1. Introduction
Apolipoproteins are proteins that bind to lipids (such as cholesterol and triglycerides) to form lipoprotein particles, which are essential for transporting lipids through the bloodstream. These proteins play crucial roles in lipid metabolism, including the transport, metabolism, and clearance of lipids from various tissues. Diabetes mellitus is characterized by high blood glucose levels. It is a condition primarily defined by the level of hyperglycemia giving rise to risk of microvascular damage (retinopathy, nephropathy and neuropathy) and macrovascular complications (ischemic heart disease, stroke and peripheral vascular disease), (Lee et al., 2022). Diabetes mellitus is a chronic metabolic disease with complex pathogenesis (Reed et al., 2021). In this disease elevated glucose levels in blood result from the defects in either secretion of insulin or its action or both. Raised blood sugar level is the main biomarker for establishing the diagnosis of diabetes (Banday et al., 2020). Diabetes causes abnormality in metabolism of fat, protein and carbohydrates and /or often leads to different microvascular and macrovascular complications that are mainly linked with increased rate of morbidity and mortality in this disease (Lee et al., 2022). It has been reported that various demographic, clinical, biochemical parameters such as age, gender, socioeconomic status, duration of onset, BMI , hypertension, BSF, HbA1c, lipidprofile, creatinine and genetic factors are also responsible for cardiovascular complications like IHD and stroke in these patients (Hassan et al., 2021; Aljulifi et al., 2021)
Increased basal metabolic index (BMI>25kg/m2) is thought to be an important factor in diabetics In past few years, it has been reported (Petrie et al., 2018) that coexistence of diabetes and hypertension exacerbates cardiovascular complications in diabetic individuals and is a significant risk for vascular complications related to type 2 diabetes. Earlier Petrie et al in 2018 observed that the frequency of hypertension is twice in diabetic patients as compared to non-diabetics and these patients have insulin resistance. Previously (Gosh et al., 2017) observed a strong link of obesity with insulin resistance and atherosclerosis leading to diabetic vascular complications.
In diabetic subjects with no past history of myocardial infarction (MI), the 7-year risk of developing MI is greater in diabetics as compared to non-diabetics. However in patients with a past history of myocardial infarction, the risk of redeveloping MI is much greater in diabetics in seven years (Wang et al ., 2017). It indicates that diabetes has a significant contribution to the development of MI. However, a study was performed on adults 30 years or older in Denmark. It reported that diabetes increases the risk of coronary heart disease (Huang et al., 2017).
Cardiovascular disease (CVD) is the most frequent cause of mortality in diabetic individuals. About 52% mortality in T2DM is due to cardiovascular complications. Many reports indicate a strong association between diabetes and cardiovascular episodes (Regassa et al., 2021).Very recently, it has been noticed that even prediabetic state with impaired fasting glycaemia (IFG) or impaired glucose tolerance (IGT), is linked with morbidity and mortality due to cardiovascular disease. (Einarson et al., 2018).
In addition to chronic heart disease( CHD), there is increased risk of developing stroke in diabetes because of damaged vessels of the body and stroke is an emergency. Diabetics have about twice the chance of developing stroke as compared to non-diabetic subjects, particularly in case of ischemic stroke and hyperglycemia is linked with poor prognosis of stroke. ( Huang et al., 2017).
Genetic factors also lead to cardiovascular complications in type 2 diabetics. Among these factors Apolipoprotein E is a highly important gene that is linked with cardiovascular complications (ischemic heart disease and stroke) due to dyslipidemia and atherosclerosis in type 2 diabetes. Apolipoprotein is a glycoprotein consisting of 299 amino acids and is present in the plasma.Various genotypes of APOE are associated with plasma lipid levels. Dyslipidemia or lipoprotein abnormalities can speed up diabetic vascular complications and can cause atherosclerotic changes in these subjects. (Zhang et al., 2017) and APOE allele ε4 might cause risk for both T2DM and CVD independently (Liu et al., 2019).
