1. Introduction
Mosquitoes are a family of insects of the order Diptera and are responsible for most insect bites worldwide [
1]. The local reaction usually produced after a mosquito bite can be sometimes severe and systemic reactions have rarely been reported [
2].
For some individuals, a large local reaction (wheal > 5 mm) occurs within minutes to hours. These individuals may be diagnosed with a mosquito allergy [
2] but unfortunately, allergic reactions to mosquito bites are underestimated due to the lack of reliable diagnostic tools.
Both “species-specif ic” and cross-reactive allergenic molecules have recently been described mainly in the saliva of yellow fever mos quito (
Aedes aegypti), Asian tiger mosquito (
Aedes albop ictus),
Aedes vexans, and
Culex quinquefasciatus [
3,
4,
5]. Few studies have been conducted on
Aedes communis (Ac) [
6,
7] and no Ac molecule has been registered to date in the WHO/IUIS database [
8].
Recently, it has been described that in individuals with severe local reactions following mosquito bites, the immune response to mosquito allergens was associated with both species-specific (Api m 1, Api m 3, and Api m 10), and cross-reactive (Api m 2 and Api m 5) bee venom components, suggesting the “Bee- Mosquito syndrome” [
9]
Unfortunately, mosquitoes and horseflies’ whole-body extracts for in vitro and in vivo diagnosis, are limited. The sensitivity and specificity of these extracts are very low due to the scarce presence of relevant salivary gland proteins and because the IgE binding is often to non-salivary proteins (e.g., tropomyosin) or to the presence of cross-reactive carbohydrate determinants (CCD)-sIgE [
10]. Several studies have demonstrated that prevalence rates for positive CCD-sIgE reactivity are approximately 20–37% in allergic patients with grass pollen exposure and Hymenoptera stings [
11,
12,
13,
14,
15]
The risk of CCD interference that compromises quantitative IgE results can be mitigated by the addition of a soluble CCD inhibitor to positive CCD-sIgE containing sera or alternatively using a non-cellulose-based sIgE assay. [
11]
Hence, it has been hypnotized that the presence of sIgE to CCD could have a role in the Bee-Mosquito syndrome.
2. Material and Methods
First Experimental procedure
2.1. Extract Preparation
100 of each mosquito species provided by Experimental Zooprophylactic Institute of Sicily were ground using a disposable spatula and thoroughly mixed by hand. Protein was extracted in 2x phosphate buffered saline + 2mM phenylmethylsulfonyl fluoride at 4oC. Briefly, samples were mixed by vortex for 30 seconds and sonicated for 15 minutes. Samples were centrifugated at 8000g for 30 minutes at 4oC. The supernatant was collected and stored at -20oC. 5mg A. mellifera extract (Citeq) was reconstituted in 5ml 0.9%NaCl, pH 7.4. The reconstituted extract was stored at -20oC.
Extracts of Mosquito and A. mellifera were quantified for total protein content using a BCA protein kit (Pierce 23225).
2.2. Serum Pool Generation
Serums from 21 different mosquito-allergic patients were combined. All patients reported immediate large local reactions characterized by hives and cutaneous angioedema lasting several hours after mosquito bites. They were all positive to sIgE to mosquitoes, A. mellifera, Api m1, Api m 2, Api m 3, Api m 5, Api m 10 and negative to sIgE to CCD (bromelain N-glycan MUXF3) (ImmunoCap Phadia Thermo Fisher, Italy). The pooled sera were aliquoted and stored at -20oC, then at -80oC for long-term storage.
2.3. SDS-PAGE
Samples were run on polyacrylamide gels according to Indoor Biotechnologies Ltd SOP IBL LAB SOP-021. Briefly, samples were prepared by mixing with Laemlli sample loading buffer with 0.1M DTT and heated for 5 minutes at 100oC. Samples were loaded at 15μg (according to concentration determined in 2.1) per well into 4–20% Mini-PROTEAN® TGXTM Precast Protein Gels (BioRad) and run at 300V for 20 minutes. Gels were taken forward for Coomassie staining by incubating with Instant Blue protein stain for a minimum of 1 hour or Immunoblotting.
