Submitted:
28 March 2024
Posted:
28 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Area and Green Roof Characteristics
2.2. Data Collection and DNA Extraction
2.3. Metabarcoding Substrate Microbial Communities
2.4. Data Analysis
3. Results
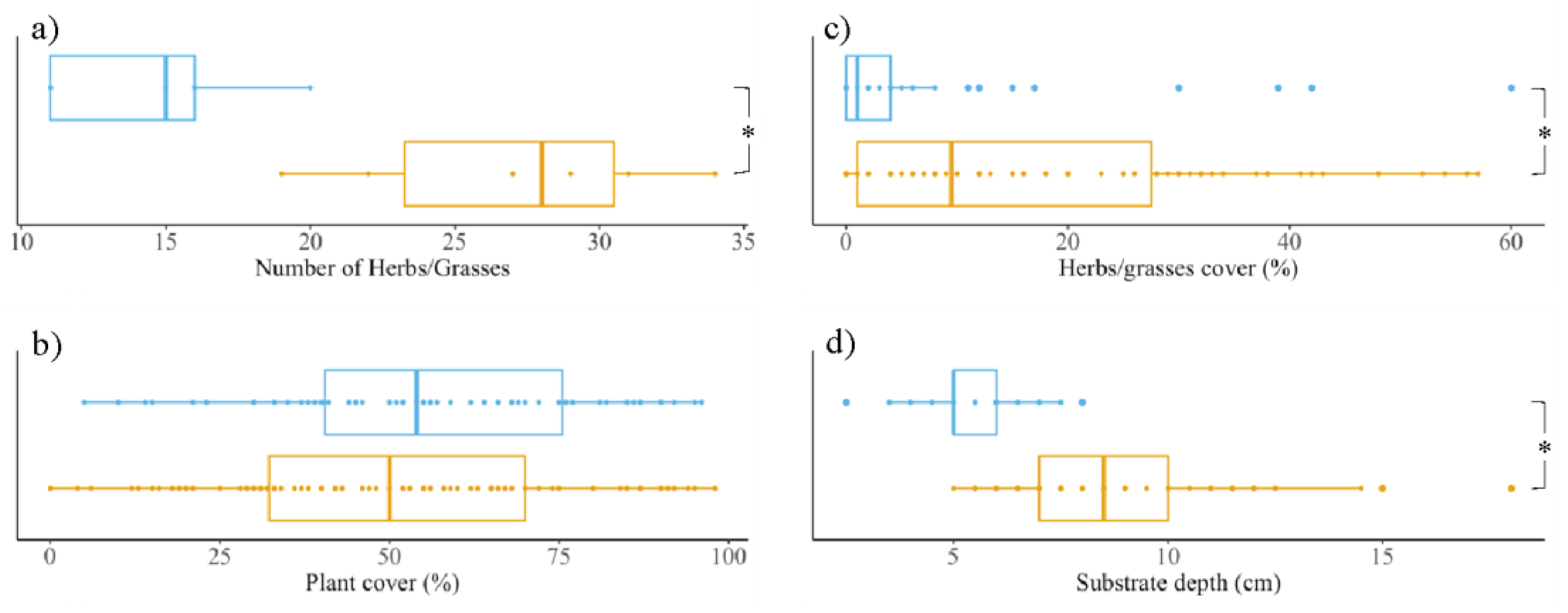
3.1. Sedum-Moss Roofs vs Sedum-Herbs-Grasses Roofs
3.2. Prokaryotic Diversity
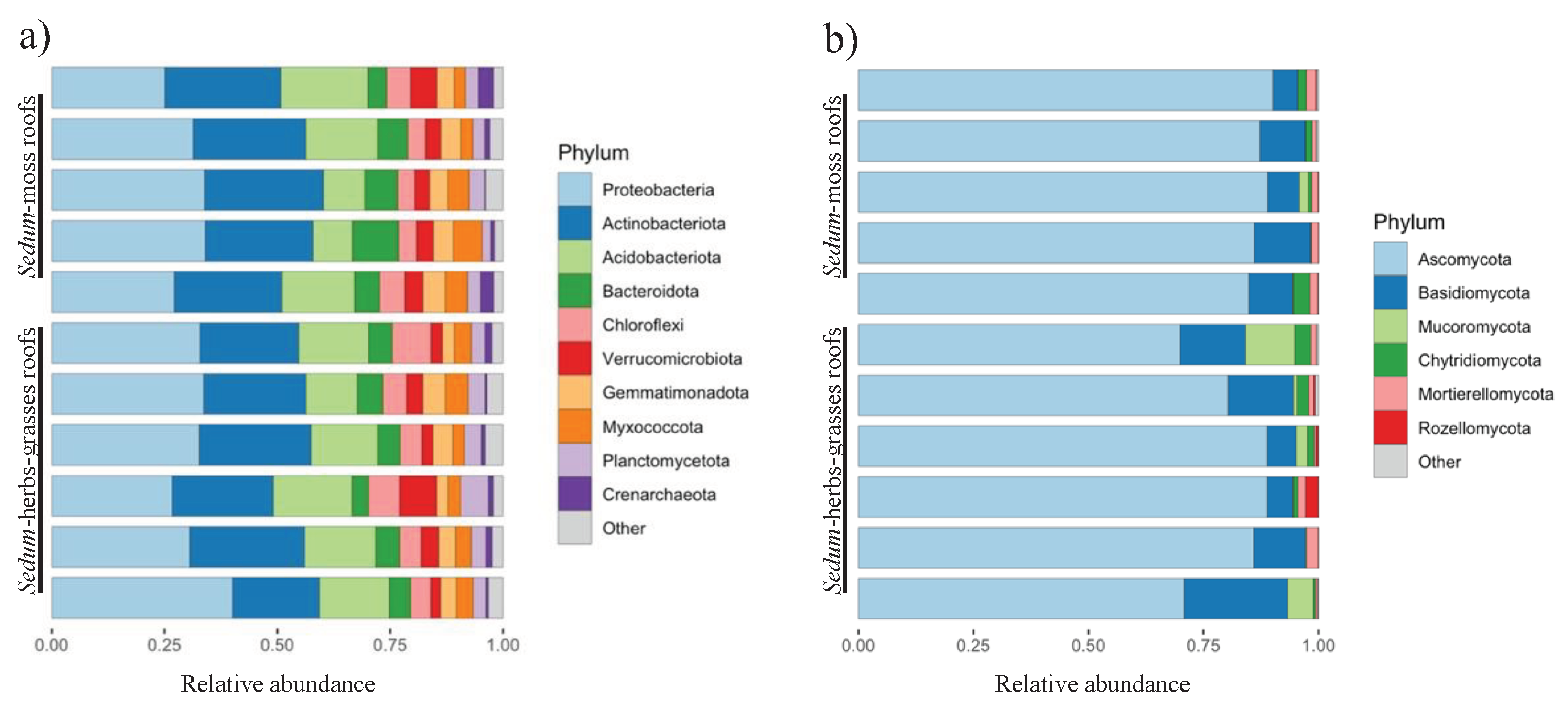
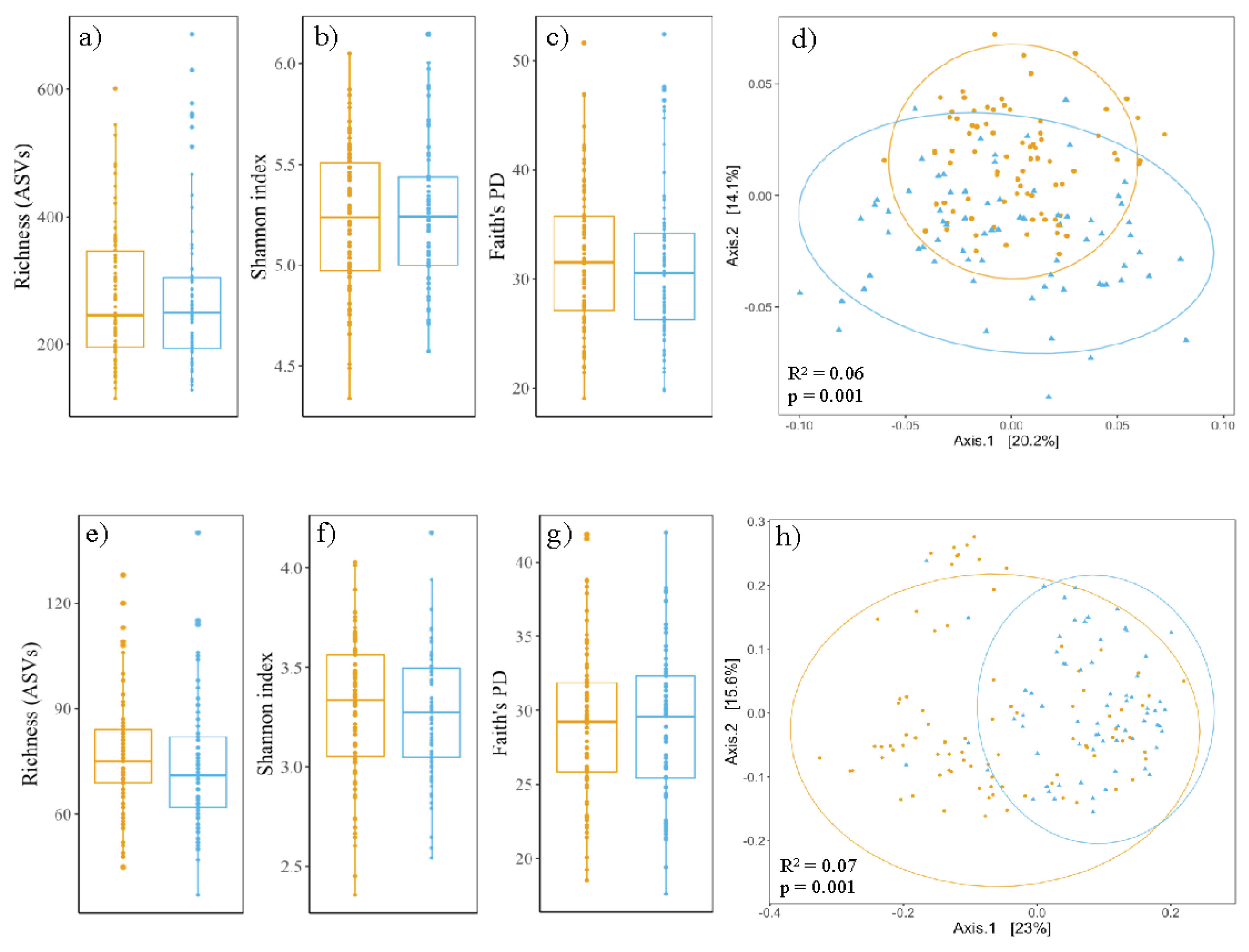
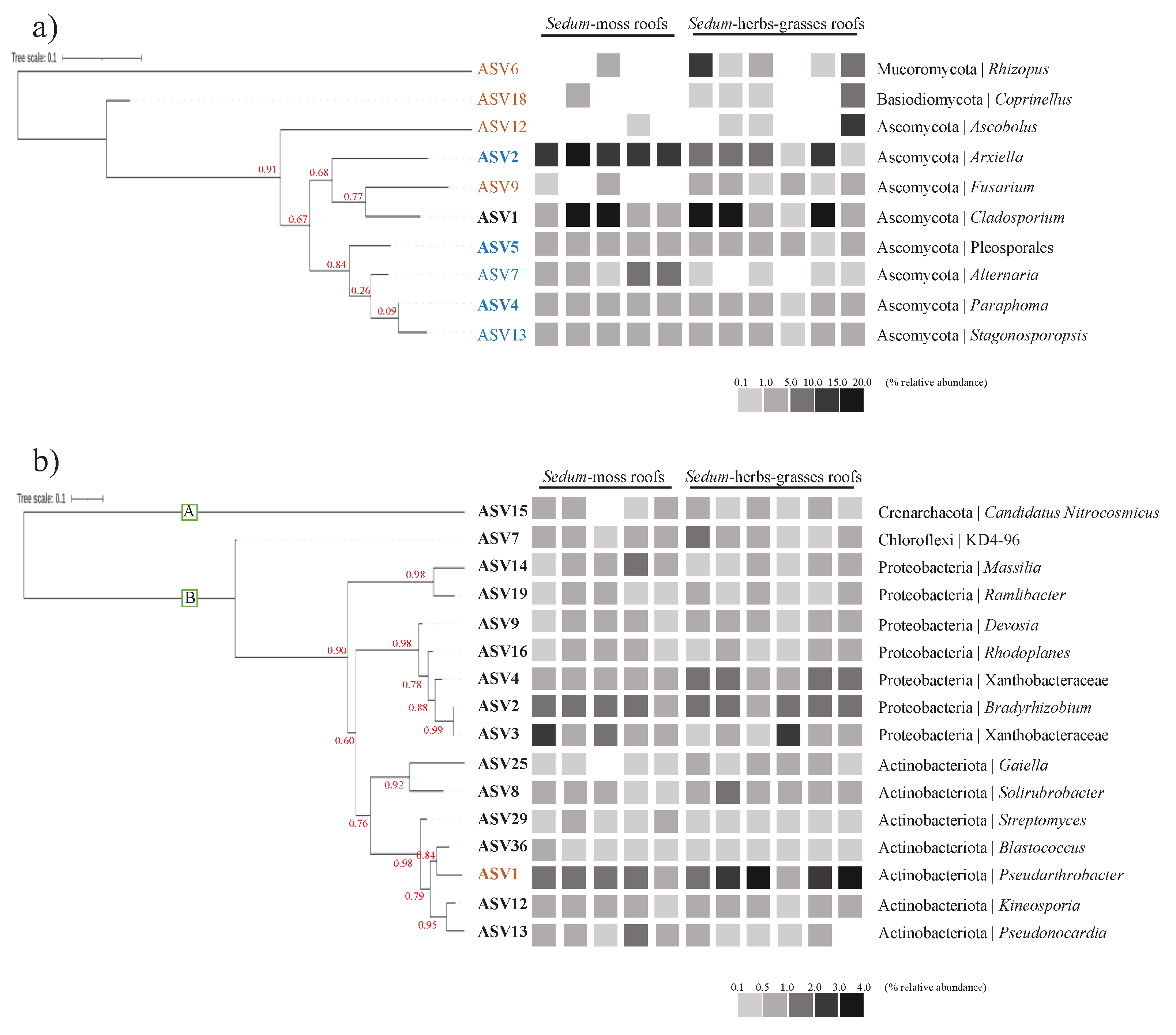
3.3. Fungal Diversity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeNardo, J.C.; Jarrett, A.R.; Manbeck, H.; Beattie, D.J.; Berghage, R. Stormwater mitigation and surface temperature reduction by green roofs. Trans. ASAE 2005, 48, 1491-1496. [CrossRef]
