1. Introduction
Sunflower (
Helianthus annuus L.) is the fourth largest oil crop and was cultivated mainly in East Europe, China, Turkey ect. According to statistics from the Food and Agriculture Organization of the United Nations (FAO), the annual planting area of sunflowers was 27.3 million hm
2 in the world with a total yield of 56.1 million tons in 2021. According to statistics from the Ministry of Agriculture and Rural Affairs of the People’s Republic of China (
http://www.moa.gov.cn/), sunflower planting area was 0.92 million hm
2 in 2022, Inner Mongolia is the most biggest sunflower planting region, its planting areas is around 0.45 million hm
2.
At present, there are at least 30 sunflower diseases reported in the world. Among them, three types of Sunflower Head Rot was reported and severely affect Sunflower yield.
Pectobacterium carotovorum and
P.atrosepticum were reported to cause Bacterial Head Rot [
1,
2], the
R.stolonifer /
R.oryzae (syn.R.arrhizus) and
R.microsporus causing Rhizopus Head Rot [
3,
4]. The
Sclerotinia sclerotiorum could cause the Sclerotinia Head Rot [
5]. In fact, in addition to Head Rot caused by bacteria, it is difficult to distinguish symptom between Head Rot caused by two kind of different fungi. In 2020 and 2021, a disease survey was conducted in four major sunflower-planting areas of Inner Mongolia (bayannur, Erdos, Ulanqab, and Baotou) during R1 ~ R8 stages of sunflower. The symptoms were rather similar to sunflower head rot caused by
Rhizopus spp. The typical symptom is circular dark-brown lesions were observed at the border of the bracts and also the backside of the receptacle. The leison size on receptacle was 7.5 ~ 9.6 cm (length) ×5.5~8.6 cm (width). The leisons was expanded quickly under high perciptation condition, thus causing the wilt of the bracts and the soft rot of the receptacle. White hyphae were always observed at the infection sites.
In order to identify the pathogen caused SDR, Koch’s postulate combined with molecular technique were performed. The phylogeny tree was also constructed with sequence of PCR bands which was obtained with primers against ITS and also EF-1a so as to identify the isolate specifically. The growth rate of myclium was measured to screen the effective fungicides to control the isolated strains. The results obtained in this study is the first report of the causal agent caused SDR and this will enrich the sunflower disease list of and alert the researcher to pay attention to this new disease.
2. Materials and Methods
2.1. Isolation and Culture
The SDR samples were collected from different fields of Guyang county (110°21′16″E, 41°12′50″N), BaoTou city, Inner Mongolia. All samples were packed in paper bags, and brought back to the laboratory for pathogen isolation.The boundaries tissues of the lesions were cut into 3~ 5mm slices, surface sterilized with 5% sodium hypochlorite for 1 minute, followed by rinsed with sterilized distilled water for 3 times. The sterilized slices was placed on Water Ager (WA) plates (five pieces per plate) for culture. Mycelia around the disinfected tissues were picked out with sterilized needle, and pure culture were obtained from the single spore, which were picked up after serials dilution on WA medium.
For pathogen identification, morphological characteristics of the isolates were recorded. Morphological characteristics were observed and photographed using an electron microscope (OLYMPUs, Chongqing, China).The colony morphology and colour of the pure cultures were recorded after six days of incubation at 25 ℃ on PDA plates. The isolated strains were preliminarily identified as Fusarium spp. according to the ‘Fungi Identification Manual’ [
6] and ‘Fusarium’ [
7].
2.2. DNA Extraction, PCR Amplification and DNA Sequencing
Mycelia were harvested from plate for DNA extraction. DNA was extracted according to the CTAB method and the quality was checked on 1.2% agarose gel. The primers ITS1(5’-TCCGTAGGTGAACCTGCGG-3’) and ITS4 (5’-TCCTCCGCTTATTGAT--ATGC-3’) [
8] and EF1α-F(5’-ATGGGTAAGGA(A/G)GACAAGAC-3’) and EF1α-R (5’-GGA(G/A)GTACCAGT(G/C)ATCATGTT-3’) [
9] were used to perform amplification. A 25 μL reaction mixture containing 0.5 μL taq DNA polymerase, 2.5 μL Taq buffer (2.5 mM ), 2 μL dNTPs (2.5 mM each), 1 μL of each primer (10 pm each) and 50 ng genomic DNA. For both reaction, the cycle parameters were an initial denaturation step at 94 °C for 5 min, 35 cycles at 94 °C for 40 s, 55 °C for 40 s, 72°C for 40 s, and final extension at 72 °C for 10 min. The annealing temperatures were 55 °C for ITS and 56 °C for EF-1α, seperately. The annealing temperatures were 55 °C and 56 °C for ITS and EF-1α primer pair seperately. The PCR products were purified using a PCR product purification kit (Life Technologies, Carlsbad, CA, USA) and submit to Beijing Hooseen Biotechnology Co., Ltd. for sequencing. The obtained sequences were compared with the sequences of related species which were retrieved from GenBank (
Table 1). The phylogenetic tree was constructed by using MEGA 7.0 software with the Maximum likelihood method [
10]. The bootstrap test was set to 1000 times.