2.4. Immunoblot
Western Blots were carried out using an adapted version of Indoor Biotechnologies Ltd SOP IBL LAB SOP-023 to account for the use of human sera and an alternative secondary antibody. Blocking, antibody wash, diluent solutions, and chemiluminescent substrate were obtained and prepared using reagents from the Western Breeze blot detection kit (Invitrogen WB7104). Proteins were transferred to PVDF membranes using Trans-Blot Turbo Transfer Pack, mini gel, 0.2μm PVDF (Bio-Rad, Cat No. 1704156) according to manufacturer instruction. Membranes were blocked following transfer for 1 hour at room temperature and washed with distilled water. Pooled sera were applied to the blot at a 1:5 dilution in antibody diluent and incubated overnight at 4o C. A negative control assay was also incubated overnight with antibody diluent at 4o C without sera. Membranes were washed using antibody wash solution before the addition of Goat anti-Human IgE-Alkaline phosphatase conjugate (BioRad STAR147A) at 1:5000 in antibody diluent and incubated for 1 hour at room temperature before washing with antibody wash solution and incubation for 5 minutes with a chemiluminescent substrate.
When typically running an immunoblot, it was used Streptactin-Alkaline phosphatase conjugate to detect molecular weight markers in chemiluminescent western blots. During optimization, it was discovered that Streptactin-Alkaline phosphatase conjugate was bound to samples and caused interference. Therefore, this reagent was left out, and western blots required two reads – one to detect samples by chemiluminescent read and a second colorimetric read to detect standards.
3. Results
3.1. Total Protein Content
Samples were quantified for total protein content by BCA protein kit (2.1). The results of the analyses are summarized below in Table 1.
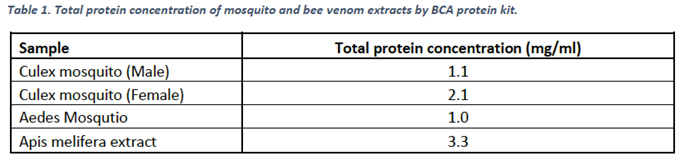
3.2. SDS-PAGE
Mosquito and bee venom extracts prepared in 2.1 were run on SDS-PAGE (2.3), with data presented in Figure 1.
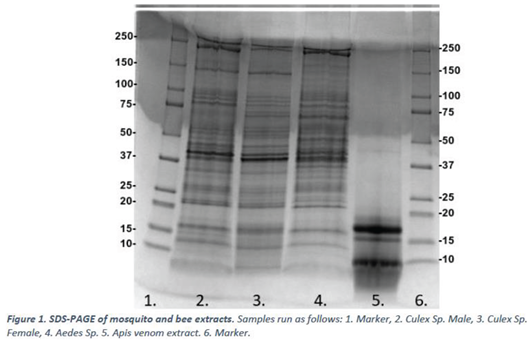
3.3. Immunoblot
Mosquito and bee venom extracts prepared in 2.1 were subject to immunoblotting (2.4) with pooled sera from 21 mosquito-allergic donors. Data is presented below in Figure 2. A negative control blot run without the addition of primary antibody (sera) can be found in the appendices – Figure 4.
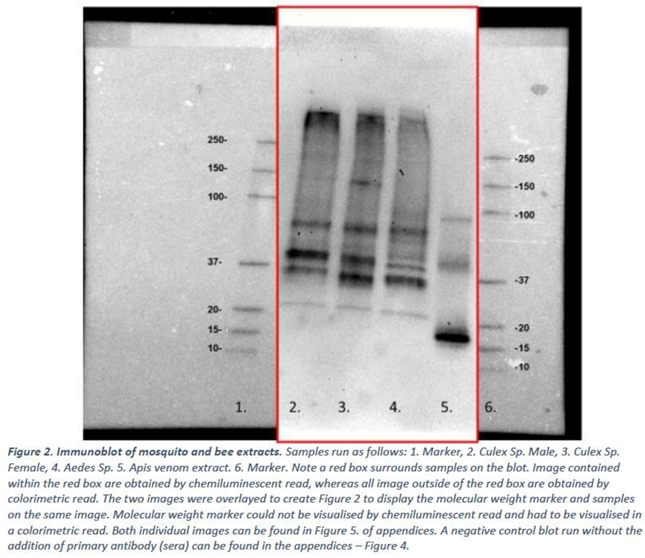
Figure 3. The SDS-PAGE and Immunoblot data were aligned as below.
The immunoblot experiments show that the IgE from the mosquito allergic sera pool binds to multiple protein bands present in Culex male, Culex female, Aedes species mosquitos and to A. mellifera venom extract (Figure 3)
A follow-on experiment was performed to assess the use of CCD-Inhibitor and whether this would prevent IgE binding in immunoblot.