- VanWoert, N.D.; Rowe, D.B.; Andresen, J.A.; Rugh, C.L.; Fernandez, R.T.; Xiao, L. Green roof stormwater retention: Effects of roof surface, slope, and media depth. J. Environ. Qual. 2005, 34, 1036-1044. [CrossRef]
- Mentens, J.; Raes, D.; Hermy, M. Green roofs as a tool for solving the rainwater runoff problem in the urbanized 21st century? Landscape and Urban Planning 2006, 77, 217-226. [CrossRef]
- Yang, J.; Yu, Q.; Gong, P. Quantifying air pollution removal by green roofs in Chicago. Atmos. Environ. 2008, 42, 7266-7273. [CrossRef]
- Del Barrio, E.P. Analysis of the green roofs cooling potential in buildings. Energy and Buildings 1998, 27, 179-193.
- Jaffal, I.; Ouldboukhitine, S.-E.; Belarbi, R. A comprehensive study of the impact of green roofs on building energy performance. Renewable Energy 2012, 43, 157-164. [CrossRef]
- Alexandri, E.; Jones, P. Temperature decreases in an urban canyon due to green walls and green roofs in diverse climates. Building and Environment 2008, 43, 480-493. [CrossRef]
- Santamouris, M. Cooling the cities - A review of reflective and green roof mitigation technologies to fight heat island and improve comfort in urban environments. Solar Energy 2014, 103, 682-703. [CrossRef]
- Williams, N.; Lundholm, J.; MacIvor, J.S. FORUM: Do green roofs help urban biodiversity conservation? Journal of Applied Ecology 2014, 51. [CrossRef]
- Jacobs, J.; Beenaerts, N.; Artois, T. Green roofs and pollinators, useful green spots for some wild bee species (Hymenoptera: Anthophila), but not so much for hoverflies (Diptera: Syrphidae). Sci. Rep. 2023, 13, 1449. [CrossRef]
- Van Dijck, T.; Klerkx, H.; Thijs, S.; Rineau, F.; Van Mechelen, C.; Artois, T. Sedum as host plants for caterpillars? Introducing gut content metabarcoding to green roof research. Urban Ecosystems 2023, 26, 955-965. [CrossRef]
- FLL (Landscape Development and Landscaping Research Society e.V). Green roof guidelines: guidelines for the planning. Construction and Maintenance of Green Roofs 2018.
- Dunnett, N.; Kingsbury, N. Planting Green Roofs and Living Walls; Timber Press: Portland, Oregon, USA, 2004.