2.3. Pathogenicity Test
To test the pathogenicity of isolates, the conidia suspension which were produced on wheat bran medium and the concentration was adjusted to 5 × 106 spores/mL for inoculation on leaves and roots. PDA plug which cut from margin of the fresh colony were inoculated on stem. The inoculated plant were obtained by sowing three seeds of LD5009 (provided by Kafry Technology Co., Ltd, Beijing) in pot (13cm height × l3cm diameter), which contained nutrient soil and field soil (1/5, v/v) and kept in a chamber with 25 to 28 °C, 70% RH with a 12 h photoperiod. During V4 stage, the sunflower leaves and stems were wiped with 75% alcohol for surface sterilization, then, 20μL conidia suspension(5 × 106 spores/mL)was dropped using a hypodermic needle.
For the roots inoculation, 100 μL conidia suspension (5×106 spores/mL) were poured into pot, the sterilized water was poured into pot as a blank control. The experiment was repeated for three times.
The pathogenicity of isolates on flower disk was performed in field. Briefly, in R-5 stage of LD5009, 100mL conidia suspension (5×106 spores/mL)was injected into the backside of the flower disk by using a sterile syringe. Three infection sites equally distributed on the backside of flower disk was set up as replicates. The inoculated flower disk were covered with a dark plastic bag for overnight to keep moisture. After 7 days post inoculation, the disease leisons was recorded and the size were measured precisly.
2.4. Re-Isolation of Pathogens on Seeds
When sunflowers are fully mature, 1/8 of the disk is used to collect seeds to detect pathogens. Regarding the isolation of fungi from sunflower seeds, 100 sunflower seeds were randomly selected and cracked open to obtain the seed inside. Surface sterilization of the sunflower seeds was carried out by placing on freshly prepared PDA medium after it was dried in a laminar flow hood. For each petri dish, 10 seeds were placed randomly and incubated at room temperature of 25 ± 2 ℃ for 7 days in a dark chamber. The number of seeds with fungal colonies growing around was counted and calculated the contamination ratio. Fungal colonies were then transferred to new PDA plate for purification and morphological identification.
2.5. Fungicides Sensitivity Evaluation
A total of six fungicide combined with five different concentration were set up in this experiment, the detail information of the treatmets was listed in
Table 2. Each concentration set up five replicates, and the plate without fungicide was used as control. The effects of each fungicide on inhibiting the mycelial growth of the tested isolates were determined by the method described by Chen [
11]. Basically, the PDA plug were cut from the edges of colony of tested isolate and upside down inoculated in the center of the plates amending with different concentration of the tested fungicide. After seven days culture at 25 ℃, the colony diameters were measured by the cross method, and the inhibition rate of mycelial growth was calculated with following formula. The inhibitory effects of the different fungicides on isolates growth were compared and the half-maximal effective concentration value (EC50) was determined based on the measure described by Addrah et al [
12].
Growth inhibition rate (%) = (control colony diameter-treated colony diameter) /control colony diameter)× 100%.
The logarithm of the concentration (X) and the percentage probability value (Y) of inhibition effects on the colony growth was calculated. The virulence regression equation, correlation coefficient, and EC50 value for each fungicide against the isolates were obtained by the least squares method [
13].
3. Results
3.1. Symptoms of Sunflower Disk Rot (SDR)
In 2021, SDR was observed on R6 stage of sunflower in Inner Mongolia, China. Margins of the bract was firstly infected and brown irregular leisons were observed, then the leisons were expanded to the the other parts of bract. Dark brown leisons were also observed at the back side of flower disk (
Figure 1a–c). The tissues of flower disk under the lesions became rot and caused the enlarge of the lesions at the infection sites. (
Figure 1a). After infection, the bract of sunflower became wilt and the flower disk showed the early deteriorate. The average disease incidence was estimated to 8.5~ 11.67% in the investigated fields.
