1. Introduction
Recently it was pointed out that over 750 invasive trees and shrub species have been recorded worldwide [
1]. Among them is
Prosopis juliflora (Sw.) DC. (Fabaceae; mesquite) which is native to Central and South America [
2,
3,
4]. But later it was introduced worldwide [
2,
4,
5]. The plant was intentionally introduced to new environments across the world in the late 1970s to early 1980s in an attempt to reverse deforestation and desertification [
6,
7,
8]. The first introductions into Africa where in South Africa in 1880s [
9] and in Khartoum, Sudan in 1917 [
10]. After introduction, the species rapidly naturalized and expanded into new locations [
2], including a wide spread across the Middle East from Saudi Arabia [
11,
12] to the UAE [
13,
14,
15], Africa and Asia [
16].
P. juliflora is an aggressive invasive weed in Asia, Africa and Australia, both in the dry and humid tropics, listed as an invasive species in 64 countries [
16]. Several incidences of occurrence and its impacts, have been reported in many parts of Africa, including the Horn of Africa [
17], and countries in the Sub-Sahara, particularly Kenya [
6,
22], Ethiopia [
2,
4], and Tanzania [
18,
32]. Both domestic and wild animals aid in the dispersal of
P. juliflora seeds, which are embedded in an attractive succulent fruit [
20]. Besides, anthropogenic disturbances such as land preparation for agriculture, livestock grazing and environmental phenomena particularly floods favor further colonization [
21,
22]. Among its major impacts include out competing native species [
3,
19,
22], reducing the carrying capacity of grazing lands [
23], destruction of water catchments [
24], and encroachment of inhabited areas, water catchments, farmlands and pastures [
25]. Soil erosion and degradation are also mentioned as consequences of
P. juliflora invasion, leading to reduced quality of rangelands and farmlands [
26,
27], causing severe losses in crop production.
P. juliflora is also linked with human and animal fatalities due to injuries caused by its thorns [
6,
25].
Due to its global distribution and impacts, various multidisciplinary actions have been initiated to manage it and reduce current and future impacts [
29,
30,
31,
32]. Durigan et al. [
33] highlighted the necessity of prevention and control actions to avoid or reverse negative effects of biological invasions. Similarly, attempts to control the spread of
P. juliflora through management by utilization have been undertaken in Ethiopia [
34,
35,
36] and Kenya [
37]. However, control through utilization does not work. Mbaabu et al. [
28] pointed out that “Although the management of
Prosopis by utilization has been promoted in Baringo for 10–15 years, the spread of
Prosopis has not stopped or slowed down”.
In Tanzania, mainly baseline research studies have been carried out [
18,
32]. Recent data indicate that Tanzania accounts for 23.9% of all invasive trees and shrub species worldwide 41]. Furthermore, about one third (32%) of the potential invasive alien plants are found in protected areas [
38]. Based on this finding, it is evident that the rest of the potential invasive trees and shrub species (68%) occupy other areas including rangelands, farmlands, roadsides and residential areas. Among the aggressive invasive species is
P. juliflora, which is also categorized in the list of priority species of national concern [
39]. There have been some efforts to control
P. juliflora invasion in the agroecosystems in Northern Tanzania [
18,
32]. Kilawe et al. [
18] established preliminary insights on the invasion status of
P. juliflora in Northern Tanzania while Eschen et al. [
32] tested different
P. juliflora management methods in combination with grassland restoration.
However, despite these interventions and a broad survey in Moshi and Simanjiro districts, focusing on agroecosystems and rangelands [
40], still not much has been achieved to control the spread of
P. juliflora in Northern Tanzania. This study aimed at testing novel and hopefully more efficient control measures against
P. juliflora. The two districts are mainly inhabited by poor subsistence farmers and livestock keepers, of which the majority cannot afford to buy modern, commercial herbicides to control
P. juliflora. The findings of an ongoing survey found that the majority of farmers (>80.0%) are not willing to spend more than 50,000 TZS per ha (equivalent to 18 Euro/ha) on herbicides to be used for
P. juliflora management [
40]. Hence, our study focused on the efficacy of cheaper methods: manual uprooting and novel mixtures consisting of water, dish soap, salt, bleach, vinegar and diesel, in comparison to a commercial herbicide (glyphosate). Previous studies have shown that mixtures containing diesel [
32], salt and vinegar [
41] may have phytotoxic effects on weeds, by impairing the cellular activity, which eventually may lead to their death [
42]. Dish liquid soap acts as a surfactant to increase the spread of the mixtures and its absorption through the stem [
41].
The study was confined to rangelands and agroecosystems in a 15km×15km area in Northern Tanzania. The following research hypotheses were tested: (i) The performance of the novel spray mixtures against P. juliflora is comparable to uprooting and commercial herbicide control methods; (ii) the performance of control methods for P. juliflora is not affected by land use; and (iii) the performance of control methods is the same in the two districts.
