1. Introduction
Urolithiasis ranks among the most common global urological conditions, with a prevalence estimated at 8-13% [
1]. Incidence varies regionally due to differences in drinking water quality, dietary patterns, climate, obesity rates, and other health conditions [
1].
Understanding regional epidemiology aids in identifying risk factors, choosing management options, and implementing preventive approaches. Sri Lanka, with its tropical climate, dietary habits, and higher insulin resistance, is /likely to be part of Asia’s "Stone Belt," where urolithiasis prevalence is elevated [
2].
Considering variations in water quality and climate within Sri Lanka, stone chemical compositions might differ across regions. Analyzing stone composition is clinically crucial and impacts treatment, secondary prevention, and prognostication [
3]. While calcium oxalates are common in urinary stones, but composition varies due to factors like diet, genetics, and underlying medical conditions [
4]. This study aims to assess diverse chemical compositions of urinary stones in northern Sri Lanka and determine the prevalence of metabolic disorders among urolithiasis patients seeking treatment at the region’s sole tertiary treatment center.
2. Methodology
This study is a prospective, laboratory-based, cross-sectional investigational study conducted among urolithiasis patients who underwent surgical interventions at Teaching Hospital Jaffna over one year from July 2022 to June 2023. Ethical clearance was obtained from the ethics review committee of the institution (J/ERC/22/133/NDR/270) and informed written consent was obtained from participants.
