Submitted:
27 March 2024
Posted:
28 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
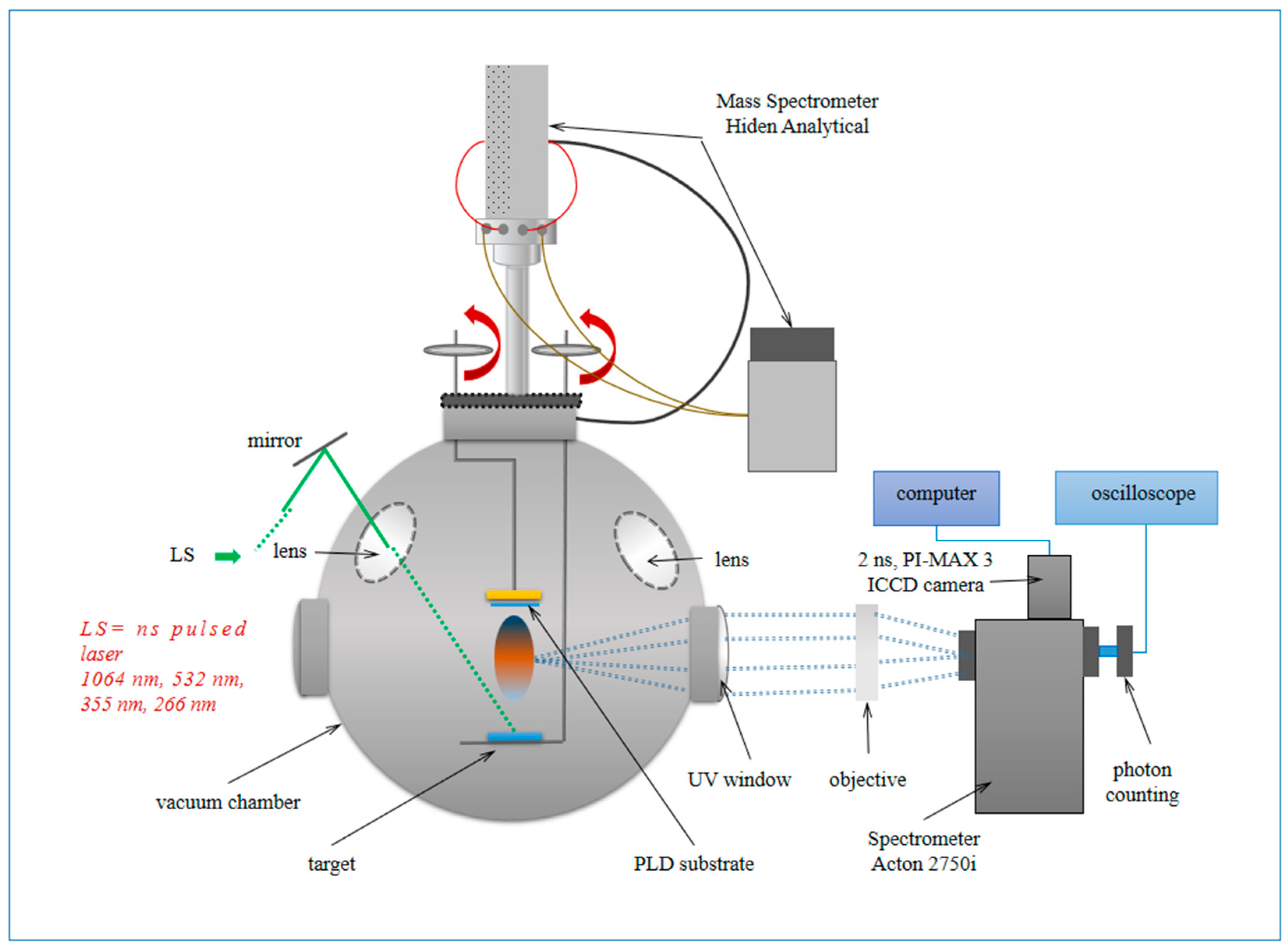
2. Materials and Methods
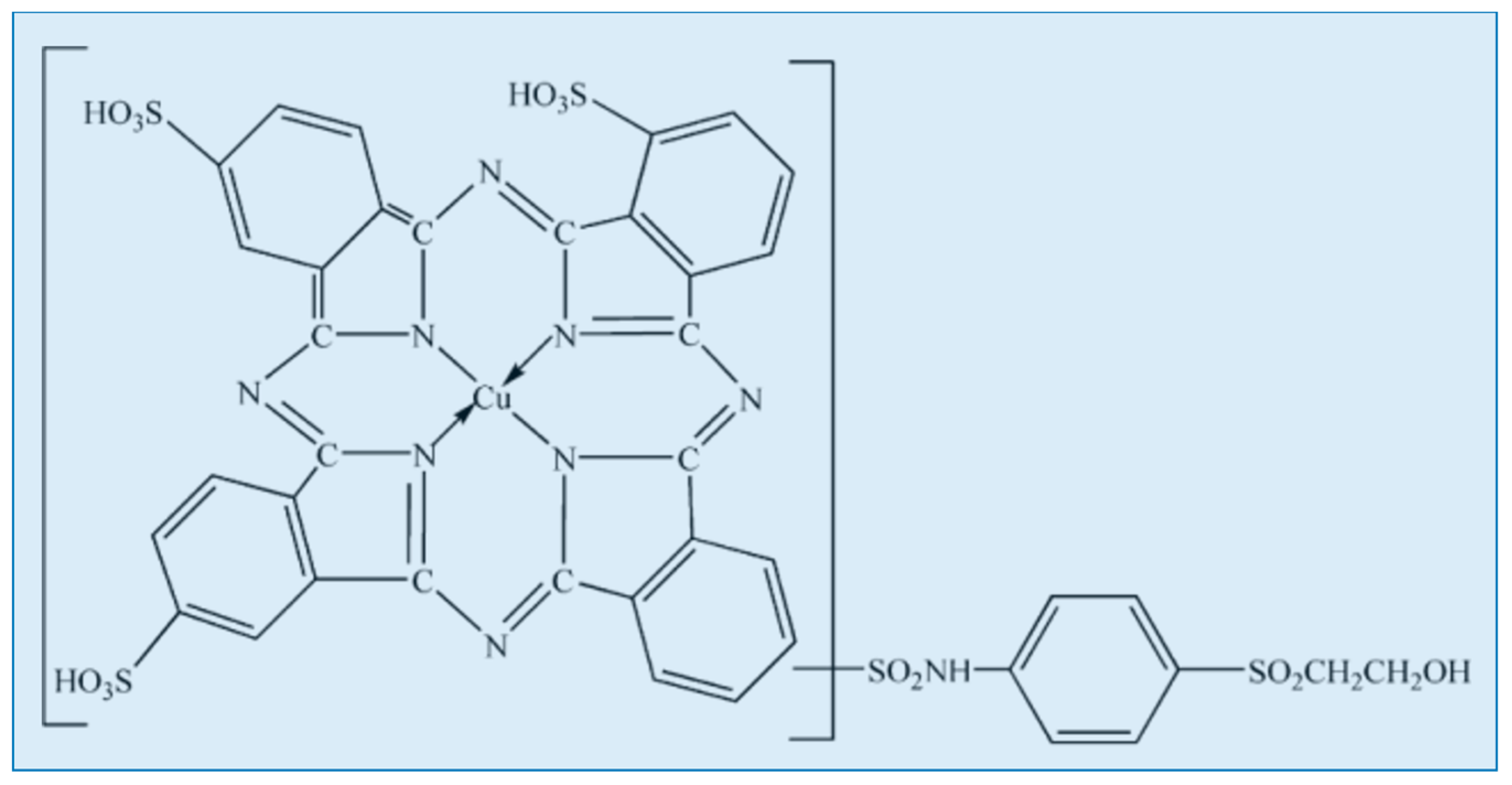
2.1. Materials
2.2. Method of Work
2.3. Methods of Analysis
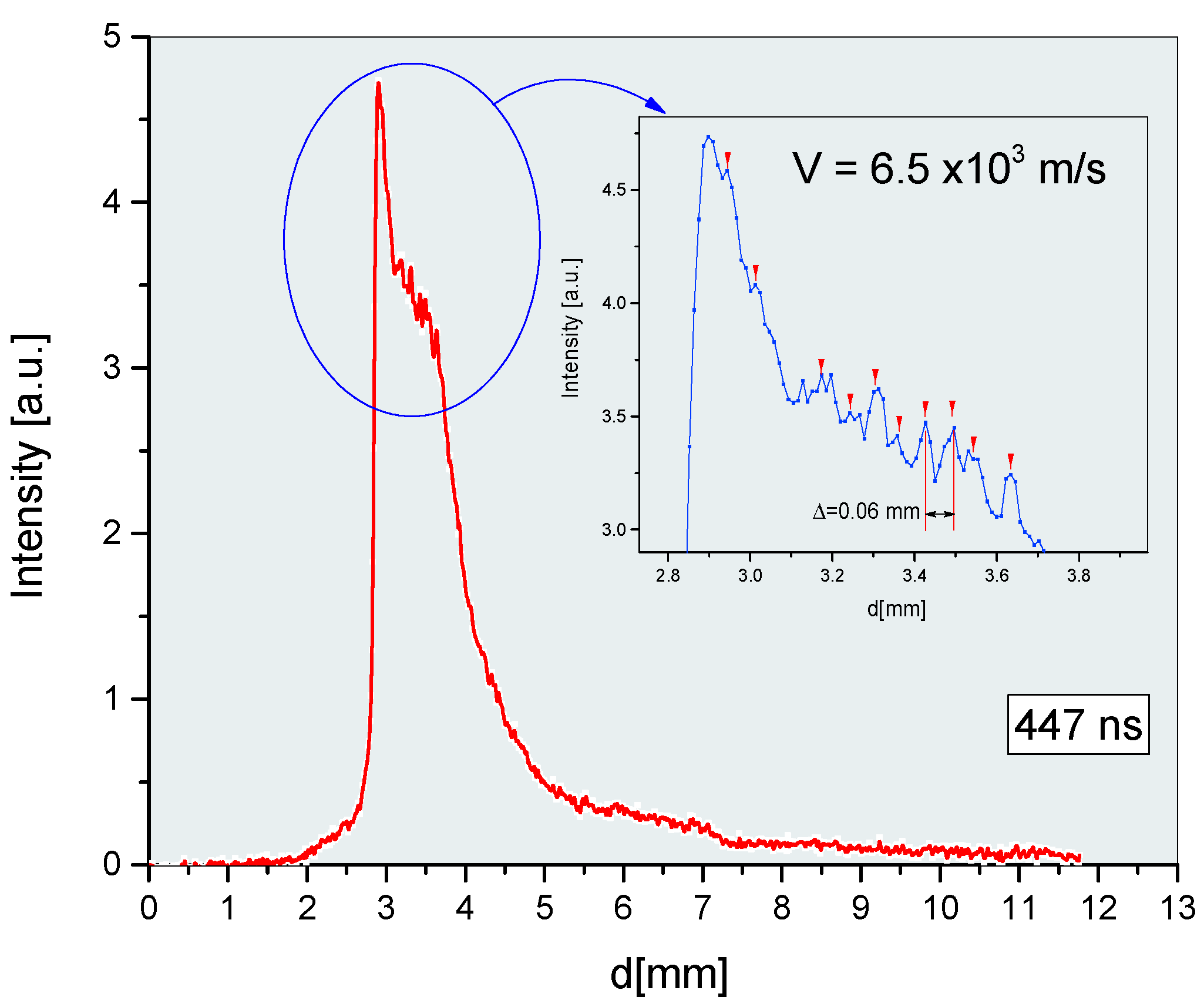
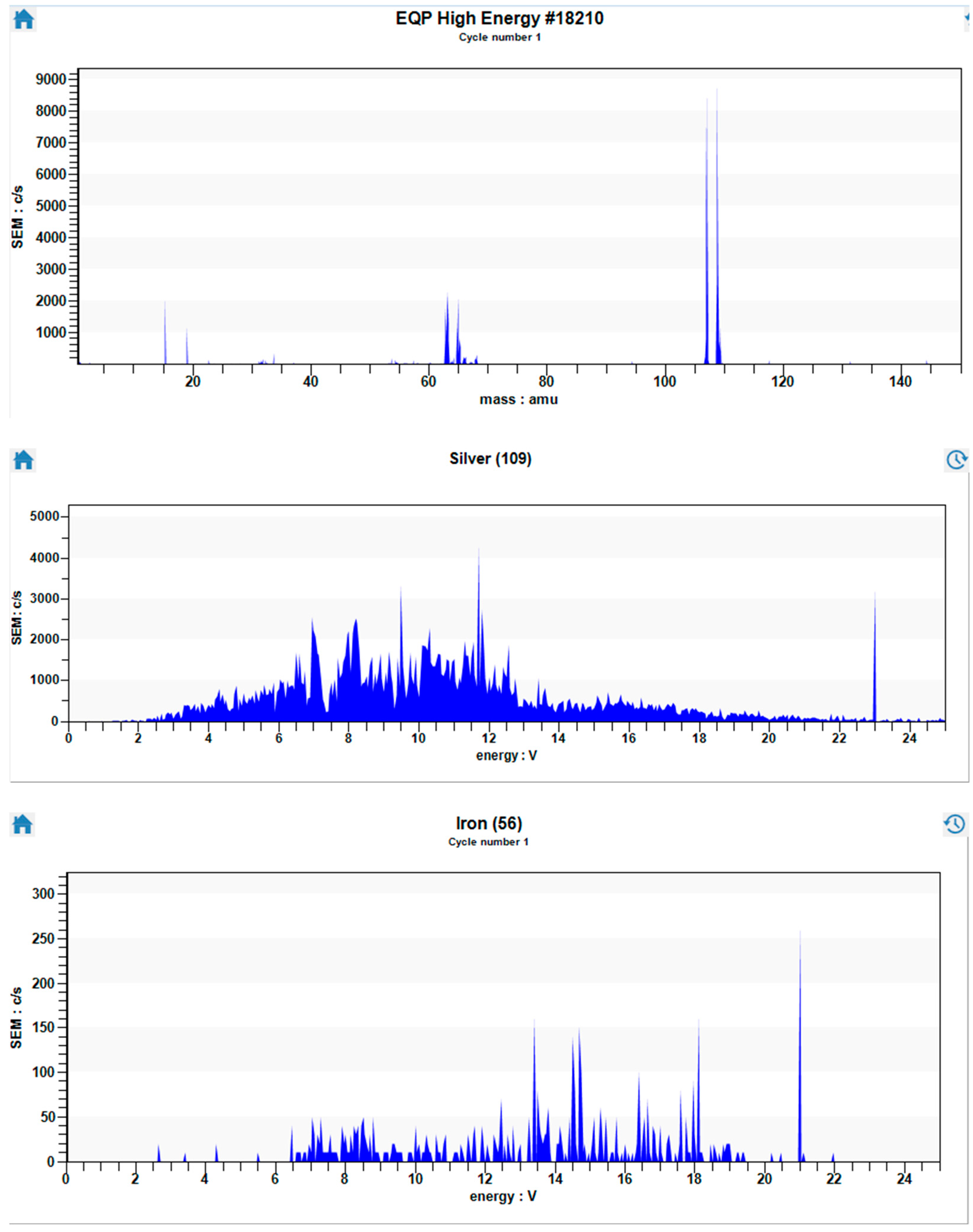
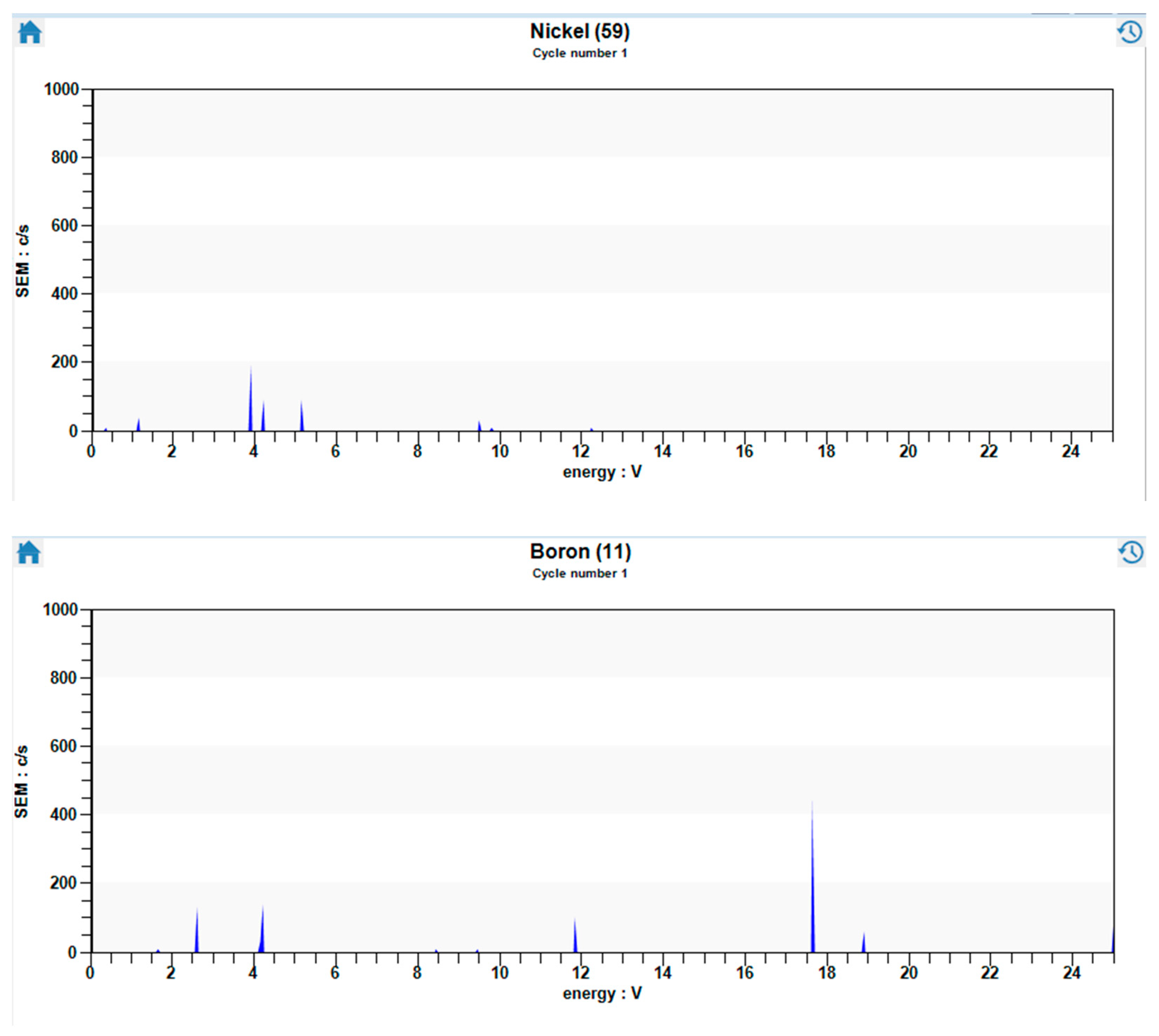
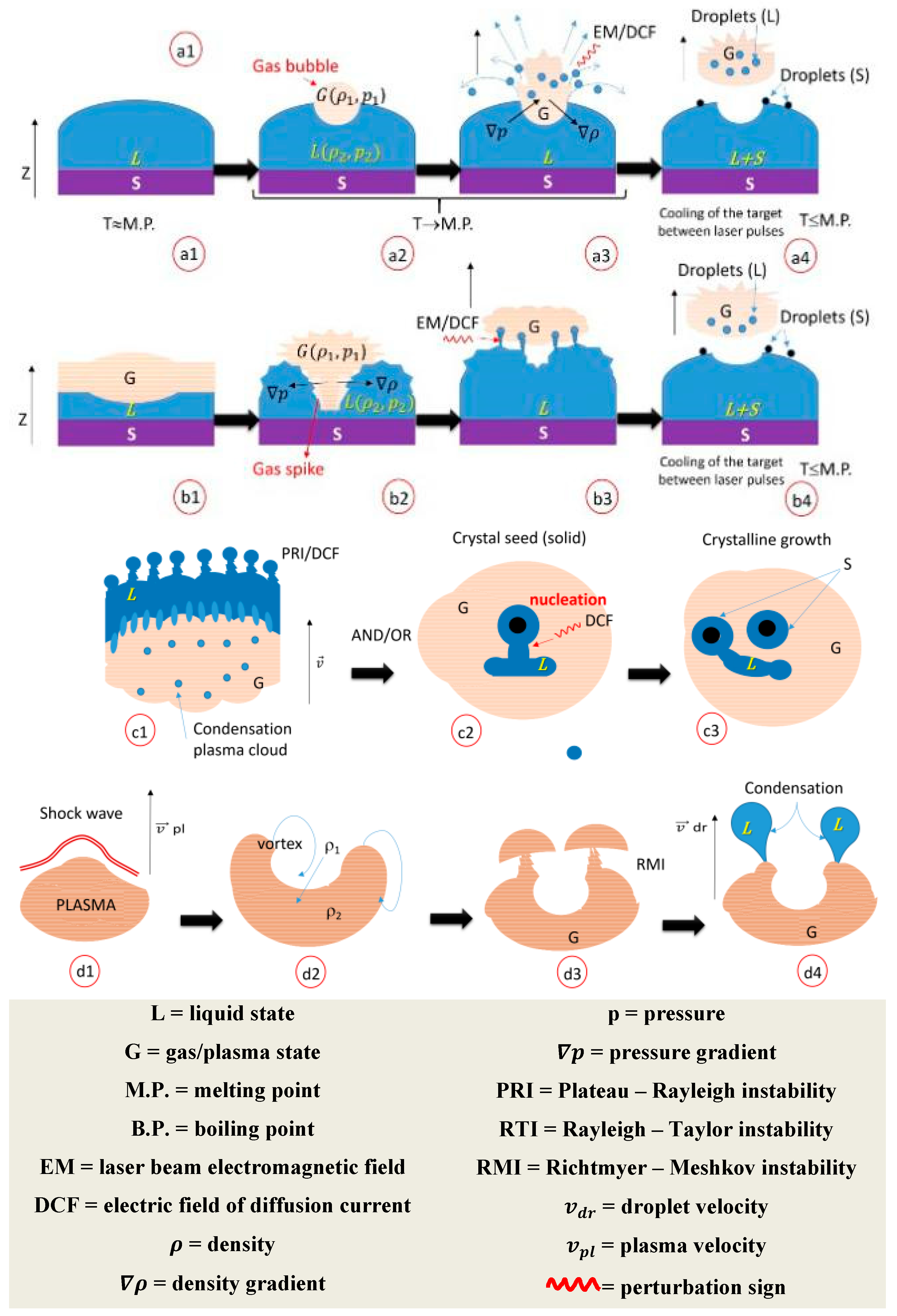
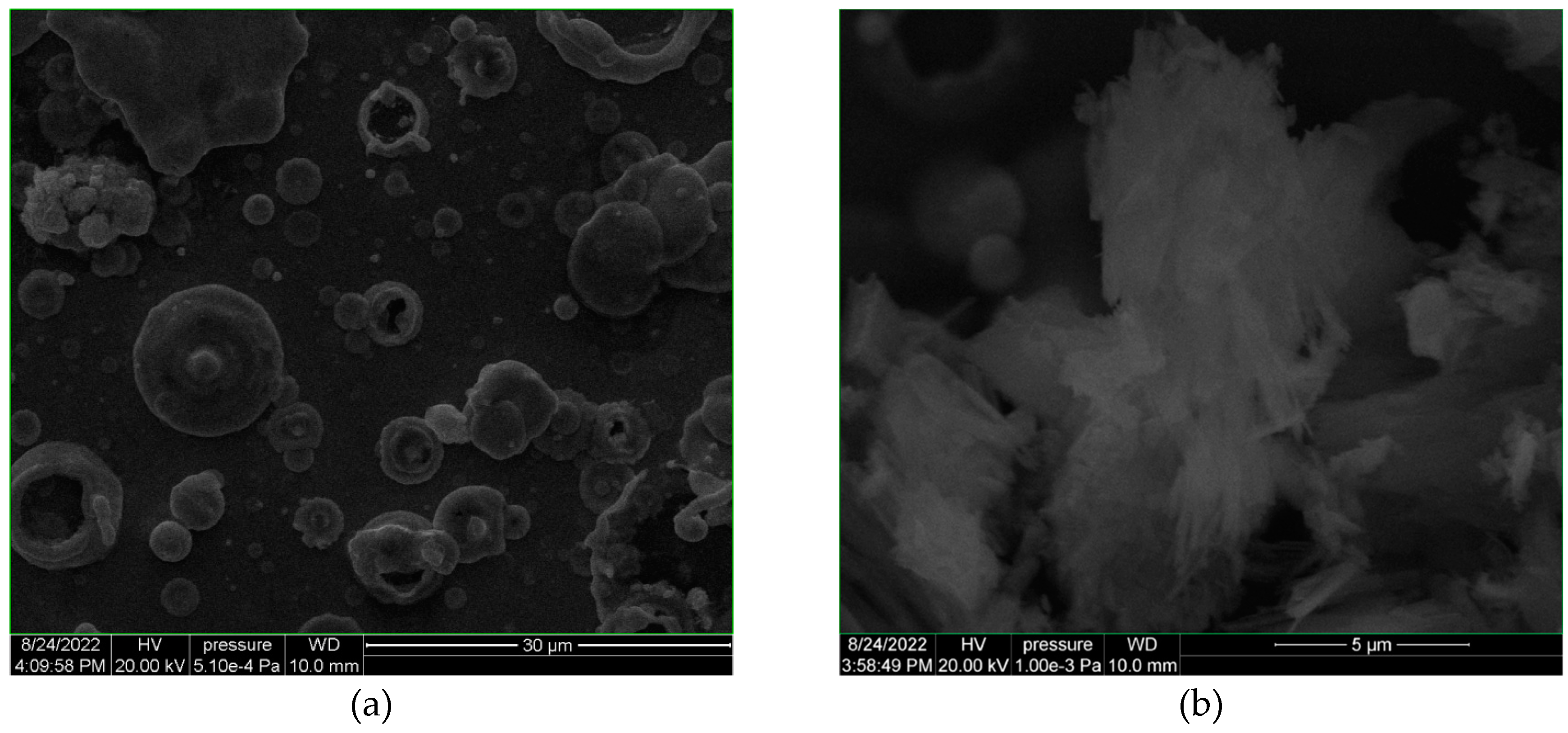
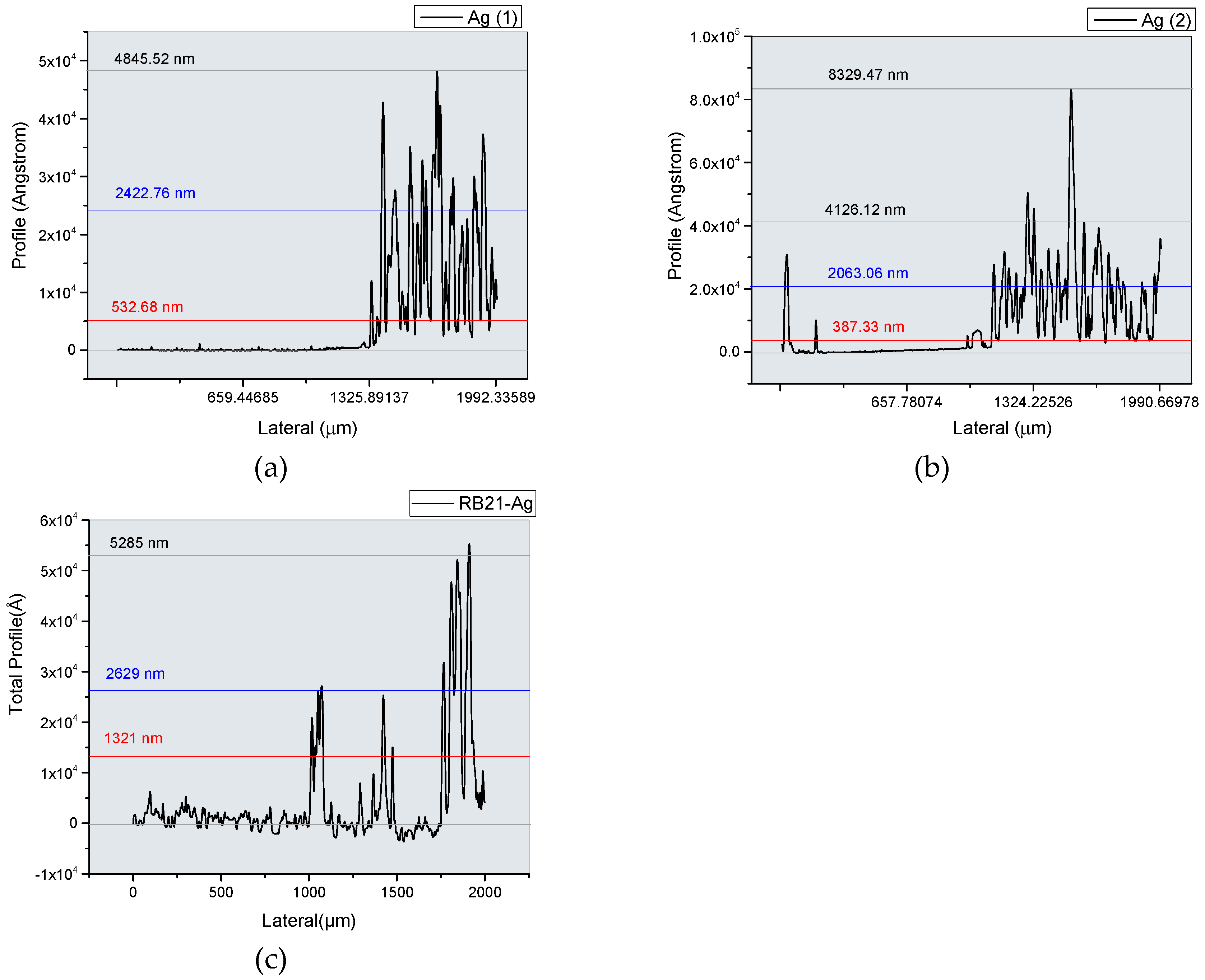
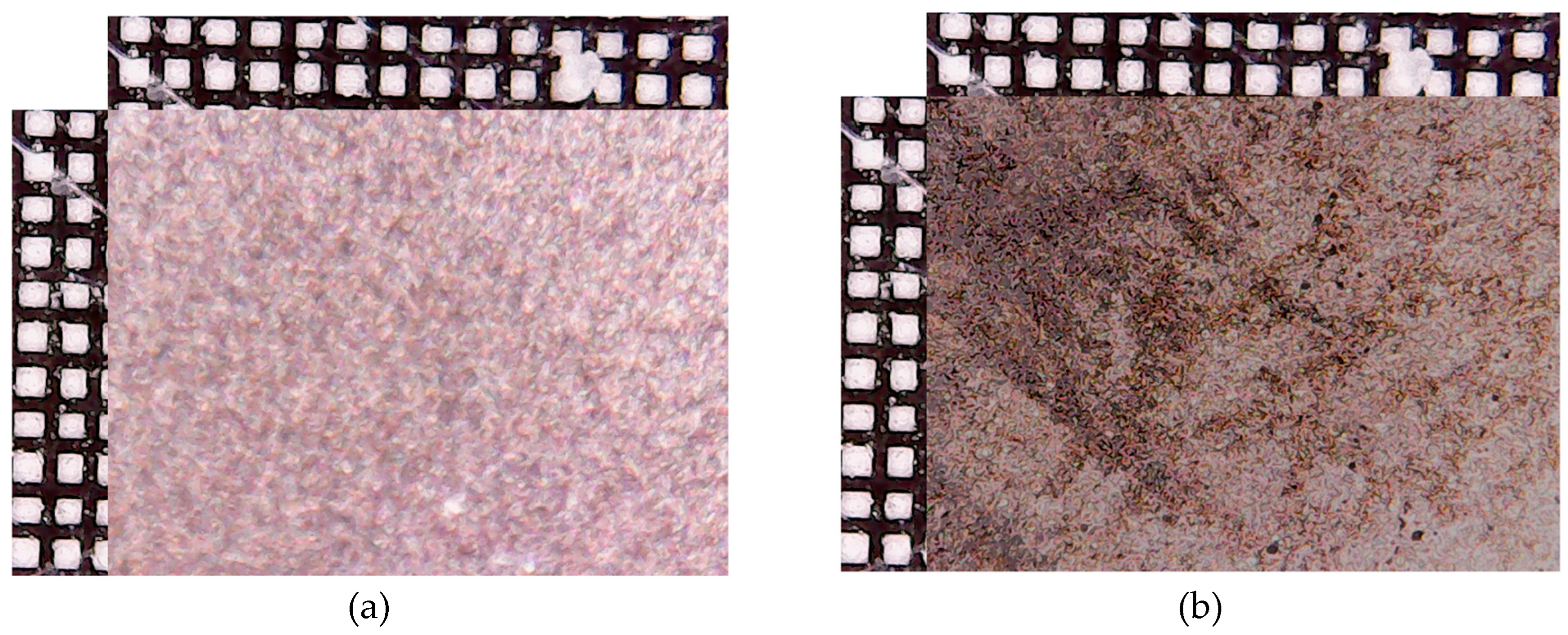
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anabitarte, F.; Cobo, A.; Lopez-Higuera, J.M. Laser-Induced Breakdown Spectroscopy: Fundamentals, Applications, and Challenges. Int. Sch. Res. Notices 2012, 2012, 285240. [CrossRef]
- Tango, W.J.; Link, J.K.; Zare, R.N. Spectroscopy of K2 Using Laser-Induced Fluorescence. J. Chem. Phys. 1968, 49, 4264-4268. [CrossRef]
- Aguilera, J.A.; Aragón, C.; Peñalba, F. Plasma shielding effect in laser ablation of metallic samples and its influence on LIBS analysis. Appl. Surf. Sci. 1998, 127–129, 309–314. [CrossRef]
- Fazio, B.; Trusso, S.; Fazio, E.; Neri, F.; Ossi, P.M.; Santo, N. Nanostructured silver thin films deposited by pulsed laser ablation. Radiat. Eff. Defects Solids 2008, 163, 673-683. [CrossRef]