3.2. Pathogen Isolation and Characterization
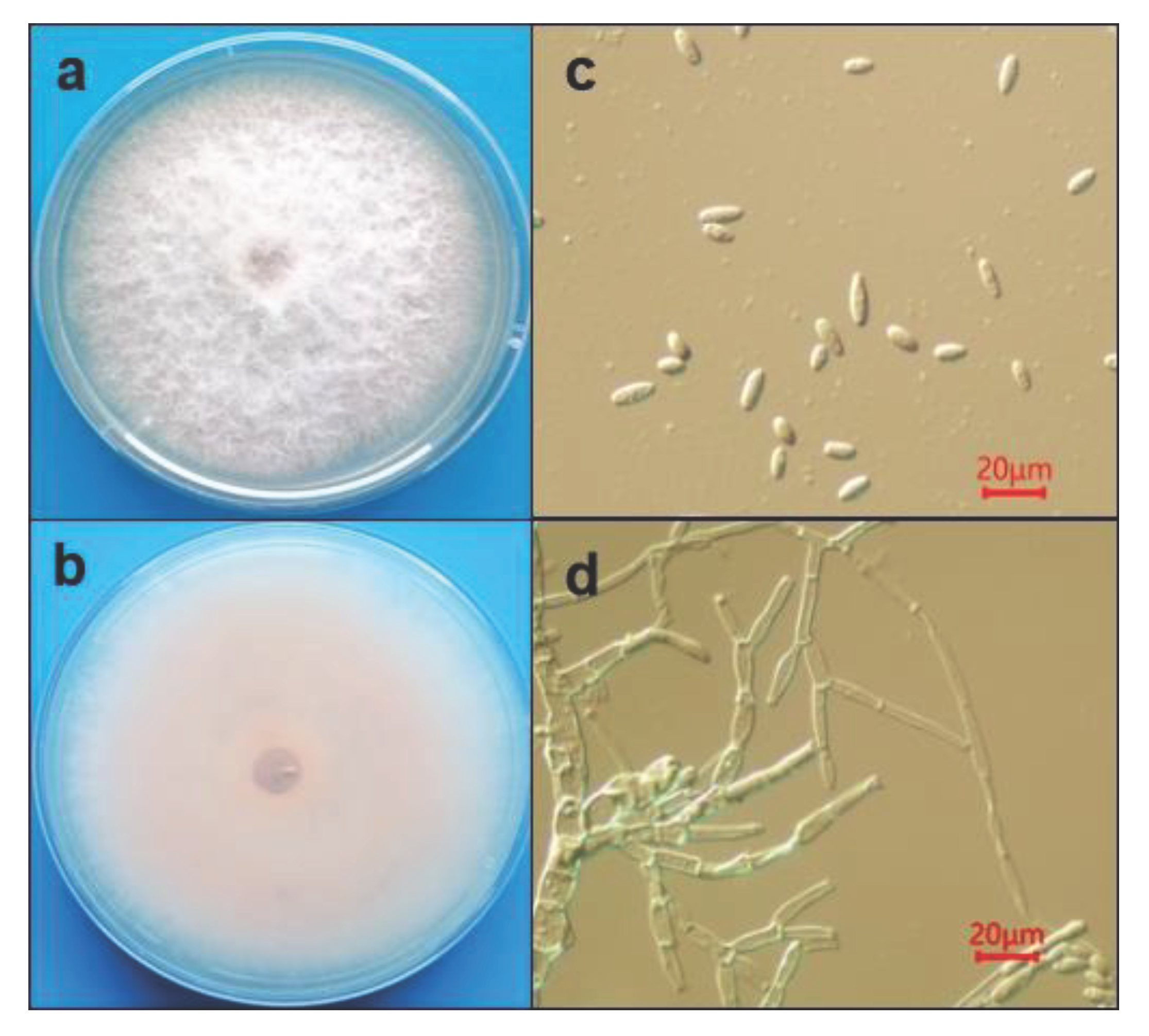
A total of 16 strains were isolated from the diseased bracts. After purification, the white and fluffy morphology of the colonies were observed after 7 days culture (
Figure 2a,b). The light pink color. Was observed on the backside of plate. After 7 days culture, the surface of the colony turned to gray. The aerial hyphae was observed after 36 h cultured, and the filamentous is highly branched and the conidiophores formed at the top of branched hyphae. Conidia produced on the conidiophores is oval or ellipsoidal shape, but no septate, the avegare size of conidia is 12.6 ~ 25.2 μm in length and 3.6 ~ 6.3 μm in width (
Figure 2c,d). Based on the morphology of both colony and conidia, the isolates were tentatively identified as Fusarium sp. [
14]. However, sickle-shaped large conidia were rarely observed on PDA medium.
