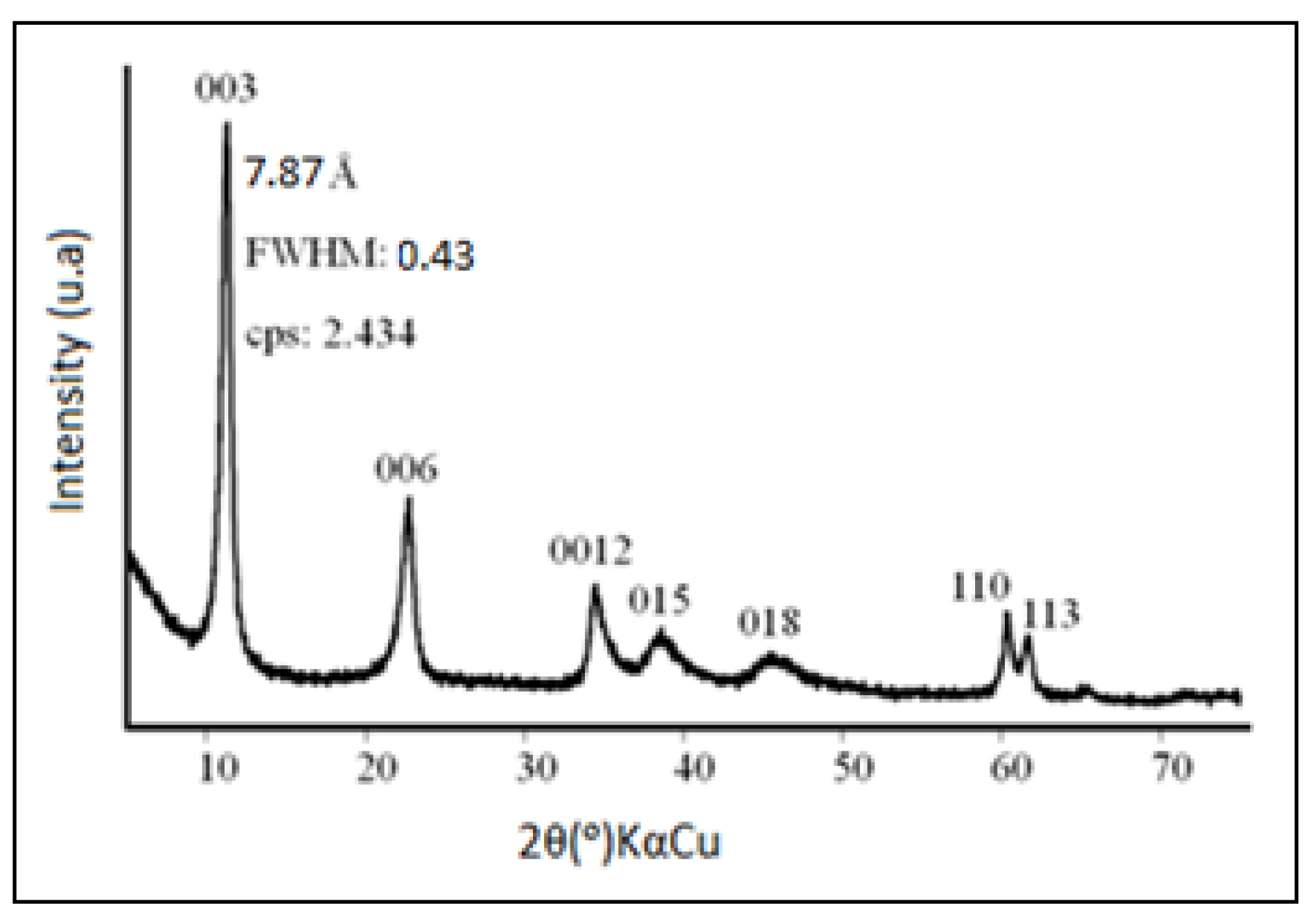
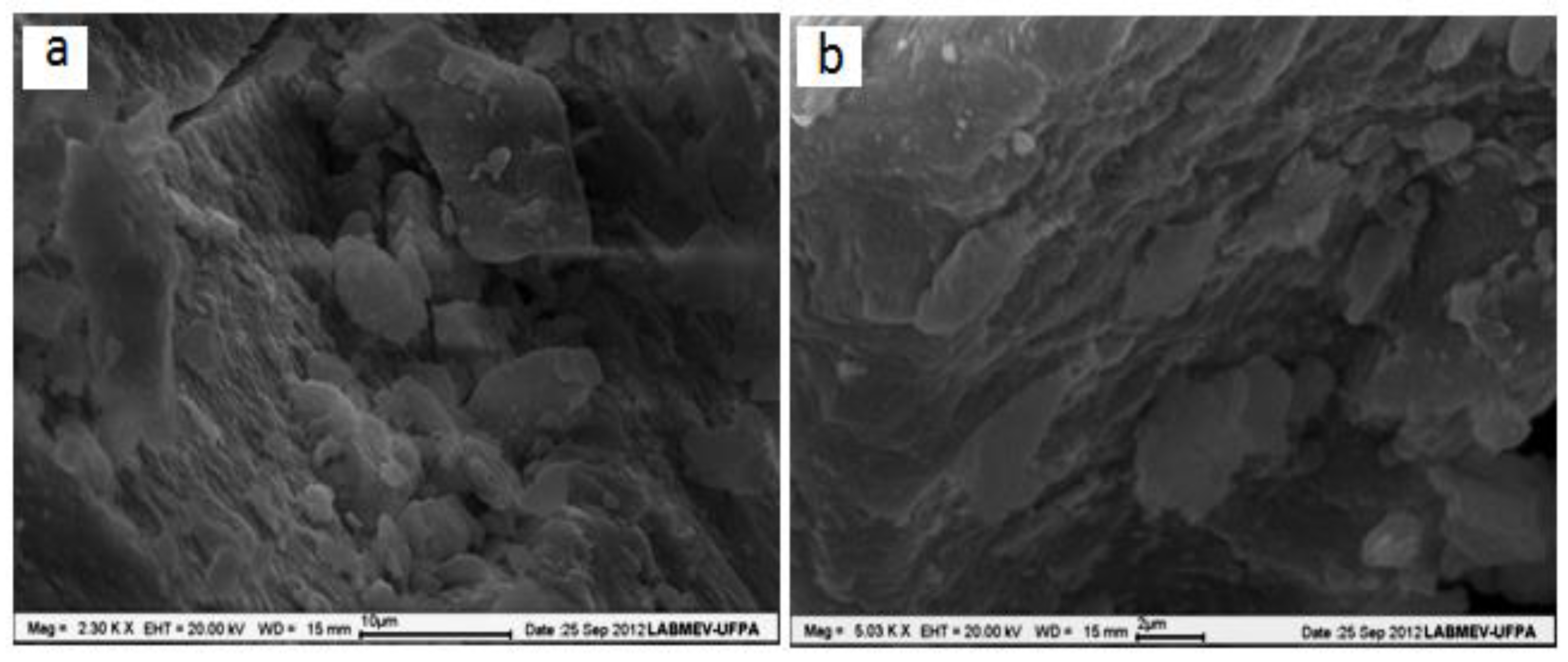
2.1. Hydrotalcite Characterization
2.2. Oil Characterization
2.3. Catalytic Tests of the Oil with Water and Hydrotalcite
3. Materials and Methods
3.1. Mineral Material
3.2. Plant Material and Processing
3.3. Oil Composition Analysis
3.4. Hydrotalcite Characterization
3.5. Catalytic Reaction with the Oil and Hydrotalcite
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Solkina, Y.S.; Rechetnikov, S.I.; Estrada, M.; Simakov, A.; Murzin, D.Y.; Simakova, I.L. Evaluation of gold on alumina catalyst deactivation dynamics during α-pinene isomerization. Chem. Eng. J. 2011, 176–177, 42–48. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Andrade, E.H.A. Database of the Amazon aromatic plants and their essential oils. Quim. Nova 2009, 32, 595–622. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Zoghbi, M.G.B.; Andrade, E.H.A. Plantas aromáticas da Amazônia e seus óleos essenciais. Museu Paraense Emílio Goeldi, Coleção Adolpho Ducke, Belém, 2001.