- Machmudah, S.; Wahyudiono; Kuwahara, Y.; Sasaki, M.; Goto, M. Pulsed laser ablation in pressurized CO2 for nanoparticles fabrication. In Proceedings of the TENCON 2011 – 2011 IEEE Region 10 Conference, Bali, Indonesia, 21-24 November 2011, pp. 792-796. [CrossRef]
- Mohammed, A.Z.; Menazea, A.A.; Mostafa, A.M.; Al-Ashkar, E.A. Ultra-thin silver nanoparticles film prepared via pulsed laser deposition: Synthesis, characterization, and its catalytic activity on reduction of 4-nitrophenol. Surf. Interfaces 2020, 19, 100438. [CrossRef]
- Becker, J.S.; Jakubowski, N. The synergy of elemental and biomolecular mass spectrometry: new analytical strategies in life sciences. Chem. Soc. Rev. 2009, 38, 1969-1983. [CrossRef]
- Gurlui, S.; Sanduloviciu, M.; Strat, M.; Strat, G.; Mihesan, C.; Ziskind, M.; Focsa, C. Dynamic space charge structures in high fluence laser ablation plumes. J. Optoelectron. Adv. Mater. 2006, 8, 148-151.
- Alonso, J.C.; Diamant, R.; Castillo, P.; Acosta–García, M.C.; Batina, N.; Haro-Poniatowski, E. Thin films of silver nanoparticles deposited in vacuum by pulsed laser ablation using a YAG:Nd laser. Appl. Surf. Sci. 2009, 255, 4933-4937. [CrossRef]
- Stafe, M.; Vladoiu, I.; Negutu, C.; Popescu, I.M. Experimental investigation of the nanosecond laser ablation rate of aluminum. Rom. Rep. Phys. 2008, 60, 789-796.
- Cocean, A.; Cocean, I.; Gurlui, S.; Iacomi, F. Study of the pulsed laser deposition phenomena by means of Comsol Multiphysics. U.P.B. Sci. Bull. A 2017, 79, 263-274.
