Submitted:
28 March 2024
Posted:
28 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Differential Expression of NRG2 in ASD Patients and Controls
2.2. Three Single-Nucleotide Polymorphisms Were Identified in NRG2
2.3. Differences in Allele and Genotype Frequencies between Super-Controls and ASD Patients
2.4. Functional Prediction of the Indel GCCCGGC Variant of NRG2
2.5. Power Analysis
3. Discussion
4. Materials and Methods
4.1. Subjuects
4.2. Preparation of the Lymphoblastoid Cell Line and cDNA
4.3. Real-Time Quantitative PCR
4.4. DNA Purification
4.5. PCR Amplification
| Amplicon | Forward | Reverse | Ta (℃) | Size (bp) |
|---|---|---|---|---|
| Exon 1.1 | TTACGCTGTTTCCGGTTTTC | TGGTCCTGCACTGACTTGAG | 60 | 458 |
| Exon 1.2 | GCTTCTCCATGCTGCTCTTC | ttcttctctccaaccccaac | 58 | 586 |
| Exon 2 | acagtggcccttactctcca | ctggttccatgggtgagtct | 63 | 372 |
| Exon 3 | agggaatctccttcccatct | gttgagtgcgagatggatca | 63 | 356 |
| Exon 4 | gagatgattcctggggccta | acttctgacccagcatctcc | 60 | 250 |
| Exon 5 | ccaagtgcctgacttggttt | tgcacccagaagctttctaa | 63 | 244 |
| Exon 6 | aaggggtctctgcaccacta | acattcttggaggcccatc | 67 | 238 |
| Exon 7-8 | gaagttcatcgttggcgagt | ggtgtgctgtgattcctgtg | 67 | 786 |
| Exon 9 | gagtggagaagggcattgag | atggagatgaggctctttgg | 67 | 468 |
| Exon 10 | gttatgcccgcgtaacagat | CCCAGATGAGCATACAGCAA | 60 | 1343 |
| SNP | Location | groups | n | Genotype | P value | Allele | P value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| A/A | A/G | G/G | A | G | ||||||
| rs889022 | IVS3+13 A>G |
ASD control |
231 1495 |
197 (85.3%) 1193 (79.8%) |
30 (13.0%) 284 (19.0%) |
4 (1.7%) 18 (1.2%) |
0.08 | 424 (91.8%) 2670 (89.3%) |
38 (8.2%) 320 (10.7%) |
0.10 |
| T/T | T/A | A/A | T | A | ||||||
| rs182642591 | IVS9+32 T>A |
ASD control |
299 1495 |
298 (99.7%) 1473 (98.5%) |
1 (0.3%) 22 (1.5%) |
0 0 |
0.11 | 597 (99.8%) 2968 (99.3%) |
1 (0.2%) 22 (0.7%) |
0.11 |
| C/C | C/indelGCCCGGC | indelGCCCGGC/indelGCCCGGC | C | GCCGGGC | ||||||
| rs1323957797 | IntelGCCCGGC | ASD | 264 | 233 (88.3%) | 31 (11.7%) | 0 | <0.0001 | 497 (94.1%) | 31 (5.9%) | <0.0001 |
| controls | 1495 | 1492 (99.8%) | 3 (0.2%) | 0 | 2987 (99.9%) | 3 (0.1%) | ||||
| location | a.a positon | variants | Consequence type | In silico analysis | Case | Control | ||
