Submitted:
27 March 2024
Posted:
28 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
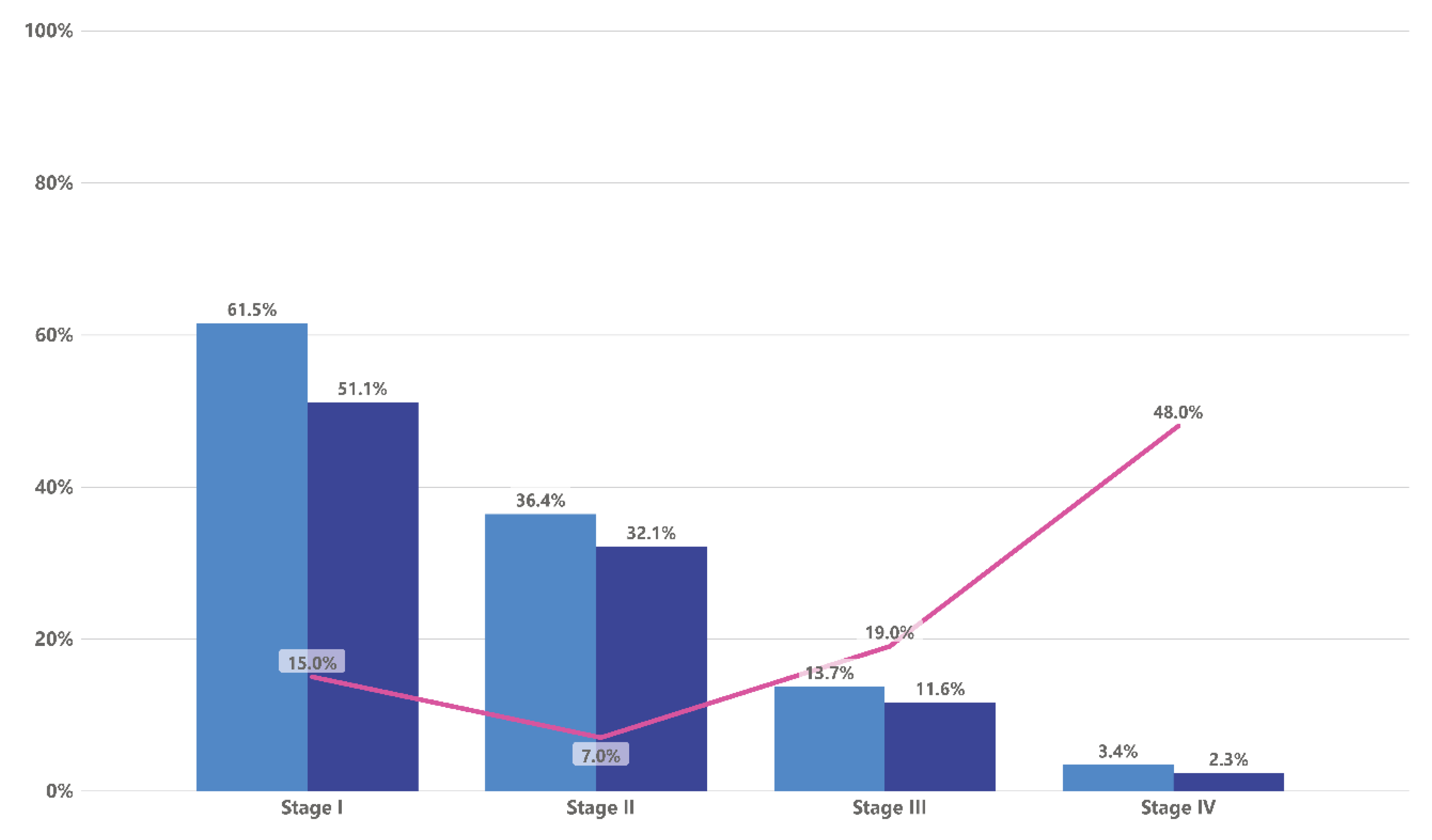
2. Incidental Lung Nodules
3. Molecular Pathology of NSCLC
4. Tumour Antigens as Biomarkers for Cancer
4.1. Cytokeratin 19 Fragment (CYFRA 21-1)
4.2. Carcinoembryonic Antigen (CEA)
4.3. CA19-9
4.4. CA125
4.5. SCC Antigen
4.6. Neuron Specific Enolase (NSE)
4.7. Serum Amyloid A (SAA)
4.8. Osteopontin (OPN)
4.9. Human Epididymis 4 (HE4)
4.10. Heat Shock Protein (HSP70)
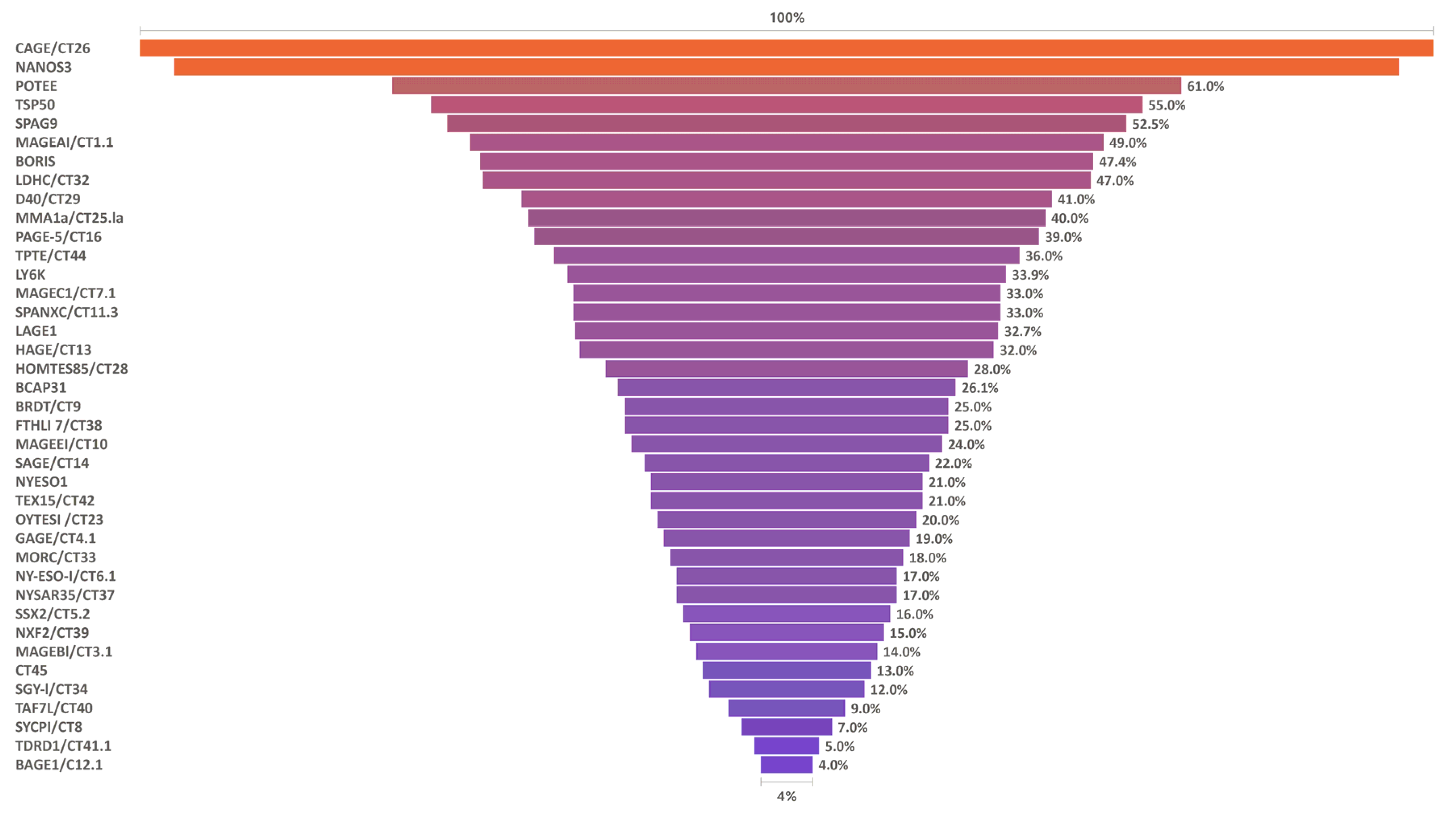
5. Cancer Testis Antigen Expression in NSCLC
5.1. The Cancer-Associated Gene (CAGE)
5.2. NANOS3
5.3. Melanoma-Associated Antigen Gene (MAGE)
5.4. XAGE-1 Gene
6. The State-of-the-Art
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | adenocarcinoma | NSCLC | non-small cell lung carcinoma |
| CAGE | cancer-associated gene | NSE | neuron specific enolase |
| CEA | carcinoembryonic antigen | OPN | Osteopontin |
| CT | computer tomography | PET | positron emission tomography |
| CTA | cancer testis antigen | SAA | serum amyloid A |
| CYFRA21-1 | cytokeratin 19 fragments | SCC | squamous cell carcinoma |
| HE4 | human epididymis 4 | SCCA | squamous cell carcinoma antigen |
| HSP70 | heat shock protein 70 | TA | tumour antigens |
| LC | lung cancer | TAA | tumour associated antigens |
| MAGE | melanoma-associated antigen gene |
References
- Verma, V.; Simone, C.B.; Werner-Wasik, M. Acute and late toxicities of concurrent chemoradiotherapy for locally-advanced non-small cell lung cancer. Cancers 2017, 9, 120. [CrossRef]
- O'Dowd, E.L.; McKeever, T.M.; Baldwin, D.R.; Anwar, S.; Powell, H.A.; Gibson, J.E.; Iyen-Omofoman, B.; Hubbard, R.B. What characteristics of primary care and patients are associated with early death in patients with lung cancer in the UK? Thorax 2015, 70, 161-168.
- Balata, H.; Evison, M.; Sharman, A.; Crosbie, P.; Booton, R. CT screening for lung cancer: Are we ready to implement in Europe? Lung Cancer 2019, 134, 25-33. [CrossRef]