2. Materials and Methods
2.1. Study Sites
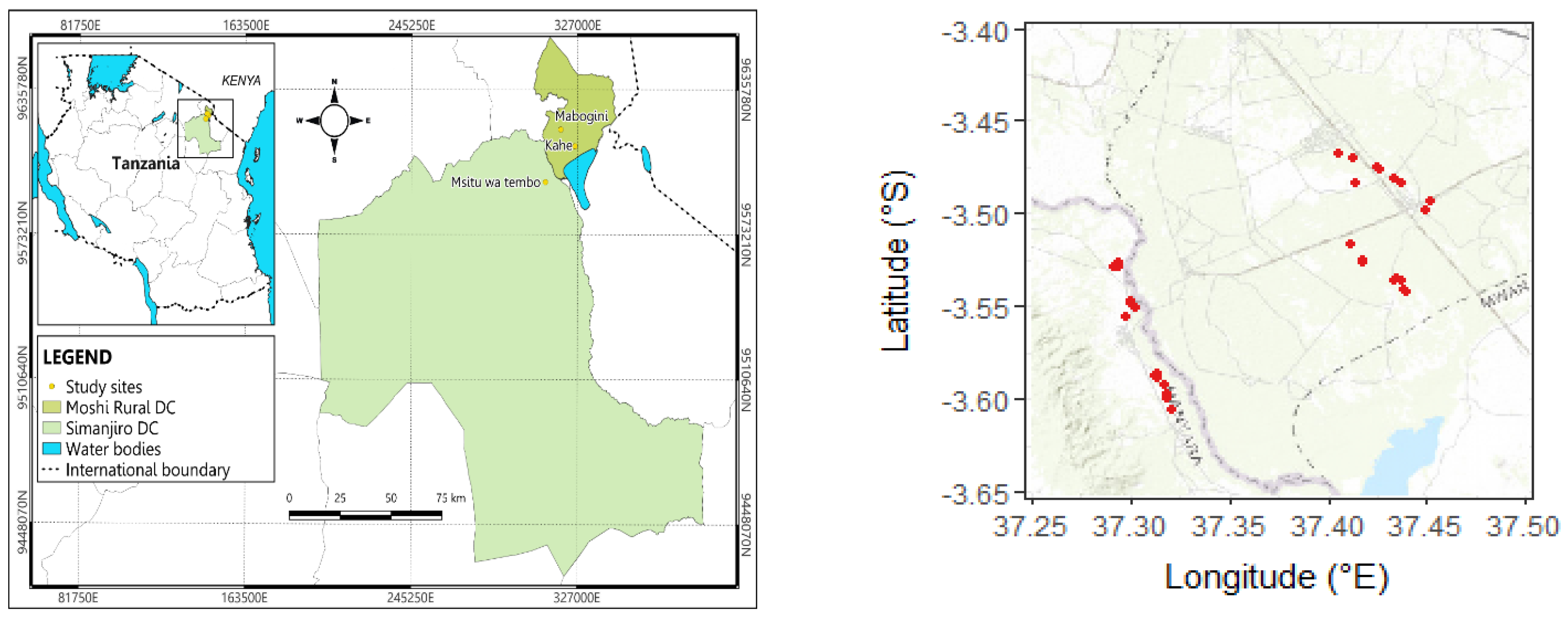
The study was conducted between May and December 2022 in the Simanjiro and Moshi districts of Northern Tanzania (
Figure 1), where
P. juliflora is known to be invasive. Experimental plots (20m×30m) were laid out nearby 3 villages in each district with a total of 14 plots in Simanjiro and 18 plots in Moshi (
Figure 1). The plots were placed aiming for
P. juliflora infested areas with no signs of recent, direct human impact on the trees, such as coppicing and burning of charcoal. Moreover, plots were distinguished into two land use types (grazing land vs. farmland). It was attempted, as far as possible, to have the two land use types equally represented among the plots around each village. Permanent pasture was categorised as grazing land, while areas under regular cultivation were categorised as farmland. The elevation of the plots was 690-790 m ASL in Simanjiro and 640-740 m ASL in Moshi.
The study area borders a mountainous topography towards Kilimanjaro Mountain in the north, while stretching towards the lowland in the south.
P. juliflora is typically confined to the lowland (yearly rainfall 400-800 mm) where it infests both farmland and grazing land [
43]. Socio-economically, the study area is dominated by farmers engaged in rice production, sorghum, maize and open cattle grazing. Due to an increasing human population and climate change, the landscape is subject to massive anthropogenic disturbances and exploitation by local and commercial use [
44].
2.2. Experimental Design
Each of the 32 plots were divided into 2×3 sub-plots of 10m×10m, thus taking up the whole 20m×30m plot area. The six treatments (
Table 1) were assigned randomly to the six sub-plots within each plot. The plots were visited twice (May and December 2022). On both occasions, two measurements were taken in each sub-plot: (i) the total number of
P. juliflora stems and (ii) the height and diameter (at 1.3 m) of all stems with a diameter ≥ 1 cm. Height (
; m) and diameter (
; m) were subsequently used to calculate tree volume (
; m
3/ha) for each sub-plot,
where
=0.5 is the form factor for
P. juliflora [
45,
46],
=100 m
2 is the size of the sub-plot area, and the constant scales from m
2 to ha units.
Treatments were carried out just after the measurements had been taken on the first visit, allowing ca. 6 months for the treatments to take effect. On the second visit, it was found that some sub-plots had been disturbed, e.g., by charcoal burning and farming operations. These sub-plots were discarded from the analysis. This reduced the number of sub-plots to 185 out of the original 192.
Treatment responses were analyzed by comparing the stem count before and after treatment ( and ; no. of stems per 100 m2 sub-plot) and the volume before and after treatment ( and ; m3/ha). Land use (grazing land vs. farmland) and district (Moshi vs. Simanjiro) were considered fixed factors possibly influencing the efficacy of the treatments.
2.3. Data Analysis
All analyses were carried out using R statistical software [
48]. Throughout a significance level of 5% was applied. The input data and R scripts can be found in the Electronic Appendix.
The growth rate of an undisturbed
P. juliflora population is an indication of its resilience against control. This was estimated for the Control plots as
, where
denotes either volume (
) or stem count (
). Plots were removed for which
. Differences in
and
between districts and between land use types would indicate whether
P. juliflora would be a more troublesome weed according to locality and land use. To test for differences, four separate Median Tests were carried out [
47] (using R function
fisher.test).
The response in tree volume was heavily dominated by zeros (). Hence, this response was assessed by visual interpretation of vs. plots.
The response to treatments in terms of stem counts (
) was analysed with a generalized linear model using the R function
glm with
as a co-variate. The full model was
with discrete factors: Treatment (6 levels), District (2 levels) and LandUse (2 levels). The asterisks denote main effects and all their interactions. Prior to analysis, experimental plots without any weeds before treatment (
) were discarded, thereby reducing the data set from
to
.
The regression (eq. 1) was carried out alternatively with Poisson and quasi-Poisson residuals. Since the deviance of the Poisson model divided by the degrees of freedom was 26.6, which is much larger than 1, the quasi-Poisson model was chosen for the analysis [
49].
The model was reduced stepwise, starting with the full model (eq.1), in each step removing non-significant interactions and factors and merging indifferent factor levels [
50]. In each step the validity of model reduction was tested by the R
anova function using a significance level of 5%. A multiple comparisons test of regression coefficients was carried out on the final model using the R packages
emmeans [
51] and
multcomp [
52].
Note that the model (eq. 1) implies a family of lines with on the y-axis and on the x-axis. The regression coefficients will designate the slopes and intercepts of those lines, possibly differing between different levels of the three factors. If all slopes turn out to be non-significantly different then the family consists of parallel lines with a common slope. The lines will then differ only in their displacement along the y-axis, as defined by any significant differences between their intercepts.
