1. Introduction
Mycobacterium tuberculosis (Mtb) and the human immunodeficiency virus (HIV) are syndemic interaction pathogens [
1,
2,
3]. They synergize an accelerated progression to tuberculosis (TB) and to acquired immune deficiency syndrome (AIDS) during coinfection [
2,
4,
5]. Both are responsible for a paradoxical effect observed in coinfected patients after the initiation of antiretroviral therapy (ART), referred to as immune reconstitution inflammatory syndrome (IRIS), a severe local and systemic inflammatory response [
6]. Approximately 13 million people are estimated to be coinfected with both pathogens, accounting for 250,000 deaths in 2022, with about 1.3 million new infections by HIV (
https://www.unaids.org/en) and 1.4 million with Mtb [
5]. While antibiotic therapy to treat TB exists, as well as an established ART for controlling HIV chronic infection, the rising resistance to both treatments and drug-drug interactions are posing serious concerns for the effective control of pathogens and instructing an urgent need for new therapies [
7,
8,
9,
10,
11,
12,
13,
14].
TB is a leading cause of death among HIV-infected people (
https://www.unaids.org/en/topic/tuberculosis). The interactions between HIV and Mtb that contribute to tuberculosis have been more extensively studied than those that Mtb uses to enhance virus replication and persistence [
3,
7,
15,
16,
17,
18].
Deciphering the pathways of these interactions may contribute to controlling both pathogens and identifying new targets for the effective development of new effective therapeutics, particularly during coinfection.
Our group has investigated the role of lysosomal cathepsins and their inhibitors, cystatins, during Mtb infection [
19,
20,
21] and during HIV coinfection [
22,
23]. The results revealed that Mtb can block cathepsin proteolytic activity, which contributes to its intracellular survival in macrophages (Mϕ) and poor activation of T lymphocytes. Concerning the natural inhibitors, there was a significant increase in gene expression for cystatins C, A, and SN during the early stages of infection, which was evident and common in both Mϕ mono-infection and coinfection with Mtb and HIV [
22]. Cystatin F (CstF) showed the highest upregulation among the inhibitors, but it was limited to Mtb mono-infection [
22]. Indeed, we have developed various strategies to overcome the blockade induced by Mtb, including the regulation of gene expression with microRNAs [
24] or using RNA silencing for cystatins [
21,
22,
25]. In addition, we have demonstrated that saquinavir, an HIV protease inhibitor used in ART, can impact cathepsin enzymatic activity, and overcome the Mtb-induced blockade. This finding suggests that saquinavir could be repurposed to control the bacterial infection including in multidrug-resistant Mtb [
26,
27].
Mtb infects Mϕ, where it establishes intracellular niches [
28,
29,
30]. Appropriate immune responses for their intracellular clearance require helper T lymphocytes, particularly TH1, as well as cytotoxic cells, mainly CD8+ T cells (CTLs) and conventional or unconventional natural killer cells (NK, NKT) [
31,
32]. Infected Mϕ and lymphocytes come into close contact in one structure, the granuloma, which is a hallmark of TB, or in nearby tissues during the establishment of the infection. Most of the effects of HIV-1 and Mtb coinfection are based on interferon gamma (IFN 𝛾) mediated Mϕ activation, leading to a more microbicidal state [
33]. HIV-1 extensively infects CD4+ T cells and establishes intracellular reservoir sanctuaries in Mϕ [
34]. Although simultaneous coinfection of Mϕ with both pathogens is possible
in vitro, it has not been demonstrated
in vivo [
2,
22]. Cytotoxic NK, NKT, and CTLs induce the death of infected cells, constituting a pivotal viral control response. However, an ineffective viral clearance occurs during HIV infection [
35,
36,
37,
38].
One of the most frequent manifestations of TB during HIV coinfection is pleurisy [
39]. Both the pleural milieu and the granuloma structure provide the appropriate contact between Mtb-infected cells and HIV-infected lymphocytes in a particular environment of cytokines and other factors that, all together, help viral replication and spread [
40,
41]. Surprisingly, CstF levels were found to be significantly increased in the pleural fluids of TB patients compared to other inflammatory conditions [
42].
The aim of this study was to decipher the role of CstF during coinfection. To achieve this, we manipulated its gene expression by siRNA in Mϕ infected with Mtb, which are known to be the highest source of secreted protease inhibitors. We also evaluated the trans effects on lymphocytes infected with HIV. Overall, the results of this study contribute to revealing a mechanism of pathogen evasion to immune responses and indicates future directions for controlling both syndemic pathogens through CstF manipulation.
2. Materials and Methods
2.1. Cell Isolation and Culture Conditions
Primary human monocyte-derived Mϕ were isolated and then differentiated from buffy coats of healthy human donors, which were provided by the National Blood Institute (Instituto Português do Sangue e da Transplantação, I.P., Lisbon, Portugal) following a previously described protocol [
22]. Autologous lymphocytes were obtained from the peripheral blood mononuclear cells (PBMCs) fractions by lysing red blood cells. The lymphocytes were then stimulated with 3 μg/mL of Phytohemagglutinin-L
(PHA-L) (ThermoFisher) for three days. It was further cultured in a 75 cm
2 flask at 2 × 10
6 cells per mL in a Roswell Park Memorial Institute (RPMI) medium (RPMI-1640) (Hyclone, GE Healthcare) supplemented with 15% (v/v) Fetal Bovine Serum (FBS) (Hyclone, GE Healthcare), 2 mM L-glutamine (Gibco), and 20 UI/mL of human recombinant interleukin-2 (BioLegend, San Diego, CA, USA).
2.2. Bacterial Cultures and HIV Isolates
M. tuberculosis H37Rv (ATCC 27294) (American Type Culture Collection) (Mtb) was grown in Middlebrook’s 7H9 medium supplemented with 10% Oleic acid-Albumin-Dextrose-Catalase enrichment (OADC) (Difco), 0.02% glycerol, and 0.05% tyloxapol at 37 °C. The primary HIV-1
UCFL1032 isolate was obtained by coculturing PBMCs isolated from the infected patient with PBMCs from uninfected individuals as described [
43]. After isolation, viral stocks were established in PBMCs from low-passaged supernatants of original cultures and stored at −80 °C until further use. All experimental procedures using Mtb and HIV were performed in the biosafety level 3 laboratory at the Faculty of Pharmacy of the University of Lisbon, maintaining the national and European containment level 3 laboratory management and biosecurity standards based on applicable EU directives.
2.3. Macrophage Infection
Before infection, Mtb was cultivated at 37 °C, 5% CO2, until the exponential growth phase was reached. On the day of infection, the bacterial suspensions were centrifuged and washed in phosphate-buffered saline (PBS) and resuspended in RPMI culture medium without antibiotics. Clumps of bacteria in the suspension were disrupted by ultrasonic bath treatment for 5 min and removed by centrifugation at a low speed of 500× g for 1 min. The obtained single-cell suspension was verified by fluorescence microscopy and quantified by measuring optical density at 600 nm. The infection was performed with a multiplicity of infection (MOI) of 1 bacterium per Mϕ for 3 h at 37 °C, 5 % CO2. Following this incubation period, cells were washed with PBS to remove free bacteria and added with fresh complete medium.
2.4. Transfection
Mϕ were transfected 72 h before infection to achieve maximum RNA silencing. Transfection with anti-CstF siRNA or with scramble control siRNA was performed with ScreenFect A (ScreenFect GmbH, Eggenstein-Leopoldshafen, Germany) transfection reagent according to the manufacturer’s protocol as previously described [
25]. Mϕ were incubated for 72 h with the transfection reagent and SMARTpool ON-TARGETplus Human CST7 siRNA (Dharmacon, USA; target sequences: AGUGAAAGGCCUGAAAUAU, GAAAUUGGCAGAACUACCU, GGAUGACUGUGACUUCCAA, and CAAGGGCCCUAGUUCAGAU) or the respective siRNA non-targeting (scramble) control (Dharmacon, USA; target sequences: UGGUUUACAUGUCGACUAA, UGGUUUACAUGUUGUGUGA, UGGUUUACAUGUUUUCUGA, and UGGUUUACAUGUUUUCCUA) in the medium without antibiotic.