- El-Khoury, V.; Béland, M.; Schritz, A.; Kim, S.-Y.; Nazarov, P.V.; Gaboury, L.; Sertamo, K.; Bernardin, F.; Batutu, R.; Antunes, L.; et al. Identification of beta-arrestin-1 as a diagnostic biomarker in lung cancer. British journal of cancer 2018, 119, 580-590. [CrossRef]
- Tammemagi, M.C.; Mayo, J.R.; Lam, S. Cancer in pulmonary nodules detected on first screening CT. The New England journal of medicine 2013, 369, 2060. [CrossRef]
- Gould, M.K.; Donington, J.; Lynch, W.R.; Mazzone, P.J.; Midthun, D.E.; Naidich, D.P.; Wiener, R.S. Evaluation of individuals with pulmonary nodules: When is it lung cancer?: Diagnosis and management of lung cancer: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e93S-e120S. [CrossRef]
- Snoeckx, A.; Reyntiens, P.; Desbuquoit, D.; Spinhoven, M.J.; Van Schil, P.E.; van Meerbeeck, J.P.; Parizel, P.M. Evaluation of the solitary pulmonary nodule: Size matters, but do not ignore the power of morphology. Insights into imaging 2018, 9, 73-86. [CrossRef]
- Callister, M.; Baldwin, D.; Akram, A.; Barnard, S.; Cane, P.; Draffan, J.; Franks, K.; Gleeson, F.; Graham, R.; Malhotra, P. British Thoracic Society guidelines for the investigation and management of pulmonary nodules: Accredited by NICE. Thorax 2015, 70, ii1-ii54. [CrossRef]
- Wahidi, M.M.; Govert, J.A.; Goudar, R.K.; Gould, M.K.; McCrory, D.C. Evidence for the treatment of patients with pulmonary nodules: When is it lung cancer?: ACCP evidence-based clinical practice guidelines. Chest 2007, 132, 94S-107S. [CrossRef]
- Seemann, M.D.; Seemann, O.; Luboldt, W.; Bonél, H.; Sittek, H.; Dienemann, H.; Staebler, A. Differentiation of malignant from benign solitary pulmonary lesions using chest radiography, spiral CT and HRCT. Lung cancer 2000, 29, 105-124. [CrossRef]
- Chikwe, J.; Cooke, D.; Weiss, A. Cardiothoracic surgery; OUP Oxford: 2013.