- Lundholm, J.; MacIvor, J.S.; MacDougall, Z.; Ranalli, M. Plant Species and Functional Group Combinations Affect Green Roof Ecosystem Functions. PLoS One 2010, 5, e9677. [CrossRef]
- Van Mechelen, C.; Dutoit, T.; Kattge, J.; Hermy, M. Plant trait analysis delivers an extensive list of potential green roof species for Mediterranean France. Ecological Engineering 2014, 67, 48-59. [CrossRef]
- Emilsson, T. Vegetation development on extensive vegetated green roofs: Influence of substrate composition, establishment method and species mix. Ecological Engineering 2008, 33, 265-277. [CrossRef]
- Olszewski, M.; Holmes, M.; Young, C. Assessment of Physical Properties and Stonecrop Growth in Green Roof Substrates Amended with Compost and Hydrogel. HortTechnology 2010, 20. [CrossRef]
- Ondoño, S.; Martínez-Sánchez, J.J.; Moreno, J. The inorganic component of green roof substrates impacts the growth of Mediterranean plant species as well as the C and N sequestration potential. Ecol. Indic. 2015, 61. [CrossRef]
- Kowalchuk, G.A.; Buma, D.S.; de Boer, W.; Klinkhamer, P.G.L.; van Veen, J.A. Effects of above-ground plant species composition and diversity on the diversity of soil-borne microorganisms. Antonie van Leeuwenhoek 2002, 81, 509-520. [CrossRef]
- Santos-González, J.C.; Finlay, R.D.; Tehler, A. Seasonal dynamics of arbuscular mycorrhizal fungal communities in roots in a seminatural grassland. Appl. Environ. Microbiol. 2007, 73, 5613-5623. [CrossRef]
- Bever, J.; Platt, T.; Morton, E. Microbial Population and Community Dynamics on Plant Roots and Their Feedbacks on Plant Communities. Annu. Rev. Microbiol. 2012, 66, 265-283. [CrossRef]
- Haichar, F.Z.; Marol, C.; Berge, O.; Rangel-Castro, J.I.; Prosser, J.I.; Balesdent, J.; Heulin, T.; Achouak, W. Plant host habitat and root exudates shape soil bacterial community structure. ISME J 2008, 2, 1221-1230. [CrossRef]
- Reynolds, H.; Packer, A.; Bever, J.; Clay, K. Grassroots ecology: Plant-microbe-soil interactions as drivers of plant community structure and dynamics. Special Feature Ecology 2003, 84, 2281-2291. [CrossRef]
- van der Heijden, M.G.A.; Klironomos, J.N.; Ursic, M.; Moutoglis, P.; Streitwolf-Engel, R.; Boller, T.; Wiemken, A.; Sanders, I.R. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 1998, 396, 69-72. [CrossRef]
- Van der Heijden, M.; Bardgett, R.; van Straalen, N.M. Van Der Heijden MGA, Bardgett RD, Van Straalen NM.. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecology letters 2008, 11, 296-310. [CrossRef]
- Klironomos, J.; McCune, J.; Hart, M.; Neville, J. Klironomos JN, McCune J, Hart M, Neville J.. The influence of arbuscular mycorrhizae on the relationship between plant diversity and productivity. Ecology Letters 2000, 3, 137-141. [CrossRef]
- Rodriguez, R.J.; Redman, R.S.; Henson, J.M. The Role of Fungal Symbioses in the Adaptation of Plants to High Stress Environments. Mitigation and Adaptation Strategies for Global Change 2004, 9, 261-272. [CrossRef]
- Yang, J.; Kloepper, J.W.; Ryu, C.M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009, 14, 1-4. [CrossRef]
- Friesen, M.L.; Porter, S.S.; Stark, S.C.; Wettberg, E.J.v.; Sachs, J.L.; Martinez-Romero, E. Microbially Mediated Plant Functional Traits. Annual Review of Ecology, Evolution, and Systematics 2011, 42, 23-46. [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: the microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789-799. [CrossRef]
- McGuire, K.L.; Payne, S.G.; Orazi, G.; Palmer, M.I. In Green Roof Ecosystems. Springer International Publishing AG, 2015; Chapter 7, p 175-191.