3.3. Pathogen Identification Molecularly
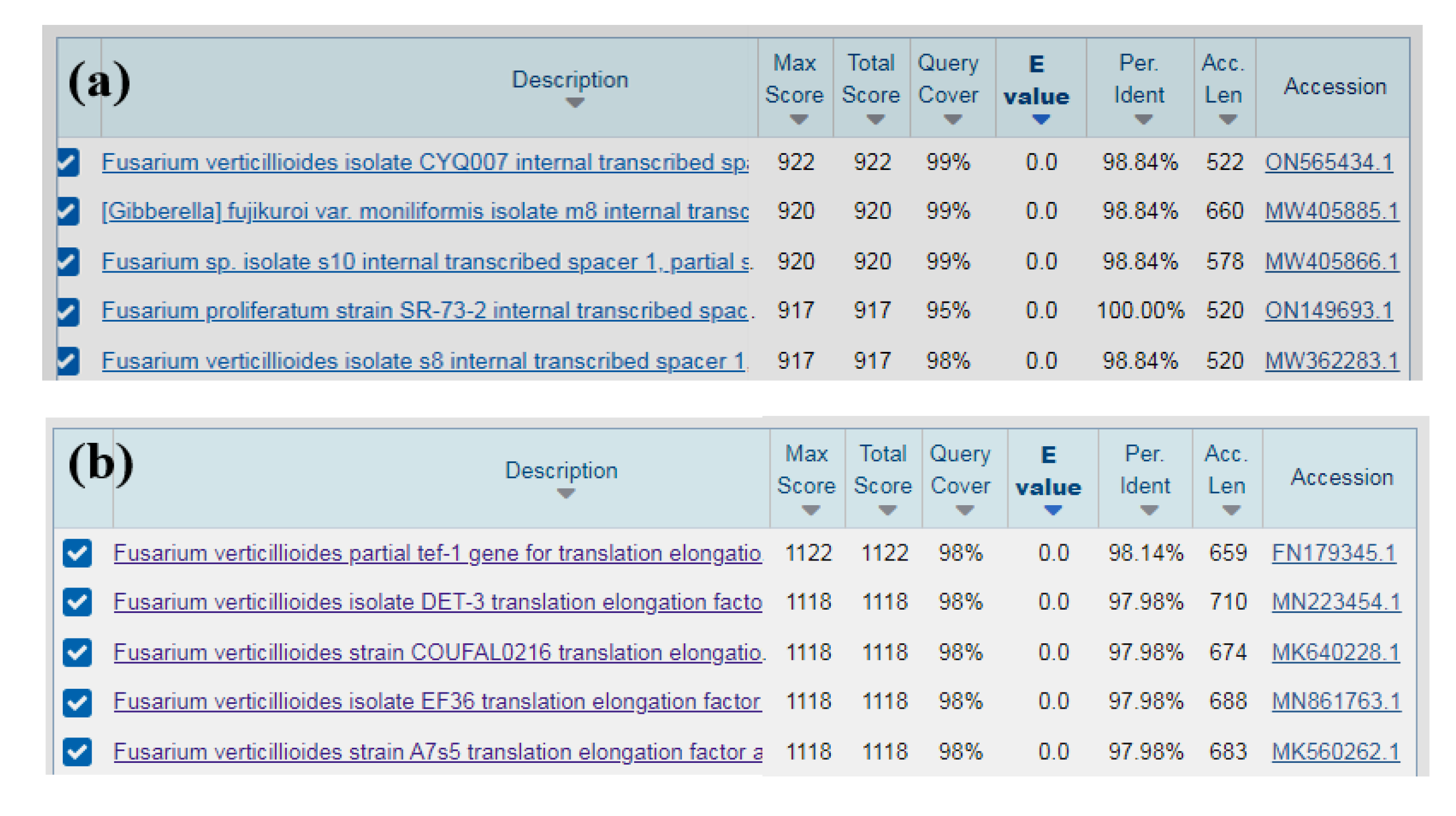
To identified isolate specifically, PCR was performed by using the fungal universal primers ITS1/ITS4 and the Fusarium specific specific primers EF1/EF2. The resulted 520 bp and 682 bp amplicons respectively, were sequenced and querried in GenBank.The results showed that the sequence of the ITS region of isolates (RH-2) is highly similarity (between 98% and 100%) with sequence of
Fusarium verticillioides,
F. proliferatum and
Gibberella sp. (
Figure 3a). The sequences of ITS were submitted to GenBank with the accession No. OP020562. However, the sequence obtained from EF1/EF2 primers showed the 98% identity with
F. verticillioides (Accession No. MN223454.1, MK640228.1 and FN179345.1ect.) (
Figure 3b). From the results of the sequence comparison we believe that the isolate was
F. verticillioides.
3.4. Phylogenetic Analyses
The phylogenetic tree was generated using sequence of EF1-α genes of the isolate (RH-2), together with the other eight Fusarium sp. which were retrieved from Genbank. According to the phylogenetic tree (
Figure 4), the isolate RH-2 was grouped into the same cluster with F. verticillioides, which isolated from both soybean and rice and caused the soybean root rot and rice bakanae disease seperately.