Second Experimental procedure
4.1. Sample Preparation
Mosquito and be venom extracts, as well as pooled sera were prepared as described in the first experimental procedure. These were taken forward for use in SDS-PAGE and Immunoblot procedures.
4.2. Incubation of Sera with CCD-Inhibitor
Pooled sera were incubated with CCD-Inhibitor (R-Biopharm, Cat No. ZA0601) according to manufacturer instructions. Briefly, 2ml of pooled sera was incubated with 50μl reconstituted CCD-Inhibitor and incubated for 1 hour at room temperature in an orbital incubator at 65rpm. CCD-Inhibited sera were used immediately after 1 hour of incubation for overnight incubation in immunoblot.
4.4. SDS-PAGE
Mosquito and bee venom extracts prepared in the first experiment were run on SDS-PAGE, with data presented in Figure 1.
4.5. Immunoblot
Mosquito and bee venom extracts prepared in the first experiment were subject to immunoblotting with pooled sera from 21 mosquito allergic donors. Sera was incubated with CCD-Inhibitor prior to use. Data is presented in Figure 2.
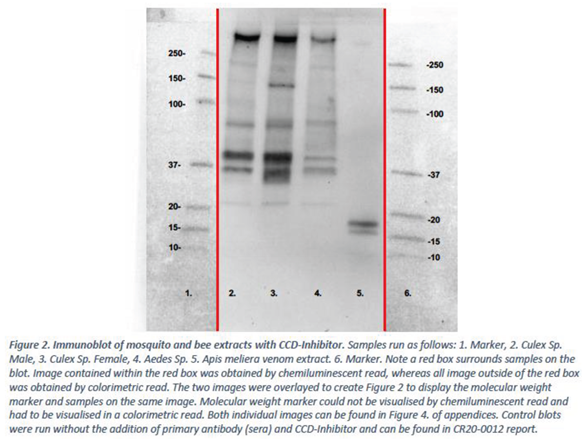
Figure 2 was aligned with the immunoblot carried out in the first experiment without CCD-Inhibitor. Aligned blots are presented in Figure 3.
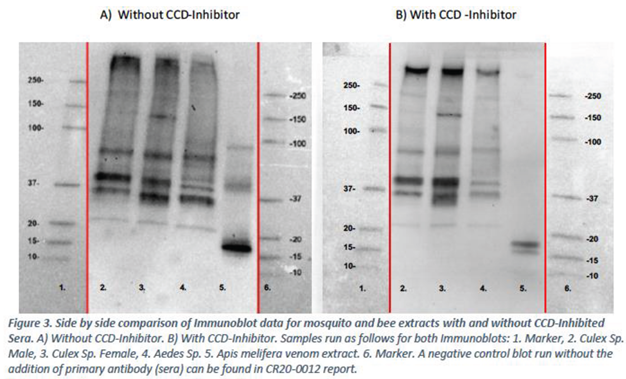
The immunoblot experiments (Figure 3) demonstrate that the use of a CCD-Inhibitor results in differences in IgE binding. Specifically, bands that were previously observed in the Apis melifera venom sample without use of CCD-Inhibitor at 40kDa and 90kDa are no longer observed when CCD-Inhibitor is used. Two bands of the Apis melifera venom remain present between 15-20kDa.
No major differences in banding pattern were observed for the mosquito samples with or without CCD-Inhibitor, although the blot appears cleaner with less background when CCD-Inhibitor is used.
5. Conclusions
Immunoblot data suggests that the use of CCD-Inhibitor prevents binding of IgE mosquito allergic individuals to multiple bands from Apis melifera venom. It may be inferred that bands originally present at 40kDa and 90kDa therefore may have been due to a CCD interaction. Two bands remain present in the Apis melifera sample between 15-20kDa, however these do not align with any bands present in the mosquito extracts.
Future experiments are necessary to determine whether the reactive bands from Apis melifera are unrelated proteins or whether the proteins are related homologues of varying molecular weight.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: title; Table S1: title; Video S1: title.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Does, A.V.; Labib, A.; Yosipovitch, G. Does, A.V.; Labib, A.; Yosipovitch, G. Update on mosquito bite reaction: Itch and hypersensitivity, pathophysiology, prevention, and treatment. Front. Immunol. 2022, 13, 1024559, . [CrossRef]
- Martinez-Molero MI: Urticaria caused by arthropod. Bites and stings (excluding Hymenoptera). Allergol Immunopathol (Madrid). 1999;27:96-104.