Complicated molecular pathways that involve raised blood sugar levels, resistance to insulin actions in addition to hypertension , obesity and dyslipidemia defined by high levels of total cholesterol, triglycerides, LDL and VLDL with low levels of HDL (good cholesterol) lead to cardiovascular complications in diabetic individuals (Javed et al., 2016; Sarfraz et al., 2016 and Beckman et al., 2016). Apolipoprotein E gene polymorphism is also responsible for diabetic macrovascular complications (Hou et al., 2020).
The role of Apolipoprotein E gene polymorphism has not been observed in Pakistani diabetics as regard to demographic, clinical and biochemical parameters. The objectives of the present study was to: assess the effect of age, duration of onset of diabetes blood pressure, body mass index (BMI)) lipid profile, BSF, HbA1c, creatinine and smoking on complications like IHD and stroke in type 2 diabetics.in addition to evaluating the Apolipoprotein gene polymorphism in these patients
2. Materials and Methods
2.1. Study Design
This is a “Case-control study”. Both disease cases and controls were selected from the Social Security Hospital, Lahore. Study subjects were segregated into control (C) and the diabetic group (D1-D4) Group 1, D1 (Diabetics without any accompanying comorbidities (DM only ), Group 2, D2 (Diabetics with ischemic heart disease (DM+IHD), Group 3, D3 (Diabetics with stroke (DM +STROKE) and Group 4, D4(Diabetics with ischemic heart disease and stroke(DM+IHD+STROKE). Both inclusion and exclusion criteria were carefully taken into consideration while selecting the control and diseased samples for the present study. All the participants were male and female above 40 years of age (Huebschmann et al., 2019; Nanyakara et al., 2021)
2.1.1. Inclusion Criteria for Healthy and Diabetic Individuals
Healthy Individuals (controls) had no diabetes, for diabetic individuals: Selection based on medical reports, family history, and physical examination. Type 2 diabetes (T2DM) patients with hypertension have blood pressure of more than 140/90 (Qun et al., 2016). T2DM patients with complications of hypertension e.g. ischemic heart disease (IHD) and stroke (CVA) as evident from medical records.