An interviewer-administered questionnaire was used to collect patient details, including age, gender, presenting complaint, family history, and co-morbidities.
A separate data extraction form was utilized to record investigational findings, encompassing ultrasound scans (USS/KUB), computer tomography results (NCCT/KUB), stone analysis, serum analysis (Serum Calcium, Serum Phosphate, Serum Uric Acid, and Serum Creatinine), and urinalysis findings (urine full report, 24-hour urine analysis of Calcium, Oxalate, Citrate, Uric acid, Magnesium and Phosphate).
The chemical composition of stones was analyzed using Fourier transform infrared spectroscopy and classified according to the system adopted by Abdel Halim et al [
5].
3. Results
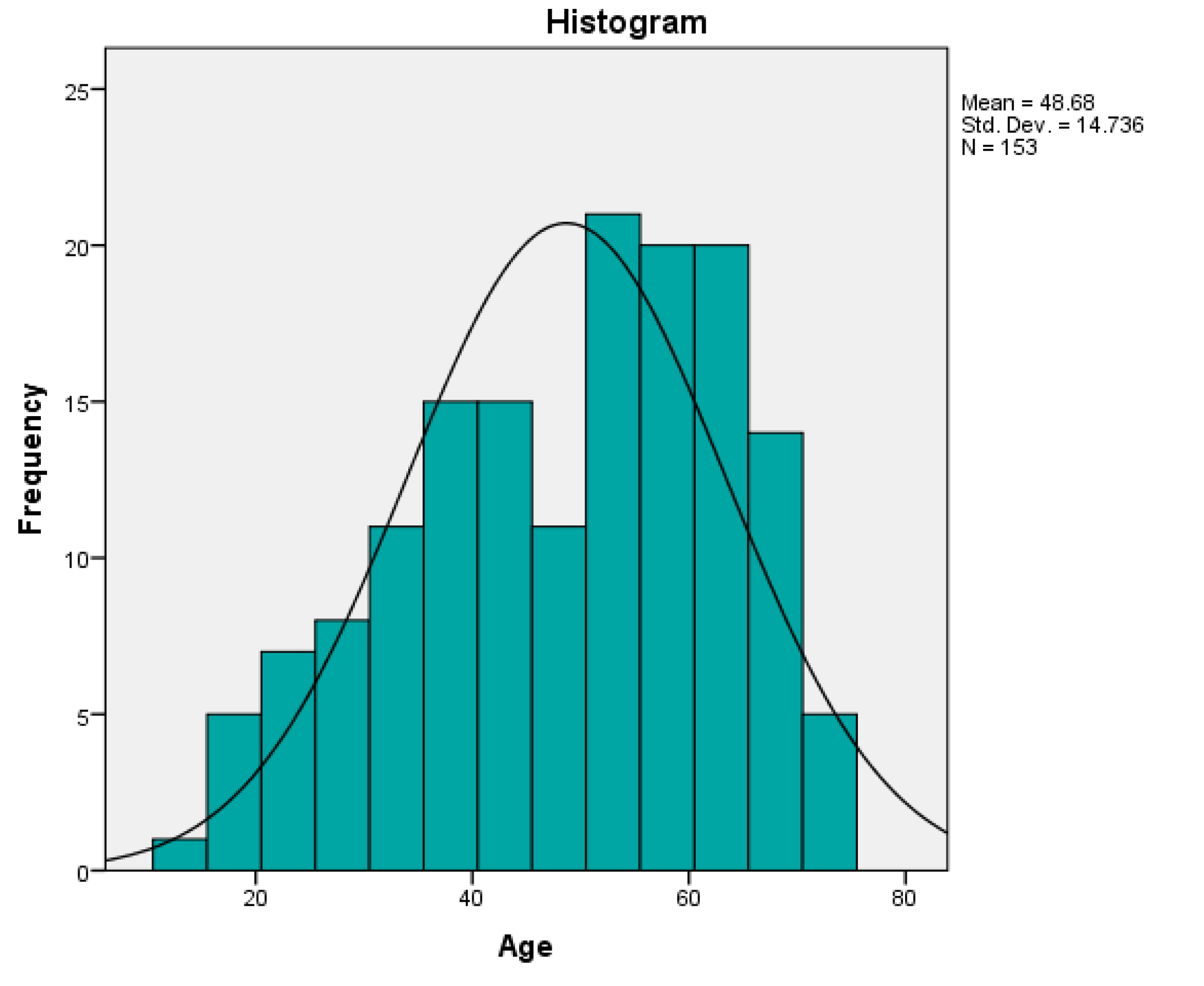
A total of 153 surgically treated urolithiasis patients were recruited over one year period for the study and majority were men -64.3%). The mean age of the patients was 48.64(SD=14.7). The majority of patients fell into 40-59 age group (46.4%) (
Table 1).
Diabetes mellitus (23.5%) was the most common comorbidity associated with urolithiasis patients, followed by hypertension and hyperlipidemia (