- Chen, Z.; Fillmore, C.M.; Hammerman, P.S.; Kim, C.F.; Wong, K.-K. Non-small-cell lung cancers: A heterogeneous set of diseases. Nature Reviews Cancer 2014, 14, 535-546. [CrossRef]
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagavatula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Supko, J.G.; Haluska, F.G. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. New England Journal of Medicine 2004, 350, 2129-2139. [CrossRef]
- Soma, S.; Tsuta, K.; Takano, T.; Hatanaka, Y.; Yoshida, A.; Suzuki, K.; Asamura, H.; Tsuda, H. Intratumoral distribution of EGFR-amplified and EGFR-mutated cells in pulmonary adenocarcinoma. Pathology-Research and Practice 2014, 210, 155-160. [CrossRef]
- Mitsudomi, T. Molecular epidemiology of lung cancer and geographic variations with special reference to EGFR mutations. Translational lung cancer research 2014, 3, 205. [CrossRef]
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.-i.; Watanabe, H.; Kurashina, K.; Hatanaka, H. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature 2007, 448, 561-566. [CrossRef]
- Sanders, H.R.; Albitar, M. Somatic mutations of signaling genes in non-small-cell lung cancer. Cancer Genetics and Cytogenetics 2010, 203, 7-15. [CrossRef]
- Gridelli, C.; Rossi, A.; Carbone, D.P.; Guarize, J.; Karachaliou, N.; Mok, T.; Petrella, F.; Spaggiari, L.; Rosell, R. Non-small-cell lung cancer. Nature reviews Disease primers 2015, 1, 1-16.
- Lokhandwala, T.; Bittoni, M.A.; Dann, R.A.; D'Souza, A.O.; Johnson, M.; Nagy, R.J.; Lanman, R.B.; Merritt, R.E.; Carbone, D.P. Costs of diagnostic assessment for lung cancer: A medicare claims analysis. Clinical lung cancer 2017, 18, e27-e34. [CrossRef]
- Patz Jr, E.F.; Campa, M.J.; Gottlin, E.B.; Kusmartseva, I.; Guan, X.R.; Herndon, J.E. Panel of serum biomarkers for the diagnosis of lung cancer. Journal of Clinical Oncology 2007, 25, 5578-5583. [CrossRef]
- Schneider, J. Tumor markers in detection of lung cancer. Advances in clinical chemistry 2006, 42, 1-41. [CrossRef]
- Hanagiri, T.; Sugaya, M.; Takenaka, M.; Oka, S.; Baba, T.; Shigematsu, Y.; Nagata, Y.; Shimokawa, H.; Uramoto, H.; Takenoyama, M. Preoperative CYFRA 21-1 and CEA as prognostic factors in patients with stage I non-small cell lung cancer. Lung cancer 2011, 74, 112-117. [CrossRef]
- Kulpa, J.; Wojcik, E.; Reinfuss, M.; Kołodziejski, L. Carcinoembryonic antigen, squamous cell carcinoma antigen, CYFRA 21-1, and neuron-specific enolase in squamous cell lung cancer patients. Clinical chemistry 2002, 48, 1931-1937. [CrossRef]
- Schneider, J.; Philipp, M.; Velcovsky, H.-G.; Morr, H.; Katz, N. Pro-gastrin-releasing peptide (ProGRP), neuron specific enolase (NSE), carcinoembryonic antigen (CEA) and cytokeratin 19-fragments (CYFRA 21-1) in patients with lung cancer in comparison to other lung diseases. Anticancer research 2003, 23, 885.
- Ayan, A.K.; Erdemci, B.; Orsal, E.; Bayraktutan, Z.; Akpinar, E.; Topcu, A.; Turkeli, M.; Seven, B. Is there any correlation between levels of serum ostepontin, CEA, and FDG uptake in lung cancer patients with bone metastasis? Revista Española de Medicina Nuclear e Imagen Molecular (English Edition) 2016, 35, 102-106. [CrossRef]
- Hammarström, S. The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. Seminars in Cancer Biology 1999, 9, 67-81. [CrossRef]
- Berge, G.; Pettersen, S.; Grotterød, I.; Bettum, I.J.; Boye, K.; Mælandsmo, G.M. Osteopontin—An important downstream effector of S100A4-mediated invasion and metastasis. International journal of cancer 2011, 129, 780-790. [CrossRef]
- Yu, Z.; Zhang, G.; Yang, M.; Zhang, S.; Zhao, B.; Shen, G.; Chai, Y. Systematic review of CYFRA 21-1 as a prognostic indicator and its predictive correlation with clinicopathological features in Non-small Cell Lung Cancer: A meta-analysis. Oncotarget 2017, 8, 4043. [CrossRef]
- Isgrò, M.A.; Bottoni, P.; Scatena, R. Neuron-specific enolase as a biomarker: Biochemical and clinical aspects. In Advances in Cancer Biomarkers; Springer: 2015; pp. 125-143. [CrossRef]
- Urieli-Shoval, S.; Linke, R.P.; Matzner, Y. Expression and function of serum amyloid A, a major acute-phase protein, in normal and disease states. Current opinion in hematology 2000, 7, 64-69. [CrossRef]
- Biaoxue, R.; Hua, L.; Wenlong, G.; Shuanying, Y. Increased serum amyloid A as potential diagnostic marker for lung cancer: A meta-analysis based on nine studies. BMC cancer 2016, 16, 836. [CrossRef]
- Okamura, K.; Takayama, K.; Izumi, M.; Harada, T.; Furuyama, K.; Nakanishi, Y. Diagnostic value of CEA and CYFRA 21-1 tumor markers in primary lung cancer. Lung Cancer 2013, 80, 45-49. [CrossRef]
- Kosacka, M.; Jankowska, R. Comparison of cytokeratin 19 expression in tumor tissue and serum CYFRA 21-1 levels in non-small cell lung cancer. Polskie Archiwum Medycyny Wewnetrznej 2009, 119, 33-37. [CrossRef]
- Wang, P.; Piao, Y.; Zhang, X.; Li, W.; Hao, X. The concentration of CYFRA 21-1, NSE and CEA in cerebro-spinal fluid can be useful indicators for diagnosis of meningeal carcinomatosis of lung cancer. Cancer Biomarkers 2013, 13, 123-130. [CrossRef]
- Grunnet, M.; Sorensen, J. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung cancer 2012, 76, 138-143. [CrossRef]
- Thomas, S.N.; Zhu, F.; Schnaar, R.L.; Alves, C.S.; Konstantopoulos, K. Carcinoembryonic antigen and CD44 variant isoforms cooperate to mediate colon carcinoma cell adhesion to E-and L-selectin in shear flow. Journal of biological chemistry 2008, 283, 15647-15655. [CrossRef]
- Duffy, M.J. Carcinoembryonic antigen as a marker for colorectal cancer: Is it clinically useful? Clinical chemistry 2001, 47, 624-630.