- Cocean, A.; Cocean, I.; Gurlui, S. Influence of the impurities to the composite materials in laser ablation phenomena, U.P.B. Sci. Bull. A 2021, 83, 225-238.
- Cocean, A.; Cocean, I.; Cocean, G.; Postolachi, C.; Pricop, D.A.; Munteanu, B.S.; Cimpoesu, N.; Gurlui, S. Study of Physico-Chemical Interactions during the Production of Silver Citrate Nanocomposites with Hemp Fiber. Nanomaterials 2021, 11, 2560. [CrossRef]
- Yang, Y.; Long, C.-L.; Yang, Z.-G.; Li, H.-P.; Wang, Q. Characterization and Determination of Silver Nanoparticle Using Single Particle-Inductively Coupled Plasma-Mass Spectrometry. Chinese J. Anal. Chem. 2014, 42, 1553-1560. [CrossRef]
- Becker, J.S.; Becker, J.S. Imaging of metals, metalloids, and non-metals by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) in biological tissues. Methods Mol Biol. 2010, 656, 51-82. [CrossRef]
- Wu, B.; Zoriy, M.; Chen, Y.; Becker, J.S. Imaging of nutrient elements in the leaves of Elsholtzia splendens by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). Talanta 2009, 78, 132-137. [CrossRef]
- Pisonero, J.; Günther, D. Femtosecond laser ablation inductively coupled plasma mass spectrometry: fundamentals and capabilities for depth profiling analysis. Mass Spectrom. Rev. 2008, 27, 609-623. [CrossRef]
- Seaman, C. Laser Ablation Inductively Coupled Plasma Mass Spectrometry Imaging of Plant Metabolites. Methods Mol. Biol. 2017, 1618, 125-135. [CrossRef]