2.5. Enzymatic Activities of Cathepsin C and Granzyme B
After 48 h of infection, Mϕ cultures in 96-well plates were lysed with chilled lysis buffer 25 mM 2-(N-morpholino)ethanesufonic acid (MES) (MP Biomedicals), 100 mM NaCl, 5 mM cysteine, pH 6 for cathepsin C and 50 mM Tris-HCl, 100 mM NaCl, pH 7.4 for granzyme B. Cells were centrifuged at 16,000× g for 20 min at 4 °C to recover the supernatant and further added with reaction buffer for 15 min at room temperature for cathepsin C or for 30 min at 37 °C for granzyme B. The specific fluorogenic substrates: 70 μM H-Gly-Phe-7-amino- 4-methylcoumarin (AMC) (Bachem) for cathepsin C, 50 μM acetyl-Ile-Glu-Pro-Asp-AMC for granzyme B (Bachem) were then added and formation of fluorescent degradation products was measured continuously with excitation at 370 nm and emission at 460 nm in a Tecan M200. The activity of the control sample was set to 100% and activities for the other samples were adjusted accordingly.
2.6. Lymphocytes Infection with HIV and Co-Culture with Macrophages Infected with Mtb
Autologous lymphocytes were obtained from the PBMC fractions, stimulated, and further cultured according to the protocol described above. On the day of infection, lymphocytes were infected with 1000 TCID 50/mL of HIV-1UCFL1032 or left uninfected as controls. Briefly, viruses were added and incubated for 3 h in the presence of 3 μg/mL of polybrene (Sigma–Aldrich, MO, USA). Cells were then washed with PBS to remove any unadsorbed virus particles and cultured in an appropriate medium (500 μL/well). Mϕ were allowed to internalize Mtb for 3 h. After this chase period they were washed with PBS to remove extracellular bacteria and cocultivated with the HIV-infected lymphocytes at a ratio of 1:2. Culture supernatants were collected at days 3 and 9 to recover virus particles and quantified by reverse transcriptase activity.
2.7. HIV Quantification
Supernatants collected from cocultures with lymphocytes infected with HIV, as described above, were used for viral replication quantification. This was assessed by using a colorimetric enzyme immunoassay (Roche, Merck KGaA, Darmstadt, Germany) for the quantitative determination of retroviral reverse transcriptase activity by incorporation of digoxigenin- and biotin-labeled dUTP into DNA. Absorbance was measured by Tecan M200 spectrofluorometer at 405 and 490 nm.
2.8. Cell death and Viability Assays Using Flow Cytometry
For assessment of apoptotic or necrotic cells the Apotracker Green and Zombie Red (Biolegend, San Diego, CA, USA) dyes were used, respectively. Mϕ were allowed to internalize Mtb and lymphocytes HIV particles for 3 h. After the internalization step, extracellular bacteria were removed by washing Mϕ cultures with PBS, and extracellular viruses were eliminated as described previously. Monocultures or co-cultures were further incubated for additional timing until 12 h, 24 h, 48 h, and 72 h post-infection (p.i) with the recommended cell death kit experiments reagents. The corresponding non-infected cells treated or not with transfection reagents and siRNAs were evaluated in parallel using the same kit. After those timings, cultured cells were detached using 5 mM EDTA. Human peripheral blood lymphocytes were stained with Alexa Fluor® 700 anti-human CD3 antibody (Biolegend, San Diego, CA, USA). Cells were fixed in 4% paraformaldehyde for one hour and then analyzed in a Cytek® Aurora flow cytometer (Cytek® Biosciences, Fremont, CA, USA). Data analysis was performed in FCS Express 7 (De Novo Software, Pasadena, CA, USA).
2.9. Statistical Analysis
Statistical analysis was performed in GraphPad Prism 9. Multiple group comparisons were conducted using one-way ANOVA followed by a Holm–Sidak post hoc test. Two group comparisons were made using Student’s t-test. Differences were considered statistically significant when the calculated adjusted p-value was equal to or below the alpha level of 0.05 (p ≤ 0.05)
3. Results
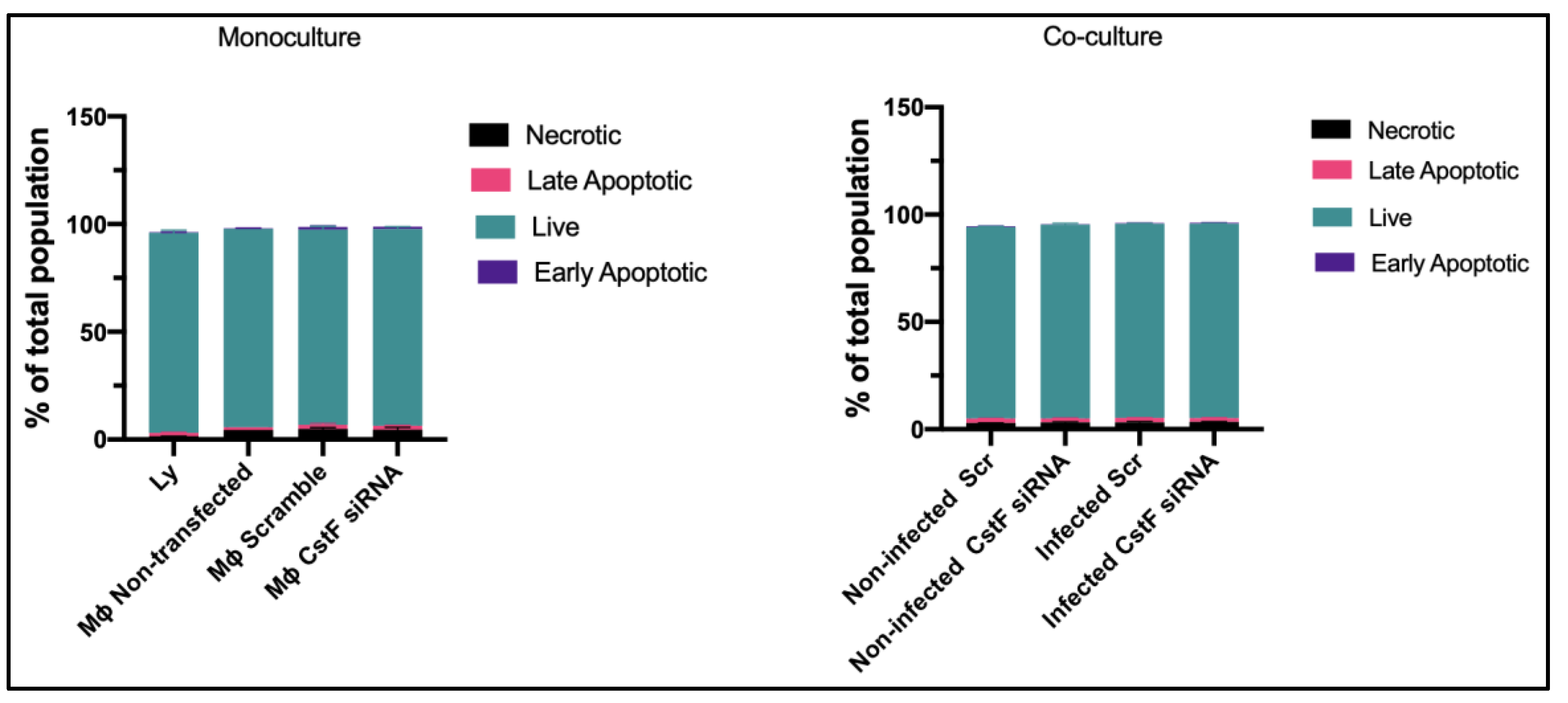
3.1. Experimental Conditions for Transfection and Infection Produce Comparable Low Levels of Cell Death
We established the experimental conditions that produce comparable and low levels of cell death between infected and non-infected cells. Mtb-infected Mϕ and HIV-infectedlymphocytes were analyzed for their effect on cell viability after transfection and 12 h of infection. Infected cells in monocultures were also compared to those in co-cultures. Cell death was evaluated by flow cytometry using markers for apoptosis and necrosis, namely Apotracker Green and Zombie Red, respectively. Lymphocytes were distinguished from monocytes using CD3-specific Alexa Fluor
® 700 antibodies.