- Weniger, B.; Robledo, S.; Arango, G.J.; Deharo, E.; Aragón, R.; Muñoz, V.; Anton, R. Antiprotozoal activities of Colombian plants. J. Ethnopharmacol. 2001, 78, 193–200. [Google Scholar] [CrossRef]
- Morais, A.A.; Mourão, J.C.; Gottlieb, O.R.; Silva, M.L.; Marx, M.C.; Maia, J.G.S.; Magalhães, M.T. Óleos essenciais da Amazônia contendo timol. Acta Amaz. 1972, 2, 45–46. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Zoghbi, M.G.B.; Andrade, E.H.A.; Silva, M.H.L. Essential oils from Conobea scoparioides (Cham. & Schltdl.) Benth. Flav. Fragr. J. 2000, 15, 413–414. [Google Scholar]
- Rebelo, M.M.; Silva, J.K.R.; Andrade, E.H.A.; Maia, J.G.S. Antioxidant capacity and biological activity of essential oil and methanol extract of Conobea scoparioides (Cham. & Schltdl.) Benth. J. Braz. Chem. Soc. 2009, 20, 1031–1038. [Google Scholar] [CrossRef]
- Costa, R.G.; Faria, L.J.G.; Gusmão, S.A.L.; Silva, J.K.R.; Andrade, E.H.A.; Maia, J.G.S. Essential oil of pataqueira (conobea scoparioides Benth. ): from natural occurrence and produced by hydroponics Adv. Plants Agric. Res. 2014, 1, 90–94. [Google Scholar] [CrossRef]
- Figueras, F.; Lopez, J.; Sanchez-Valente, J.; Vu, T.T.H.; Clacens, J.-M.; Palomeque, J. Isophorone isomerization as model reaction for the characterization of solid bases: application to the determination of the number of sites. J. Catal. 2002, 211, 144–149. [Google Scholar] [CrossRef]
- Climent, M.; Corma, A.; Iborra, S.; Velty, A. Activated hydrotalcites as catalysts for the synthesis of chalcones of pharmaceutical interest. J. Catal. 2004, 221, 474–482. [Google Scholar] [CrossRef]
- Takehira, K.; Shishido, T.; Wang, P.; Kosaka, T.; Takaki, K. Autothermal reforming of CH4 over supported Ni catalysts prepared from Mg–Al hydrotalcite-like anionic clay. J. Catal. 2004, 221, 43–51. [Google Scholar] [CrossRef]
- Antunes, W.; Veloso, C.O.; Henriques, C.A. Transesterification of soybean oil with methanol catalyzed by basic solids. Catal. Today 2008, 133–135, 548–554. [Google Scholar] [CrossRef]
- Nakatsuka, T.; Kawasaki, H.; Yamashita, S.; Kohjiya, S. The polymerization of β-propiolactone by calcined synthetic hydrotalcite. Bull. Chem. Soc. Jpn. 1979, 52, 2449–2450. [Google Scholar] [CrossRef]
- Reichle, W.T. Catalytic reactions by thermally activated, synthetic, anionic clay minerals. J. Catal. 1985, 94, 547–557. [Google Scholar] [CrossRef]
- Kannan, S. Catalytic applications of hydrotalcite-like materials and their derived forms. Catal. Surv. Asia 2006, 10, 117–137. [Google Scholar] [CrossRef]