- Sawabata, N.; Ohta, M.; Takeda, S.-i.; Hirano, H.; Okumura, Y.; Asada, H.; Maeda, H. Serum carcinoembryonic antigen level in surgically resected clinical stage I patients with non-small cell lung cancer. The Annals of thoracic surgery 2002, 74, 174-179. [CrossRef]
- Seemann, M.D.; Beinert, T.; Fürst, H.; Fink, U. An evaluation of the tumour markers, carcinoembryonic antigen (CEA), cytokeratin marker (CYFRA 21-1) and neuron-specific enolase (NSE) in the differentiation of malignant from benign solitary pulmonary lesions. Lung Cancer 1999, 26, 149-155. [CrossRef]
- Pioglitazone Hydrochloride in Treating Patients With Stage IA-IIIA Non-small Cell Lung Cancer.
- Hsu, W.-H.; Huang, C.-S.; Hsu, H.-S.; Huang, W.-J.; Lee, H.-C.; Huang, B.-S.; Huang, M.-H. Preoperative serum carcinoembryonic antigen level is a prognostic factor in women with early non-small-cell lung cancer. The Annals of thoracic surgery 2007, 83, 419-424. [CrossRef]
- Nonaka, M.; Kataoka, D.; Yamamoto, S.; Bito, A.; Matsuoka, J.; Kawada, T.; Takaba, T. Pre-and post-operative serum carcinoembryonic antigen in primary lung adenocarcinoma. Ann Thorac Cardiovasc Surg 2004, 10, 281-284.
- Doseeva, V.; Colpitts, T.; Gao, G.; Woodcock, J.; Knezevic, V. Performance of a multiplexed dual analyte immunoassay for the early detection of non-small cell lung cancer. J Transl Med 2015, 13, 55. [CrossRef]
- Schmiegel, W.; Kreiker, C.; Eberl, W.; Arndt, R.; Classen, M.; Greten, H.; Jessen, K.; Kalthoff, H.; Soehendra, N.; Thiele, H. Monoclonal antibody defines CA 19-9 in pancreatic juices and sera. Gut 1985, 26, 456-460. [CrossRef]
- Herlyn, M.; Sears, H.F.; Steplewski, Z.; Koprowski, H. Monoclonal antibody detection of a circulating tumor-associated antigen. I. Presence of antigen in sera of patients with colorectal, gastric, and pancreatic carcinoma. Journal of clinical immunology 1982, 2, 135-140. [CrossRef]
- Kawai, T.; Suzuki, M.; Kase, K.; Ozeki, Y. Expression of carbohydrate antigens in human pulmonary adenocarcinoma. Cancer 1993, 72, 1581-1587. [CrossRef]
- Toumbis, M.; Rasidakis, A.; Passalidou, E.; Dimitroulis, J.; Gaga, M.; Alchanatis, M.; Orphanidou, D.; Jordanoglou, J. Diagnostic usefulness of 5 tumor-markers in patients with primary lung-cancer. Oncology reports 1995, 2, 1135-1140. [CrossRef]
- Tsumatori, G.; Ozeki, Y.; Takagi, K.; Ogata, T.; Tanaka, S. Relation between the Serum E-Selectin Level and the Survival Rate of Patients with Resected Non-small Cell Lung Cancers. Japanese journal of cancer research 1999, 90, 301-307. [CrossRef]
- Pejovic, T.; Nezhat, F. Effect of screening on ovarian cancer mortality: The prostate, lung, colorectal and ovarian (PLCO) cancer screening randomized controlled trial. Journal of Minimally Invasive Gynecology 2011, 18, 823-825. [CrossRef]
- Bottoni, P.; Scatena, R. The role of CA 125 as tumor marker: Biochemical and clinical aspects. Advances in Cancer Biomarkers: From biochemistry to clinic for a critical revision 2015, 229-244.
- Clevers, M.R.; Kastelijn, E.A.; Peters, B.J.; Kelder, H.; Schramel, F.M. Evaluation of serum biomarker CEA and Ca-125 as immunotherapy response predictors in metastatic non-small cell lung cancer. Anticancer Research 2021, 41, 869-876. [CrossRef]
- Cedrés, S.; Nuñez, I.; Longo, M.; Martinez, P.; Checa, E.; Torrejón, D.; Felip, E. Serum tumor markers CEA, CYFRA21-1, and CA-125 are associated with worse prognosis in advanced non–small-cell lung cancer (NSCLC). Clinical lung cancer 2011, 12, 172-179. [CrossRef]
- Yu, D.; Du, K.; Liu, T.; Chen, G. Prognostic Value of Tumor Markers, NSE, CA125 and SCC, in Operable NSCLC Patients. International Journal of Molecular Sciences 2013, 14, 11145-11156. [CrossRef]
- Kagohashi, K.; Satoh, H.; Ishikawa, H.; Ohtsuka, M.; Sekizawa, K. A re-evaluation of squamous cell carcinoma antigen (SCC) as a serum marker for non-small cell lung cancer. Medical Oncology 2008, 25, 187-189. [CrossRef]
- Barlési, F.; Gimenez, C.; Torre, J.-P.; Doddoli, C.; Mancini, J.; Greillier, L.; Roux, F.; Kleisbauer, J.-P. Prognostic value of combination of Cyfra 21-1, CEA and NSE in patients with advanced non-small cell lung cancer. Respiratory medicine 2004, 98, 357-362. [CrossRef]
- Altintas, Z.; Tothill, I. Biomarkers and biosensors for the early diagnosis of lung cancer. Sensors and Actuators B-Chemical 2013, 188, 988-998. [CrossRef]
- Isgro, M.A.; Bottoni, P.; Scatena, R. Neuron-Specific Enolase as a Biomarker: Biochemical and Clinical Aspects. Adv Exp Med Biol 2015, 867, 125-143. [CrossRef]
- Dittadi, R.; Gion, M. Re: Biological variation of neuroendocrine tumor markers chromogranin A and neuron-specific enolase. Clinical Biochemistry 2013, 12, 1145. [CrossRef]
- Urieli-Shoval, S.; Linke, R.P.; Matzner, Y. Expression and function of serum amyloid A, a major acute-phase protein, in normal and disease states. Curr Opin Hematol 2000, 7, 64-69. [CrossRef]
- Moshkovskii, S. Why do cancer cells produce serum amyloid A acute-phase protein? Biochemistry (Moscow) 2012, 77, 339-341. [CrossRef]