- Gurlui, S.; Agop, M.; Nica, P.; Ziskind, M.; Focsa, C. Experimental and theoretical investigations of a laser produced aluminum plasma, Phys. Rev. E 2008, 78, 026405. [CrossRef]
- Cocean, I.; Cocean, A.; Postolachi, C.; Pohoata, V.; Cimpoesu, N.; Bulai, G.; Iacomi, F.; Gurlui, S. Alpha keratin amino acids BEHVIOR under high FLUENCE laser interaction. Medical applications. Appl. Surf. Sci. 2019, 488, 418-426. [CrossRef]
- Cook, M.M.; Lander, J.A. Use of Sodium Borohydride to Control Heavy Metal Discharge in the Photographic Industry. J. Appl. Photogr. Eng. 1979, 5, 144-147.
- Cotton, F.A.; Wilkinson, G. Advanced Inorganic Chemistry, 4th ed.; Interscience: New York, USA, 1980.
- Lide, D.R. (Ed.) Handbook of Chemistry and Physics, 73rd ed.; CRC Press: Boca Raton, FL, USA, 1992; pp. 10-211.
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C7440224&Units=SI&Mask=20#Ion-Energetics (accessed on 10 January 2024).
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C7439896&Units=SI&Mask=20#Ion-Energetics (accessed on 10 January 2024).
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C7440020&Units=SI&Mask=20#ref-5 (accessed on 10 January 2024).
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C7440428&Units=SI&Mask=20#Ion-Energetics (accessed on 10 January 2024).
- Cocean, A.; Pelin, V.; Cazacu, M.M.; Cocean, I.; Sandu, I.; Gurlui, S.; Iacomi, F. Thermal Effects Induced by Laser Ablation in Non-Homogeneous Limestone Covered by an Impurity Layer. Appl. Surf. Sci. 2017, 424, 324-329. [CrossRef]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2015, 9, 217-227. [CrossRef]