Exclusion Criteria: Pregnant women, Lactating women, Patients with malignant tumors
2.2. Patients with Renal Disease
2.2.1. Collection of Demographic and Clinical Data
Demographic data was gathered from the selected subjects whereas the examination was performed by the concerned medical professional in a clinical setting. All the subjects were recruited from the Department of Medicine, Social Security Hospital, and Lahore. It also included patients admitted to the ward as well as those visiting the outpatient department regularly.
2.2.2. Samples Collection
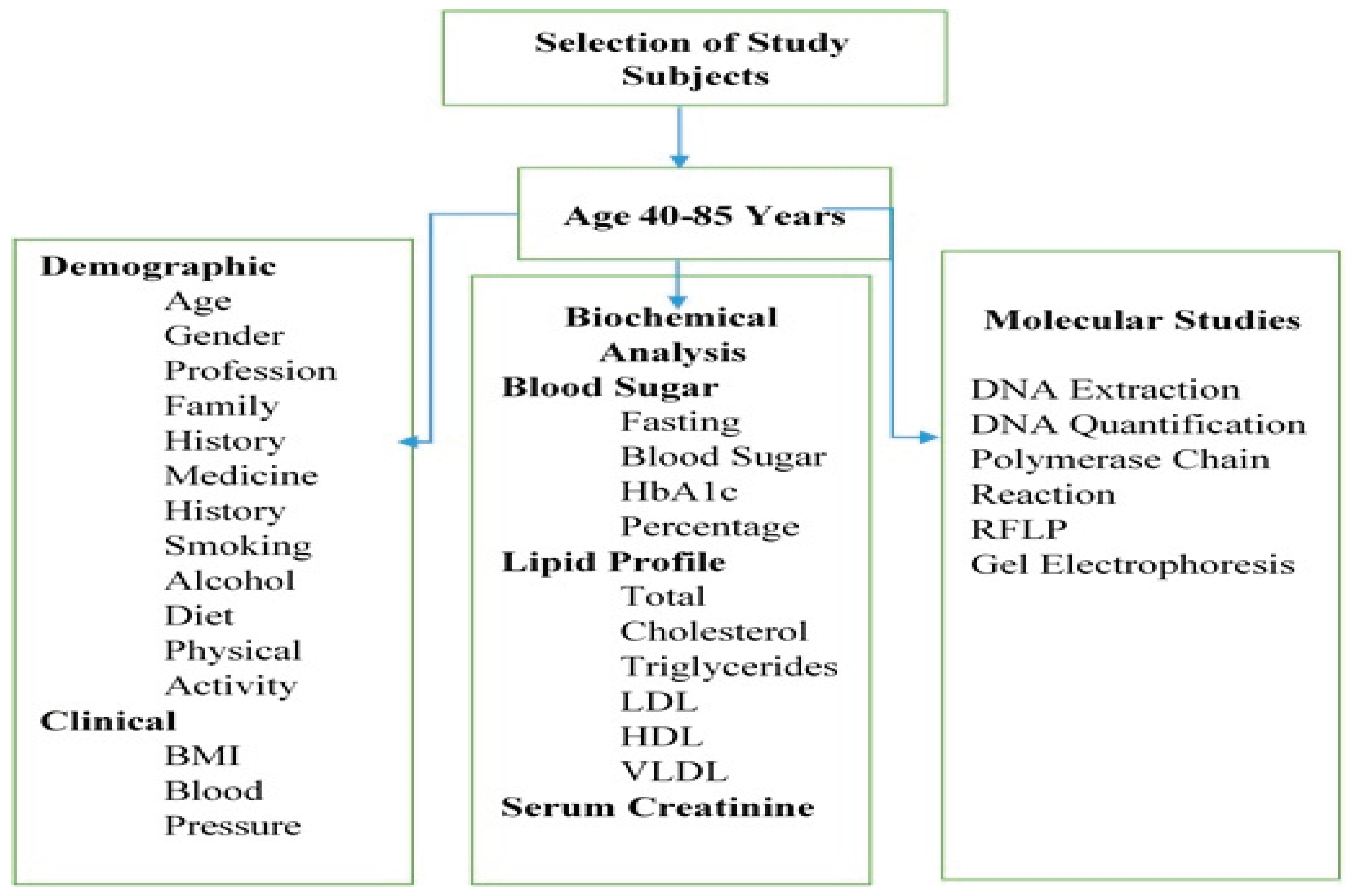
Venus blood samples were taken aseptically from all study subjects. For this, ten (10) ml blood sample was collected through venipuncture and distributed equally in two (02) customized blood collecting tubes, with and without anti-coagulants such as ethylenediamine tetraacetic acid (EDTA). These samples were transported under cold conditions to the laboratory at the Institute of Molecular Biology and Biotechnology (IMBB), The University of Lahore. The tubes containing the coagulated blood sample were centrifuged at 5000 rpm for 10-15 minutes. Supernatant plasma was pipetted out and stored at -20o C for later use. Whereas, uncoagulated blood was used for DNA extraction. The schematic diagram of the experimental plan is presented in Figure 1.
Figure 1.
Experimental plan showing demographic, clinical, biochemical and genetic analysis.
Figure 1.
Experimental plan showing demographic, clinical, biochemical and genetic analysis.
Anthropometric measurements are noninvasive quantitative measurements of the body. In this regard, the core elements of anthropometry i.e. height and body weight, were taken into account to calculate the body mass index (BMI). Weight and height of the subjects were obtained from the study subjects and BMI was calculated according to the standard formula [BMI = weight (kg)/ [height (m)] 2 described by (Bhupathiraju et al., 2016). Blood pressure assessment of the study subjects was measured by sphygmomanometer as described earlier (Muntner et al., 2019; Maqbool et al., 2019; Mansoor et al., 2022).