Figure 1 shows that there were no differences observed in Mϕ monoculture conditions when comparing CstF-silenced phagocytes to cells transfected with scramble or non-transfected. Notably, during co-culture, non-infected (scramble) or CstF siRNA-treated cells displayed similar viability to the respective infected conditions (
Figure 1, co-culture). Indeed, monocultures of HIV-infected lymphocytes displayed high viability by the end of 12 h p.i. Therefore, it can be concluded that our experimental conditions produce cells with similar viability and low impact at the early time points of the coinfection, resulting in low interference for the next co-culture assays.
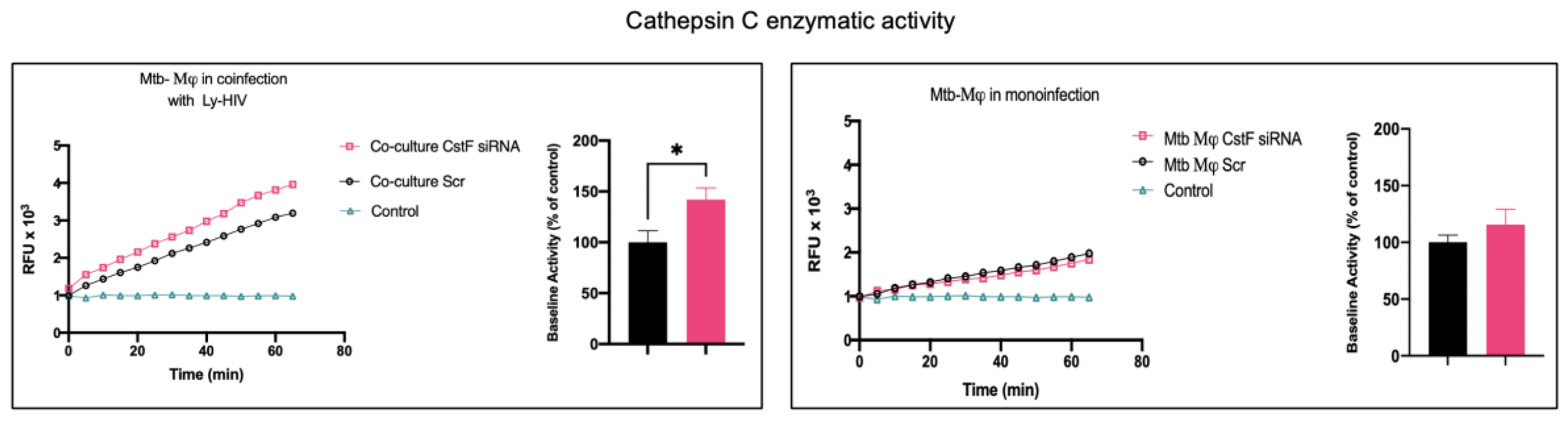
3.2. Decreased CstF Levels from Mtb-Infected Macrophages are Correlated with Increased Enzymatic Activity of Cathepsin C in Lymphocytes During Coinfection
CstF can be internalized from the extracellular milieu into the endocytic pathway of cytotoxic immune cells, where the inhibition of the pro-granzyme convertase cathepsin C occurs [
44,
45]. As we previously observed high expression of CstF during Mϕ infection with Mtb [
22], we depleted CstF using siRNA on infected phagocytes and evaluated its effects on cathepsin C during co-culture with lymphocytes. The enzymatic activity was measured by continuously monitoring the formation of fluorescent degradation products using a specific fluorogenic substrate for cathepsin C. A cathepsin-specific inhibitor was used as negative control.
Figure 2 shows an increase in enzymatic activity of cathepsin C when co-cultivated Mϕ were treated with CstF siRNA, compared to the respective scramble control. No detectable effects on cathepsin C activity in response to CstF silencing were observed for Mϕ monocultures infected with Mtb (
Figure 2). Overall, the results indicate that countering CstF overexpression by depleting CstF in Mtb-infected Mϕ impacts the enzymatic activity of cathepsin C in lymphocytes, since these effects were only observed in the co-cultures [
46,
47].
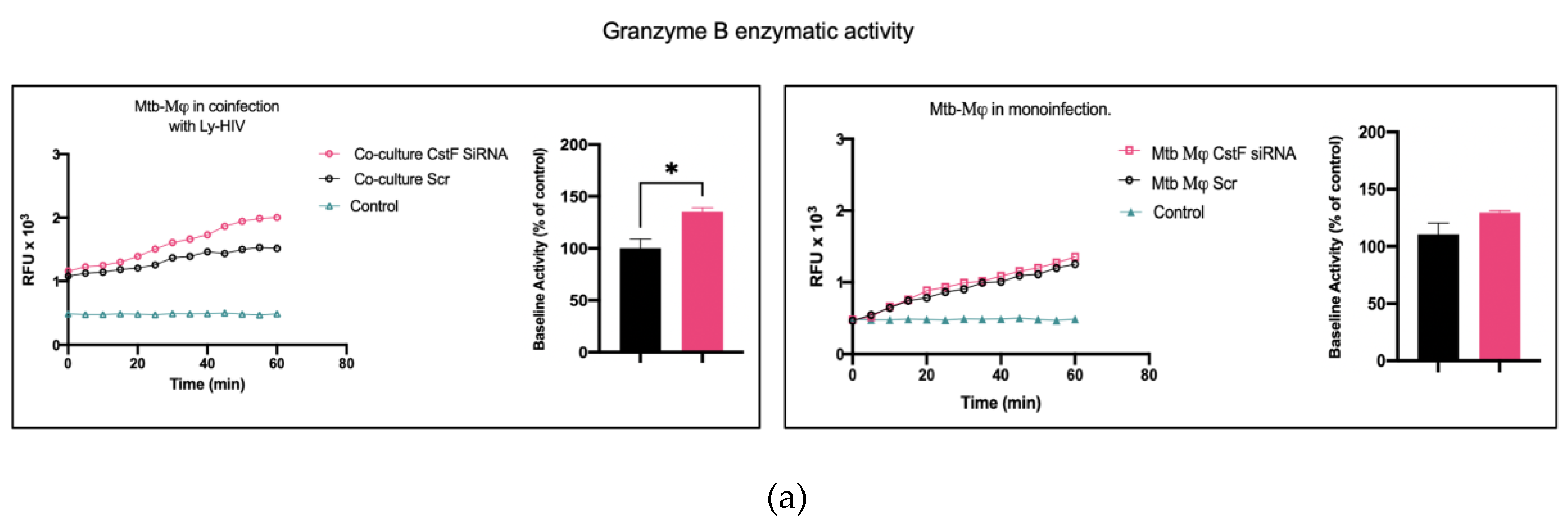
3.3. CstF Depletion is Correlated with Increased Cathepsin C-Granzyme B Driven Cytotoxic Effects
It was next investigated whether higher levels of cathepsin C-induced proteolysis lead to increased granzyme B activity in cytotoxic lymphocytes. As expected, granzyme B activity was significantly higher in CstF silenced conditions during co-culture compared to the scramble control (
Figure 3a). However, no effects were observed during monoculture of Mtb-infected Mϕ since the depletion of CstF did not impact the granzyme B activity of those cells, compared to the scramble control (
Figure 3a) [
48,
49,
50].