Table 2). Nearly a quarter of patients (26.1%) had high serum creatinine at the time of the surgical procedure, and in the majority of them, renal function improved during follow-up.
Ureteric colic was by far the most common complaint with 48.4% of the patients presenting with it, followed by loin pain (16.3%) and lower urinary tract symptoms (LUTS) (13.1%). A smaller proportion presented with non specific abdominal pain or haematuria. 6 (3.9%) patients were detected incidentally (
Table 3)
The most prevalent stone location was kidney with 45.8% of patients having renal stones. Ureteric stones were also a common diagnosis with 43.8% of patients, while bladder/urethral stones were less common (10.5%). Notably majority of patients (57.52%) had recurrent stones.
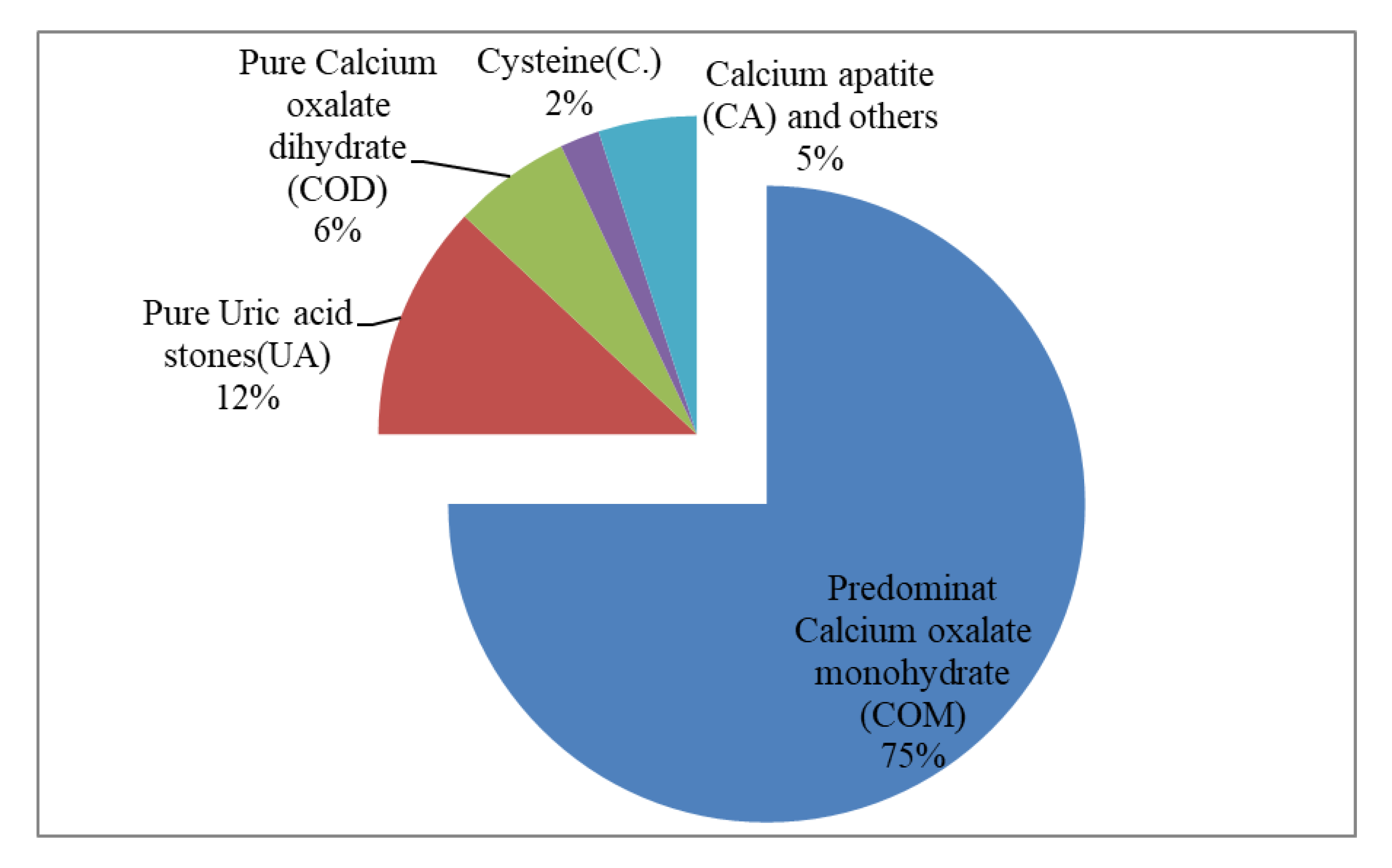
The distribution of the types of stones based on chemical composition is illustrated in
Figure 2. The majority of the stones were calcium oxalate monohydrate (COM) predominant stones accounting for more than three-quarters of the cases (78.4%). Pure Uric acid stones amounted to (12.4%) of the cases. Pure Calcium oxalate dihydrate (COD), cysteine, and calcium apatite (CA) stones were found in the remaining cases.
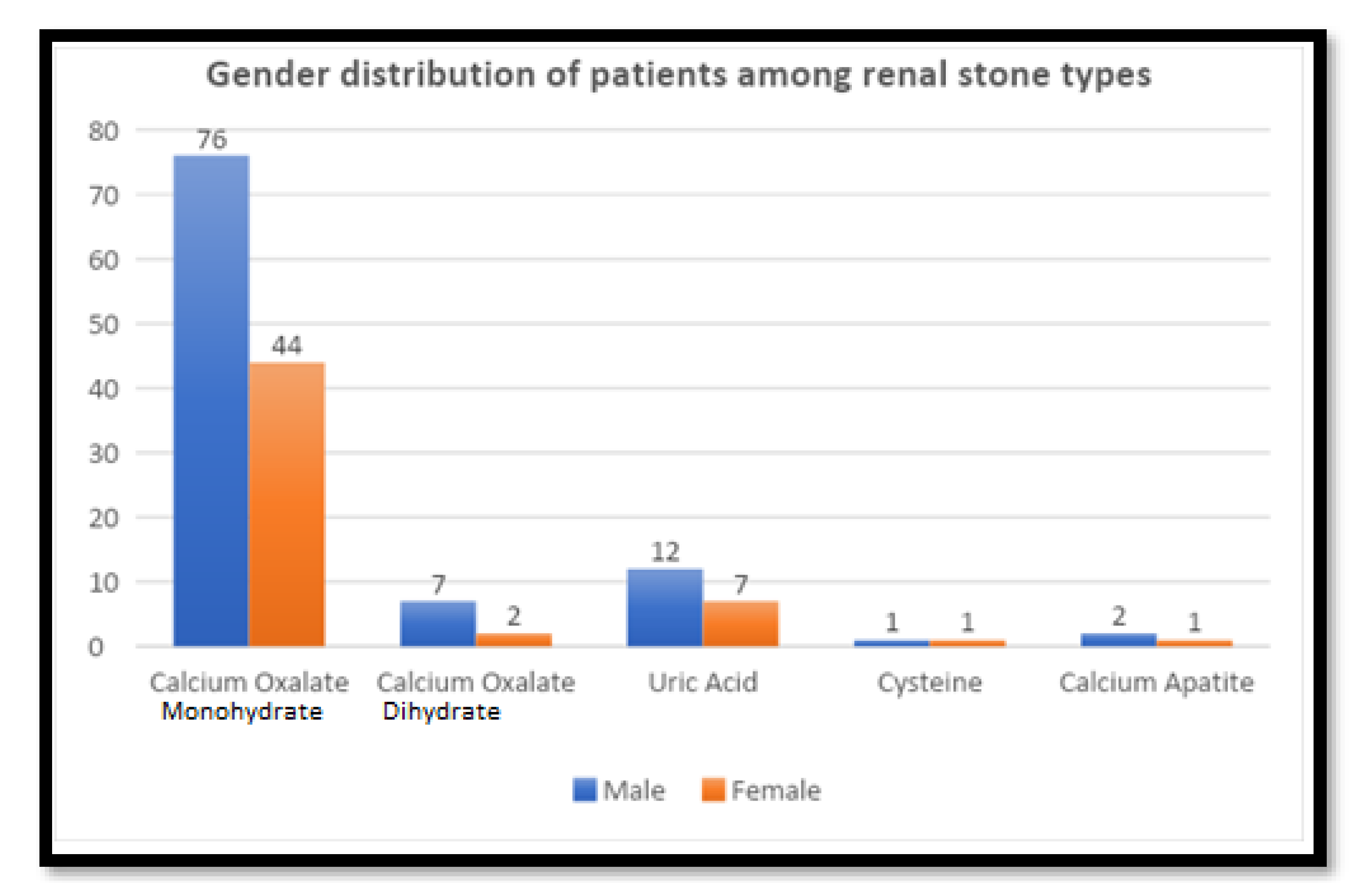
Overall, the dataset indicates a male predominance in urolithiasis cases, with males having a higher prevalence across all stone types compared to females. However, there was no statistically significant association between the type of stone and gender (p = 0.917) (
Figure 3).