- Bagherzade, G.; Tavakoli, M.M.; Namaei, M.H. Green synthesis of silver nanoparticles using aqueous extract of saffron (Crocus sativus L.) wastages and its antibacterial activity against six bacteria. Asian Pac. J. Trop. Biomed. 2017, 7, 227–233. [CrossRef]
- Karthik, L.; Kumar, G.; Vishnu Kirthi, A.; Rahuman, A.A.; Bhaskara Rao, K.V. Streptomyces sp. LK3 mediated synthesis of silver nanoparticles and its biomedical application. Bioprocess Biosyst. Eng. 2014, 37, 261-267. [CrossRef]
- Hajakbari, F.; Ensandoust, M. Study of Thermal Annealing Effect on the Properties of Silver Thin Films Prepared by DC Magnetron Sputtering, Acta Phys. Pol. A 2016, 129, 680-682. [CrossRef]
- Taylor, G.I. The instability of liquid surfaces when accelerated in a direction perpendicular to their planes. I. Proc. R. Soc. Lond. A 1950, 201, 192-196. [CrossRef]
- Chavaraddi, K.B.; Chandaragi, P.I.; Gouder, P.M.; Marali, G.B. Effect of Electromagnetic field on Rayleigh-Taylor instability in a power-law fluid in presence of boundary roughness. J. Phys.: Conf. Ser. 2021, 1849, 012019. [CrossRef]
- Abarzhi, S.I.; Herrmann, M. New type of the interface evolution in the Richtmyer-Meshkov instability. Available online: https://ntrs.nasa.gov/api/citations/20040027954/downloads/20040027954.pdf (accessed on 19 March 2024).