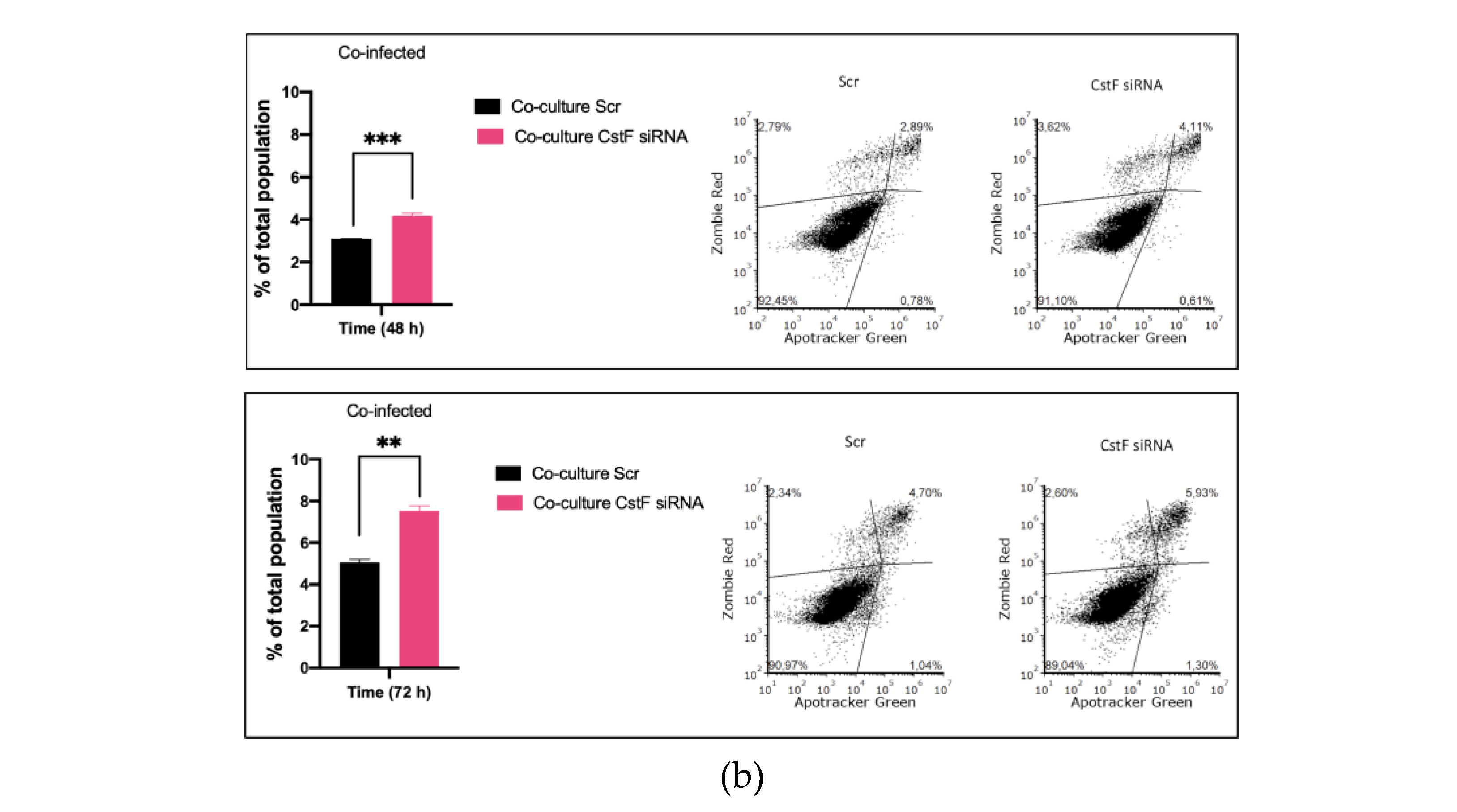
Next, the impact of CstF manipulation on granzyme B-driven apoptosis was evaluated.
Figure 3b shows that apoptosis was more prominent in CstF-silenced co-cultures of Mtb-infected Mϕ and HIV-infected lymphocytes when compared to the scramble control. By the end of 48 h, the effect was already significantly high and it remained so until at least 72 h p.i. There were no differences in cell death observed between non-infected or infected CstF-silenced Mϕ in co-culture conditions when compared with the correspondent scramble by 12 h p.i (
Figure 1). Overall, the results indicate a significant impact of CstF depletion from Mtb-infected Mϕ on cathepsin C/Granzyme B-driven apoptosis of lymphocytes infected with HIV over time, at least until 3 days p.i.
3.4. CstF Depletion Improves Cathepsin C/Granzyme B-Driven Reduction of Viral Replication During Mtb-HIV Coinfection
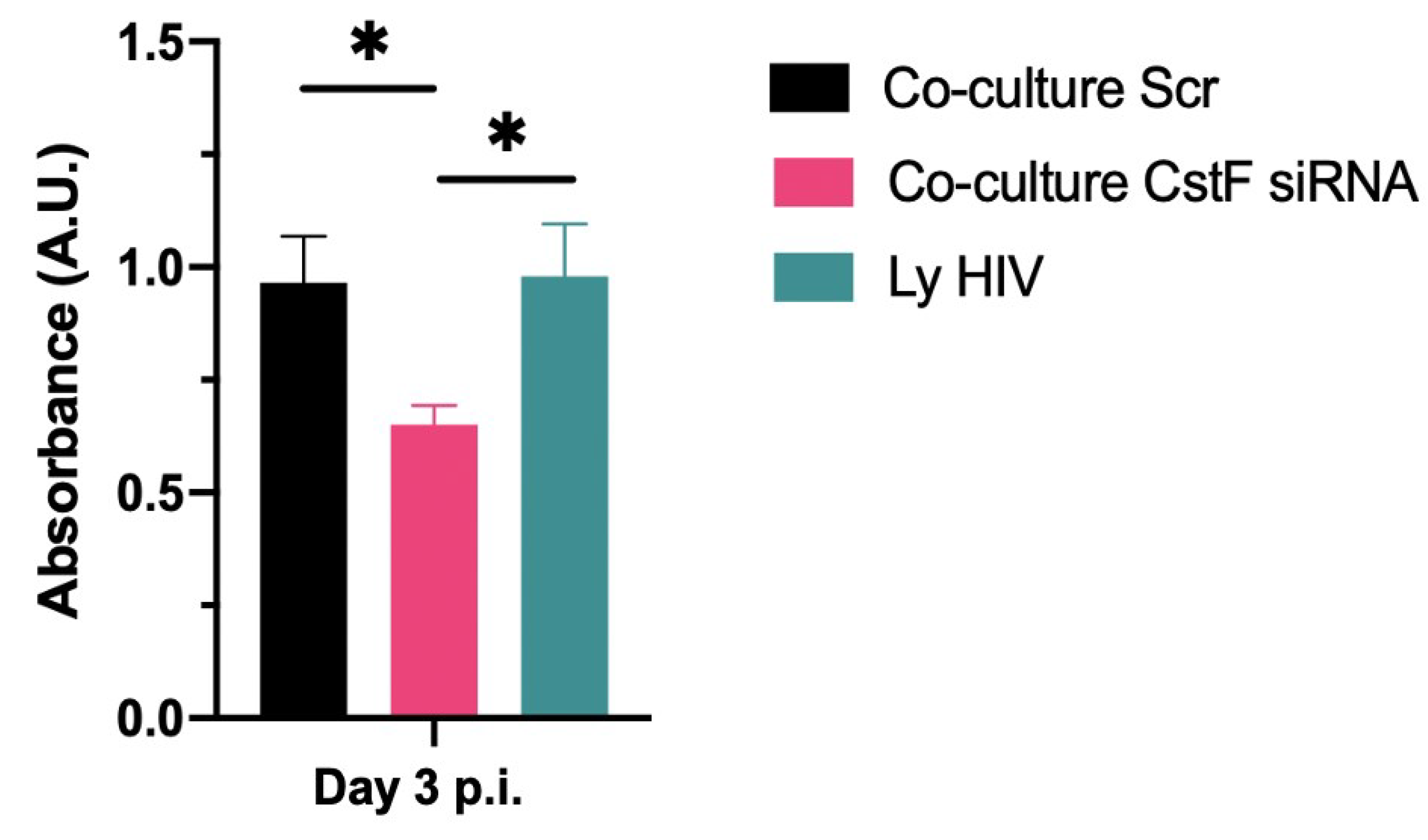
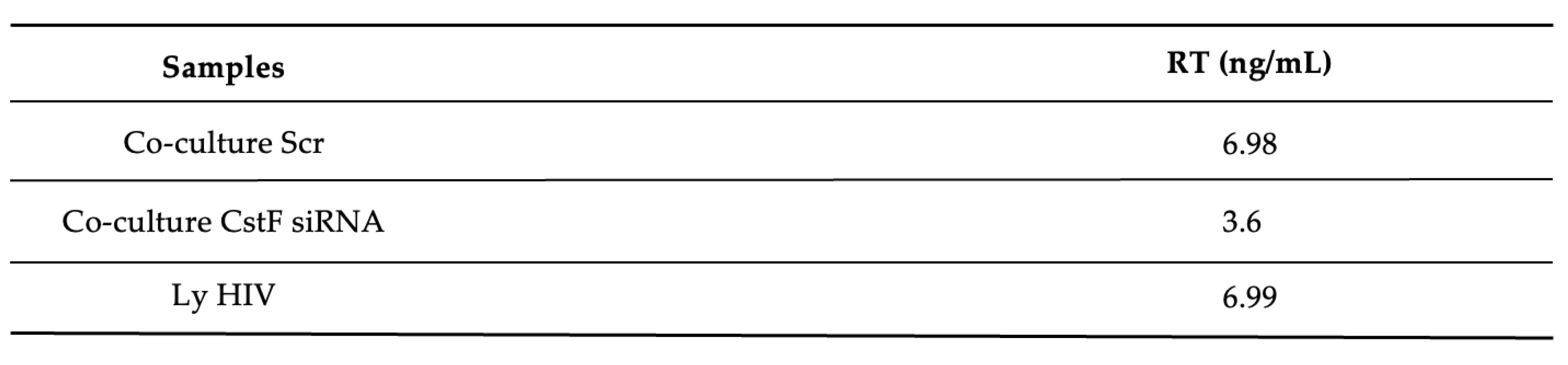
We next aimed to determine whether the increased cytotoxic effects of granzyme B could decrease HIV replication in Mtb-HIV co-cultures. As shown in
Figure 4 and
Table 1, there was a significant decrease in viral particles production at the end of 72 h p.i., as assessed by reverse transcriptase analysis of culture supernatants.
Overall, the results demonstrate that the depletion of CstF in Mtb-infected cells correlates with increased activity of cathepsin C and granzyme B, resulting in higher cytotoxicity of lymphocytes towards HIV-infected cells and leading to a reduction in viral replication.
4. Discussion
Previous work from the group revealed that CstF, a protease inhibitor of lysosomal cathepsins, plays a significant role in Mtb infection, contributing for the intracellular survival of Mtb in human Mϕ [
25]. Depletion of CstF resulted in the control of the infection, even in clinical strains of Mtb that are resistant to first-line antibiotics used to treat TB [
25]. Moreover, a significant increase in the expression of the protease inhibitor during Mtb infection was previously demonstrated, which impacts the proteolytic activity of lysosomal proteases [
22]. In contrast, HIV infection did not contribute to an increase in CstF gene expression. These results are consistent with previous studies showing a general decrease in CstF gene expression in CD4+ T lymphocytes infected with HIV, as well as in genes related to cytotoxicity [
51] .