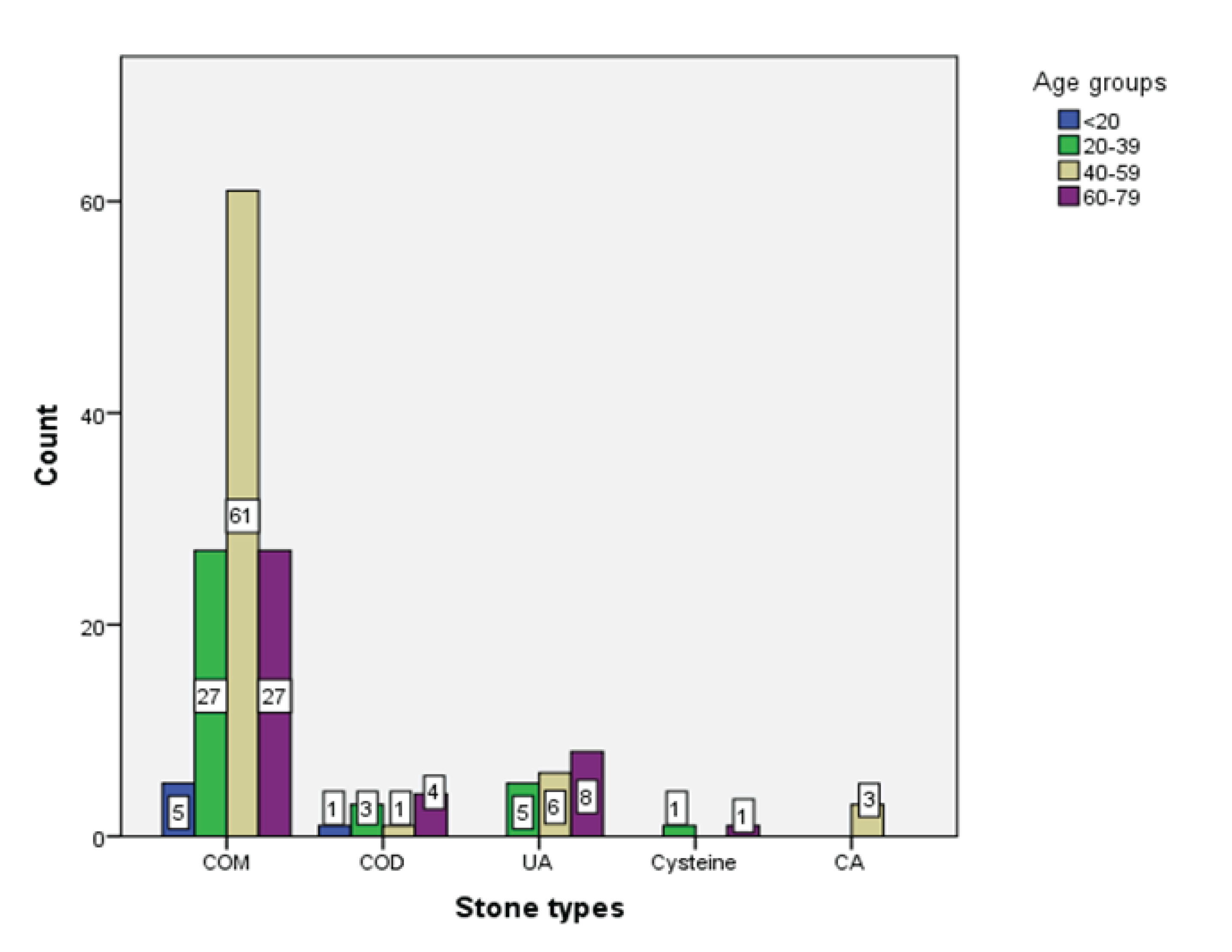
The most prevalent stone type is Calcium Oxalate Monohydrate (COM) across all age groups, with the highest count in the 40-59 age group (n=61), followed by Uric Acid (UA) stones which showed an increasing trend with age, peaking in the 60-79 age group with 8 cases. All cases of Calcium Apatite (CA) belonged to the 40-59 age group. Overall, the dataset suggested that the prevalence of different stone types varied across age groups. There was no significant association between the type of stones and the age groups of patients (p = 0.222 (
Figure 4).