- Berge, G.; Pettersen, S.; Grotterød, I.; Bettum, I.J.; Boye, K.; Mælandsmo, G.M. Osteopontin—An important downstream effector of S100A4-mediated invasion and metastasis. International Journal of Cancer 2011, 129, 780-790. [CrossRef]
- Rud, A.K.; Boye, K.; Øijordsbakken, M.; Lund-Iversen, M.; Halvorsen, A.R.; Solberg, S.K.; Berge, G.; Helland, Å.; Brustugun, O.T.; Mælandsmo, G.M. Osteopontin is a prognostic biomarker in non-small cell lung cancer. BMC cancer 2013, 13, 1-10. [CrossRef]
- Li, J.; Li, Y.; Huo, L.; Sun, R.; Liu, X.; Gu, Q.; Li, A.; Han, S.; Liu, H.; Li, Y.; et al. Detection of serum HE4 levels contributes to the diagnosis of lung cancer. Oncol Lett 2023, 25, 255. [CrossRef]
- Tokuishi, K.; Yamashita, S.-i.; Ohbo, K.; Kawahara, K. Splice variant HE4-V3 expression is associated with favorable prognosis in pulmonary adenocarcinoma. Tumor Biology 2012, 33, 103-109. [CrossRef]
- Nagy, B.; Bhattoa, H.P.; Steiber, Z.; Csobán, M.; Szilasi, M.; Méhes, G.; Müller, M.; Lázár, J.; Kappelmayer, J.; Antal-Szalmás, P. Serum human epididymis protein 4 (HE4) as a tumor marker in men with lung cancer. Clinical Chemistry and Laboratory Medicine (CCLM) 2014, 52, 1639-1648. [CrossRef]
- Bingle, L.; Singleton, V.; Bingle, C.D. The putative ovarian tumour marker gene HE4 (WFDC2), is expressed in normal tissues and undergoes complex alternative splicing to yield multiple protein isoforms. Oncogene 2002, 21, 2768-2773. [CrossRef]
- Drapkin, R.; Von Horsten, H.H.; Lin, Y.; Mok, S.C.; Crum, C.P.; Welch, W.R.; Hecht, J.L. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer research 2005, 65, 2162-2169. [CrossRef]
- Iwahori, K.; Suzuki, H.; Kishi, Y.; Fujii, Y.; Uehara, R.; Okamoto, N.; Kobayashi, M.; Hirashima, T.; Kawase, I.; Naka, T. Serum HE4 as a diagnostic and prognostic marker for lung cancer. Tumour biology: The journal of the International Society for Oncodevelopmental Biology and Medicine 2012, 33, 1141-1149. [CrossRef]
- Mayer, M.; Bukau, B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cellular and molecular life sciences 2005, 62, 670-684. [CrossRef]
- Morimoto, R.I. Regulation of the heat shock transcriptional response: Cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes & development 1998, 12, 3788-3796. [CrossRef]
- Chuma, M.; Saeki, N.; Yamamoto, Y.; Ohta, T.; Asaka, M.; Hirohashi, S.; Sakamoto, M. Expression profiling in hepatocellular carcinoma with intrahepatic metastasis: Identification of high-mobility group I (Y) protein as a molecular marker of hepatocellular carcinoma metastasis. The Keio journal of medicine 2004, 53, 90-97. [CrossRef]
- Garg, M.; Kanojia, D.; Saini, S.; Suri, S.; Gupta, A.; Surolia, A.; Suri, A. Germ cell-specific heat shock protein 70-2 is expressed in cervical carcinoma and is involved in the growth, migration, and invasion of cervical cells. Cancer 2010, 116, 3785-3796. [CrossRef]
- Wang, B.; Lan, T.; Xiao, H.; Chen, Z.-H.; Wei, C.; Chen, L.-F.; Guan, J.-F.; Yuan, R.-F.; Yu, X.; Hu, Z.-G. The expression profiles and prognostic values of HSP70s in hepatocellular carcinoma. Cancer Cell International 2021, 21, 1-17. [CrossRef]
- Tang, T.; Yang, C.; Brown, H.E.; Huang, J. Circulating Heat Shock Protein 70 Is a Novel Biomarker for Early Diagnosis of Lung Cancer. Dis Markers 2018, 2018, 6184162. [CrossRef]
- Gjerstorff, M.F.; Andersen, M.H.; Ditzel, H.J. Oncogenic cancer/testis antigens: Prime candidates for immunotherapy. Oncotarget 2015, 6, 15772. [CrossRef]
- Fratta, E.; Coral, S.; Covre, A.; Parisi, G.; Colizzi, F.; Danielli, R.; Nicolay, H.J.M.; Sigalotti, L.; Maio, M. The biology of cancer testis antigens: Putative function, regulation and therapeutic potential. Molecular oncology 2011, 5, 164-182. [CrossRef]
- O'Leary, K.; Shia, A.; Schmid, P. Epigenetic regulation of EMT in non-small cell lung cancer. Current cancer drug targets 2018, 18, 89-96. [CrossRef]
- Gure, A.O.; Chua, R.; Williamson, B.; Gonen, M.; Ferrera, C.A.; Gnjatic, S.; Ritter, G.; Simpson, A.J.; Chen, Y.-T.; Old, L.J. Cancer-testis genes are coordinately expressed and are markers of poor outcome in non–small cell lung cancer. Clinical Cancer Research 2005, 11, 8055-8062. [CrossRef]
- John, T.; Starmans, M.H.; Chen, Y.T.; Russell, P.A.; Barnett, S.A.; White, S.C.; Mitchell, P.L.; Walkiewicz, M.; Azad, A.; Lambin, P.; et al. The role of Cancer-Testis antigens as predictive and prognostic markers in non-small cell lung cancer. PLoS ONE 2013, 8, e67876. [CrossRef]
- Cho, B.; Lim, Y.; Lee, D.-Y.; Park, S.-Y.; Lee, H.; Kim, W.H.; Yang, H.; Bang, Y.-J.; Jeoung, D.-I. Identification and characterization of a novel cancer/testis antigen gene CAGE. Biochemical and biophysical research communications 2002, 292, 715-726. [CrossRef]
- Iwata, T.; Fujita, T.; Hirao, N.; Matsuzaki, Y.; Okada, T.; Mochimaru, H.; Susumu, N.; Matsumoto, E.; Sugano, K.; Yamashita, N. Frequent Immune Responses to a Cancer/Testis Antigen, CAGE, in Patients with Microsatellite instability–Positive Endometrial Cancer. Clinical cancer research 2005, 11, 3949-3957. 10.1158/1078-0432.CCR-04-1702.