- Rebelo, M.M.; Cunha, M.V.P.O.; Corrêa, J.A.M. Hidróxidos duplos lamelares à base de escória de alto forno. Quim. Nova 2012, 35, 883–888. [Google Scholar] [CrossRef]
- Kishore, D.; Kannan, S. Isomerization of eugenol and safrole over MgAl hydrotalcite, a solid base catalyst. Green Chem. 2012, 4, 607–610. [Google Scholar] [CrossRef]
- Rachwalick, R.R.; Olejniczak, Z.; Jiao, J.; Huang, J.; Hunger, M.; Sulikowski, B. Isomerization of α-pinene over dealuminated ferrierite-type zeolites. J. Catal. 2007, 252, 161–170. [Google Scholar] [CrossRef]
- Gscheidmeier, M.; Häberlein, H.; Häberlein, H.H.; Häberlein, J.T.; Häberlein, M.C. Process for the preparation of camphene by the rearrangement of α-pinene US Pat. 582 6202, 1998. [Google Scholar]
- Aeschbach, R.; Löliger, J.; Scott, B. C.; Murcia, A.; Butler, J.; Halliwell, B.; Aruoma, O. I. Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem. Toxicol. 1994, 32, 31–36. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Yousuf, S.; Khan, L.A.; Manzoor, N. Proton translocating ATPase mediated fungicidal activity og eugenol and thymol. Fitoterapia 2010, 81, 1157–1162. [Google Scholar] [CrossRef]
- Mathela, C.S.; Singh, K.K.; Gupta, V.K. Synthesis and in vitro antibacterial activity of thymol and carvacrol derivatives. Acta Pol. Pharm. 2010, 67, 375–380. [Google Scholar] [PubMed]
- Riella, K.R.; Marinho, R.R.; Santos, J.S.; Pereira-Filho, R.N.; Cardoso, J.C.; Albuquerque-Junior, R.L.C.; Thomazzi, S.M. Anti-inflammatory and cicatrizing activities of thymol, a monoterpene of the essential oil from Lippia gracilis, in rodents. J. Ethnopharmacol. 2012, 143, 656–663. [Google Scholar] [CrossRef]
- Miyata, S. Anion-exchange properties of hydrotalcite-like compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Ross, G. J.; Kodama, H. Properties of a synthetic magnesium-aluminum carbonate hydroxide and its relationship to magnesium-aluminum double hydroxide, manasseite and hydrotalcite. Am. Mineral. 1967, 52, 1036–1047. [Google Scholar]
- Miyata, S. The Syntheses of hydrotalcite-like compounds and their structures and physico-chemical properties—I: the systems Mg2+-Al3+-NO−3, Mg2+-Al3+-Cl−, Mg2+-Al3+-ClO−4, Ni2+-Al3+-Cl− and Zn2+-Al3+-Cl−. Clays Clay Miner. 1975, 23, 369–375. [Google Scholar] [CrossRef]
- NIST/EPA/HIH. Mass Spectral Library - Mass Spectral Search Program, NIST 05, Version 2.0d; The NIST Mass Spectrometry Data Center: Gaithersburg, 2005. [Google Scholar]
- Adams, R.P. ; Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th Edition; Allured Publishing Corporation, Carol Stream, 2007.