3.5. Pathogenicity Identification
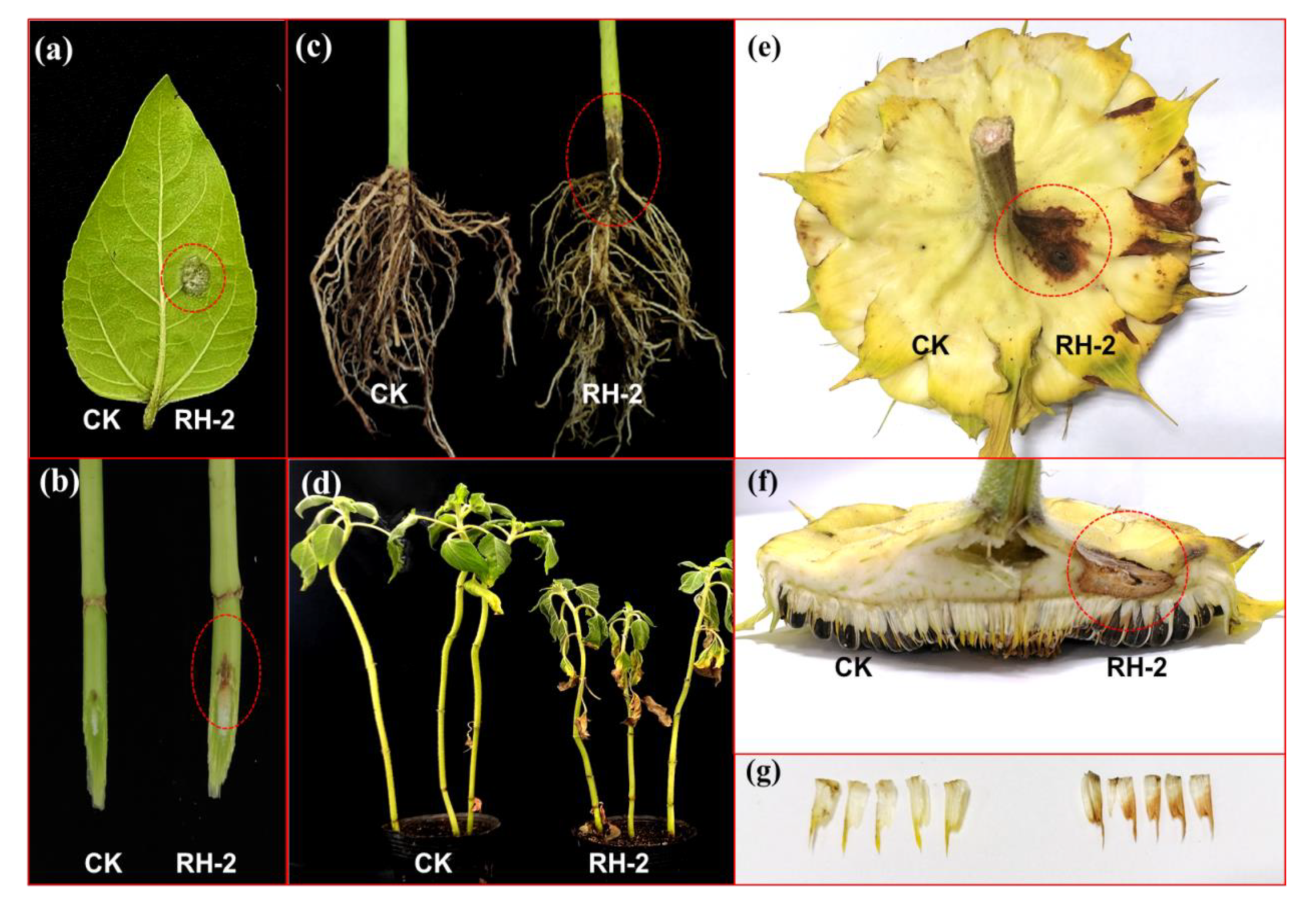
To verify the pathogenicity of the isolate RH-2, conidia suspension was inoculated on sunflower leave, the lesion was observed after 2 dpi, the white to gray mold (mycelium) was observed at the inoculation site 3 dpi, whereas, no symptom was observed on the control site (
Figure 5a). Regarding to the stem inoculation, the expanding of the lesions vertically along the epidermis layer of the stem was observed at the infection sites (
Figure 5b). After inoculation the condia in roots, the basal stem became dark to brown , and the constriction of basal stem were also observed after 15dpi. The sunflower leaves became yellowing and the height of plant is much lower than that of the control. If the disease became severe, the inoculated plant is easily pull out due to the root rot (
Figure 5c). After inoculation the conidia suspension on the bract of flower disk at R5 stage in the field, the brownish leisons were observed at the margin of bract after 7 dpi. The leison also appeared at the infection site of the back side of flower disk.The flower disk rot was observed during R7 stage with high humidity (
Figure 5e,f).Eventually, we also observed browning on small bracts(
Figure 5g).