|---|---|---|---|---|---|---|---|---|
| SIFT | PolyPhen-2 | UniProt | ||||||
| Exon 10 | 661-663 | IndelGCCCGGC | Frameshift | neutral | unknown | Likely Benign | 31/248 (12.5%) | 3/1495 (0.2%) |
4.6. Direct PCR Autosequencing
4.7. Statistical Analysis
4.8. In Silico Analysis
5. Conclusions
Supplementary Materials
Authors contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- America Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-V); American Psychiatric Association Press Inc: Washington, DC, 2013. [Google Scholar]
- Chen, Y.L.; Chen, W.J.; Lin, K.C.; Shen, L.J.; Gau, S.S. Prevalence of DSM-5 mental disorders in a nationally representative sample of children in Taiwan: methodology and main findings. Epidemiol. Psychiatr. Sci. 2019, 29, e15. [Google Scholar] [CrossRef]
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. MMWR Surveill Summ 2023, 72, 1–14. [Google Scholar] [CrossRef]
- Fombonne, E. Epidemiological surveys of autism and other pervasive developmental disorders: an update. J. Autism. Dev. Disord. 2003, 33, 365–382. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Fombonne, E. Pervasive developmental disorders in preschool children: confirmation of high prevalence. Am. J. Psychiatry 2005, 162, 1133–1141. [Google Scholar] [CrossRef]
- Lichtenstein, P.; Carlstrom, E.; Rastam, M.; Gillberg, C.; Anckarsater, H. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am. J. Psychiatry 2010, 167, 1357–1363. [Google Scholar] [CrossRef]
- Gupta, A.R.; State, M.W. Recent advances in the genetics of autism. Biol. Psychiatry 2007, 61, 429–437. [Google Scholar] [CrossRef]
- Holt, R.; Monaco, A.P. Links between genetics and pathophysiology in the autism spectrum disorders. EMBO Mol. Med. 2011, 3, 438–450. [Google Scholar] [CrossRef]
- Genovese, A.; Butler, M.G. Clinical Assessment, Genetics, and Treatment Approaches in Autism Spectrum Disorder (ASD). Int. J. Mol. Sci. 2020, 21. [Google Scholar]
- Eapen, V. Genetic basis of autism: is there a way forward? Curr. Opin. Psychiatr. 2011, 24, 226–236. [Google Scholar] [CrossRef]
- State, M.W.; Levitt, P. The conundrums of understanding genetic risks for autism spectrum disorders. Nat. Neurosci. 2011, 14, 1499–1506. [Google Scholar] [CrossRef]