- Granger, R.; Passet, J.; Verdier, R. Gamma-terpinene, precursor of p-cymene in Thymus vulgaris L. CR Hebd. Seances Acad. Sci. 1964, 258, 5539–5541. [Google Scholar]
- Poulose, A.J.; Croteau, R. Biosynthesis of aromatic monoterpenes: conversion of γ-terpinene to p-cymene and thymol in Thymus vulgaris L. Arch. Biochem. Biophys. 1978, 187, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Poulose, A.J. Croteau, R. Gamma-Terpinene synthetase: a key enzyme in the biosynthesis of aromatic monoterpenes. Arch. Biochem. Biophys. 1978, 191, 400–411. [Google Scholar] [CrossRef]
- Bohlmann, J.; Meyer-Guaen, G.; Croteau, R. Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc. Natl. Acad. Sci. USA 1998, 95, 4126–4133. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiró, F.; Vaccari, A. Hydrotalcite-type anionic clays: preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
| Constituents | IR | S-1 | S-2 | S-3 | |
| Fresh plant | Dried plant | ||||
| (Z)-3-Hexenol | 858 | 0.1 | 0.2 | ||
| α-Thujene | 930 | 0.2 | 0.3 | ||
| α-Pinene | 937 | 0.1 | 0.1 | ||