TB remains a significant public health concern, with one of the contributing factors being the synergistic effect of the coinfection with HIV. Although HIV can also infect Mϕ, CD4+ T lymphocytes are the primary target cells. While our recent work has demonstrated
in vitro Mϕ infection with both pathogens [
22], this has not yet been observed
in vivo [
2]. Here our experiments were designed to replicate the conditions found
in vivo in the lungs of patients simultaneously infected with Mtb and HIV. Therefore, Mϕ were infected with Mtb and cocultured with autologous lymphocytes infected with HIV. To achieve this, lymphocytes were isolated from the blood of healthy donors, including CD4+ and CD8+ naive T lymphocytes, conventional and unconventional NK cells. Since the Portuguese population has been vaccinated for BCG until the last 5 years, it is expected that PBMCs from healthy donors also contain effector and memory T lymphocytes that recognize autologous Mϕ infected with Mtb.
CstF was shown to be secreted from immune cell producers into the extracellular milieu and internalized by bystander cells [
44,
52,
53,
54]. A key target of the protease inhibitor is cathepsin C a major progranzyme convertase [
55]. Likewise, internalization of CstF was observed to have an inhibitory effect on cytotoxic cells, both in NK [
44] and in CD8+ cytotoxic T lymphocytes (CTL) [
45], leading to anergy split, a condition where these cells lose the ability to secrete granzyme- and perforin [
55]. Additionally, human NK cells displayed a 30-fold increase in CstF compared to CTL [
56]. However, it was not demonstrated whether this difference resulted from accelerated synthesis and/or increased internalization of secreted CstF by closely interacting immune cells [
55]. In this study, we depleted CstF from Mtb-infected Mϕ by siRNA, as they are the primary sources of the protease inhibitor in the coculture conditions. In previous work we provided evidence of successful CstF silencing at the gene expression and protein synthesis levels [
25]. The present results indicate that the depletion cathepsin C activity in cocultured lymphocytes has an impact on granzyme activation. However, no effects of CstF depletion were observed in an autocrine way in monocultures of Mϕ either on cathepsin C [
46,
47] or on granzyme B [
48,
49,
50] as these phagocytes were shown, in some inflammatory conditions, to display a cytotoxic effect. We conclude that the depletion of CstF from infected phagocytes has an impact on the cytotoxic activity of lymphocytes during coculture conditions.