- Ebrahimi-Fakhari, D.; Sahin, M. Autism and the synapse: emerging mechanisms and mechanism-based therapies. Curr. Opin. Neurol. 2015, 28, 91–102. [Google Scholar] [CrossRef]
- Schena, M.; Shalon, D.; Heller, R.; Chai, A.; Brown, P.O.; Davis, R.W. Parallel human genome analysis: microarray-based expression monitoring of 1000 genes. Proc. Natl. Acad. Sci. U. S. A. 1996, 93, 10614–10619. [Google Scholar] [CrossRef]
- Nishimura, Y.; Martin, C.L.; Vazquez-Lopez, A.; Spence, S.J.; Alvarez-Retuerto, A.I.; Sigman, M.; Steindler, C.; Pellegrini, S.; Schanen, N.C.; Warren, S.T.; et al. Genome-wide expression profiling of lymphoblastoid cell lines distinguishes different forms of autism and reveals shared pathways. Hum. Mol. 2007, 16, 1682–1698. [Google Scholar] [CrossRef]
- Hu, V.W.; Nguyen, A.; Kim, K.S.; Steinberg, M.E.; Sarachana, T.; Scully, M.A.; Soldin, S.J.; Luu, T.; Lee, N.H. Gene expression profiling of lymphoblasts from autistic and nonaffected sib pairs: altered pathways in neuronal development and steroid biosynthesis. PloS One 2009, 4, e5775. [Google Scholar] [CrossRef]
- Chien, W.H.; Gau, S.S.; Chen, C.H.; Tsai, W.C.; Wu, Y.Y.; Chen, P.H.; Shang, C.Y. Increased gene expression of FOXP1 in patients with autism spectrum disorders. Mol. Autism 2013, 4, 23. [Google Scholar] [CrossRef]
- Falls, D.L. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res 2003, 284, 14–30. [Google Scholar] [CrossRef]
- Olayioye, M.A.; Neve, R.M.; Lane, H.A.; Hynes, N.E. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000, 19, 3159–3167. [Google Scholar] [CrossRef]
- Benzel, I.; Bansal, A.; Browning, B.L.; Galwey, N.W.; Maycox, P.R.; McGinnis, R.; Smart, D.; St Clair, D.; Yates, P.; Purvis, I. Interactions among genes in the ErbB-Neuregulin signalling network are associated with increased susceptibility to schizophrenia. Behav. Brain. Funct. 2007, 3, 31. [Google Scholar] [CrossRef]
- Buonanno, A.; Fischbach, G.D. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr. Opin. Neurobiol. 2001, 11, 287–296. [Google Scholar] [CrossRef]
- Buonanno, A. The neuregulin signaling pathway and schizophrenia: from genes to synapses and neural circuits. Brain Res Bull 2010, 83, 122–131. [Google Scholar] [CrossRef]