| Sabinene | 975 | 0.2 | |||
| β-Pinene | 979 | 0.3 | 0.2 | ||
| 3-Octanone | 982 | 2.0 | 2.1 | ||
| α-Phellandrene | 1002 | 11.6 | 12.1 | 12.1 | 14.3 |
| p-Cymene | 1020 | 1.6 | 1.6 | 1.5 | 1.7 |
| Limonene | 1028 | 0.1 | 0.1 | ||
| β- Phellandrene | 1030 | 0.5 | 0.5 | 0.2 | 0.2 |
| (E)-β-Ocimene | 1048 | 0.2 | 0.5 | 0.7 | |
| γ-Terpinene | 1059 | 0.3 | 0.4 | 0.4 | 0.3 |
| Linalool | 1098 | 0.3 | 0.3 | 0.2 | 0.2 |
| cis-p-Menth-2-en-1-ol | 1120 | 0.1 | 0.1 | ||
| allo-Ocimene | 1130 | 0.1 | 0.1 | ||
| Karahanaenone | 1154 | 0.1 | 0.1 | ||
| p-Cymen-8-ol | 1181 | 0.6 | 0.6 | 0.4 | 0.7 |
| Thymol methyl ether | 1232 | 39.2 | 38.3 | 39.6 | 47.7 |
| Thymol | 1290 | 41.2 | 41.1 | 40.0 | 26.4 |
| Thymol acetate | 1355 | 0.1 | 0.1 | ||
| Eugenol | 1360 | 0.2 | 0.2 | 0.2 | 0.2 |
| Viridiflorene | 1496 | 0.5 | 1.2 | ||
| α-Selinene | 1498 | 0.2 | 0.9 | ||
| (E,E)-α-Farnesene | 1508 | 0.3 | 0.3 | 1.2 | 1.3 |
| (E)-Nerolidol | 1563 | 0.1 | 0.1 | ||
| Unidentified sesquiterpenes | 0.2 | 0.3 | 0.4 | 1.4 | |
| Total | 98.7 | 98.8 | 98.2 | 97.8 | |