- Fulthorpe, R.; MacIvor, J.S.; Jia, P.; Yasui, S.-L.E. The Green Roof Microbiome: Improving Plant Survival for Ecosystem Service Delivery. Frontiers in Ecology and Evolution 2018, 6. [CrossRef]
- Dixon, R.; Kahn, D. Genetic regulation of biological nitrogen fixation. Nat. Rev. Microbiol. 2004, 2, 621-631. [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429-448. [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and Fungal Contributions to Carbon Sequestration in Agroecosystems. Soil Sci. Am. J. 2006, 70, 555-569. [CrossRef]
- Trivedi, P.; Anderson, I.C.; Singh, B.K. Microbial modulators of soil carbon storage: integrating genomic and metabolic knowledge for global prediction. Trends Microbiol. 2013, 21, 641-651. [CrossRef]
- Schimel, J.P.; Schaeffer, S.M. Microbial control over carbon cycling in soil. Frontiers in Microbiology 2012, 3, 348. [CrossRef]
- Molineux, C.; Connop, S.; Gange, A. Manipulating soil microbial communities in extensive green roof substrates. Sci. Total Environ.2014, 493, 632-638. [CrossRef]
- Molineux, C.; Gange, A.; Newport, D. Using soil microbial inoculations to enhance substrate performance on extensive green roofs. Sci. Total Environ.2016, 580. [CrossRef]
- Rumble, H.; Gange, A. Microbial inoculants as a soil remediation tool for extensive green roofs. Ecological Engineering 2017, 102, 188-198. [CrossRef]
- Rumble, H.; Finch, P.; Gange, A.C. Can microbial inoculants boost soil food webs and vegetation development on newly constructed extensive green roofs? Urban Forestry & Urban Greening 2022, 75, 127684. [CrossRef]
- Xie, L.; Lehvävirta, S.; Timonen, S.; Kasurinen, J.; Niemikapee, J.; Valkonen, J.P.T. Species-specific synergistic effects of two plant growth—promoting microbes on green roof plant biomass and photosynthetic efficiency. PLoS One 2019, 13, e0209432. [CrossRef]
- Xie, L.; Lehvävirta, S.; Valkonen, J.P.T. Case study: Planting methods and beneficial substrate microbes effect on the growth of vegetated roof plants in Finland. Urban Forestry & Urban Greening 2020, 53, 126722. [CrossRef]
- Young, T.; Cameron, D.D.; Phoenix, G.K. Using AMF inoculum to improve the nutritional status of Prunella vulgaris plants in green roof substrate during establishment. Urban Forestry & Urban Greening 2015, 14, 959-967. [CrossRef]
- McGuire, K.L.; Payne, S.G.; Palmer, M.I.; Gillikin, C.M.; Keefe, D.; Kim, S.J.; Gedallovich, S.M.; Discenza, J.; Rangamannar, R.; Koshner, J.A.; et al. Digging the New York City Skyline: Soil Fungal Communities in Green Roofs and City Parks. PLoS One 2013, 8, e58020. [CrossRef]
- Gill, A.S.; Purnell, K.; Palmer, M.I.; Stein, J.; McGuire, K.L. Microbial Composition and Functional Diversity Differ Across Urban Green Infrastructure Types. Frontiers in Microbiology 2020, 11, 912. [CrossRef]
- Droz, A.G.; Coffman, R.R.; Eagar, A.C.; Blackwood, C.B. Drivers of fungal diversity and community biogeography differ between green roofs and adjacent ground-level green space. Environ. Microbiol. 2022, 24, 5809-5824. [CrossRef]
- Hénault, A.; Heim, A.; Brisson, J.; Dagenais, D.; De Bellis, T.; Chagnon, P.L. Stressful, isolated, yet diverse: Green roofs have rich microbiomes that are not dominated by oligotrophic taxa. Environ. Microbiol. 2022, 14, 766-774. [CrossRef]
- Mitchell, M.E.; Hamilton, T.L.; Uebel-Niemeier, C.; Hopfensperger, K.N.; Buffam, I. Nitrogen cycling players and processes in green roof ecosystems. Applied Soil Ecology 2018, 132, 114-125. [CrossRef]
- Hoch, J.M.; Rhodes, M.E.; Shek, K.L.; Dinwiddie, D.; Hiebert, T.C.; Gill, A.S.; Salazar Estrada, A.E.; Griffin, K.L.; Palmer, M.I.; McGuire, K.L. Soil microbial assemblages are linked to plant community composition and contribute to ecosystem services on urban green roofs. Frontiers in Ecology and Evolution 2019, 7, 198. [CrossRef]
- KMI (Koninklijk Metereologisch Instituut van België). Analyse van het jaar 2019. Available online: https://www.meteobelgie.be/klimatologie/waarnemingen-en-analyses/jaar-2019/2254-analyse-van-het-jaar-2019. (accessed on 29 December 2023).
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A.; et al. Improved Bacterial 16S rRNA Gene (V4 and V4-5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. mSystems 2016, 1. [CrossRef]
- Vancov, T.; Keen, B. Amplification of soil fungal community DNA using the ITS86F and ITS4 primers. FEMS Microbiol. Lett. 2009, 296, 91-96. [CrossRef]
- R Core Team. R: A language and environment for statistical computing. Available online: https://www.R-project.org/ (accessed on 20 March 2024).