3. Results
The Median tests showed that the growth rate of uncontrolled P. juliflora in the Moshi vs. Simanjiro locality was 2.59 vs. 1.19 (P=0.13) by volume () and 1.66 vs. 1.11 (P=0.03) by stem count (), which means that P. juliflora grew faster in Moshi than in Simanjiro by a factor of 2.2 by volume and 1.5 by stem count. Thus one would expect that P. juliflora would be more difficult to control and would spread faster in Moshi than in Simanjiro. A comparison of Farming vs. Grazing land uses gave 2.04 vs. 1.19 (P=0.10) for and 1.28 vs. 1.32 for (P=1.00). Hence, there was no effect of land use on the growth potential of P. juliflora.
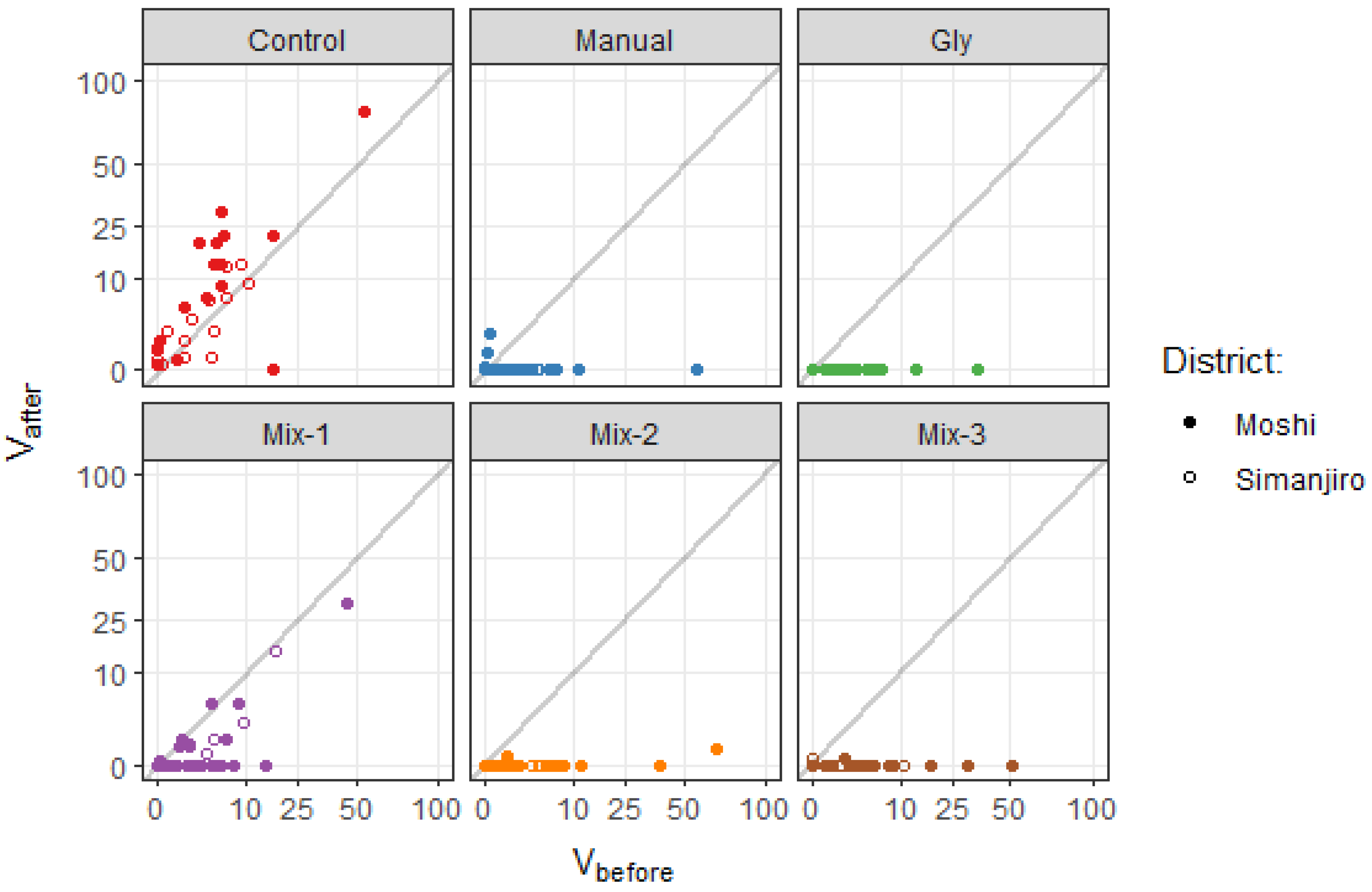
Tree volume was reduced to zero (
) in nearly all the treated sub-plots (
Figure 2), which made tree volume a rather uninformative response variable. It should be born in mind that
was estimated disregarding all stems thinner than 1cm. If a longer response period than 6 months had been chosen, stems would have grown thicker and tree volume might have become a more sensitive response variable.
) and after () treatment. Grey lines show 1:1 equality. Axes are square root-scaled. Uninfested sub-plots () not included ().