- Mei, L.; Nave, K.A. Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron 2014, 83, 27–49. [Google Scholar] [CrossRef]
- Mostaid, M.S.; Lloyd, D.; Liberg, B.; Sundram, S.; Pereira, A.; Pantelis, C.; Karl, T.; Weickert, C.S.; Everall, I.P.; Bousman, C.A. Neuregulin-1 and schizophrenia in the genome-wide association study era. Neurosci. Biobehav. Rev. 2016, 68, 387–409. [Google Scholar] [CrossRef]
- Neddens, J.; Fish, K.N.; Tricoire, L.; Vullhorst, D.; Shamir, A.; Chung, W.; Lewis, D.A.; McBain, C.J.; Buonanno, A. Conserved interneuron-specific ErbB4 expression in frontal cortex of rodents, monkeys, and humans: implications for schizophrenia. Biol. Psychiatry 2011, 70, 636–645. [Google Scholar] [CrossRef]
- Shamir, A.; Kwon, O.B.; Karavanova, I.; Vullhorst, D.; Leiva-Salcedo, E.; Janssen, M.J.; Buonanno, A. The importance of the NRG-1/ErbB4 pathway for synaptic plasticity and behaviors associated with psychiatric disorders. J Neurosci 2012, 32, 2988–2997. [Google Scholar] [CrossRef]
- Brennand, K.J.; Simone, A.; Jou, J.; Gelboin-Burkhart, C.; Tran, N.; Sangar, S.; Li, Y.; Mu, Y.; Chen, G.; Yu, D.; et al. Modelling schizophrenia using human induced pluripotent stem cells. Nat. 2011, 473, 221–225. [Google Scholar] [CrossRef]
- Ring, H.Z.; Chang, H.; Guilbot, A.; Brice, A.; LeGuern, E.; Francke, U. The human neuregulin-2 (NRG2) gene: cloning, mapping and evaluation as a candidate for the autosomal recessive form of Charcot-Marie-Tooth disease linked to 5q. Hum. Genet. 1999, 104, 326–332. [Google Scholar] [CrossRef]
- Busfield, S.J.; Michnick, D.A.; Chickering, T.W.; Revett, T.L.; Ma, J.; Woolf, E.A.; Comrack, C.A.; Dussault, B.J.; Woolf, J.; Goodearl, A.D.; et al. Characterization of a neuregulin-related gene, Don-1, that is highly expressed in restricted regions of the cerebellum and hippocampus. Mol. Cell. Biol. 1997, 17, 4007–4014. [Google Scholar] [CrossRef]
- Carraway, K.L.; Weber, J.L.; Unger, M.J.; Ledesma, J.; Yu, N.; Gassmann, M.; Lai, C. Neuregulin-2, a new ligand of ErbB3/ErbB4-receptor tyrosine kinases. Nat. 1997, 387, 512–516. [Google Scholar] [CrossRef]
- Chang, H.; Riese, D.J.; Gilbert, W.; Stern, D.F.; McMahan, U.J. Ligands for ErbB-family receptors encoded by a neuregulin-like gene. Nat. 1997, 387, 509–512. [Google Scholar] [CrossRef]