HIV infection has the ability to evade the early immune response, resulting in ineffective viral clearance. Both NK cells and HIV-specific CTLs are crucial for the outcome of infection and arise shortly after infection [
1,
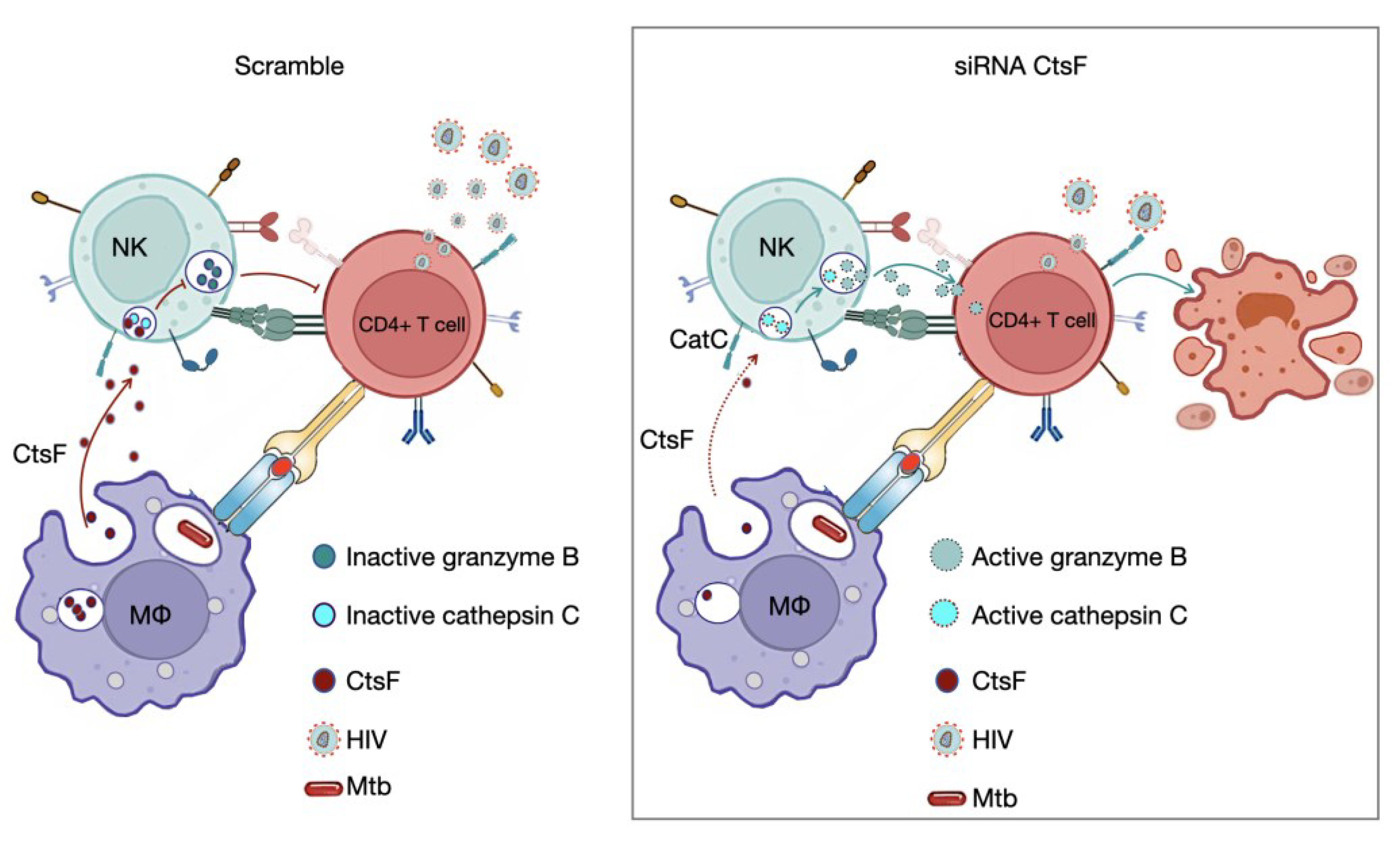
38]. Since the lymphocytes used in this study were from healthy donors, not infected with HIV, it is less likely that CD8+ lymphocytes are already primed to CTLs in the coculture environment. It is probable that NK cells lacking conventional receptors for antigens, such as surface Ig or TCR, are responsible for the paracrine internalization of CstF during scramble control conditions (
Figure 5). Additionally, high levels of NK recruitment have been observed in tuberculous pleural effusions and in early innate granulomas [
57]. Pleurisy is a common manifestation of TB, often observed during the primo-infection [
41]. It is also frequently observed in HIV-coinfected patients, where high levels of virus particles are present at the sites of Mtb infection [
40,
41]. Coincidently, higher levels of CstF were found in pleural effusion of TB patients than in other inflammatory conditions [
42]. CD4+ effector T lymphocytes responding to Mtb and infected with HIV are expected to establish an immune synapse with Mϕ, and NK cells, through a variety of ligands and receptors, can also be connected to HIV-infected cells. Altogether, our results demonstrate a clear impact of CstF depletion on cytotoxic effects directed at lymphocytes, with consequences on HIV replication and viral loads (
Figure 5)
The results also suggest the existence of an evasion mechanism that enables early HIV replication during infection through the axis CstF/ cathepsin C/ granzyme B, primarily mediated by NK cells. Overall, the results presented here indicate a mechanism of Mtb/HIV evasion of the cytotoxic-mediated pathogen killing, which contributes to this syndemic interaction. Ultimately, this knowledge can be crucial for developing new therapeutic approaches to control both pathogens based on the manipulation of CstF.
Author Contributions
Conceptualization, E.A.; methodology, E.A., J.M.A.-P., D.P. and M.M.; validation, E.A., D.P. and M.M.; formal analysis, E.A., D.P., J.M.A.-P. and M.M.; investigation, M.M., M.C., and D.P.; data curation, E.A.; writing—original draft preparation, E.A., M.M. and D.P.; writing—review and editing, E.A., D.P., J.M.A.-P. and M.M., visualization, E.A.; supervision, E.A.; project administration, E.A.; funding acquisition, E.A. and D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Fundação para a Ciência e a Tecnologia (FCT)—grant numbers PTDC/SAU-INF/28182/2017 to E.A., EXPL/SAU-INF/0742/2021 to D.P., UIDB/04138/2020 to iMed-ULisboa, UIDB/04279/2020 to the Center for Interdisciplinary Research in Health and CEECINST/00070/2021 to Universidade Católica Portuguesa. M.M. and M.C. are supported by a PhD fellowship from FCT with the references 2021.07978.BD and SFRH/BD/131948/2017, respectively.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge Associação para o Ensino e Investigação em Microbiologia (ADEIM).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Waters, R.; Ndengane, M.; Abrahams, M.-R.; Diedrich, C.R.; Wilkinson, R.J.; Coussens, A.K. The Mtb -HIV Syndemic Interaction: Why Treating M. tuberculosis Infection May Be Crucial for HIV-1 Eradication. Future Virol 2020, 15, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.C.K.; Noursadeghi, M. Pathogenesis of HIV-1 and Mycobacterium tuberculosis Co-Infection. Nat Rev Microbiol 2018, 16, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Azevedo-Pereira, J.M.; Pires, D.; Calado, M.; Mandal, M.; Santos-Costa, Q.; Anes, E. HIV/Mtb Co-Infection: From the Amplification of Disease Pathogenesis to an “Emerging Syndemic”. Microorganisms 2023, 11, 853. [Google Scholar] [CrossRef] [PubMed]
- Montales, M.T.; Chaudhury, A.; Beebe, A.; Patil, S.; Patil, N. HIV-Associated TB Syndemic: A Growing Clinical Challenge Worldwide. Front Public Health 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2023; Geneva, 2023. [Google Scholar]
- Lai, R.P.J.; Meintjes, G.; Wilkinson, R.J. HIV-1 Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome. Semin Immunopathol 2016, 38, 185–198. [Google Scholar] [CrossRef]
- Bares, S.H.; Swindells, S. Latent Tuberculosis and HIV Infection. Curr Infect Dis Rep 2020, 22, 17. [Google Scholar] [CrossRef]
- Jones, R.M.; Adams, K.N.; Eldesouky, H.E.; Sherman, D.R. The Evolving Biology of Mycobacterium tuberculosis Drug Resistance. Front Cell Infect Microbiol 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Gui, X.; Wu, Z.; Zhang, Y.; Yan, L. Prediction of Drug Resistance Profile of Multidrug-Resistant Mycobacterium tuberculosis (MDR-MTB) Isolates from Newly Diagnosed Case by Whole Genome Sequencing (WGS): A Study from a High Tuberculosis Burden Country. BMC Infect Dis 2022, 22, 499. [Google Scholar] [CrossRef] [PubMed]
- Navasardyan, I.; Miwalian, R.; Petrosyan, A.; Yeganyan, S.; Venketaraman, V. HIV–TB Coinfection: Current Therapeutic Approaches and Drug Interactions. Viruses 2024, 16, 321. [Google Scholar] [CrossRef]
- World Health Organization HIV DRUG RESISTANCE HIV DRUG RESISTANCE REPORT 2021. 2021.