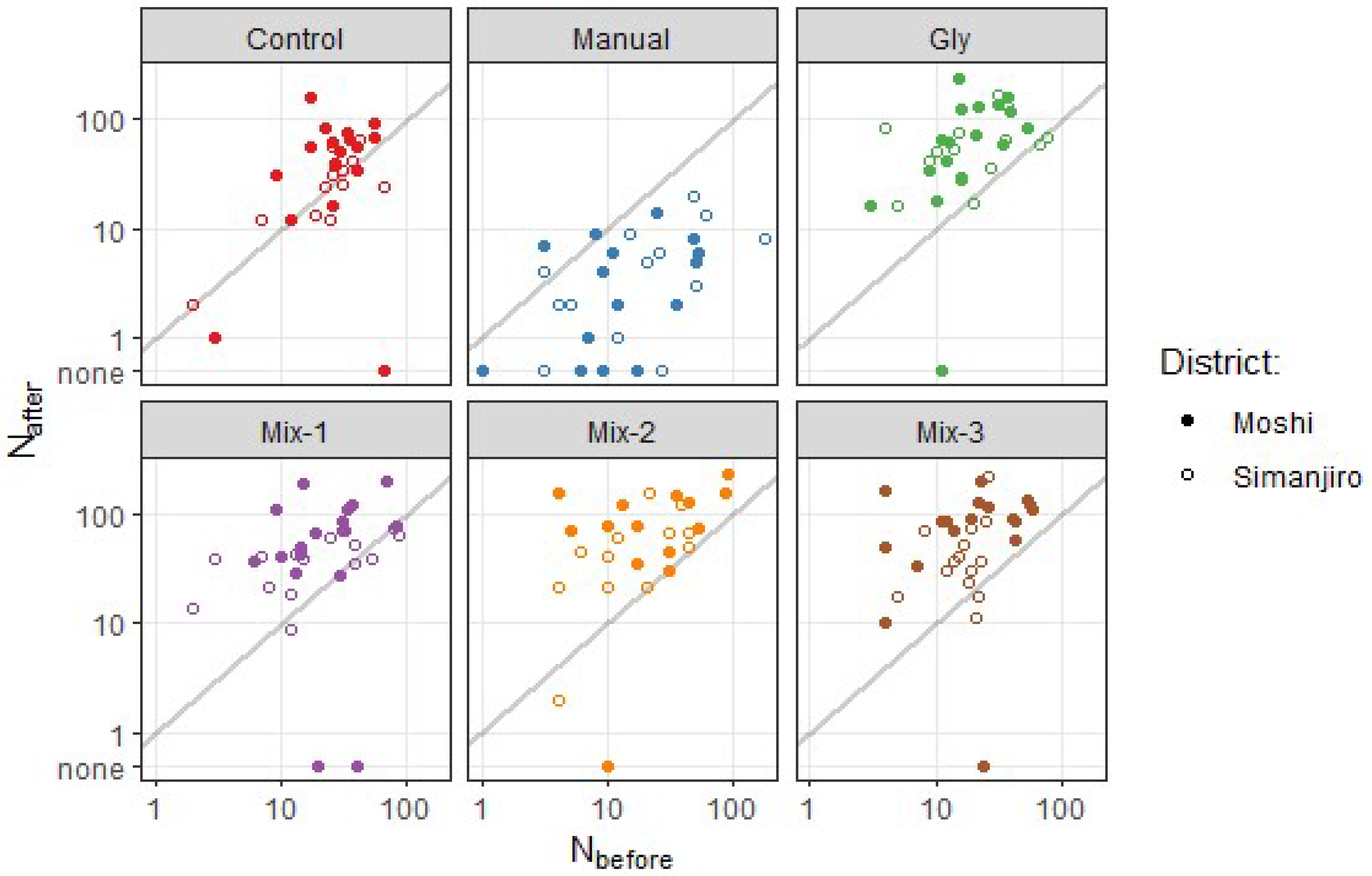
Judged from the scatter plots (
Figure 3), the number of stems increased during the experiment in all treaments, except when trees were uprooted (Manual treatment), as most data points lie above the 1:1 line. Treatments other than Manual seem to have have stimulated stem production, since the points are placed even further away from the 1:1 line than for the Control treatment.
) and after () treatment. Grey lines show 1:1 equality. Axes are log-scaled. Uninfested sub-plots () not included (). Data points with shown at the arbitrary position labelled ‘none’.
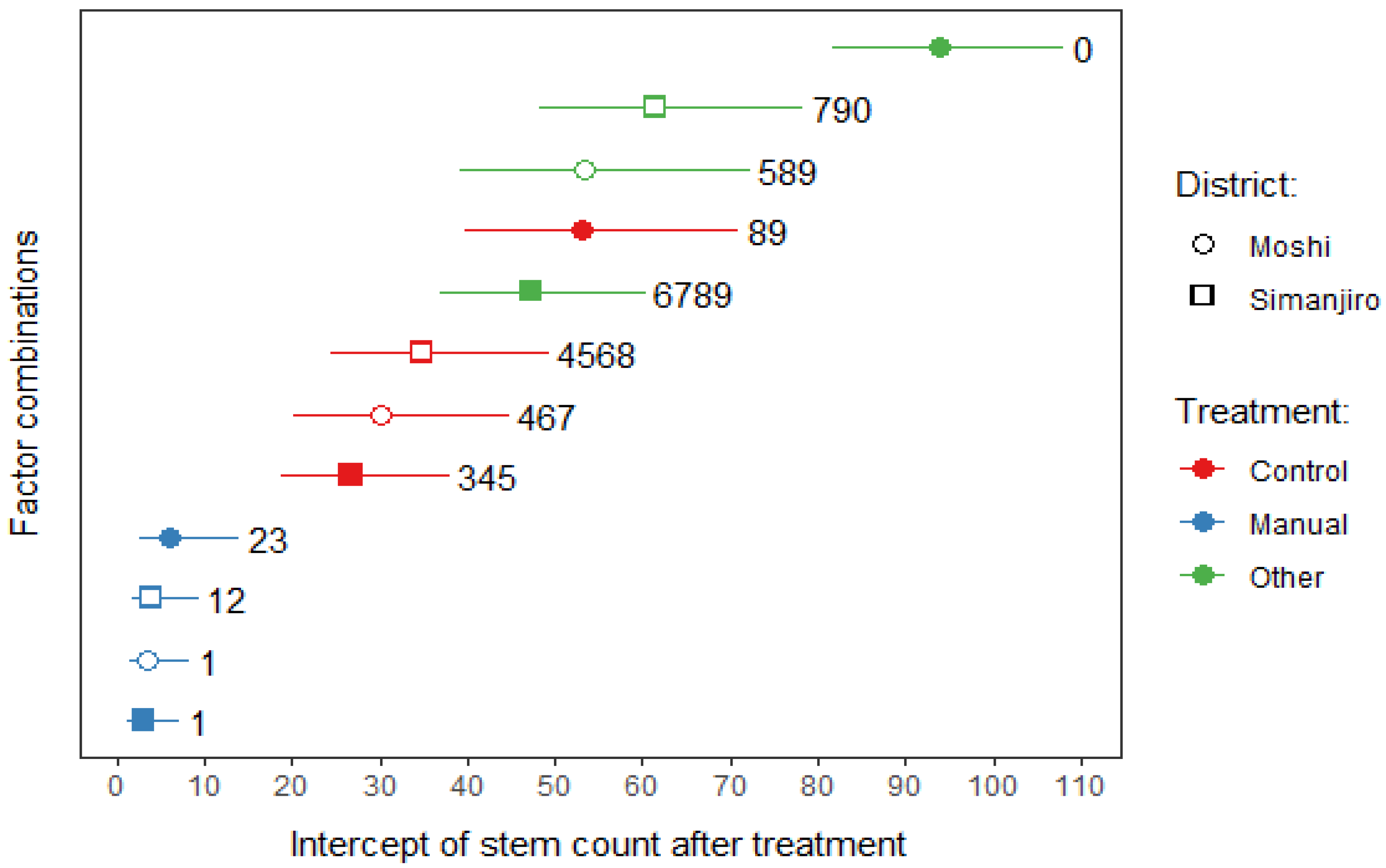
The regression analysis reduced the full model (eq. 1) to four main effects and one interaction (shown by the colon operator),
The Treatment factor was reduced from six to three levels (Control, Manual, Other), where Other included the Gly, Mix-1, Mix-2 and Mix-3 levels, since their effects were not significantly different from each other. A positive common slope (0.00996±0.0022; P<0.001) indicated that, in general, the more stems that were counted before treatment, the more stems would be counted at the end of the experiment six months later. The differences between treatments, districts and land uses were thus expressed solely by differences in intercepts among this family of parallel lines (
Figure 4).