- Yeon, M.; Lee, H.; Yeo, J.; Jeong, M.S.; Jung, H.S.; Lee, H.; Shim, K.; Jo, H.; Jeon, D.; Koh, J.; et al. Cancer/testis antigen CAGE mediates osimertinib resistance in non-small cell lung cancer cells and predicts poor prognosis in patients with pulmonary adenocarcinoma. Scientific Reports 2023, 13, 15748. [CrossRef]
- Grelet, S.; Andries, V.; Polette, M.; Gilles, C.; Staes, K.; Martin, A.P.; Kileztky, C.; Terryn, C.; Dalstein, V.; Cheng, C.W. The human NANOS3 gene contributes to lung tumour invasion by inducing epithelial–mesenchymal transition. The Journal of Pathology 2015, 237, 25-37. [CrossRef]
- Kusz, K.; Tomczyk, L.; Sajek, M.; Spik, A.; Latos-Bielenska, A.; Jedrzejczak, P.; Pawelczyk, L.; Jaruzelska, J. The highly conserved NANOS2 protein: Testis-specific expression and significance for the human male reproduction. Molecular human reproduction 2009, 15, 165-171. [CrossRef]
- Janic, A.; Mendizabal, L.; Llamazares, S.; Rossell, D.; Gonzalez, C. Ectopic expression of germline genes drives malignant brain tumor growth in Drosophila. Science 2010, 330, 1824-1827. [CrossRef]
- Miles, W.O.; Korenjak, M.; Griffiths, L.M.; Dyer, M.A.; Provero, P.; Dyson, N.J. Post-transcriptional gene expression control by NANOS is up-regulated and functionally important in pR b-deficient cells. The EMBO Journal 2014, 33, 2201-2215. [CrossRef]
- Bonnomet, A.; Polette, M.; Strumane, K.; Gilles, C.; Dalstein, V.; Kileztky, C.; Berx, G.; Van Roy, F.; Birembaut, P.; Nawrocki-Raby, B. The E-cadherin-repressed hNanos1 gene induces tumor cell invasion by upregulating MT1-MMP expression. Oncogene 2008, 27, 3692-3699. [CrossRef]
- Campagnolo, C.; Meyers, K.J.; Ryan, T.; Atkinson, R.C.; Chen, Y.-T.; Scanlan, M.J.; Ritter, G.; Old, L.J.; Batt, C.A. Real-Time, label-free monitoring of tumor antigen and serum antibody interactions. Journal of biochemical and biophysical methods 2004, 61, 283-298. [CrossRef]
- Krishnadas, D.K.; Bai, F.; Lucas, K.G. Cancer testis antigen and immunotherapy. ImmunoTargets and therapy 2013, 2, 11. [CrossRef]
- Doyle, J.M.; Gao, J.; Wang, J.; Yang, M.; Potts, P.R. MAGE-RING protein complexes comprise a family of E3 ubiquitin ligases. Molecular cell 2010, 39, 963-974. [CrossRef]
- Fanipakdel, A.; Seilanian Toussi, M.; Rezazadeh, F.; Mohamadian Roshan, N.; Javadinia, S.A. Overexpression of cancer-testis antigen melanoma-associated antigen A1 in lung cancer: A novel biomarker for prognosis, and a possible target for immunotherapy. Journal of Cellular Physiology 2019, 234, 12080-12086. [CrossRef]
- Tyagi, P.; Mirakhur, B. MAGRIT: The largest-ever phase III lung cancer trial aims to establish a novel tumor-specific approach to therapy. Clinical lung cancer 2009, 10, 371-374. [CrossRef]
- Morgan, R.A.; Chinnasamy, N.; Abate-Daga, D.D.; Gros, A.; Robbins, P.F.; Zheng, Z.; Feldman, S.A.; Yang, J.C.; Sherry, R.M.; Phan, G.Q. Cancer regression and neurologic toxicity following anti-MAGE-A3 TCR gene therapy. Journal of immunotherapy (Hagerstown, Md.: 1997) 2013, 36, 133. [CrossRef]
- Egland, K.A.; Kumar, V.; Duray, P.; Pastan, I. Characterization of overlapping XAGE-1 transcripts encoding a cancer testis antigen expressed in lung, breast, and other types of cancers. Molecular Cancer Therapeutics 2002, 1, 441-450.
- Nakagawa, K.; Noguchi, Y.; Uenaka, A.; Sato, S.; Okumura, H.; Tanaka, M.; Shimono, M.; Ali Eldib, A.M.; Ono, T.; Ohara, N. XAGE-1 expression in non–small cell lung cancer and antibody response in patients. Clinical cancer research 2005, 11, 5496-5503. [CrossRef]
- Sato, S.; Noguchi, Y.; Ohara, N.; Uenaka, A.; Shimono, M.; Nakagawa, K.; Koizumi, F.; Ishida, T.; Yoshino, T.; Shiratori, Y. Identification of XAGE-1 isoforms: Predominant expression of XAGE-1b in testis and tumors. Cancer Immunity 2007, 7, 5.
- Kikuchi, E.; Yamazaki, K.; Nakayama, E.; Sato, S.; Uenaka, A.; Yamada, N.; Oizumi, S.; Dosaka-Akita, H.; Nishimura, M. Prolonged survival of patients with lung adenocarcinoma expressing XAGE-1b and HLA class I antigens. Cancer Immun 2008, 8, 13.