3.6. Pathogen Reisolation
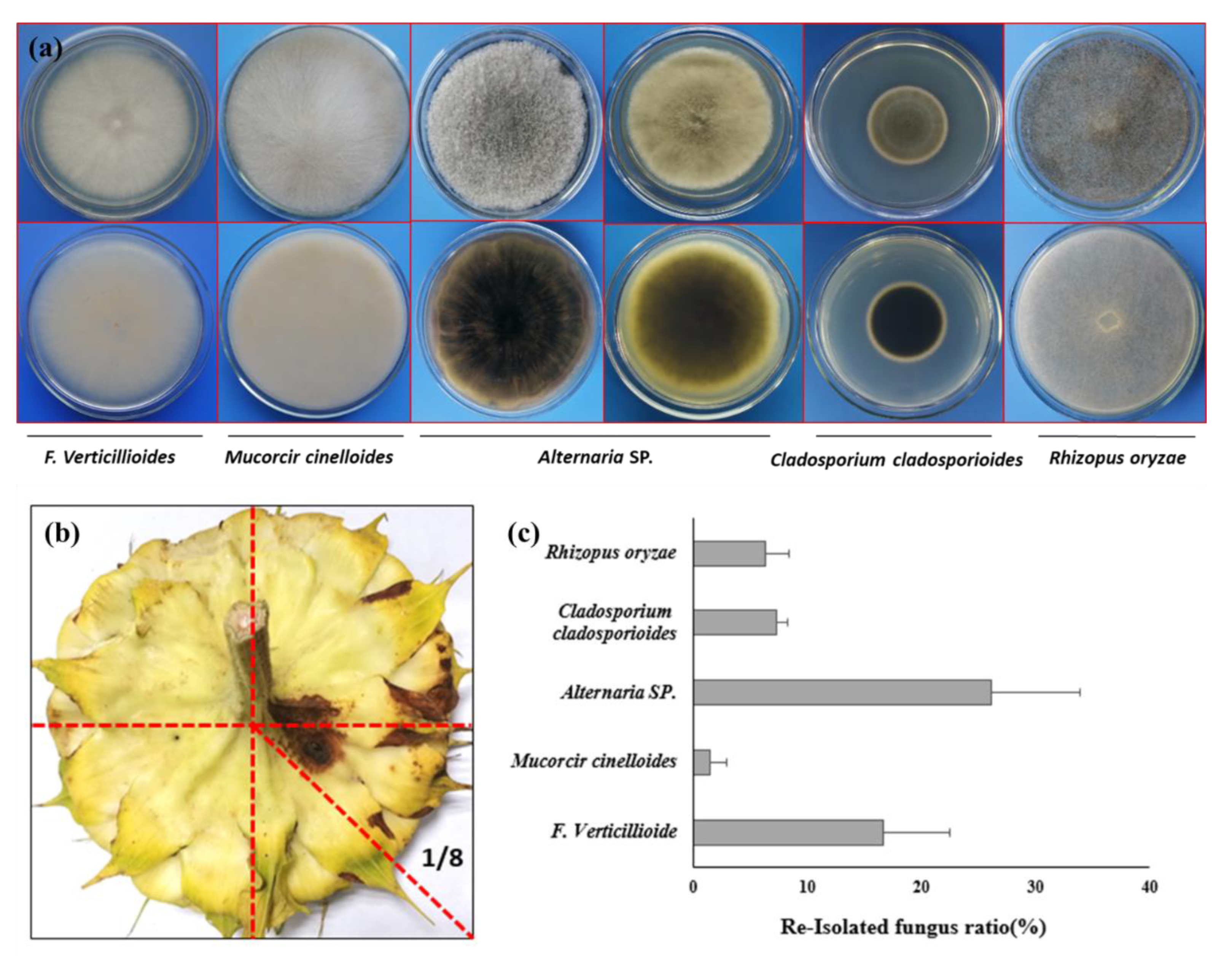
Due to the infection on both bract and also back side of flower disk, it will raising the question that if the pathogen can also caused the contamination of the sunflower seeds after SDR. To verify the hypothesis, we re-isolated the strain from both the infection site and also the seed coat. Just as what we expected, the morphology of isolated strain is completely same with the inoculated one (
Figure 6a). To verify if the sunflower seeds also can be contaminated by inoculated strain, we harvested the seeds from 1/8 of the flower disk (
Figure 6b), where the leison formed in the backside of the flower dish. To our surprise, beside the inoculate strain
F. verticillioides, the other 4 microorganism, such as
Mucorcir cinelloide,
Cladosporium cladosporioides,
Rhizopus oryzare,
Alternaria spp. were also obtained from the seeds coats of harvest sunflower seeds (
Figure 6a). The average isolation frequency of
F. verticillioides from sunflower seeds is 16.9% (
Figure 6c).
3.7. Fungicides Sensitivity of F. verticillioides
The inhibitory effects of selected fungicides on the
F. verticillioides showed that all tested fungicides could inhibit the growth of
F. Verticillioides, but the inhibition ratio is variable due to the concentration of test fungicide is different (
Table 3). The diameters of the colony of
F. verticillioides increased with the dilution ratio of the fungicide increased. The most significant inhibition effects were observed after adding 280 µg/mL of Tebuconazole·dimetachlone and 128 µg/mL Pyraclostrobine in medium, the inhibition rate reach to 86.54% and 84.16% seperately, followed by Flutolanil (160 µg/mL ) and Fludioxonil (5 µg/mL), the inhibition rate are 76.39%, and 76.39%, respectively. The inhibitory effects of Iprodione (500 µg/mL), and Hymexazol (32µg/mL ) were relatively weak, the inhibition rates is only 59.11% and 49.88%.