- Martin, M. CUTADAPT removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 2011, 17. [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581-583. [CrossRef]
- Abarenkov, K.; Nilsson, R.H.; Larsson, K.-H.; Taylor, Andy F.S.; May, Tom W.; Frøslev, T.G.; Pawlowska, J.; Lindahl, B.; Põldmaa, K.; Truong, C.; et al. The UNITE database for molecular identification and taxonomic communication of fungi and other eukaryotes: sequences, taxa and classifications reconsidered. Nucleic Acids Res. 2023, 52, D791-D797. [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590-596. [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 2013, 8, e61217. [CrossRef]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2013, 41, D36-42. [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.; O'Hara, B.; Simpson, G.; Solymos, P.; Stevens, H.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.2-1 2015, 2, 1-2.
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463-1464. [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228-8235. [CrossRef]
- Wickham, H. (2009). ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York. [CrossRef]
- Cao, Y.; Dong, Q.; Wang, D.; Zhang, P.; Liu, Y.; Niu, C. microbiomeMarker: an R/Bioconductor package for microbiome marker identification and visualization. Bioinformatics 2022, 38, 4027-4029. [CrossRef]
- Ji, P.; Sæbø, A.; Stovin, V.; Hanslin, H.M. Sedum root foraging in layered green roof substrates. Plant and Soil 2018, 430, 1-14. [CrossRef]
- Smalla, K.; Wieland, G.; Buchner, A.; Zock, A.; Parzy, J.; Kaiser, S.; Roskot, N.; Heuer, H.; Berg, G. Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: plant-dependent enrichment and seasonal shifts revealed. Appl. Environ. Microbiol. 2001, 67, 4742-4751. [CrossRef]
- Tan, F.; Wang, J.; Chen, Z.; Feng, Y.; Chi, G.; Rehman, S.U. Assessment of the arbuscular mycorrhizal fungal community in roots and rhizosphere soils of Bt corn and their non-Bt isolines. Soil Biol. Biochem. 2011, 43, 2473-2479.
- Fierer, N.; Leff, J.W.; Adams, B.J.; Nielsen, U.N.; Bates, S.T.; Lauber, C.L.; Owens, S.; Gilbert, J.A.; Wall, D.H.; Caporaso, J.G. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 21390-21395. [CrossRef]
- Maestre, F.T.; Delgado-Baquerizo, M.; Jeffries, T.C.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Quero, J.L.; García-Gómez, M.; Gallardo, A.; Ulrich, W.; et al. Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 15684-15689. [CrossRef]
- Delgado-Baquerizo, M.; Oliverio, A.; Brewer, T.; Benavent-González, A.; Eldridge, D.; Bardgett, R.; Maestre, F.; Singh, B.; Fierer, N. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320-325. [CrossRef]
- Barberán, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2012, 6, 343-351. [CrossRef]
- Waite, D.W.; Chuvochina, M.; Pelikan, C.; Parks, D.H.; Yilmaz, P.; Wagner, M.; Loy, A.; Naganuma, T.; Nakai, R.; Whitman, W.B.; et al. Proposal to reclassify the proteobacterial classes Deltaproteobacteria and Oligoflexia, and the phylum Thermodesulfobacteria into four phyla reflecting major functional capabilities. Int. J. Syst. Evol. Microbiol. 2020, 70, 5972-6016. [CrossRef]
- Wehner, J.; Powell, J.R.; Muller, L.A.H.; Caruso, T.; Veresoglou, S.D.; Hempel, S.; Rillig, M.C. Determinants of root-associated fungal communities within Asteraceae in a semi-arid grassland. J. Ecol. 2014, 102, 425-436. [CrossRef]
- Egidi, E.; Delgado-Baquerizo, M.; Plett, J.M.; Wang, J.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K. A few Ascomycota taxa dominate soil fungal communities worldwide. Nat. Commun. 2019, 10, 2369. [CrossRef]