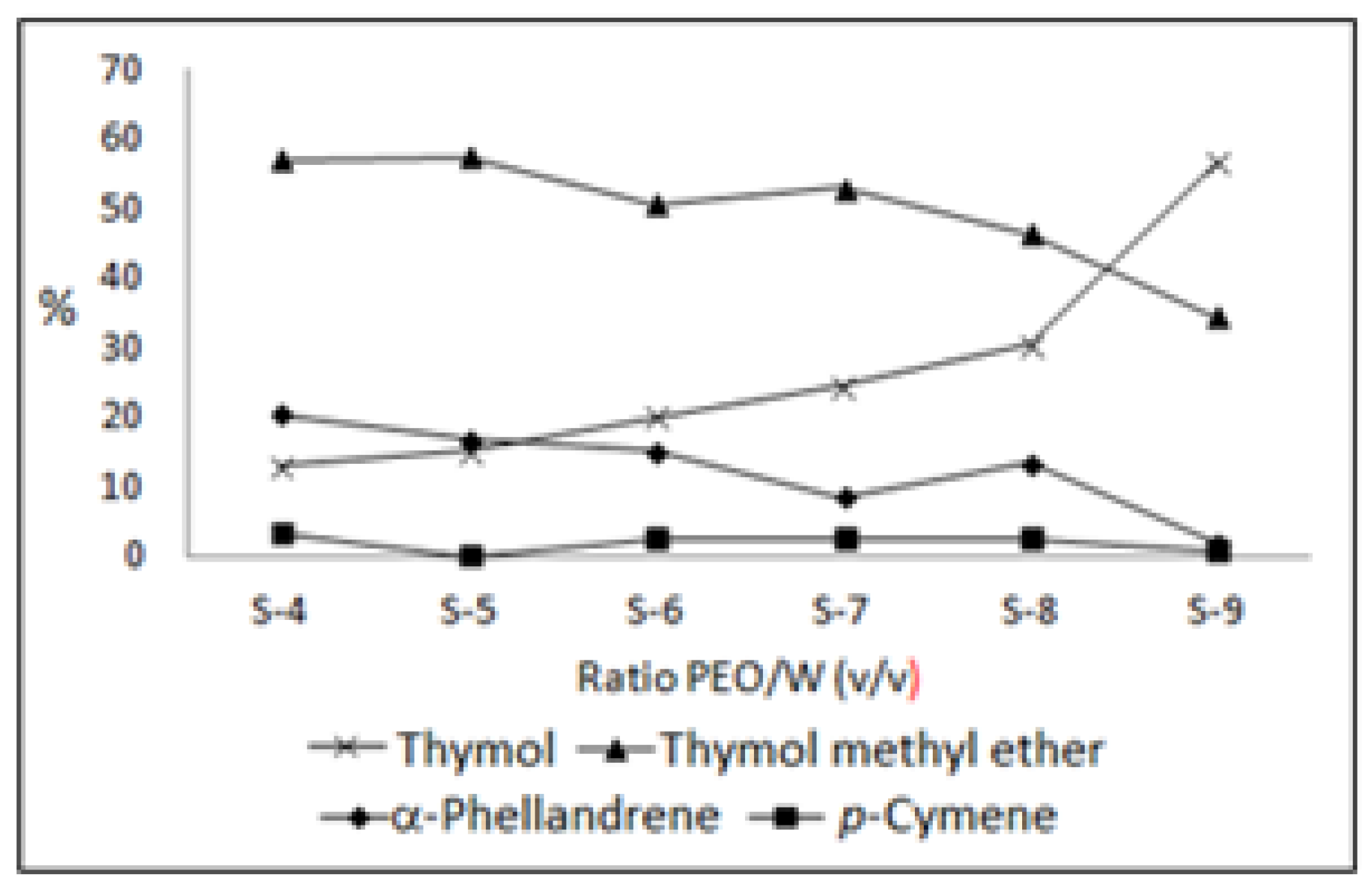
| Constituents | IR | S-4 | S-5 | S-6 | S-7 | S-8 | S-9 |
| α-Phellandrene | 1002 | 20.4 | 16.8 | 15.2 | 8.5 | 13.5 | 1.9 |
| p-Cymene | 1020 | 3.1 | 2.8 | 2.5 | 2.3 | 2.3 | 0.6 |
| γ-Terpinene | 1059 | 0.8 | 0.7 | 0.6 | 0.7 | 0.5 | 0.1 |
| p-Cymen-8-ol | 1181 | 0.2 | 0.2 | 0.2 | 0.3 | 0.4 | 0.6 |
| Thymol methyl ether | 1232 | 56.9 | 57.2 | 50.5 | 52.9 | 46.3 | 34.3 |
| Thymol | 1290 | 13.1 | 15.0 | 20.0 | 24.4 | 30.3 | 56.6 |
| Total | 94.5 | 92.7 | 89.0 | 89.1 | 93.3 | 94.1 | |
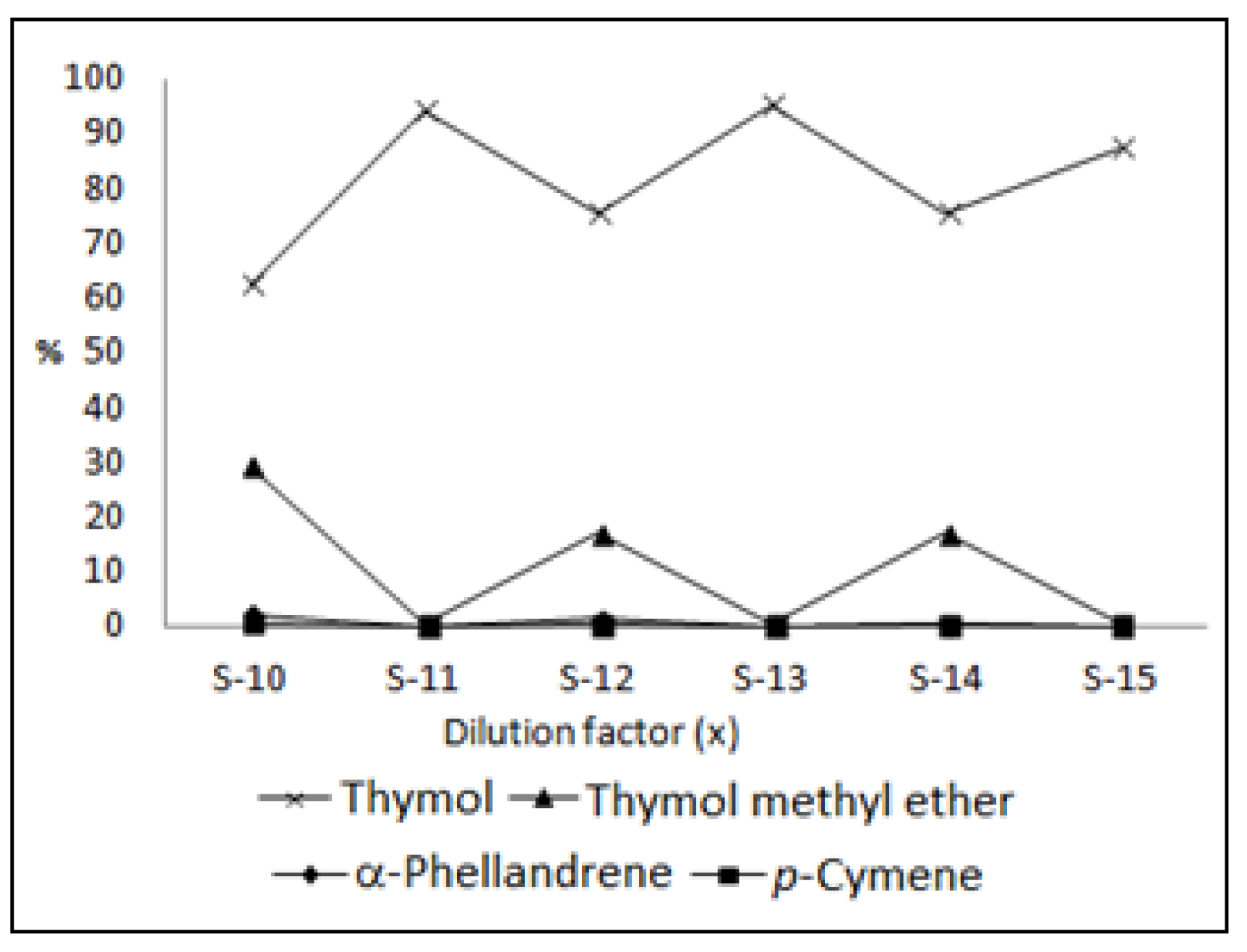
| Constituents | IR | S-10 | S-11 | S-12 | S-13 | S-14 | S-15 |
| α-Phellandrene | 1002 | 2.0 | 0.1 | 1.3 | 0.3 | ||
| p-Cymene | 1020 | 0.5 | 0.1 | 0.4 | 0.2 | ||
| γ-Terpinene | 1059 | 0.1 | 0.1 | ||||
| p-Cymen-8-ol | 1181 | 0.8 | 1.4 | 0.9 | 1.3 | 0.9 | 1.1 |
| Thymol methyl ether | 1232 | 29.2 | 0.5 | 16.9 | 0.4 | 16.9 | 0.3 |
| Thymol | 1290 | 62.3 | 94.2 | 75.4 | 95.0 | 75.5 | 87.2 |
| Total | 94.9 | 96.3 | 95.0 | 96.7 | 93.8 | 88.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