The mean urine oxalate levels were within the normal range for both male and female cohorts. However, the variability as indicated by the standard deviation (SD) and interquartile range (IQR) was higher in males than in females. The mean urine calcium level was below the midpoint of the normal range, while the urine mean phosphorus level was below the lower end. The mean urine uric acid level was within the normal range and was relatively consistent as indicated by the smaller IQR compared to the mean. Overall, these indicated most components of urinary metabolic profiles were within normal range with some components nearing the limits of the normal range in individuals (
Table 4). A small percentage of the patients (13.1%) had no metabolic abnormality and majority (86.9%) had at least a single metabolic abnormality (
Table 5).
Hypocitraturia is the most prevalent metabolic abnormality in the population (60.1%), followed by Hypomagnesuria (35.3%) and Hyperoxaluria (14.4%). Hyper-phosphaturia (11.1%) Hyperuricosuria (6.5%) and Hypercalciuria (5.9%) were the other metabolic abnormalities seen with lower prevalence levels. 23% (n=35) had both hypocitraturia and hypomagnesuria.
The distribution of these abnormalities varies between males and females, with some abnormalities being more prevalent in males than females. However, there was no significant association between gender and metabolic disorders. (p=0.061). There was a marginal association (p < 0.061) between age and metabolic disorders.
3.1. Figure and Tables
Table 1.
Demographic characteristics.
Table 1.
Demographic characteristics.
| Characteristics |
n |
% |
| Sex |
Male |
98 |
64.3 |
| |
Female |
55 |
35.7 |
| Age in years |
< 20 |
6 |
3.92 |
| |
20 -39 |
36 |
23.53 |
| |
40 -59 |
71 |
46.40 |
| |
60 -79 |
40 |
26.14 |
| Occupation |
Students |
3 |
2 |
| |
Skill workers |
93 |
60.8 |
| |
Non-skill workers |
57 |
37.2 |
Figure 1.
Prevalence of urolithiasis with age.
Figure 1.
Prevalence of urolithiasis with age.
Table 2.
Co-morbidities associated with urolithiasis.
Table 2.
Co-morbidities associated with urolithiasis.
Table 3.
Clinical characteristics.
Table 3.
Clinical characteristics.
| Presenting Complaints |
No of patients |
| Ureteric colic |
74 (48.4) |
| Loin pain |
25 (16.3) |
| Abdominal pain (non specific) |
14 ( 9.2) |
| LUTS |
20 (13.1) |
| Haematuria |
3 (2.0) |
| Asymptomatic |
6 ( 3.9) |
| Diagnosis |
|
| Renal stone |
69 (45.8) |
| Stag horn calculi |
17 (11.1) |
| Non-stag horn calculi |
52 (34.0) |
| Ureteric stone |
67 (43.8) |
| Bladder/urethral stone |
16 (10.5) |
| Recurrent stone |
|
| Yes |
88 (57.52) |
| No |
65 (42.48) |
Figure 2.
Chemical composition of stones.
Figure 2.
Chemical composition of stones.
Figure 3.
Gender distribution and stone chemical composition types.
Figure 3.
Gender distribution and stone chemical composition types.
Figure 4.
Age distribution versus chemical composition of urolithiasis.
Figure 4.
Age distribution versus chemical composition of urolithiasis.
Table 4.
Metabolic parameters measured in 24 hours urine analysis.
Table 4.
Metabolic parameters measured in 24 hours urine analysis.
| Parameters |
Mean ± SD |
Inter quartile range |
Urine oxalate
Male (0.08-0.49 mmol/24Hrs) |
0.31 ± 0.48 |
0.20 |
| Female (0.04-0.32 mmol/24Hrs) |
0.21 ± 0.17 |
0.16 |
| Urine calcium (100-300 mg/24Hrs) |
152.89± 98.19 |
132.795 |
| Urine phosphorus (500-750mg/24Hrs |
443.46 ± 205.18 |
273.43 |
| Urine citrate (288-902 mg/24Hrs) |
272.11 ± 149.93 |
176.075 |
| Urine Magnesium (60-210 mg/24Hrs) |
70.61 ± 32.00 |
27.37 |
Table 5.