- Cantillo JF, Puerta L, Puchalska P, Lafosse-Marin S, Subiza JL, Fernandez-Caldas E: Allergenome characterization of the mosquito Aedes aegypti. Allergy 2017; 72: 1499–1509.
- Kulthanan K, Wongkamchai S, Triwongwaranat D: Mosquito allergy: clinical features and natural course. J Dermatol 2010; 37: 1025–1031.
- Peng Z, Simons FE: Mosquito allergy: immune mechanisms and recombinant salivary allergens. Int Arch Allergy Immunol 2004;133: 198–209.
- Brummer-Korvenkontio H, Lappalainen P, Reunala T, Palosuo T: Detection of mosquito saliva-specific IgE and IgG4 antibodies by immunoblotting. J Allergy Clin Immunol 1994; 93: 551–555.
- Brummer-Korvenkontio H, Palosuo K, Palosuo. T, Brummer-Korvenkontio M, Leinikki P, Reunala T: Detection of mosquito saliva specific IgE antibodies by capture ELISA. Allergy 1997; 52: 342–345.
- Allergen nomenclature WHO/IUIS Allergen Nomenclature Sub-Committee 2023. https://allergen.org/index.php.
- Scala, E.; Pirrotta, L.; Uasuf, C.G.; Mistrello, G.; Amato, S.; Guerra, E.C.; Locanto, M.; Meneguzzi, G.; Giani, M.; Cecchi, L.; et al. Scala, E.; Pirrotta, L.; Uasuf, C.G.; Mistrello, G.; Amato, S.; Guerra, E.C.; Locanto, M.; Meneguzzi, G.; Giani, M.; Cecchi, L.; et al. Aedes communis Reactivity Is Associated with Bee Venom Hypersensitivity: An in vitro and in vivo Study. Int. Arch. Allergy Immunol. 2018, 176, 101–105, . [CrossRef]
- Hemmer, W.; Wantke, F. Hemmer, W.; Wantke, F. Insect hypersensitivity beyond bee and wasp venom allergy. Allergologie 2020, 4, 97–104, . [CrossRef]
- Sinson, E.; Ocampo, C.; Liao, C.; Nguyen, S.; Dinh, L.; Rodems, K.; Whitters, E.; Hamilton, R.G. Sinson, E.; Ocampo, C.; Liao, C.; Nguyen, S.; Dinh, L.; Rodems, K.; Whitters, E.; Hamilton, R.G. Cross-reactive carbohydrate determinant interference in cellulose-based IgE allergy tests utilizing recombinant allergen components. PLOS ONE 2020, 15, e0231344, . [CrossRef]
- Mari, A.; Iacovacci, P.; Afferni, C.; Barletta, B.; Tinghino, R.; Di Felice, G.; Pini, C. Mari, A.; Iacovacci, P.; Afferni, C.; Barletta, B.; Tinghino, R.; Di Felice, G.; Pini, C. Specific IgE to cross-reactive carbohydrate determinants strongly affect the in vitro diagnosis of allergic diseases. J. Allergy Clin. Immunol. 1999, 103, 1005–1011, . [CrossRef]
- Kochuyt, A.; Van Hoeyveld, E.M.; Stevens, E.A.M. Kochuyt, A.; Van Hoeyveld, E.M.; Stevens, E.A.M. Prevalence and clinical relevance of specific immunoglobulin E to pollen caused by sting- induced specific immunoglobulin E to cross-reacting carbohydrate determinants in Hymenoptera venoms. Clin. Exp. Allergy 2005, 35, 441–447, . [CrossRef]
- Mari, A. Mari, A. IgE to Cross-Reactive Carbohydrate Determinants: Analysis of the Distribution and Appraisal of the in vivo and in vitro Reactivity. Int. Arch. Allergy Immunol. 2002, 129, 286–295, . [CrossRef]
- Holzweber, F.; Svehla, E.; Fellner, W.; Dalik, T.; Stubler, S.; Hemmer, W.; Altmann, F. Holzweber, F.; Svehla, E.; Fellner, W.; Dalik, T.; Stubler, S.; Hemmer, W.; Altmann, F. Inhibition of IgE binding to cross-reactive carbohydrate determinants enhances diagnostic selectivity. Allergy 2013, 68, 1269–1277, . [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).