- Olivença, F.; Nunes, A.; Macedo, R.; Pires, D.; Silveiro, C.; Anes, E.; Miragaia, M.; Gomes, J.P.; Catalão, M.J. Uncovering Beta-Lactam Susceptibility Patterns in Clinical Isolates of Mycobacterium tuberculosis through Whole-Genome Sequencing. Microbiol Spectr 2022, 10. [Google Scholar] [CrossRef]
- Pires, D.; Valente, E.; Simões, M.F.; Carmo, N.; Testa, B.; Constantino, L.; Anes, E. Esters of Pyrazinoic Acid Are Active against Pyrazinamide-Resistant Strains of Mycobacterium tuberculosis and Other Naturally Resistant Mycobacteria In Vitro and Ex Vivo within Macrophages. Antimicrob Agents Chemother 2015, 59, 7693–7699. [Google Scholar] [CrossRef] [PubMed]
- Pais, J.P.; Magalhães, M.; Antoniuk, O.; Barbosa, I.; Freire, R.; Pires, D.; Valente, E.; Testa, B.; Anes, E.; Constantino, L. Benzoic Acid Derivatives as Prodrugs for the Treatment of Tuberculosis. Pharmaceuticals (Basel) 2022, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Toor, J.S.; Singh, S.; Sharma, A.; Arora, S.K. Mycobacterium tuberculosis Modulates the Gene Interactions to Activate the HIV Replication and Faster Disease Progression in a Co-Infected Host. PLoS One 2014, 9, e106815. [Google Scholar] [CrossRef] [PubMed]
- Dupont, M.; Rousset, S.; Manh, T.-P.V.; Monard, S.C.; Pingris, K.; Souriant, S.; Vahlas, Z.; Velez, T.; Poincloux, R.; Maridonneau-Parini, I.; et al. Dysregulation of the IFN-I Signaling Pathway by Mycobacterium tuberculosis Leads to Exacerbation of HIV-1 Infection of Macrophages. J Leukoc Biol 2022, 112, 1329–1342. [Google Scholar] [CrossRef]
- Dupont, M.; Souriant, S.; Balboa, L.; Vu Manh, T.-P.; Pingris, K.; Rousset, S.; Cougoule, C.; Rombouts, Y.; Poincloux, R.; Ben Neji, M.; et al. Tuberculosis-Associated IFN-I Induces Siglec-1 on Tunneling Nanotubes and Favors HIV-1 Spread in Macrophages. Elife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Souriant, S.; Balboa, L.; Dupont, M.; Pingris, K.; Kviatcovsky, D.; Cougoule, C.; Lastrucci, C.; Bah, A.; Gasser, R.; Poincloux, R.; et al. Tuberculosis Exacerbates HIV-1 Infection through IL-10/STAT3-Dependent Tunneling Nanotube Formation in Macrophages. Cell Rep 2019, 26, 3586–3599.e7. [Google Scholar] [CrossRef]
- Pires, D.; Marques, J.; Pombo, J.P.; Carmo, N.; Bettencourt, P.; Neyrolles, O.; Lugo-Villarino, G.; Anes, E. Role of Cathepsins in Mycobacterium tuberculosis Survival in Human Macrophages. Sci Rep 2016, 6, 32247. [Google Scholar] [CrossRef] [PubMed]
- Anes, E.; Pires, D.; Mandal, M.; Azevedo-Pereira, J.M. Spatial Localization of Cathepsins: Implications in Immune Activation and Resolution during Infections. Front Immunol 2022, 13, 955407. [Google Scholar] [CrossRef]
- Pires, D.; Mandal, M.; Matos, A.I.; Peres, C.; Catalão, M.J.; Azevedo-Pereira, J.M.; Satchi-Fainaro, R.; Florindo, H.F.; Anes, E. Development of Chitosan Particles Loaded with SiRNA for Cystatin C to Control Intracellular Drug-Resistant Mycobacterium tuberculosis. Antibiotics (Basel) 2023, 12, 729. [Google Scholar] [CrossRef]
- Pires, D.; Calado, M.; Velez, T.; Mandal, M.; Catalão, M.J.; Neyrolles, O.; Lugo-Villarino, G.; Vérollet, C.; Azevedo-Pereira, J.M.; Anes, E. Modulation of Cystatin C in Human Macrophages Improves Anti-Mycobacterial Immune Responses to Mycobacterium tuberculosis Infection and Coinfection With HIV. Front Immunol 2021, 12, 1–16. [Google Scholar] [CrossRef]
- Anes, E.; Azevedo-Pereira, J.M.; Pires, D. Cathepsins and Their Endogenous Inhibitors in Host Defense During Mycobacterium tuberculosis and HIV Infection. Front Immunol 2021, 12, 726984. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.; Bernard, E.M.; Pombo, J.P.; Carmo, N.; Fialho, C.; Gutierrez, M.G.; Bettencourt, P.; Anes, E. Mycobacterium tuberculosis Modulates MiR-106b-5p to Control Cathepsin S Expression Resulting in Higher Pathogen Survival and Poor T-Cell Activation. Front Immunol 2017, 8, 1819. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.; Pires, D.; Catalão, M.J.; Azevedo-Pereira, J.M.; Anes, E. Modulation of Cystatin F in Human Macrophages Impacts Cathepsin-Driven Killing of Multidrug-Resistant Mycobacterium tuberculosis. Microorganisms 2023, 11, 1861. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.; Valente, S.; Calado, M.; Mandal, M.; Azevedo-Pereira, J.M.; Anes, E. Repurposing Saquinavir for Host-Directed Therapy to Control Mycobacterium tuberculosis Infection. Front Immunol 2021, 12, 647728. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.; Mandal, M.; Pinho, J.; Catalão, M.J.; Almeida, A.J.; Azevedo-Pereira, J.M.; Gaspar, M.M.; Anes, E. Liposomal Delivery of Saquinavir to Macrophages Overcomes Cathepsin Blockade by Mycobacterium tuberculosis and Helps Control the Phagosomal Replicative Niches. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Mwandumba, H.C.; Russell, D.G.; Nyirenda, M.H.; Anderson, J.; White, S.A.; Molyneux, M.E.; Squire, S.B. Mycobacterium tuberculosis Resides in Nonacidified Vacuoles in Endocytically Competent Alveolar Macrophages from Patients with Tuberculosis and HIV Infection. The Journal of Immunology 2004, 172, 4592–4598. [Google Scholar] [CrossRef] [PubMed]
- Anes, E.; Kühnel, M.P.; Bos, E.; Moniz-Pereira, J.; Habermann, A.; Griffiths, G. Selected Lipids Activate Phagosome Actin Assembly and Maturation Resulting in Killing of Pathogenic Mycobacteria. Nat Cell Biol 2003, 5, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Jordao, L.; Bleck, C.K.E.; Mayorga, L.; Griffiths, G.; Anes, E. On the Killing of Mycobacteria by Macrophages. Cell Microbiol 2008, 10, 529–548. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.E. Vaccine Development against Tuberculosis before and after Covid-19. Front Immunol 2023, 14. [Google Scholar] [CrossRef]
- Portevin, D.; Via, L.E.; Eum, S.; Young, D. Natural Killer Cells Are Recruited during Pulmonary Tuberculosis and Their Ex Vivo Responses to Mycobacteria Vary between Healthy Human Donors in Association with KIR Haplotype. Cell Microbiol 2012, 14, 1734–1744. [Google Scholar] [CrossRef]