Prevalence of metabolic abnormalities.
Table 5.
Prevalence of metabolic abnormalities.
| Metabolic abnormality |
Male (n) |
Female (n) |
Total [n (%)] |
| Hyperoxaluria |
11 |
11 |
22 (14.4) |
| Hypercalciuria |
7 |
2 |
9 (5.9) |
| Hyperuricosuria |
8 |
2 |
10 (6.5) |
| Hypocitraturia |
60 |
32 |
92 (60.1) |
| Hypomagnesuria |
27 |
27 |
54 (35.3) |
| Hyperphosphaturia |
10 |
7 |
(11.1) |
4. Discussion
Age and sex have been shown to have an association with the prevalence of urolithiasis [
6]. In our study, we observed a male predominance with a male-to-female ratio of 2:1 among patients treatment for urolithiasis in our institution. The majority of these patients belonged to the age category 40 -59 years.
According to a study based on global burden of diseases data, Males had higher rates of global incidence for all age groups in 2019, with the highest rates occurring from ages 50 to 69 years for both males and females [
7]. A study analyzing the epidemiology of urolithiasis in Asia found a predisposition in Asian countries, with a male-to-female ratio ranging from 1.3 to 5. The study highlighted certain anatomical contrariety; role of testosterone difference in certain dietary habits and climatic conditions are possible reasons for further exploration. The peak incidence of urolithiasis was observed in the age group of 30-60 years [
6].
When looking at the Sri Lankan data, a study among 50 patients from different climatic zones found that the male-to-female ratio was 2.6: 1 [
8]. A study done in Northern Sri Lanka found that 74% of patients seeking surgical treatment for renal stones were males [
9]. The majority of the patients belonged to the age group 40-59. Overall, our findings on the socio-demographic epidemiology of urolithiasis resemble the findings of the regional and national studies.
Diabetes mellitus (23.5%) was the most common co-morbidity among urolithiasis patients in this cohort, followed by hypertension, hyperlipidemia, and ischemic heart disease. This finding aligns with and supports the significance of insulin resistance in urinary tract stone formation. Nearly a quarter of patients (26.1%) had high serum creatinine at the time of the surgical procedure, which significantly improved in the majority following the unblocking of the renal system through surgical interventions. This underscores the importance of early intervention in urolithiasis patients with impaired renal function.
The prevalence of different types of stones based on chemical composition provides key insights into the major risk factors for stone disease in the population and the possible prevention strategies [
10,
11].
Calcium oxalate monohydrate (COM) stones (78.4%) were by far the most prevalent stone type among our patients. Uric acid stones, Calcium oxalate dihydrate (COD), cysteine, and calcium apatite (CA) stones were also found at lower percentages.
The prevalence of different types of stones varies significantly among countries and regions. In most countries of Asia, calcium oxalate (75% - 90%) is the most common component of stones, followed by uric acid (5% - 20%), calcium phosphate (6% - 13%), struvite (2% - 15%), apatite(1%) and cysteine (0.5% - 1%) [
6]. In a Chinese study among 507 patients from Southern China found Calcium Oxalate (78.3%) to be the most prevalent stone type in China. Uric acid stones were found in 3.6%, and calcium phosphate in 3.4% of the patients [
12].
According to an Indian study that analyzed 1050 stones, (93.04%) were calcium oxalate stones, out of which 80% were calcium oxalate monohydrate (COM) and 20% were calcium oxalate dihydrate (COD). Uric acid stones accounted for only (0.95%) of the stones [
13]. Our study aligns with the majority of the aforementioned findings from different studies, indicating that calcium oxalate stones are the most prevalent type, with calcium phosphate and cysteine constituting smaller percentages. However, our study observed a higher proportion of uric acid stones.
When looking at Sri Lankan studies that analyzed the chemical composition of stones in 50 patients from different climate zones of Sri Lanka revealed that Calcium Oxalate stones constituted 86% of renal stones [
8]. Phosphate and struvite calculi amounted to 2% each. Another study that analyzed the mineralogical and chemical composition of stones also found that the majority of urinary calculi were Calcium Oxalate stones. Struvite calculi, Uric acid calculi, and Hydroxy Apatite calculi were other prevailing types [
14].
Focusing in Northern Sri Lanka, a study carried out among 104 patients with renal stones revealed that 75.9% of the patients had calcium oxalate and 13.5 % had uric acid stones [
15].
Our findings align with those of other Sri Lankan studies, highlighting consistency despite variations in climate and water quality. Notably, there was no significant regional difference in the prevalence of different types of stones, as observed by Hareendra et al. interestingly, our study and a previous one conducted in the northern region revealed a higher proportion of uric acid stones compared to other Sri Lankan studies. It is noteworthy that the mean uric acid level among the patients was within the normal range, and only 6.5% of the patients exhibited hyper-uricosuria.