- Vullhorst, D.; Mitchell, R.M.; Keating, C.; Roychowdhury, S.; Karavanova, I.; Tao-Cheng, J.H. Buonanno A: A negative feedback loop controls NMDA receptor function in cortical interneurons via neuregulin 2/ErbB4 signalling. Nat. Commun. 2015, 6, 7222. [Google Scholar] [CrossRef]
- Yan, L.; Shamir, A.; Skirzewski, M.; Leiva-Salcedo, E.; Kwon, O.B.; Karavanova, I.; Paredes, D.; Malkesman, O.; Bailey, K.R.; Vullhorst, D.; et al. Neuregulin-2 ablation results in dopamine dysregulation and severe behavioral phenotypes relevant to psychiatric disorders. Mol. Psychiatry 2018, 23, 1233–1243. [Google Scholar] [CrossRef]
- Konte, B.; Leicht, G.; Giegling, I.; Pogarell, O.; Karch, S.; Hartmann, A.M.; Friedl, M.; Hegerl, U.; Rujescu, D.; Mulert, C. A genome-wide association study of early gamma-band response in a schizophrenia case-control sample. World J. Biol. Psychiatry 2018, 19, 602–609. [Google Scholar] [CrossRef]
- Seshadri, S.; Kamiya, A.; Yokota, Y.; Prikulis, I.; Kano, S.; Hayashi-Takagi, A.; Stanco, A.; Eom, T.Y.; Rao, S.; Ishizuka, K.; et al. Disrupted-in-Schizophrenia-1 expression is regulated by beta-site amyloid precursor protein cleaving enzyme-1-neuregulin cascade. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 5622–5627. [Google Scholar] [CrossRef]
- Mostaid, M.S.; Lee, T.T.; Chana, G.; Sundram, S.; Shannon Weickert, C.; Pantelis, C.; Everall, I.; Bousman, C. Peripheral Transcription of NRG-ErbB Pathway Genes Are Upregulated in Treatment-Resistant Schizophrenia. Front. Psychiatry 2017, 8, 225. [Google Scholar] [CrossRef]
- Diaz-Moran, S.; Palencia, M.; Mont-Cardona, C.; Canete, T.; Blazquez, G.; Martinez-Membrives, E.; Lopez-Aumatell, R.; Sabariego, M.; Donaire, R.; Moron, I.; et al. Gene expression in hippocampus as a function of differential trait anxiety levels in genetically heterogeneous NIH-HS rats. Behav. Brain Res. 2013, 257, 129–139. [Google Scholar] [CrossRef]
- Ma, D.Q.; Cuccaro, M.L.; Jaworski, J.M.; Haynes, C.S.; Stephan, D.A.; Parod, J.; Abramson, R.K.; Wright, H.H.; Gilbert, J.R.; Haines, J.L.; et al. Dissecting the locus heterogeneity of autism: significant linkage to chromosome 12q14. Mol. Psychiatry 2007, 12, 376–384. [Google Scholar] [CrossRef]