Regarding to the EC50 values, the tested fungicides showed different EC50 values, the most sensitive fungicide toward F. Verticillioides is Flutolanil, its EC50 value is only 0.05 µg/mL (R = 0.9825), followed by Flutolanil and Pyraclostrobine , the value are 0.96µg/mL (R = 0.9964) and 8.44 µg/mL (R = 0.9907) respectively. Iprodione showed the most highest EC50 value, it is 186.21 µg/mL (R = 0.9919), indicating that Fludioxonil, Flutolanil and Hymexazol can be the best candidate for controlling F. verticillioides.
4. Discussion
Authors should discuss the results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted.The sunflower disk rot can be caused by different pathogen, such as the
R.stolonifer/
R.oryzae (syn.
R.arrhizus) and
R.microsporus causing Rhizopus Head Rot [
3,
4]. The
Sclerotinia sclerotiorum causing Sclerotinia Head Rot [
5], thus affect the seed quality and yield dramatically. In this study, we identified a new pathogen
F. verticillioides, which caused sunflower disk rot (SDR) via Kock’s postulate. The symptoms of SDR is not similar with the symptom caused by
Sclerotinia sclerotirum, which the rot of flower disk dropped easilly. The SDR caused the tissue rot around the infection sites only under high perciptation condition, and the pathogen can expand from the bract to the backside of flower disk, and also can reach to the inside tissue of flower disk thus caused the rot of seeds. To our surprise, the pathogen isolated from flower disk is not only infect flower disk, but also caused symptom on leave, stem and also root. This is stand in line with the report in China, that
F. verticillioide can cause root rot not only on wheat [
15] maize [
16], soybean [
17],tobacco [
18], but also on sugar beet [
19].
In fact, there also have reports on the different
Fusarium spp. caused sunfower wilt. Sunflower wilt is caused by
Fusarium infestation, which starts at the roots of the plant and reaches the stems destroying the cells of the vascular system, ultimately leading to total wilting of the plant. Currently, many
Fusarium species causing sunflower wilt have been reported from all over the world, such as
F. oxysporum f.sp
helianthi [
20],
F. solani [
21],
F. equiseti and
F. culmorum [
22]. However,
F. moniliforme does not cause rotting of the rhizomes and causes yellowing and discoloration of the leaves and drooping [
23].
F. tabactinum [
24] can cause sunflower stems to break easily at the base. Root-rot diseases are still the most important diseases affecting sunflowers.
Besides that, the
F. verticillioides caused damping-off of sunflower also was reported [
25], indicating the
Fusarium spp. infected sunflower is also rather popular in sunflower planting region. Based on the previous report,
Fusarium could infected sunflower root via both vascular system and also epidermis layer. In principle, the seeds can be contaminated by
Fusarium spp. and also the multiple pathogens, such as
V. dahliae,
Alternaria spp., and
Rhizopus spp [
12]. In this study, we also tesed the seeds contanimation collected from the infection sites and identified that beside F. verticillioide, several pathogens could also colonize on the seeds coat of sunflower, indicating that seeds transmission will be the main primiary infection resources for SDR. However, the infection resources of SDR is still a question mark. We hypothothed that the conidia on the diseased sunflower basel stem may be the resouces for the SDR. At least, the infection of SDR is not systematially, it was supposed to be infected by conidia. Therefore, the sunflower seeds coated with fungicides, such as Fludioxonil, Flutolanil and Hymexazol is rather important to control sunflower wilt caused by
Fusarium spp, but also can control SDR to keep the seeds quality and yield.
In a word, this is the first report of SDR caused by F. verticillioide in China. This study will not only broaden the disease list of sunflower in China, but also alert sunflower breeder to pay attention on generating the new sunflower variety against Fusarium sp.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, J.Y. and J.Z.; methodology, J.Y. and J.Z.; software, W.Z.; validation, A.E., B.L. and J.Z.; formal analysis, J.Y. and W.Z.; investigation, J.Y. B.L. and J.Z; resources, B.L. and J.Z.; data curation, J.Y. W.Z. and J.Z.; writing—original draft preparation, J.Y.; writing—review and editing, J.Z.; visualization, J.Y.and J.Z.; supervision, J.Z. and J.Z.; project administration, J.Z. and J.Z; funding acquisition, J.Z. and J.Z. All authors have read and agreed to the published version of the manuscript.”
Funding
“This research was funded by China Ministry of Agriculture, China Agricultural Research System(CARS-14), Central Guided Local Science and Technology Development Funds(2022ZY0075) and Inner Mongolia Key Fund for Science and Technology.
Data Availability Statement
Not applicable.
Conflicts of Interest
“The all authors declare no conflicts of interest, We declare that we have no financial and personal relationships with other people or organizations that inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
References
- Jeong, Ho. Kim.; Yong, Ho. Joen.; Sang, Gyu. Kim.; Young, Ho. Kim.. First report on bacterial soft rot of graftcactus chamaecereus silvestrii caused by pectobacterium carotovorum subsp. carotovorum in korea. Plant Pathology Journal.2007, 23(4), 314-317.