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015, 4, 1. [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. Bmj 2015, 350, g7647. [CrossRef]
- Mohamed, E.; García Martínez, D.J.; Hosseini, M.S.; Yoong, S.Q.; Hart, S.; Guinn, B.A. Identification of biomarkers for the early detection of non-small cell lung cancer: A systematic review and meta-analysis. Carcinogenesis 2023. [CrossRef]
- Field, J.K.; Duffy, S.; Baldwin, D.R.; Whynes, D.; Devaraj, A.; Brain, K.E.; Eisen, T.; Gosney, J.; Green, B.; Holemans, J. UK Lung Cancer RCT Pilot Screening Trial: Baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016, 71, 161-170. [CrossRef]
- Nasrullah, N.; Sang, J.; Alam, M.S.; Mateen, M.; Cai, B.; Hu, H. Automated Lung Nodule Detection and Classification Using Deep Learning Combined with Multiple Strategies. Sensors (Basel) 2019, 19, 3722. [CrossRef]
- MacMahon, H.; Naidich, D.P.; Goo, J.M.; Lee, K.S.; Leung, A.N.; Mayo, J.R.; Mehta, A.C.; Ohno, Y.; Powell, C.A.; Prokop, M. Guidelines for management of incidental pulmonary nodules detected on CT images: From the Fleischner Society 2017. Radiology 2017, 284, 228-243. [CrossRef]
- Welch, H.G.; Prorok, P.C.; O’Malley, A.J.; Kramer, B.S. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. New England Journal of Medicine 2016, 375, 1438-1447. [CrossRef]
- Bretthauer, M.; Kaminski, M.F.; Hassan, C.; Kalager, M.; Holme, Ø.; Hoff, G.; Løberg, M.; Regula, J.; Castells, A.; Adami, H.-O. America, we are confused: The updated US Preventive Services Task Force recommendation on colorectal cancer screening. 2017. [CrossRef]
- Huang, H.; Wang, W.; Lin, T.; Zhang, Q.; Zhao, X.; Lian, H.; Guo, H. Comparison of the complications of traditional 12 cores transrectal prostate biopsy with image fusion guided transperineal prostate biopsy. BMC urology 2016, 16, 68. [CrossRef]
- Wallace, M.B.; Pascual, J.M.; Raimondo, M.; Woodward, T.A.; McComb, B.L.; Crook, J.E.; Johnson, M.M.; Al-Haddad, M.A.; Gross, S.A.; Pungpapong, S. Minimally invasive endoscopic staging of suspected lung cancer. Jama 2008, 299, 540-546. [CrossRef]
- Saenger, A.; Beyrau, R.; Braun, S.; Cooray, R.; Dolci, A.; Freidank, H.; Giannitsis, E.; Gustafson, S.; Handy, B.; Katus, H. Multicenter analytical evaluation of a high-sensitivity troponin T assay. Clinica chimica acta 2011, 412, 748-754. [CrossRef]
- Kammer, M.N.; Massion, P.P. Noninvasive biomarkers for lung cancer diagnosis, where do we stand? Journal of Thoracic Disease 2020, 12, 3317-3330. [CrossRef]
- Yonemori, K.; Tateishi, U.; Uno, H.; Yonemori, Y.; Tsuta, K.; Takeuchi, M.; Matsuno, Y.; Fujiwara, Y.; Asamura, H.; Kusumoto, M. Development and validation of diagnostic prediction model for solitary pulmonary nodules. Respirology 2007, 12, 856-862. [CrossRef]
- Kupert, E.; Anderson, M.; Liu, Y.; Succop, P.; Levin, L.; Wang, J.; Wikenheiser-brokamp, K.; Chen, P.; Pinney, S.M.; Macdonald, T. Plasma secretory phospholipase A2-IIa as a potential biomarker for lung cancer in patients with solitary pulmonary nodules. Bmc Cancer 2011, 11, 513. [CrossRef]
- Bigbee, W.L.; Gopalakrishnan, V.; Weissfeld, J.L.; Wilson, D.O.; Dacic, S.; Lokshin, A.E.; Siegfried, J.M. A multiplexed serum biomarker immunoassay panel discriminates clinical lung cancer patients from high-risk individuals found to be cancer-free by CT screening. Journal of thoracic oncology 2012, 7, 698-708. [CrossRef]
- Boyle, P.; Chapman, C.; Holdenrieder, S.; Murray, A.; Robertson, C.; Wood, W.; Maddison, P.; Healey, G.; Fairley, G.; Barnes, A. Clinical validation of an autoantibody test for lung cancer. Annals of Oncology 2011, 22, 383-389. [CrossRef]
- Murray, A.; Chapman, C.; Healey, G.; Peek, L.; Parsons, G.; Baldwin, D.; Barnes, A.; Sewell, H.; Fritsche, H.; Robertson, J. Technical validation of an autoantibody test for lung cancer. Annals of Oncology 2010, 21, 1687-1693. [CrossRef]
- Daly, S.; Rinewalt, D.; Fhied, C.; Basu, S.; Mahon, B.; Liptay, M.J.; Hong, E.; Chmielewski, G.; Yoder, M.A.; Shah, P.N.; et al. Development and Validation of a Plasma Biomarker Panel for Discerning Clinical Significance of Indeterminate Pulmonary Nodules. Journal of Thoracic Oncology 2013, 8, 31-36. [CrossRef]
- Tufman, A.; Tian, F.; Huber, R.M. Can microRNAs improve the management of lung cancer patients? A clinician's perspective. Theranostics 2013, 3, 953-963. [CrossRef]
- Robbins, P.F.; Lu, Y.-C.; El-Gamil, M.; Li, Y.F.; Gross, C.; Gartner, J.; Lin, J.C.; Teer, J.K.; Cliften, P.; Tycksen, E. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nature medicine 2013, 19, 747. [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69-74. [CrossRef]