- Philippi, A.; Roschmann, E.; Tores, F.; Lindenbaum, P.; Benajou, A.; Germain-Leclerc, L.; Marcaillou, C.; Fontaine, K.; Vanpeene, M.; Roy, S.; et al. Haplotypes in the gene encoding protein kinase c-beta (PRKCB1) on chromosome 16 are associated with autism. Mol. psychiatry 2005, 10, 950–960. [Google Scholar] [CrossRef]
- Neves-Pereira, M.; Muller, B.; Massie, D.; Williams, J.H.; O'Brien, P.C.; Hughes, A.; Shen, S.B.; Clair, D.S.; Miedzybrodzka, Z. Deregulation of EIF4E: a novel mechanism for autism. J. Med. Genet. 2009, 46, 759–765. [Google Scholar] [CrossRef]
- Vincent, J.B.; Noor, A.; Windpassinger, C.; Gianakopoulos, P.J.; Schwarzbraun, T.; Alfred, S.E.; Stachowiak, B.; Scherer, S.W.; Roberts, W.; Wagner, K.; et al. Characterization of a de novo translocation t(5;18)(q33.1;q12.1) in an autistic boy identifies a breakpoint close to SH3TC2, ADRB2, and HTR4 on 5q, and within the desmocollin gene cluster on 18q. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009, 150B, 817–826. [Google Scholar] [CrossRef]
- Della Monica, M.; Lonardo, F.; Faravelli, F.; Pierluigi, M.; Luquetti, D.V.; De Gregori, M.; Zuffardi, O.; Scarano, G. A case of autism with an interstitial 1q deletion (1q23.3-24.2) and a de novo translocation of chromosomes 1q and 5q. Am. J. Med. Genet. A. 2007, 143A, 2733–2737. [Google Scholar] [CrossRef]
- Philippi, A.; Tores, F.; Carayol, J.; Rousseau, F.; Letexier, M.; Roschmann, E.; Lindenbaum, P.; Benajjou, A.; Fontaine, K.; Vazart, C.; et al. Association of autism with polymorphisms in the paired-like homeodomain transcription factor 1 (PITX1) on chromosome 5q31: a candidate gene analysis. BMC Med. Genet. 2007, 8, 74. [Google Scholar] [CrossRef]
- Schwab, S.G.; Eckstein, G.N.; Hallmayer, J.; Lerer, B.; Albus, M.; Borrmann, M.; Lichtermann, D.; Ertl, M.A.; Maier, W.; Wildenauer, D.B. Evidence suggestive of a locus on chromosome 5q31 contributing to susceptibility for schizophrenia in German and Israeli families by multipoint affected sib-pair linkage analysis. Mol. Psychiatry 1997, 2, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Sklar, P.; Pato, M.T.; Kirby, A.; Petryshen, T.L.; Medeiros, H.; Carvalho, C.; Macedo, A.; Dourado, A.; Coelho, I.; Valente, J.; et al. Genome-wide scan in Portuguese Island families identifies 5q31-5q35 as a susceptibility locus