The higher incidence of uric acid stones observed in our study may be attributed to various coexisting factors, including but not limited to a higher prevalence of insulin resistance [
16], dietary habits [
17], hereditary factors, and variations in drinking water quality. The higher prevalence of diabetes mellitus and high acidic load resulting from rice consumption are two most likely reasons that need to be explored. Further research is warranted in the future to comprehensively investigate and understand the interplay of these factors in the formation of kidney stone.
Low urine pH and low urine volume are two major etiologies for uric acid stones [
18]. Since our findings did not reveal an increased prevalence of any hyperaciduric metabolic disorder among our patients, it is pertinent to assess the daily urine volumes of urolithiasis patients. This assessment might shed some light on the reasons for the higher prevalence of uric acid stones.
Urinary metabolic abnormalities are one of the most important risk factors for urinary stone disease and its recurrence. Urinary stasis, supersaturation of urine, and formation of nidus are major pathologies that underlie urolithiasis [
11]. Different metabolic factors either facilitate or inhibit the development of stone formation. Hypercalciuria, Hyperoxaluria, Hyperuricosuria, and Hypercysteinuria are important metabolic derangements that promote urinary stone formation while hypercitraturia and hypermagnesuria are deterrents [
10,
18,
19].
Our study found that a large majority (86.9%) of the urolithiasis patients suffered from at least one urinary metabolic abnormality diagnosed by 24-hour urine analysis. The most common abnormality was Hypocitraturia (60.1%), followed by Hypomagnesuria (35.3%). Hyperoxaluria(14.4%). Hyper-phosphaturia (11.1%) Hyperuricosuria (6.5%) and Hypercalciuria (5.9%).
Sri Lankan data on the prevalence of metabolic disorders among urolithiasis patients is largely lacking. A study done on 30 patients with recurrent stone disease in northern Sri Lanka revealed that 80% of the patients suffered from a single metabolic derangement or a combination of multiple derangements [
15]. Hyperoxaluria and hypomagnesuria were the most common metabolic disorders with 30% of the patients suffering from each of the conditions. Hypocitraturia was found in only 7(23.3%) patients. 10% of the patients had Hyperuricosuria. Countrywide population studies are needed to find the actual prevalence of these metabolic disorders and understand the regional differences.
5. Limitation
The study was conducted at a single tertiary care urology clinic with a sample size of 153, which may limit the generalizability of the findings to other settings. Nevertheless, this study had several strengths, including a comprehensive analysis of urolithiasis and detailed urinary metabolic evaluations for over 150 patients— a figure that surpasses the numbers reported in the national literature. Due to economic constraints, we could not perform serum analysis of electrolytes and metabolites for all patients; only 54 patients had a complete profile. This limitation suggests the need for further studies in the future.
6. Conclusions
In summary, our study elucidates significant insights into urolithiasis and its metabolic evaluation. Predominantly affecting middle-aged males, with ureteric colic as the primary symptom, our findings underscore the prevalence of recurrent stones and the predominance of calcium oxalate monohydrate and uric acid stones. Notably, metabolic abnormalities were prevalent among patients, with hypocitraturia and hypomagnesuria being the most common. While age showed no substantial association with stone type, a marginal correlation with metabolic disorders was observed. This highlights the intricate interplay between demographic factors, stone composition, and metabolic abnormalities in urolithiasis. Our study emphasizes the importance of tailored preventive strategies and personalized treatments to mitigate the burden of stone formation and recurrence effectively.
Author Contributions
Conceptualization, BB, SITS, SS, SR and SV; Methodology, BRT, SS and PS; Validation, BB and MS; Formal analysis, SS and SR; Investigation, SS and PS; Data curation, BB; Writing – original draft, BB and SS; Writing – review & editing, BB, BRT, SITS, MS, SR, SV and PS; Supervision, BB. All authors have read and agreed to the published version of the manuscript.
Funding
This research study was funded by university of Jaffna research grant (URG/2022/SEIT/10).
Informed Consent
An informed written consent was obtained from all individual participants included in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author BB upon reasonable request.
Acknowledgments
We would like to thank Vithyasagar and Jothini for their valuable contributions in the recruitment of study participants, survey administration, and manuscript preparation. We would also like to thank Asiri Laboratory Pvt Ltd for their support during the investigations. We also thank University Research Grant for the funding support to carry out our study.
Conflicts Interest
There is no conflict interest.