The Manual treatment stood out as the most effective treatment having the lowest intercept values (
Figure 4) with only a slight overlap with the Control treatment (overlapping in multiple comparisons grouping 3). The efficacy of the Other treatments was in general not different from the Control treatment. Some factor combinations even favoured stem formation at a rate above that in the control (multiple comparisons grouping 0: Moshi farming and Simanjro grazing). Hence, the treatments worsened the weed problem, at least at the six-month time scale of the experiment. For every treatment it is seen that the open vs. closed circles belong to different multiple comparison groupings (
Figure 4). This means that in the Moshi district, the efficacy of the Manual treatment was higher in the grazing than in the farming plots.
4. Discussion
The findings of this study indicated that stem count increased during the experiment for all six treatments. This shows that the P. juliflora population had not reached the local carrying capacity. Since coppicing of P. juliflora is a common method of exploiting this resource (e.g., for poles and charcoal), the status of P. juliflora stands located near human habitation likely reflect a combination of local natural conditions and extensive human management. For the manual control treatment, the increase in stem count was reduced compared to the untreated control. For all other treatments, the increase in stem count was the same or even higher compared to the untreated control. Thus, manual uprooting was the only treatment having a controlling effect. The seeming effect of all treatments on tree volume, we consider an artifact due to the method by which tree volume was estimated. Since only stems with a diameter 1cm entered the calculation, and it turned out that new stems did not achieve this diameter within six months, re-growth (and hence the effect of treatment) could not be estimated by this measure.
It is known that tree diameter and density, and the extent to which the stems have been affected by previous treatments, may affect the outcomes of treatments [
32]. According to Gonçalves [
53], extensive efforts have been made to control
P. juliflora by chemical, biological, and mechanical means. It was found that
P. juliflora spread can be halted by clear-cutting followed by burning of the stumps and application of herbicides on the cuts [
53]. However, Patniak [
8] pointed out that none of those strategies to control or eradicate the plant has achieved any enduring success. According to Eschen et al. [
32], the laborious uprooting of
P. juliflora may be the only action needed, if the subsequent land use involves plowing. It has also been pointed out that clearing of invaded lands, digging out stumps and roots are one of the common practices used to control infestation, particularly in the farming areas [
54]. Our findings are in agreement with these earlier studies, emphasising that uprooting, however costly, is a necessary first step in any control campaign.
Stem cutting, followed by application of one of the novel mixtures or glyphosate, was nullified by re-growth during the following six months. In some cases, cutting even stimulated stem formation above that in the untreated control. This demonstrated how
P. juliflora is well-suited as a resource for coppicing. We do not know to what extent repeated coppicing will prevent
P. juliflora from spreading further afield, or even reduce its density locally. Regular coppicing makes sense in pastoral societies where
P. juliflora does not get in the way for farming [
32], where cultivation without
P. juliflora is mostly preferred. It should be investigated whether regular coppicing prevents regional spreading of the weed.
Unlike Simanjiro, Moshi is on a slightly higher altitude with heavy rains and irrigation of crops. Simanjiro, is mainly inhabited by pastoralists, and farmers who only grow crops in dry season, relying on moisture accumulated in the soil during the rainy season. The water from higher mountain slopes of Mt. Kilimanjaro and adjacent elevated areas falls at Simanjiro, which is characterized by an undulated landscape with flood plains, eroded gullies and sandy clay loam soils, where
P. juliflora is widely distributed. According to Shaltout et al. [
55], livestock and flooding are two crucial dispersal agents of
P. juliflora seeds. Wakie et al. [
54] reported extensive invasion of the flatlands along the Awash River and the Alledeghie plain, due to flooding in northern Afar and the Somali region in Ethiopia. Hulme [
56] also pointed out that flooding has the effect of washing away
P. juliflora plants, to facilitate their spread. Thus, the wide distribution of
P. juliflora on the lowland areas and flood plains of the Simanjiro District, are likely attributed to the flood and grazing phenomena. According to Patnaik et al. [
8] ingestion of the seeds by grazing animals, enhances germination success and ensures dispersal of seeds over a wide terrain.