- Bauftauf, K.; K., Hekimhan, H.; Maden, S.;Tufr, M. First report of bacterial stalk and head rot disease caused by pectobacterium atrosepticum on sunflower in turkey. Plant disease. 2009,93(12), 1352.
- Rasera, K.; NM Osório, Mitchell, D. A.; Krieger, N.; Ferreira-Dias, S. Interesterification of fat blends using a fermented solid with lipolytic activity. Journal of Molecular Catalysis B Enzymatic.2002,76(none), 75-81.
- Mathew, F. M.; Prasifka, J. R.; Gaimari, S. D.; Shi, L.; Gulya, T. J. Rhizopus oryzae associated with melanagromyza splendida and stem disease of sunflowers (helianthus annuus) in california. Plant Health Progress.2015, 16(1), 39-42.
- Gregoire, T.; Lamey, A.; Hofman, V. Sclerotinia head rot of sunflower. Magnetic Resonance Imaging Clinics of North America.2010,21(2), 6-18.
- Wei, J.C. Manual of Fungal Identification; Shanghai Science and Technology Press: Shanghai, China, 1979. 609-928.
- Booth, C.; Chen, Q.. Agriculture Press: Beijing, China, 1998.
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics.PCR Protoc. Guide Methods Appl. 1990, 18, 315-322.
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Sciences. 1998, 95, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, H.; Chen, C.; Zhou, M. Sensitivity of fusarium graminearum to fungicide js399-19: In vitro determination of baseline sensitivity and the risk of developing fungicide resistance. Phytoparasitica 2008, 36(4), 326–337. [Google Scholar] [CrossRef]
- Addrah, M.E.; Zhang, Y.; Zhang, J.; Liu, L.; Zhou, H.; Chen, W.; Zhao, J. Fungicide Treatments to Control Seed-borne Fungi of Sunflower Seeds. Pathogens. 2019, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M. G.; Ye, Z. Y.; Liu, J. F. Progress of fungicide resistance. 1994.
- Leslie, J. F; Summerell, B. A. USA: Blackwell Publishing, 2006.
- 15. Dong F; Li Y; Chen X; Wu J; Wang S; Zhang X; Ma G; Lee Y W; Mokoena M.P; Olaniran A.O; Xu JH; Shi J R. Analysis of the Fusarium graminearum species complex from gramineous weeds near wheat fields in Jiangsu Province, China. Plant Disease 2021, 105(10), 3269–3275. [CrossRef] [PubMed]
- Gai, X. T.; Xuan, Y. H.; Gao, Z. G. Diversity and pathogenicity of Fusarium graminearum species complex from maize stalk and ear rot strains in northeast China. Plant Pathology 2017, 66(8), 1267–1275. [Google Scholar] [CrossRef]
- Chang, X.; Dai, H.; Wang, D.; Zhou, H.; He, W.; Fu, Y.; Ibrahim, F.; Zhou, Y.; Gong, G.; Shang, J.; et al. Identification of Fusarium species associated with soybean root rot in Sichuan Province, China. Euroual Journal Plant Pathology 2018, 151(3), 563–577. [Google Scholar] [CrossRef]
- Gai, X. T.; Jiang, N.; Lu, C. H.; Xia, Z. Y.; He, Y. S. First report of tobacco Fusarium root rot caused by Fusarium verticillioides in China. Plant Disease 2021, 105(11), 3762. [Google Scholar] [CrossRef]
- Cao, S.; Yang, N.; Zhao, C.; Liu, J.; Han, C.; and Wu, X.. Diversity of Fusarium species associated with root rot of sugar beet in China.Journal of General Plant Pathology.2018, 84(5): 321-329.
- El, Mahjoub. M.. Vascular Fusarium wilt of sunflower in Tunisia caused by Fusarium oxysporum (Sn, et H.) f. sp. helianthi nov. sp. Annales de 1’ Institut National de la Recherche Agronomique de Tunisie,1975,48(3):3-11.
- Haggag, W. M. ; Amin, A. W.. Efficiency of trichoderma species on control of fusarium rot, root knot and reniform nematodes disease complex on sunflower. Pakistan Journal of Biological ences.2001,4(6), 679-683.
- Pineda, P. J. B.;Avila, M. J. M.. Management and control of sunflower disease in Portuguesa State. FONAIAP Divulga,1991, 9(38):6-8.
- Bhargava, S. N; Shukla, D. N.; Singh, N.. Wilt of sunflower caused by Fusarium moniliforme. Indian Phytopathology,1978.
- Zazzerini, A; Tosi, L.. New sunflower disease caused by Fusarium tabacinum. Plant Disease,1987,71(11):1043-1044.
- Mahmoud, A.. Molecular and biological investigations of damping-off and charcoal-rot diseases in sunflower.2010.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).