- Pretch, E.; Bülmann, P.; Badertscher, M. Structure Determination of Organic Compounds. Tables of Spectral Data, 4th ed.; Springer: Berlin, Germany, 2009.
- Miller, F.A.; Wilkins, C.H. Infrared Spectra and Characteristic Frequencies of Inorganic Ions. Their Use in Qualitative Analysis. Anal. Chem. 1952, 24, 1253–1294. [CrossRef]
- Cocean, G.; Cocean, A.; Postolachi, C.; Garofalide, S.; Bulai, G.; Munteanu, B.S.; Cimpoesu, N.; Cocean, I.; Gurlui, S. High-Power Laser Deposition of Chitosan Polymers: Medical and Environmental Applications. Polymers 2022, 14, 1537. [CrossRef]
- Cocean, G.; Cocean, A.; Garofalide, S.; Pelin, V.; Munteanu, B.S.; Pricop, D.A.; Motrescu, I.; Dimitriu, D.G.; Cocean, I.; Gurlui, S. Dual-Pulsed Laser Ablation of Oyster Shell Producing Novel Thin Layers Deposed to Saccharomyces cerevisiae. Polymers 2023, 15, 3953. [CrossRef]
- Said, R.B.; Kolle, J.M.; Essalah, K.; Tangour, B.; Sayari, A. A Unified Approach to CO2–Amine Reaction Mechanisms. ACS Omega 2020, 5, 26125-26133. [CrossRef]
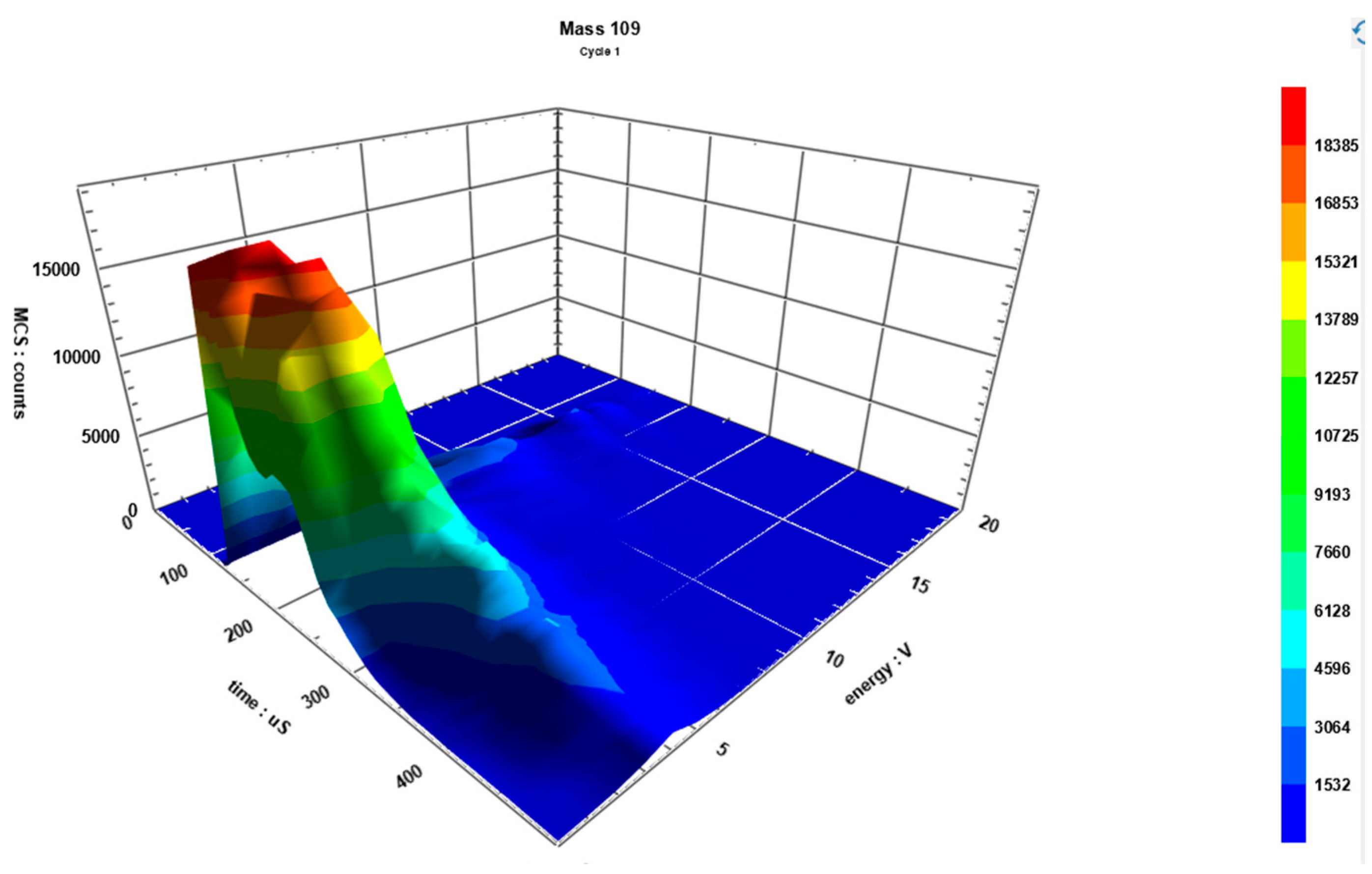
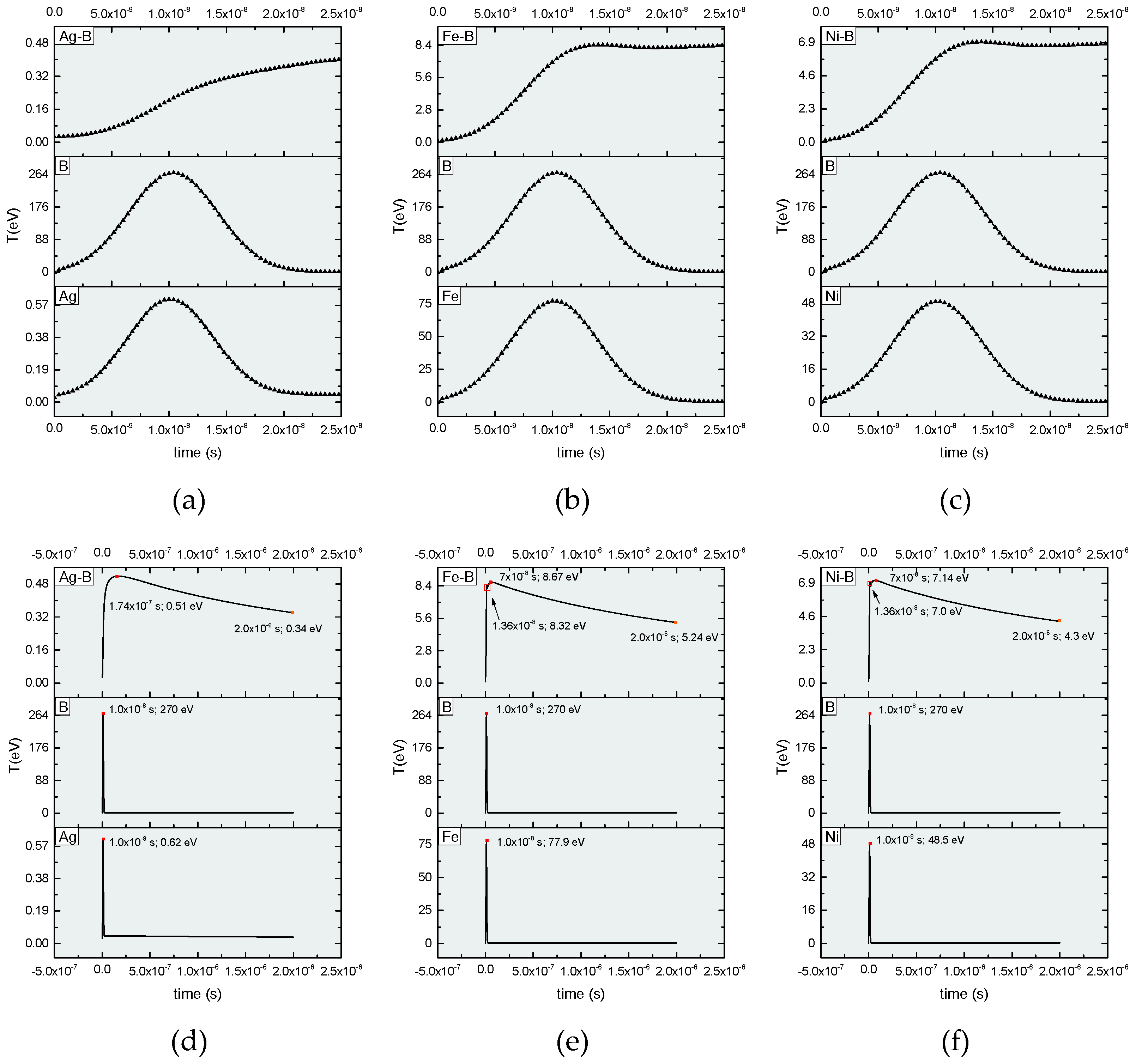
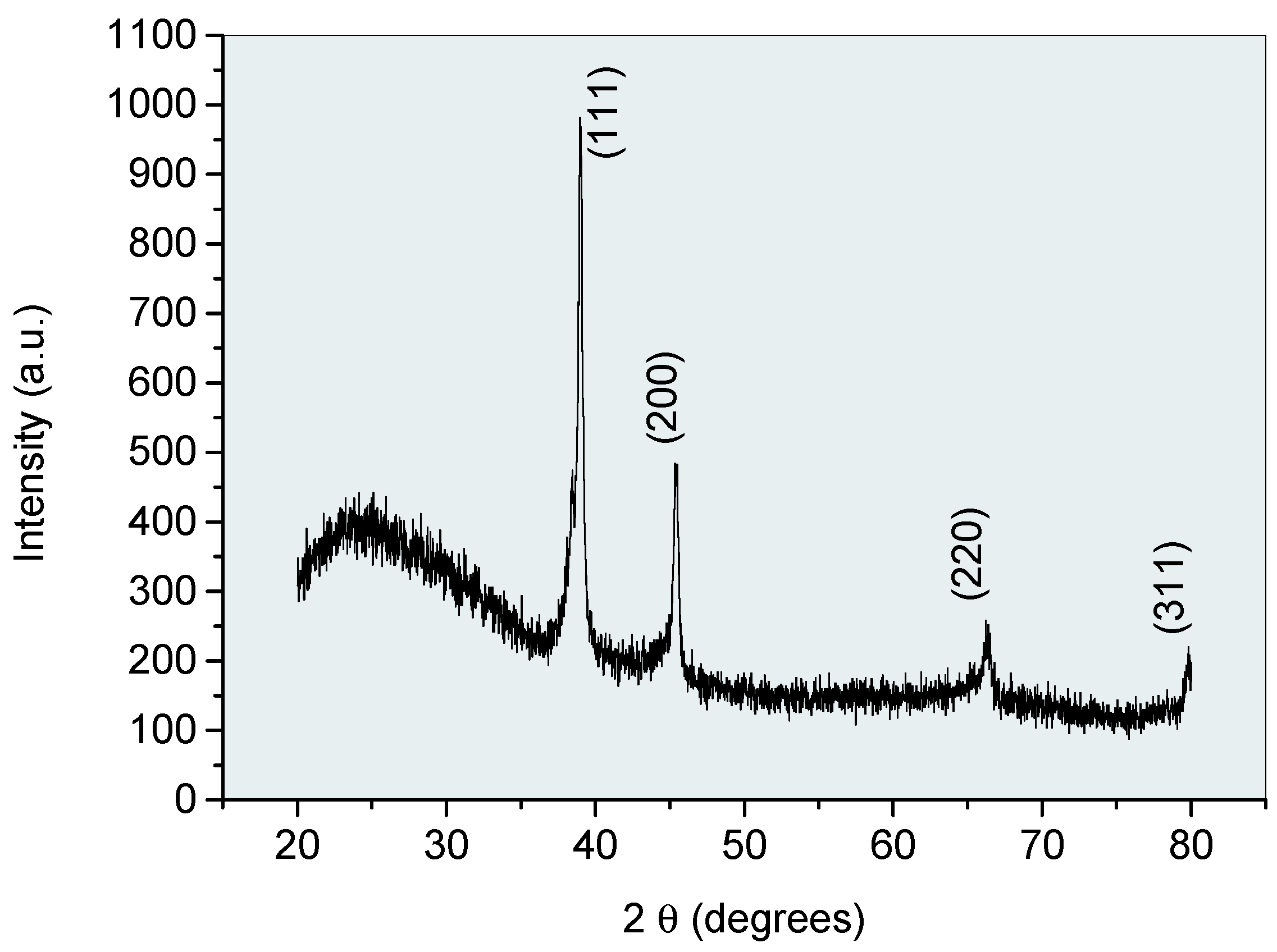
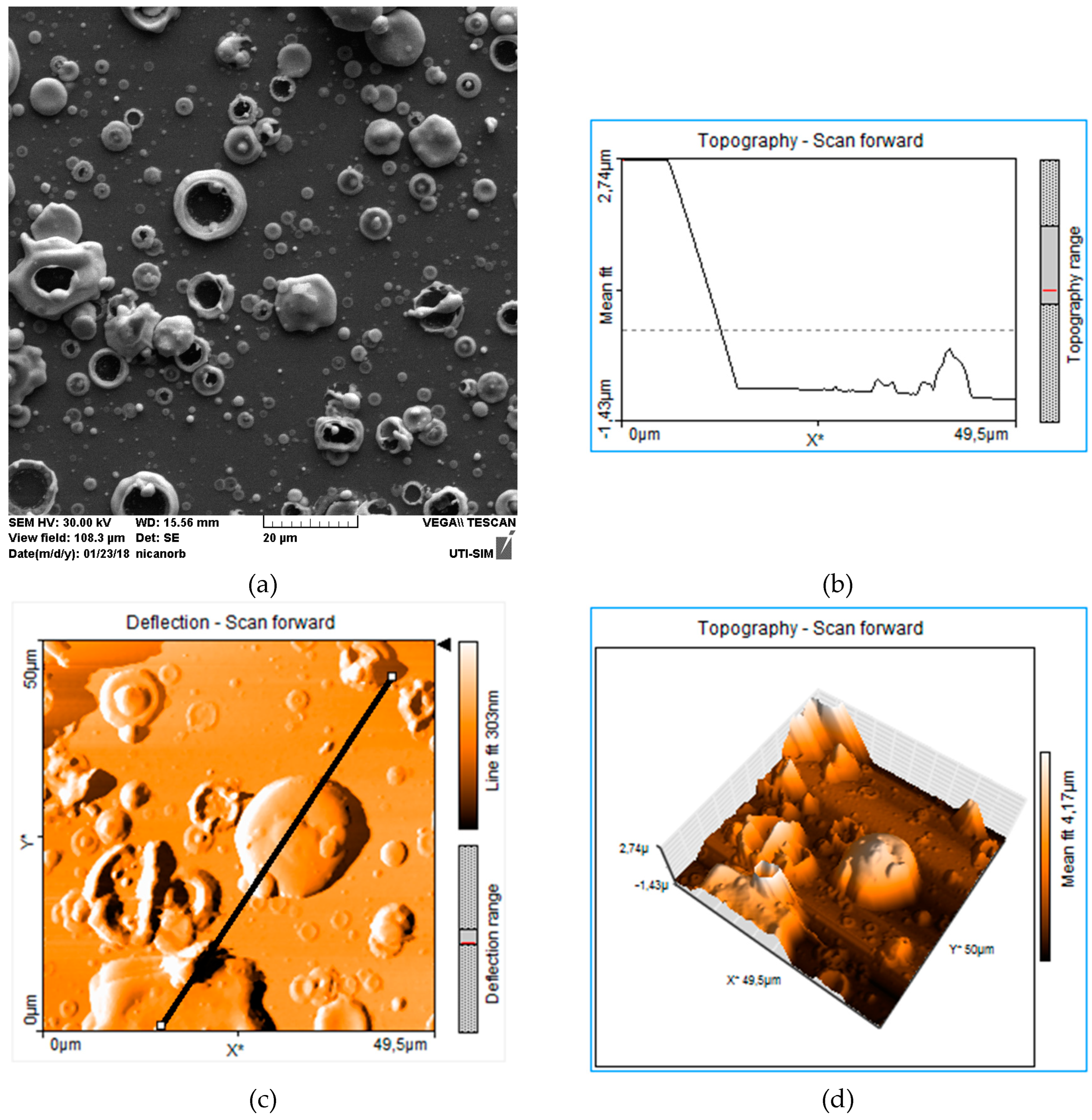