However, the relatively low number of stems on grazing land particularly Simanjiro, may be caused by frequent-intensive grazing that may limit successful germination and growth of new seedlings (
Figure 5 and
Figure 6). Likewise, the main forms of disturbances encountered on the grazing land namely‒ firewood, charcoal making and pole harvesting are less intensive compared to farmland areas where extensive clearance of
P. juliflora infested lands for farming is more prominent, thus exacerbating the sprouting. Despite the likelihood of arid conditions increasing distribution of
P. juliflora, as mentioned by Dakhil et al. [
57], drought-induced stress along with other factors may also contribute to low number of stems on grazing land. Variation in soil, landscape and levels of anthropogenic disturbances as mentioned by Kumar, and Mathur [
58] and Linders et al. [
59], can also be responsible for the observed limitations. Dakhil et al. [
57], pointed out that climatic conditions and edaphic factors can set ecological limits that restrict species distributions. Wakie et al. [
60] also underscored inclusion of soil and hydrologic parameters in order to establish the current status and distribution of
P. juliflora. Nascimento et al. [
5], earmarked the life history of the plants, soil properties and human activities as potential drivers for the distribution, proliferation, and invasion ability of
P. juliflora. Therefore, it is pertinent to study the mechanisms underlying the relatively low number of
P. juliflora stems found on grazing lands, despite the observed wide distribution. Likewise, it is necessary to understand the effect of agronomic practices such as fertilisation on
P. julifora distribution.
5. Conclusions
We estimated the effect on P. juliflora of one control treatment, leaving six months for the treatment to take effect. It turned out that within this time frame only uprooting was effective in slowing re-growth of the weed. No other treatments, whether novel mixtures or glyphosate, had any controlling effect. It remains to be seen how P. juliflora will react in the long run to repeated control treatments.
We only examined the local effect of treatments on P. juliflora but it is necessary also to study how the spread of P. juliflora can be prevented, and how local interventions, such as uprooting or regular coppicing, interacts with the population dynamics of P. juliflora on a regional scale.
Supplementary Materials
The following supporting information can be downloaded at: at the website of this paper posted on Preprints.org.
Author Contributions
“Conceptualization, G.S.; methodology, G.S., C.K.; K.P.S. and P.K.; software, G.S. and N.H.; validation, P.K., N.H., K.P.S. and C.K.; formal analysis, G.S. and N.H.; resources, P.K., K.P.S. and G.S.; data curation, G.S.; writing—original draft preparation, G.S.; writing—review and editing, G.S., P.K. and N.H.; visualization, N.H. and G.S.; supervision, P.K., N.H., K.P.S. and C.K.; project administration, K.P.S. and P.K.; funding acquisition, G.S., K.P.S. and P.K. All authors have read and agreed to the published version of the manuscript.”
Funding
This research received financial support from DANIDA, BSU 3 Programme between Sokoine University of Agriculture (SUA) and Aarhus (AU) Universities in Tanzania and Denmark respectively.
Data Availability Statement
Supplementary information is available online at xxx. Additional information may be obtained upon request.
Acknowledgments
Much appreciation is given to Aarhus University, Flakkebjerg for hosting part of the research activities. Much appreciation is also given to regional administration and local governments in Kilimanjaro and Manyara regions where the entire study was undertaken, together with the support being obtained from the ward and village governments in both Districts. Many thanks are given to Emanuel Mbazi for advice on experimental design, Joseph S. Makero for initial advice on inventory data processing, and Wilson Mugasha, Christopher Magomba, and Daniel Isidory for in-kind contribution of field equipment. Lastly, we recognize assistance by the village counterparts Ludovick Temba, Oscar Kiwale and Rafael Aloyce Makundi during data collection activities.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Richardson, D.M.; Rejmánek, M. Trees and shrubs as invasive alien species–a global review. Div. and distr., 2011,17,5, 788-809. [CrossRef]
- Wakie, T.T. Distribution and Economic Analysis of Prosopis Juliflora in Ethiopia. Unpublished Dissertation for Award of Doctor of Philosophy Degree, Colorado State University, Fort Collins, Colorado, USA, 2015. Pp 33 – 95.
- Shackleton, R.T.; Le Maitre, D.C.; Richardson, D.M. Prosopis invasions in South Africa: population structures and impacts on native tree population stability. J. of Arid Env., 2015, 114, 70–78. [CrossRef]
- Ilukor, J.; Rettberg, S.; Treydte, A.; Birner, R. To eradicate or not to eradicate? Recommendations on Prosopis juliflora management in Afar, Ethiopia, from an interdisciplinary perspective. Pastoralism 6, 2016, 14. [CrossRef]
- Nascimento, C.E.D.S.; da Silva, C.A.D.; Leal, I.R.; Tavares, W.D.S.; Serrão, J.E.; Zanuncio, J.C.; Tabarelli, M. Seed germination and early seedling survival of the invasive species Prosopis juliflora (Fabaceae) depend on habitat and seed dispersal mode in the Caatinga dry forest. Peer J 8: e9607, 2020. [CrossRef]
- Mwangi, E.; Swallow, B. Prosopis juliflora invasion and rural livelihoods in the Lake Baringo Area of Kenya. Conserv. and Soc., 2008, 6, 2, 130-140. [CrossRef]
- Dubow, J. Still-Life, After-Life: W. G. Sebald and the Demands of Landscape. In: Envisioning Landscapes, Making Worlds: Geography and the Humanities, (1st ed.). Daniels, S., DeLyser, D., Entrikin, J.N. & Richardson, D., Eds.; Routledge: London, UK, 2011. [CrossRef]
- Patnaik, P.; Abbasi, T.; Abbasi, S.A. Prosopis (Prosopis juliflora): Blessing and bane. Trop. Ecol., 2017, 58,3, 455-483.
- Broun, A.F.; Massey, R.E. Flora of the Sudan. Thomas Murby and Company: London, United Kingdom, 1929; pp. 376.
- Magid, T.D.A.; Siddig, E.N.A.E.; Ahmed, A.; Ahmed, M.T.E.S.A. (2014) Mesquite in Sudan: A Boon or Bane for Dry lands? It’s Socioeconomic and Management Aspects in Kassala State, Sudan. J. of For. Prod. and Ind., 2014, 3, 182–190.