- Castle, J.C.; Kreiter, S.; Diekmann, J.; Löwer, M.; Van de Roemer, N.; de Graaf, J.; Selmi, A.; Diken, M.; Boegel, S.; Paret, C. Exploiting the mutanome for tumor vaccination. Cancer research 2012, 72, 1081-1091. [CrossRef]
- Ding, C.; Zhou, X.; Xu, C.; Chen, J.; Ju, S.; Chen, T.; Liang, Z.; Cui, Z.; Li, C.; Zhao, J. Circulating tumor cell levels and carcinoembryonic antigen: An improved diagnostic method for lung adenocarcinoma. Thorac Cancer 2018, 9, 1413-1420. [CrossRef]
- Massion, P.P.; Antic, S.; Ather, S.; Arteta, C.; Brabec, J.; Chen, H.; Declerck, J.; Dufek, D.; Hickes, W.; Kadir, T.; et al. Assessing the Accuracy of a Deep Learning Method to Risk Stratify Indeterminate Pulmonary Nodules. Am J Respir Crit Care Med 2020, 202, 241-249. [CrossRef]
| Gene name (symbol) | Function | Healthy tissue | Expression in LC(s) | Reference(s) |
|---|---|---|---|---|
| Carcinoembryonic antigen (CEA) | Glycoprotein involved in cell adhesion and signal transduction | Low expression in colon, appendix | High expression all types in advanced stages | [25,26] |
| Osteopontin (OPN) | Cell survival and angiogenesis |
Gall bladder, placenta, brain |
High expression associated with poor prognosis |
[25,27] |
| Cytokeratin 19 fragments (CYFR A 21-1) |
Part of the cytoskeleton of epithelial cells | All epithelial cells |
NSCLC mainly SCC. High expression associated with negative prognosis rather than advanced stages |
[28] |
| Neuron specific enolase (NSE) |
Glycolytic enzyme involved in inflammatory and neurotrophic activity regulating neuronal growth, differentiation, survival and death |
Brain, adrenal, lung | Preferred for SCLC but also NSCLC and a marker of metastasis | [29] |
| Serum amyloid A (SAA) |
Secreted during acute inflammation, transports cholesterol to liver, recruits immune cells to inflammatory sites |
Housekeeping” role in normal human tissues | All types. High expression in late stages |
[30,31] |
| Gene | Probe set | Survival (mo) | P-value | Gene | Probe set | Survival (mo) | P-value | ||
| Low¶ | High¶ | Low¶ | High¶ | ||||||
| TPX2 | 210052_s_at | 96.2 | 42 | <1e-16 | TSP50 | 220126_at | 81 | 56.7 | 0.0009 |
| DNAJB11 | 223054_at | 119.87 | 52 | 8.40E-12 | CTAGE-1 | 220957_at | 79.27 | 61.3 | 0.0009 |
| MAGEA1 | 207325_x_at | 86.27 | 48.6 | 1.40E-11 | PAGE-4 | 205564_at | 76 | 60.73 | 0.001 |
| SSX2IP | 203015_s_at | 91 | 52 | 2.80E-11 | SSX3 | 211670_x_at | 78.5 | 62.2 | 0.0012 |
| DDX12 | 213378_s_at | 89 | 52 | 1.70E-10 | SYCP1 | 206740_x_at | 79.87 | 60 | 0.0018 |
| (DNAJB14) | 222850_s_at | 52 | 111 | 1.20E-09 | NXF2/CT39 | 220257_x_at | 79.87 | 62.2 | 0.0021 |
| MAGEA3 | 209942_x_at | 86.27 | 49.97 | 2.70E-09 | SSX1 | 206626_x_at | 78 | 64.1 | 0.0023 |
| DDX11/KRG2 | 208149_x_at | 88.7 | 54 | 1.10E-08 | DNAJB4 | 203811_s_at | 75.43 | 62.47 | 0.0035 |
| (GAGE3)/CT4.3 | 207663_x_at | 89 | 54.2 | 1.10E-07 | SGY-1/CT34 | 220284_at | 76 | 59 | 0.0053 |
| MAGEA12 | 210467_x_at | 84 | 52 | 2.70E-07 | MEGEA2 | 214603_at | 74 | 59.53 | 0.0058 |
| GAGE1/4/7/11 | 207086_x_at | 88 | 56 | 6.00E-07 | FATE/CT43 | 231573_at | 86.27 | 63 | 0.0085 |
| GAGE12 E | 207086_x_at | 88 | 56 | 6.00E-07 | FATE1 | 231573_at | 86.27 | 63 | 0.0085 |
| TPTE/CT44 | 220205_at | 80.03 | 59 | 1.30E-05 | GPATCH2 | 239768_x_at | 69 | 89 | 0.0094 |
| SAGE | 220793_at | 79.5 | 56.5 | 2.00E-05 | SSX2 | 216471_x_at | 76 | 63.3 | 0.01 |
| MAGEA10 | 210295_at | 86.27 | 57.33 | 2.40E-05 | SPO11/ CT35 | 222259_s_at | 76 | 62.3 | 0.0185 |
| DDX10/HRH-J8 | 204977_at | 79.54 | 57 | 8.70E-05 | (DNAJB13) | 230936_at | 70 | 90 | 0.0188 |
| NA88A/VENTXP1 | 216726_at | 81.2 | 61.2 | 0.0001 | PLU-1/ KDM5B | 211202_s_at | 63 | 77.6 | 0.019 |
| TEX15/CT42 | 221448_s_at | 79.87 | 59 | 0.0001 | LAGE1 | 215733_x_at | 73.3 | 64.1 | 0.025 |
| DNAJB2 (HSPF3) | 202500_at | 62 | 74 | 0.0002 | TAF7L | 220325_at | 76 | 63.4 | 0.0254 |
| MORC1/CT33 | 220850_at | 79.27 | 57 | 0.0003 | TDRD1/CT41.1 | 221018_s_at | 74 | 65.1 | 0.0284 |
| LDHC/CT32/ | 207022_s_at | 78 | 62.2 | 0.0004 | PAGE-1 | 206897_at | 73.2 | 65 | 0.0299 |
| DDX13 (SKIV2L) | 203727_at | 81 | 59.11 | 0.0004 | MAGE-C2 | 215932_at | 74 | 64.1 | 0.0326 |
| (LDHC)/CT32 | 207022_s_at | 78 | 62.2 | 0.0004 | (DNAJB1)/Sis1 | 200666_s_at | 72.33 | 65.57 | 0.0366 |
| MAGE-C1 | 206609_at | 79.5 | 61.2 | 0.0006 | LUZP4/CT28 | 220665_at | 73.3 | 65 | 0.0461 |
| CAGE1 | 1563787_a_at | 91 | 62 | 0.0008 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