| Element | Melting point (K) | Boiling point (K) | Atomic mass (a.m.u.) | Atomic number | Heat of vaporization (kJ/mol) | Ionization energy (eV) |
|---|---|---|---|---|---|---|
| Ag | 1234 | 2435 | 107.8682 | 47 | 254 | 7.5762 |
| Fe | 1811 | 3134 | 55.845 | 26 | 340 | 7.9024 |
| Ni | 1728 | 2002 | 58.6934 | 28 | 379 | 7.6398 |
| B | 2349 | 4200 | 10.811 | 5 | 508 | 8.29803 |
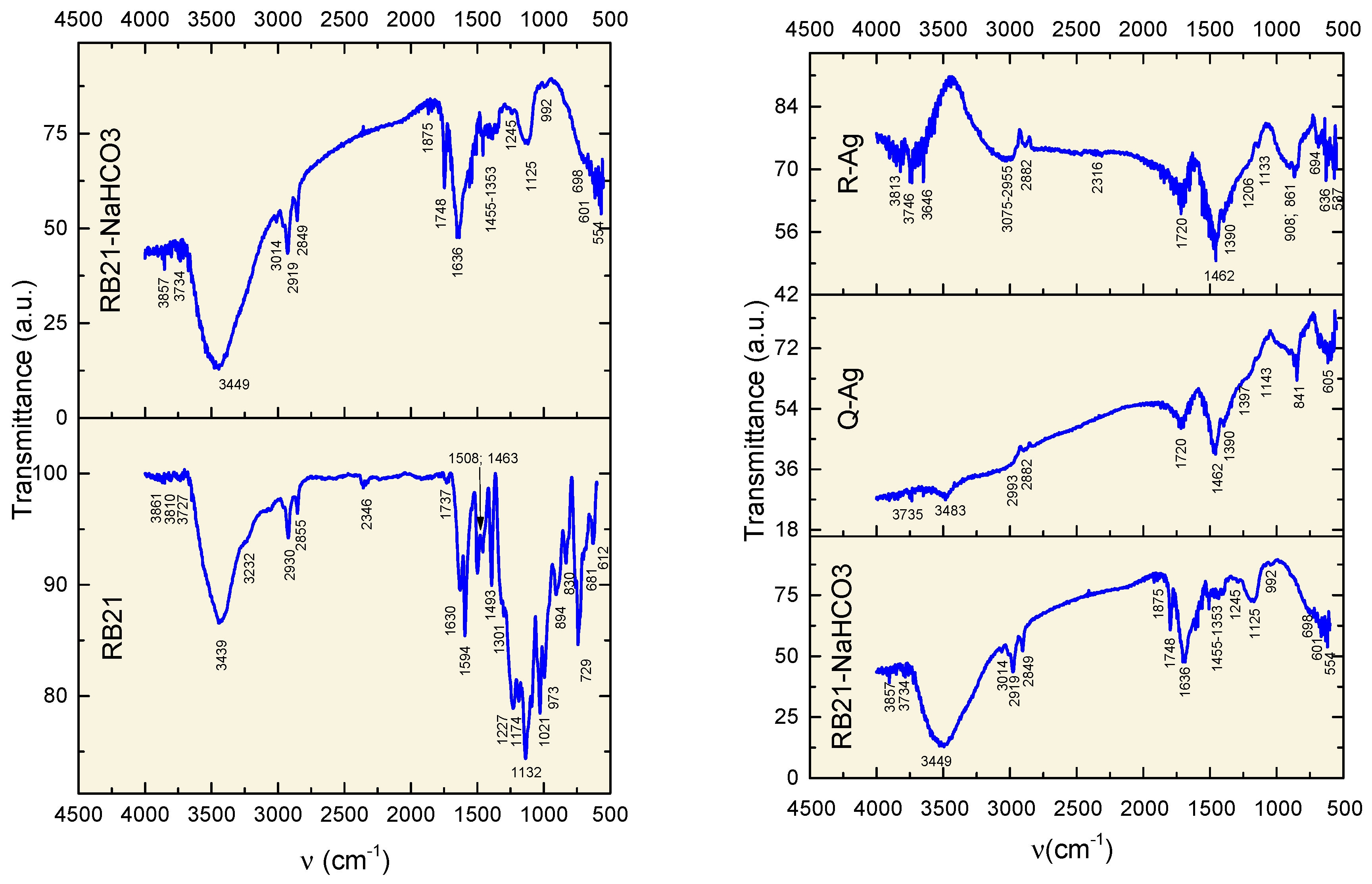
| RB21 | RB21-NaCO3 | R-Ag (dried RB21-NaCO3 aq after interaction with Ag-thin film) | Q-Ag (dried leakage of RB21-NaCO3) | Comments |
|---|---|---|---|---|
| 3861; 3810 v.w. | 3857 v.w. | 3813 v.w. | O-H free str. | |
| 3727 v.w. | 3734 v.w. | 3746 v.w. | 3735 v.w. | O-H free str. |
| 3646 v.w. | O-H free str. | |||
| 3439 s. | 3449 s. | - | 3483 v.w | O-H and N-H free and H-bonded str. |
| 3232 sh. | N-H | |||
| 3014 v.w. | 3075-2955 m. | C-H in aromatic/alkenes | ||
| 2930 m. | 2919 m. | 2993 m. | C-H alifatic | |
| 2855 m. | 2849 m. | 2882 m. | 2883 m. | C-H alifatic |
| 2346 w. | 2316 w. | O=C=O carbon dioxide | ||
| 1875 w. | C=O stretching | |||
| 1737 w. | 1748 m. | 1720 m. | 1720 m. | C=O stretching in carbonate CO3-2 |
| 1630 s. | 1636 s. | C-H aromatic; N-H bending; SO3 | ||