- Hall, M.; Llewellyn, O.A.; Miller, A.G.; Al-Abbasi, T.M.; Al-Wetaid, A.H.; Al-Harbi, R.J.; Al-Shammari, K.F. Important plant areas in the Arabian Peninsula: 2. Farasan Archipelago, Edinburgh J. Bot., 2010, 67, 2, 189–208. [CrossRef]
- Aldhebiani, A.; Elhag, M.; Alshehri, A. Consideration of hyperspectral data in intraspecific variation (spectrotaxonomy) in Prosopis juliflora (Sw.) DC, Saudi Arabia. Open Geosci., 2021, 13, 280-292. [CrossRef]
- El-Keblawy, A; Al-Rawai, A. Impacts of the invasive exotic Prosopis juliflora (Sw.) D.C. on the native flora and soils of the UAE. Plant Ecol., 2007, 190, 23-35. [CrossRef]
- Byalt, V.; Korshunov, M. Distribution of Invasive Species Prosopis juliflora (Mimosaceae) in Fujairah (UAE). Rus. J. of Biol. Inv., 2021, 12, 157-166. [CrossRef]
- Howari, F.M.; Sharma, M.; Nazzal, Y.; El-Keblawy, A.; Mir, S.; Xavier, C.M.; Salem, I.B.; Al-Taani, A.A.; Alaydaroos, F. Changes in the Invasion Rate of Prosopis juliflora and Its Impact on Depletion of Groundwater in the Northern Part of the United Arab Emirates. Plants, 2022, 11, 682. [CrossRef]
- Randall, J.; McDonald, J.; Wong, L.J.; Pagad, S. Global Register of Introduced and Invasive Species - Australia. Version 1.9. Invasive Species Specialist Group ISSG. Checklist dataset, 2022. Available online: https://doi.org/10.15468/3pz20c (accessed via GBIF.org on 2023-09-26).
- Tsegay, B.; Livingstone, J.; Fre, Z. Exploring Prosopis Management and Policy Options in the Greater Horn of Africa: In Proceedings of a Regional Conference, Desalegn Hotel, Addis Ababa, Ethiopia, 26th – 27th November, 2014.
- Kilawe, C.J.; Mbwambo, J.R.; Kajembe, G.C.; Mwakalukwa, E.E.; Amri, A.M.; Mushi, G.V.; Athumani, A.M.; Eckert, S.; Eschen, R. Mrashia: Prosopis invading pastures and agricultural lands in Tanzania. The Woody Weeds Project. INSIGHTS Report, 2017. https://woodyweeds.org/wp-content/uploads/2017/05/WW_INSIGHTS_Prosopsis_Tanzania.pdf.
- Shiferaw, H.; Teketay, D.; Nemomissa, S.; Assefa, F. Some biological characteristics that foster the invasion of Prosopis juliflora (Sw.) DC. at Middle Awash Rift Valley Area, north-eastern Ethiopia. J. of Arid Env., 2004, 58,135-154.
- Bellard C.; Cassey P.; Blackburn, TM. Alien species as a driver of recent extinctions. Biol. Lett., 2016, 12: 20150623. [CrossRef]
- Malavasi, M.; Santoro, R.; Cutini, M.; Acosta, A.T.R.; Carranza, M.L. The impact of human pressure on landscape patterns and plant species richness in Mediterranean coastal dunes. Plant Bios., 2016, 150,1, 73–82. [CrossRef]
- Muturi, G.M.; Poorter, L.; Mohren, G.M.J.; Kigomo, B.N. Ecological impact of Prosopis species invasion in Turkwel riverine forest, Kenya. J. of Arid Env., 2013, 92, 89–97. 10.1016/j.jaridenv.2013.01.010.
- Ndhlovu, T.; Milton-Dean, S.; Esler, K. (2011) Impact of Prosopis (mesquite) invasion and clearing on the grazing capacity of semiarid Nama Karoo rangeland, South Africa. African J. of Range & Forage Sci., 2011, 28, 3, 129-137. [CrossRef]
- Dzikiti, S.; Schachtschneider, K.; Naiken, V.; Gush, M.; Moses G.; Le Maitre, D.C. Water relations and the effects of clearing invasive Prosopis trees on groundwater in an arid environment in the Northern Cape, South Africa. J. of Arid Env., 2013, 90, 103-113.
- Maundu, P.; Kibet, S.; Morimoto, Y.; Imbumi, M.; Adeka, R. Impact of Prosopis juliflora on Kenya’s semi-arid and arid ecosystems and local livelihoods. Biodiversity, 2009, 10, 2-3, 33-50. [CrossRef]
- Wakie, T.; Evangelista, P.; Laituri, M. Utilization assessment of Prosopis juliflora in Afar Region, Ethiopia. US Forest Service, USDA Office of International Programs, USAID Pastoral Livelihoods Initiative II Project (PLI II), 2012. 10.13140/RG.2.2.29015.27046.
- Niguse, H.; Amare, F. Distribution and Socio-economic Impacts of Prosopis juliflora in East Shewa and West Arsi Zones, Ethiopia. Intern. J. of Afr. and Asi. Stud., 24, 2016, 31-41.
- Mbaabu, P.R.; Ng, W.-T.; Schaffner, U.; Gichaba, M.; Olago, D.; Choge, S.; Oriaso, S.; Eckert, S. Spatial Evolution of Prosopis Invasion and its Effects on LULC and Livelihoods in Baringo, Kenya. Remote Sens. 2019, 11, 1217. [CrossRef]
- Tewari, J.C.; Harris, P.J.C.; Harsh, L.N.; Cadoret, K.; Pasiecznik, N.M. (2000). Managing Prosopis juliflora (Vilayati Babul): A Technical Manual. CAZRI, Jodhpur, India and HDRA, Coventry, UK, 2000; p 94. ISBN 0 905343 27 1.
- Pyšek, P.; Richardson, D.M. Invasive species, environmental change and management, and health. Annual Rev. of Env. and Res., 35, 2010, 25-55. [CrossRef]
- Shackleton, R.T.; Le Maitre, D.C.; Pasiecznik, N.M.; Richardson, D.M. Prosopis: a global assessment of the biogeography, benefits, impacts and management of one of the world’s worst woody invasive plant taxa. AoB Plants 2014, 6, 1-18. [CrossRef]
- Eschen, R.; Bekele, K.; Jumanne, Y.; Kibet, S.; Makale, F.; Mbwambo, J.; Megersa, B.; Mijay, M.; Moyo, F.; Munishi, L.; Mwihomeke, M.; Nunda, W.; Nyangito, M.; Witt, A.; Schaffner, U. Experimental prosopis management practices and grassland restoration in three Eastern African countries. CABI Agri. Bios., 2023, 4, 21. [CrossRef]
- Durigan, G.; Ivanauskas, N.; Zakia, M.; Abreu, R. Control of Invasive Plants: Ecological and Socioeconomic Criteria for the Decision-Making Process. Natureza & Conservacao., 2013, 11. 23-30. [CrossRef]
- Admasu, D. Invasive plants and food security: the case of Prosopis juliflora in the Afar Region of Ethiopia, FARM-Africa - IUCN., 2008, 1-13.