- Põlme, S.; Abarenkov, K.; Nilsson, R.H.; Lindahl, B.; Clemmensen, K.; Kauserud, H.; Nguyen, N.; Kjøller, R.; Bates, S.; Baldrian, P.; et al. FungalTraits: a user-friendly traits database of fungi and fungus-like stramenopiles. Fungal Diversity 2020, 105, 1-16. [CrossRef]
- Boyce, K.; Andrianopoulos, A. Fungal dimorphism: The switch from hyphae to yeast is a specialized morphogenetic adaptation allowing colonization of a host. FEMS Microbio. Rev. 2015, 39. [CrossRef]
- Rai, M.; Agarkar, G. Plant-fungal interactions: What triggers the fungi to switch among lifestyles? Crit. Rev. Microbiol. 2016, 42, 428-438. [CrossRef]
- Marttinen, E.M.; Niemi-Kapee, J.; Laaka-Lindberg, S.; Valkonen, J.P.T. Fungal pathogens infecting moss green roofs in Finland. Urban Forestry & Urban Greening 2020, 55, 126812. [CrossRef]
- Bensch, K.; Braun, U.; Groenewald, J.Z.; Crous, P. The genus Cladosporium. Stud. Mycol. 2012, 72, 1-401. [CrossRef]
- Fröhlich-Nowoisky, J.; Pickersgill, D.; Despres, V.; Pöschl, U. High diversity of fungi in air particulate matter. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 12814-12819. [CrossRef]
- Núñez, A.; García, A.M.; Moreno, D.A.; Guantes, R. Seasonal changes dominate long-term variability of the urban air microbiome across space and time. Environment International 2021, 150, 106423. [CrossRef]
- Tordoni, E.; Ametrano, C.G.; Banchi, E.; Ongaro, S.; Pallavicini, A.; Bacaro, G.; Muggia, L. Integrated eDNA metabarcoding and morphological analyses assess spatio-temporal patterns of airborne fungal spores. Ecol. Indic. 2021, 121. [CrossRef]
- Marčiulynas, A.; Lynikienė, J.; Marčiulynienė, D.; Gedminas, A.; Menkis, A. Seasonal and Site-Specific Patterns of Airborne Fungal Diversity Revealed Using Passive Spore Traps and High-Throughput DNA Sequencing. Diversity 2023, 15, 539. [CrossRef]
- Gönczöl, J.; Révay, Á. Fungal spores in rainwater: Stemflow, throughfall and gutter conidial assemblages. Fungal Diversity 2004, 16.
- Bowers, R.; Clements, N.; Emerson, J.; Wiedinmyer, C.; Hannigan, M.; Fierer, N. Seasonal Variability in Bacterial and Fungal Diversity of the Near-Surface Atmosphere. Environ. Sci. Technol. 2013, 47. [CrossRef]
- Gandolfi, I.; Bertolini, V.; Ambrosini, R.; Bestetti, G.; Franzetti, A. Unravelling the bacterial diversity in the atmosphere. Appl. Microbiol. and Biotechnol. 2013, 97, 4727-4736. [CrossRef]
- Zhong, S.; Zhang, L.; Jiang, X.; Gao, P. Comparison of chemical composition and airborne bacterial community structure in PM2.5 during haze and non-haze days in the winter in Guilin, China. Sci. Total Environ. 2019, 655, 202-210. [CrossRef]
| ID | City | Coordinates | Year | Height (m) | Area (m2) | Type |
|---|---|---|---|---|---|---|
| R1 | Ghent | 51.0239N 3.7665E | 2014 | 3.2 | 25 | Sedum-herbs-grasses |
| R2 | Ghent | 51.0479N 3.7419E | 2015 | 3.4 | 76 | Sedum-herbs-grasses |
| R3 | Ghent | 51.0457N 3.7509E | 2005 | 10.5 | 110 | Sedum-moss |
| R4 | Ghent | 51.0766N 3.7211E | 2013 | 8.4 | 588 | Sedum-moss |
| R5 | Hasselt | 50.9285N 5.3430E | 2015 | 7.0 | 225 | Sedum-herbs-grasses |
| R6 | Hasselt | 50.9338N 5.3419E | 2012 | 6.0 | 81 | Sedum-moss |
| R7 | Hasselt | 50.9263N 5.3410E | 2004 | 3.0 | 175 | Sedum-herbs-grasses |
| R8 | Antwerp | 51.1927N 4.4163E | 2014 | 22.3 | 708 | Sedum-moss |
| R9 | Antwerp | 51.1693N 4.3941E | 2008 | 9?0 | 280 | Sedum-moss |
| R10 | Antwerp | 51.2507N 4.4190E | 2009 | 17.4 | 777 | Sedum-herbs-grasses |
| R11 | Antwerp | 51.2302N 4.4165E | 2015 | 9.0 | 312 | Sedum-herbs-grasses |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