for schizophrenia and psychosis. Mol. Psychiatry 2004, 9, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Arcos-Burgos, M.; Castellanos, F.X.; Pineda, D.; Lopera, F.; Palacio, J.D.; Palacio, L.G.; Rapoport, J.L.; Berg, K.; Bailey-Wilson, J.E.; Muenke, M. Attention-deficit/hyperactivity disorder in a population isolate: linkage to loci at 4q13.2, 5q33.3, 11q22, and 17p11. Am. J. Hum. Genet. 2004, 75, 998–1014. [Google Scholar] [CrossRef] [PubMed]
- Czarnek, M.; Bereta, J. Proteolytic Processing of Neuregulin 2. Mol. Neurobiol. 2020, 57, 1799–1813. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Rutter, M.; Le Couteur, A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994, 24, 659–685. [Google Scholar] [CrossRef]
- Gau, S.S.; Lee, C.M.; Lai, M.C.; Chiu, Y.N.; Huang, Y.F.; Kao, J.D.; Wu, Y.Y. Psychometric properties of the Chinese version of the social communication questionnaire. Res. Autism Spectr. Disord. 2011, 5, 809–818. [Google Scholar] [CrossRef]
- Chang, J.C.; Lai, M.C.; Chien, Y.L.; Cheng, C.Y.; Wu, Y.Y.; Gau, S.S. Psychometric properties of the Mandarin version of the Autism Diagnostic Observation Schedule-Generic. J. Formos. Med. Assoc. 2023, 122, 574–583. [Google Scholar] [CrossRef]
- Pan, W.H.; Fann, C.S.; Wu, J.Y.; Hung, Y.T.; Ho, M.S.; Tai, T.H.; Chen, Y.J.; Liao, C.J.; Yang, M.L.; Cheng, A.T.; et al. Han Chinese cell and genome bank in Taiwan: purpose, design and ethical considerations. Hum. Hered. 2006, 61, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Chen, M.L.; Tsai, Y.L.; Tsai, M.T.; Chen, C.H. Elevated adrenomedullin mRNA in lymphoblastoid cells from schizophrenic patients. Neuroreport. 2004, 15, 1443–1446. [Google Scholar] [CrossRef]
- Shi, Y.Y.; He, L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res 2005, 15, 97–98. [Google Scholar] [CrossRef]
- Buchner, A. F.F.; Erdfelder, E. G-Power: A priori, post hoc and Compromise Power Analyses for the Macintosh, Version 2.1.1; University of Trier: Trier, 1996. [Google Scholar]
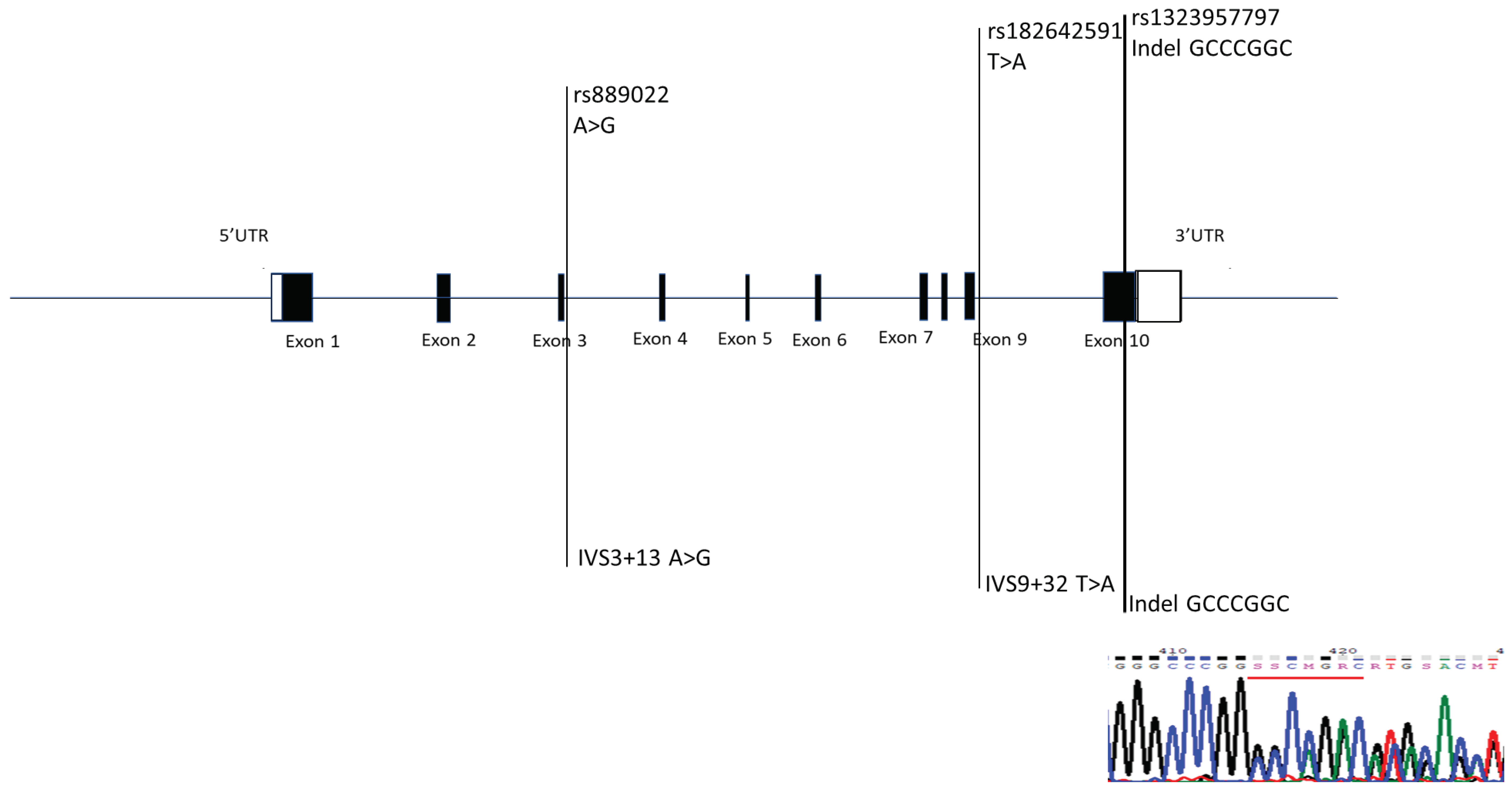
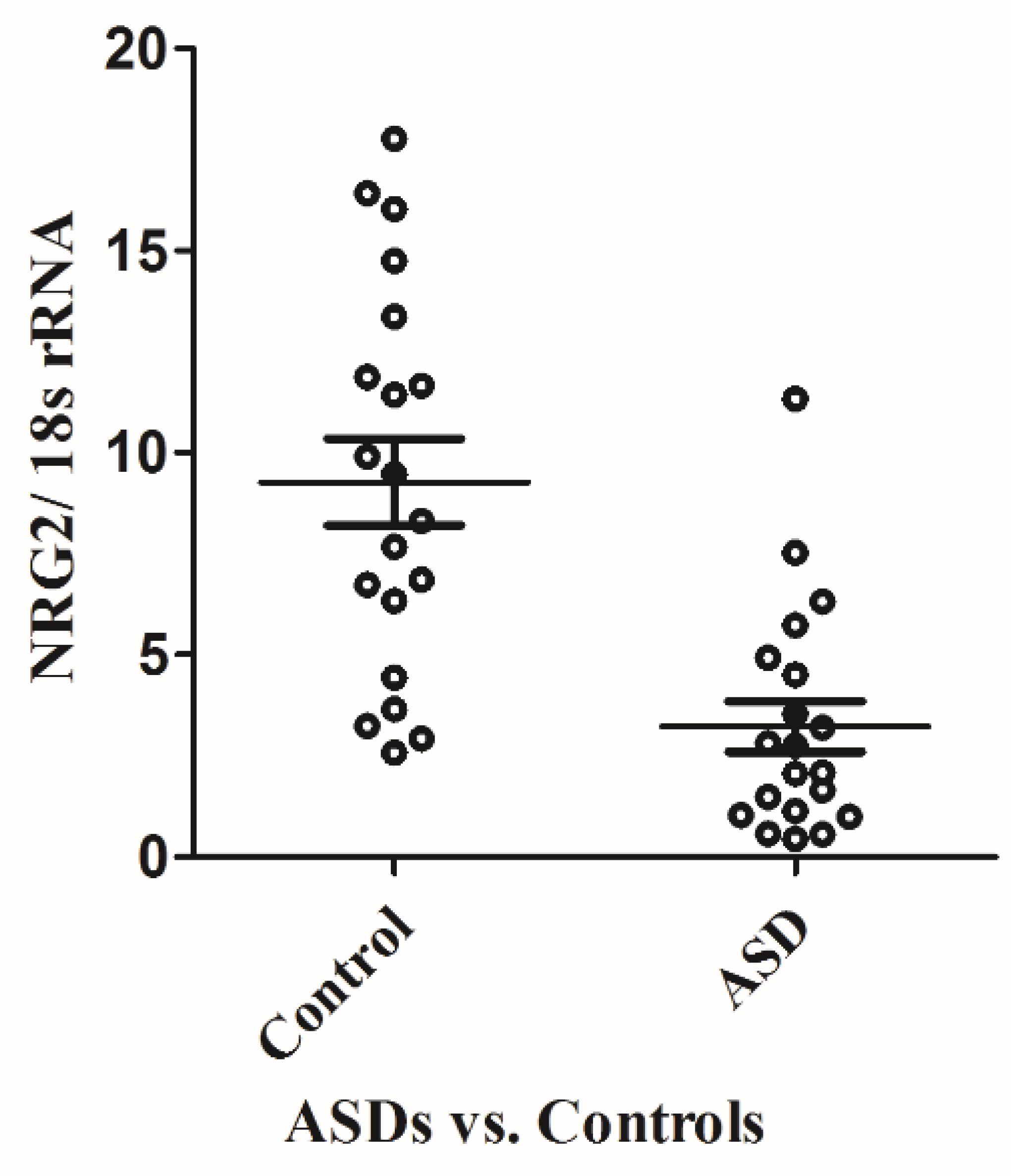
| Gene | Forward primer | Backward primer | Ta |
|---|---|---|---|
| NRG2 | CCACAGACCATGTCATCAGG | CCGACTGGGAGTCAGAAGTC | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