| 1594 s. | - | - | - | Ring skeleton in pyrrole group; N-H bending in sulfonamide group |
| 1508 s. | - | - | - | N-H bending in sulfonamide group |
| 1493s. | - | - | - | C in heterocycles |
| 1463 s. | 1455 w. | 1462 s. | 1462 s. | C-C in heterocycles; C=O in (COO)-; C-H aliphatic bending; CO3-2 lattice vibrations |
| 1301 s. | 1353 w. | 1390 m. | 1390; 1397m. | S=O stretching asymmetric in SO3 of sulfones in chromophore and sulfonamides in chromogen |
| 1227 v.s. | 1245v.w. | 1206 sh. | - | C-N stretching in amines; C-O stretching in alcohols; S=O stretching symmetric in SO3 in chromophore |
| 1174 v.s. | 1125 m. | 1133 w. | 1143 sh. | SO2 in chromogen |
| 1021 v.s. | - | - | - | C-N bending |
| 973 v.s. | 992v.w. | 908 m., wide | - | C=C bending |
| 894 s. | - | 861 m. | - | C=C bending; S-O stretching |
| 830 m. | - | - | 841 m. sharp | N-H bending in sulfonamides; C=C bending; S-O stretching |
| 729 s. | - | - | - | N-H bending; S-O stretching |
| 681 m. | 698 m. | 694 w. | - | S-O stretching |
| 612 m | 601 m. | 636 m. sharp | 605 m. (multiple peaks | S-O stretching |
| - | 554 m. | 537 m. sharp | - | S-O stretching |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