References
- Sorokin I, Mamoulakis C, Miyazawa K, Rodgers A, Talati J, Lotan Y. Epidemiology of stone disease across the world. World J Urol. 2017 Sep;35(9):1301-1320. Epub 2017 Feb 17. PMID: 28213860. [CrossRef]
- Ali, A., Khan, Q., Jafar, T.H. (2012). Kidney Stones and Chronic Kidney Disease. In: Talati, J., Tiselius, HG., Albala, D., YE, Z. (eds) Urolithiasis. Springer, London. [CrossRef]
- Blandy, J. P., & Singh, M. (1976). The case for a more aggressive approach to staghorn stones. Journal of Urology, 115(5), 505–506. [CrossRef]
- Alelign T, Petros B. Kidney Stone Disease: An Update on Current Concepts. Adv Urol. 2018 Feb 4;2018:3068365. [CrossRef]
- Abdel-Halim, R. E., Al-Sibaai, A., & Baghlaf, A. O. (1993). The structure of large lamellar urinary stones. A quantitative chemical analytic study applying a new classification scheme. Scandinavian Journal of Urology and Nephrology, 27(3), 337–341. [CrossRef]
- Liu, Y., Chen, Y., Liao, B., Luo, D., Wang, K., Li, H., & Zeng, G. (2018). Epidemiology of urolithiasis in Asia. Asian Journal of Urology, 5(4), 205–214. [CrossRef]
- Lang, J., Narendrula, A., El-Zawahry, A., Sindhwani, P., & Ekwenna, O. (2022). Global Trends in Incidence and Burden of Urolithiasis from 1990 to 2019: An Analysis of Global Burden of Disease Study Data. European Urology Open Science, 35, 37–46. [CrossRef]
- Hareendra, P. P. G. K., Hunais, M. M., Suvendiran, S., Palihakkara, S. D., & Abeygunasekera, A. M. (2015).Chemical composition of kidney stones obtained from a cohort of Sri Lankan patients. Sri Lanka Journal of Surgery, 33(2), 14. [CrossRef]
- Rajendra, S. (2020a). Is there unique difference in the type of renal stones in Northern Sri Lanka? Analysis of chemical composition of renal stones in Jaffna by infrared spectroscopy. Sri Lanka Journal of Surgery, 38(1), 28. [CrossRef]
- Singh, V. K., & Rai, P. K. (2014). Kidney stone analysis techniques and the role of major and trace elements on their pathogenesis: a review. Biophysical Reviews, 6(3–4), 291. [CrossRef]
- Moe, O. W. (2006). Kidney stones: pathophysiology and medical management. Lancet (London, England), 367(9507), 333–344. [CrossRef]
- Wu, W., Yang, D., Tiselius, H. G., Ou, L., Liang, Y., Zhu, H., Li, S., & Zeng, G. (2014). The Characteristics of the Stone and Urine Composition in Chinese Stone Formers: Primary Report of a Single-center Results.Urology, 83(4), 732–737. [CrossRef]
- Ansari, M. S., Gupta, N. P., Hemal, A. K., Dogra, P. N., Seth, A., Aron, M., & Singh, T. P. (2005). Spectrum of stone composition: structural analysis of 1050 upper urinary tract calculi from northern India.International Journal of Urology : Official Journal of the Japanese Urological Association, 12(1), 12–16. [CrossRef]
- Chandrajith R, Weerasingha A, Premaratne KM, Gamage D, Abeygunasekera AM, Joachimski MM, Senaratne A. Mineralogical, compositional and isotope characterization of human kidney stones (urolithiasis) in a Sri Lankan population. Environ Geochem Health. 2019 Oct;41(5):1881-1894. Epub 2019 Jan 22. [CrossRef] [PubMed]
- Rajendra, S. (2020b). Metabolic evaluation of patients with recurrent, multiple or bilateral renal stones in Jaffna. Ceylon Medical Journal, 65(3), 56. [CrossRef]
- Rannan-Eliya RP, Wijemunige N, Perera P SLHAS Collaborators, et al.Prevalence of diabetes and pre-diabetes in Sri Lanka: a new global hotspot–estimates from the Sri Lanka Health and Ageing Survey 2018/2019.BMJ Open Diabetes Research and Care 2023;11:e003160. [CrossRef]
- Jayawardena, R., Byrne, N. M., Soares, M. J., Katulanda, P., & Hills, A. P. (2013). Food consumption of Sri Lankan adults: an appraisal of serving characteristics. Public Health Nutrition, 16(4), 653–658. [CrossRef]
- Cloutier, J., Villa, L., Traxer, O., & Daudon, M. (2015). Kidney stone analysis: “Give me your stone, I will tell you who you are!” World Journal of Urology, 33(2), 157–169. [CrossRef]
- Pak CY, Poindexter JR, Adams-Huet B, Pearle MS. Predictive value of kidney stone composition in the detection of metabolic abnormalities. Am J Med. 2003 Jul;115(1):26-32. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).