- Russell, D.G.; Vanderven, B.C.; Glennie, S.; Mwandumba, H.; Heyderman, R.S. The Macrophage Marches on Its Phagosome: Dynamic Assays of Phagosome Function. Nat Rev Immunol 2009, 9, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, S.; Caliò, R.; Balzarini, J.; Bellocchi, M.C.; Garaci, E.; Perno, C.F. Macrophages and HIV Infection: Therapeutical Approaches toward This Strategic Virus Reservoir. Antiviral Res 2002, 55, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Alter, G.; Teigen, N.; Ahern, R.; Streeck, H.; Meier, A.; Rosenberg, E.S.; Altfeld, M. Evolution of Innate and Adaptive Effector Cell Functions during Acute HIV-1 Infection. J Infect Dis 2007, 195, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Borrow, P.; Bhardwaj, N. Innate Immune Responses in Primary HIV-1 Infection. Curr Opin HIV AIDS 2008, 3, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Asquith, B.; Edwards, C.T.T.; Lipsitch, M.; McLean, A.R. Inefficient Cytotoxic T Lymphocyte–Mediated Killing of HIV-1–Infected Cells In Vivo. PLoS Biol 2006, 4, e90. [Google Scholar] [CrossRef]
- McMichael, A.J.; Borrow, P.; Tomaras, G.D.; Goonetilleke, N.; Haynes, B.F. The Immune Response during Acute HIV-1 Infection: Clues for Vaccine Development. Nat Rev Immunol 2010, 10, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.A.; Light, R.W. Tuberculous Pleural Effusion. Turkish Thoracic Journal/Türk Toraks Dergisi 2015, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Toossi, Z.; Johnson, J.L.; Kanost, R.A.; Wu, M.; Luzze, H.; Peters, P.; Okwera, A.; Joloba, M.; Mugyenyi, P.; Mugerwa, R.D.; et al. Increased Replication of HIV-1 at Sites of Mycobacterium tuberculosis Infection: Potential Mechanisms of Viral Activation. JAIDS Journal of Acquired Immune Deficiency Syndromes 2001, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, J. Pleural Tuberculosis. European Respiratory Journal 1997, 10, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Werle, B.; Sauckel, K.; Nathanson, C.-M.; Bjarnadottir, M.; Spiess, E.; Ebert, W.; Abrahamson, M. Cystatins C, E/M and F in Human Pleural Fluids of Patients with Neoplastic and Inflammatory Lung Disorders. Biol Chem 2003, 384. [Google Scholar] [CrossRef]
- Calado, M.; Matoso, P.; Santos-Costa, Q.; Espirito-Santo, M.; Machado, J.; Rosado, L.; Antunes, F.; Mansinho, K.; Lopes, M.M.; Maltez, F.; et al. Coreceptor Usage by HIV-1 and HIV-2 Primary Isolates: The Relevance of CCR8 Chemokine Receptor as an Alternative Coreceptor. Virology 2010, 408, 174–182. [Google Scholar] [CrossRef]
- Nanut, M.P.; Sabotič, J.; Švajger, U.; Jewett, A.; Kos, J. Cystatin F Affects Natural Killer Cell Cytotoxicity. Front Immunol 2017, 8. [Google Scholar] [CrossRef]
- Prunk, M.; Perišić Nanut, M.; Jakoš, T.; Sabotič, J.; Švajger, U.; Kos, J. Extracellular Cystatin F Is Internalised by Cytotoxic T Lymphocytes and Decreases Their Cytotoxicity. Cancers (Basel) 2020, 12, 3660. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Liu, S.; Liu, Y.; Yang, X.; Liu, G.; Shimizu, T.; Ikenaka, K.; Fan, K.; Ma, J. Cathepsin C Promotes Microglia M1 Polarization and Aggravates Neuroinflammation via Activation of Ca2+-Dependent PKC/P38MAPK/NF-ΚB Pathway. J Neuroinflammation 2019, 16, 10. [Google Scholar] [CrossRef]
- Alam, S.; Liu, Q.; Liu, S.; Liu, Y.; Zhang, Y.; Yang, X.; Liu, G.; Fan, K.; Ma, J. Up-Regulated Cathepsin C Induces Macrophage M1 Polarization through FAK-Triggered P38 MAPK/NF-ΚB Pathway. Exp Cell Res 2019, 382, 111472. [Google Scholar] [CrossRef]
- Kim, W.-J.; Kim, H.; Suk, K.; Lee, W.-H. Macrophages Express Granzyme B in the Lesion Areas of Atherosclerosis and Rheumatoid Arthritis. Immunol Lett 2007, 111, 57–65. [Google Scholar] [CrossRef]
- Velotti, F.; Barchetta, I.; Cimini, F.A.; Cavallo, M.G. Granzyme B in Inflammatory Diseases: Apoptosis, Inflammation, Extracellular Matrix Remodeling, Epithelial-to-Mesenchymal Transition and Fibrosis. Front Immunol 2020, 11. [Google Scholar] [CrossRef]
- Boivin, W.A.; Cooper, D.M.; Hiebert, P.R.; Granville, D.J. Intracellular versus Extracellular Granzyme B in Immunity and Disease: Challenging the Dogma. Laboratory Investigation 2009, 89, 1195–1220. [Google Scholar] [CrossRef]
- Zhang, X.; Deshmukh, S.; Mukim, A.; Zhang, J.; Beliakova-Bethell, N. HIV Infection Elicits Differential Transcriptomic Remodeling in CD4+ T Cells with Variable Proliferative Responses to the T Cell Receptor Stimulus. Pathogens 2023, 12, 511. [Google Scholar] [CrossRef] [PubMed]
- Kos, J.; Nanut, M.P.; Prunk, M.; Sabotič, J.; Dautović, E.; Jewett, A. Cystatin F as a Regulator of Immune Cell Cytotoxicity. Cancer Immunol Immunother 2018, 67, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, A.J.; Garand, M.; Chaussabel, D.; Feng, C.G. Transcriptomic Profiling Identifies Neutrophil-Specific Upregulation of Cystatin F as a Marker of Acute Inflammation in Humans. Front Immunol 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Colbert, J.D.; Matthews, S.P.; Kos, J.; Watts, C. Internalization of Exogenous Cystatin F Supresses Cysteine Proteases and Induces the Accumulation of Single-Chain Cathepsin L by Multiple Mechanisms. Journal of Biological Chemistry 2011, 286, 42082–42090. [Google Scholar] [CrossRef] [PubMed]
- Magister, Š.; Tseng, H.C.; Bui, V.T.; Kos, J.; Jewett, A. Regulation of Split Anergy in Natural Killer Cells by Inhibition of Cathepsins C and H and Cystatin F. Oncotarget 2015, 6, 22310–22327. [Google Scholar] [CrossRef] [PubMed]
- Obata-Onai, A.; Hashimoto, S. -i.; Onai, N.; Kurachi, M.; Nagai, S.; Shizuno, K. -i.; Nagahata, T.; Matsushima, K. Comprehensive Gene Expression Analysis of Human NK Cells and CD8+ T Lymphocytes. Int Immunol 2002, 14, 1085–1098. [Google Scholar] [CrossRef]
- Ota, T.; Okubo, Y.; Sekiguchi, M. Analysis of Immunologic Mechanisms of High Natural Killer Cell Activity in Tuberculous Pleural Effusions. American Review of Respiratory Disease 1990, 142, 29–33. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).