- Wakie, T.; Evangelista, P.; Laituri, M. Utilization Assessment of Prosopis juliflora in Afar Region, Ethiopia; US Forest Service, USDA Office of International Programs USAID Pastoral Livelihoods Initiative II Project (PLI II): USA, 2012. [CrossRef]
- Bekele, M.; Girmay, Z. (2013). Reading through the Charcoal Industry in Ethiopia: Production, Marketing, Consumption and Impact. FSS Monograph, 2013, 9, p. 104.
- Syomiti, M.; Dana, H.; Getachew, G.; Beatrice, M.; Wamae, D. Medicated Prosopis spp-based feed blocks- for antihelmintic efficacy and performance of weaner lambs. Livest. Res. for R. Dev., 2015, 27, 50. http://www.lrrd.org/lrrd27/3/syom27050.html.
- The United Republic of Tanzania (URT). National Invasive Species Strategy and Action Plan (NISSAP) (2019 - 2029). Vice President’s Office, Dodoma, 2019.
- The United Republic of Tanzania (URT). National Biodiversity Strategy and Action Plan (NBSAP) (2015 - 2020). Vice President’s Office, Division of Environment, Dar es Salaam, 2015.
- Swai, G., Sibuga, K.P., Kilawe, C.J., Kudsk, P. Invasion of Rangelands and Agro-Ecosystems by Prosopis Juliflora: Factors, Control Measures and Impact in Northern Tanzania, 2023, submitted.
- Moran, P.J. and Greenberg, S.M. Winter Cover Crops and Vinegar as Weed Control Techniques in Sustainable Cotton Production: In Proceedings of Beltwide Cotton Conferences, San Antonio, Texas, 3rd – 6th, January, 2006.
- Chatterjee, D.; Kumar, R.; Kuotsu, R.; Deka, B. Validation of traditional weed control method through common salt application in hill region of Nagaland. Current science, 2015, 110, 1459-1467. [CrossRef]
- The United Republic of Tanzania (URT). Moshi District Council Strategic Plan (2016/2017-2020/2021). President’s Office, Regional Administration and Local Government, Dar es Salaam, 2016.
- Mbinile, S.D.; Munishi, L.K.; Ngondya, I.B.; Ndakidemi, P.A. Conservation and Management Challenges Facing a Medicinal Plant Zanthoxylum chalybeum in Simanjiro Area, Northern Tanzania. Sustainability 2020, 12, 4140. [CrossRef]
- Masota, A.M.; Zahabu, E.; Malimbwi, R.E.; Bollandsås, O.M.; Eid, T.H. Volume Models for Single Trees in Tropical Rainforests in Tanzania. J. of Energy and Nat. Res. 2014, 3,5, 66-76. [CrossRef]
- Kashaigili, J.; Mdemu, M.V.; Nduganda, A.R.; Mbilinyi, B.P. Integrated Assessment of Forest Cover Change and Above-Ground Carbon Stock in Pugu and Kazimzumbwi Forest Reserves, Tanzania. Adv. in Rem. Sens. 2013, 02, 1-9. [CrossRef]
- Siegel, S.; Castellan Jr., N.J. (1988). Nonparametric Statistics for the Behavioral Sciences, 2nd ed.; McGrawHill: New York, USA, 1988. [CrossRef]
- R Core Team (2023). _R: A Language and Environment for Statistical Computing_. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
- Buckley, Y.M. ‘Generalized linear models’, In Ecological Statistics: Contemporary theory and application, online ed.; Fox, G.A., Negrete-Yankelevich, S., and Sosa, V.J. Eds.; Oxford Academic, Oxford, United Kingdom, 2015. accessed 25 Sept. 2023 . [CrossRef]
- Crawley, M.J. The R Book, 1st ed.; Jown Wiley & Sons: Chishester, UK, 2007; pp. 387–448.
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Package “Emmeans”. R Package Version 4.0-3, 2018. http://cran.r-project.org/package=emmeans.
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biometrical Journal: J. of Math. M. in Biosci., 2008, 50, 3, 346-363. [CrossRef]
- Gonçalves, G.; Andrade, L.; Xavier, K; Silva, J. Control methods of Prosopis juliflora (Sw.) DC. (Fabaceae) in invaded areas in the semiarid region of Brazil. Ciência Florestal, 2015, 25, 645-653. [CrossRef]
- Wakie, T.T.; Hoag, D.; Evangelista, P.H.; Luizza, M.; Laituri, M. Is control through utilization a cost effective Prosopis juliflora management strategy? J. Environ. Manag. 2016, 168, 74–86. [CrossRef]
- Shaltout, K.H.; El-Hennawy, M.; Nafeaa, A.; Afefe, A.A.; Abo-Bakr, S.; Ghazaly, O.; Eid, E.M.; Fouda, M. National Progress Towards Targets of the Global Strategy for Plant Conservation. Egyptian Environmental Affairs Agency. Nature Conservation Section (NCS); Cairo, Egypt, 2009. [Google Scholar].
- Hulme, P.E. Invasion pathways at a crossroad: policy and research challenges for managing alien species introductions. J. of Appl. Ecol., 2015, 52, 6,1418–1424. [CrossRef]
- Dakhil, M.A.; El-Keblawy, A.; El-Sheikh, M.A.; Halmy, M.W.A.; Ksiksi, T.; Hassan, W.A. Global Invasion Risk Assessment of Prosopis juliflora at Biome Level: Does Soil Matter? Biology 2021, 10, 203. [CrossRef]
- Kumar, S. and Mathur, M. Impact of invasion by Prosopis juliflora on plant communities in arid grazing lands. Trop. Ecol., 2014, 55, 33–46.
- Linders, T.E.W.; Bekele, K.; Schaffner, U.; Allan, E.; Alamirew, T.; Choge, S.K.; Eckert S.; Haji, J.; Muturi, G.; Mbaabu, P.R.; Shiferaw, H.; Eschen, R. The impact of invasive species on social-ecological systems: relating supply and use of selected provisioning ecosystem services. Ecosyst. Serv., 2020, 41, 101055. [CrossRef]
- Wakie, T.T.; Evangelista, P.H.; Jarnevich, C.S.; Laituri, M. Mapping current and potential distribution of non-native Prosopis juliflora in the Afar region of Ethiopia. PLoS ONE, 2014, 9, 3–11. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).