2.3. Biochemical Estimations
All the biochemical estimations were carried out according to (Mansoor et al., 2022). Biochemical estimations were done on a Beckman coulter AU 480 Chemistry Analyzer (USA). Fasting blood sugar levels in the study subjects were measured by using the commercial Glucose Oxidase method known as GLU-OX) available from DIRUI, China by spectrophotometric method., Detection of Glycated Hemoglobin (HbA1c) levels, was estimated by Latex agglutination method using commercial kit GLYCOHEMOGLOBIN A 1 c), DIRU!, China. Fasting Lipid Profiles such as Total cholesterol (TC), triglycerides (TG), and high-density lipoprotein cholesterol (HDL-C), levels were estimated in the study samples using commercially available kits from DIRUI, China. Low-density lipoprotein was calculated by using the modified Friedewald formula ( LDL-C (mg/dL) = Non-HDL-C X 90% minus TG X 10) described by (Chen et al., 2010). Very low-density lipoprotein (VLDL) levels were estimated by using the Friedewald equation described as VLDL=Triglycerides / 5 by (Oliveira et al., 2013). Creatinine was measured by enzymatic method using a commercially available kit (CRE-ENZYME), DIRUI. China,
2.4. DNA Extraction and Quantification
DNA was extracted by blood genomic DNA extraction kit (QIAAMP DNA mini kit, Qiagen, Batch no. 166030545, Briogene Pvt. Ltd., Germany) according to the manufacturer's guidelines.. 20 µL of this mixture as a DNA source for amplification.DNA concentration was measured by the Nanodrop (U Zafar et al., 2016). In some cases, DNA concentration was measured by ultraviolet absorbance spectrometry at 260 nm. This wavelength corresponds to 50 µg of double-stranded DNA per ml. The ratio of absorbencies at 260 nm and 280 nm was taken as 1.8 with a pure sample. A ratio of less than 1.8 indicates that the preparation is contaminated with phenol or protein.
2.4.1. Polymerase Chain Reaction (PCR) Amplification
Conventional PCR was performed according to (Maqbool et al., 2019), with 100 ng purified genomic DNA, 0.5 µM of APOE P1 and APOE P2 primers; P1 Forward sense (5’GGCACGGCTGTCCAAGGA-3’, and P2 forward sense (5’ CCCACCTGCGCAAGCTGCGC 3’) and P3 Reverse Antisense 5’-CTCGCGGATGGCGCTGAG-3’.The reaction mixture contained (200 µM of dNTP mixture, 1 M betaine, 2 MgCl2, 2.5 U Taq DNA polymerase (BioQuest), and reaction buffer in a 50-µl reaction volume. Each PCR reaction mixture comprised 100ng (almost 2 µL) of DNA, 0.8 µL of both primers (10 pmol/µL each), 8 µL master mix and 5 µL of distilled water. The following steps were performed. The amplification was initiated at 95◦C for 2 min, followed by 45 cycles of 95◦C for 30 s, 68◦C for 30 s, and 72◦C for 30 s, and then a final extension at 72◦C for 7 min.
2.4.1. Restriction Fragment Length Polymorphism (RFLP)
The resulting PCR products (20 µl) were treated with 5 U Hha1, restriction enzyme for 3 hr. The restricted fragments were separated on 12.5% discontinuous polyacrylamide gel electrophoresis (Haas et al., 1994) and 3 percent agarose gel.
Statistical Analysis: Data was analyzed by using SPSS version 26.0. Data were described as mean ± standard deviation (SD) or frequency and percentages as applicable. One-way analysis of variance was used within the group and Two-way analysis of variance (ANOVA) was used between the groups. P value ≤ 0.05 was considered significant.
3. Results
In the present study, subjects with type 2 diabetes from Lahore region were investigated with or without ischemic heart disease or stroke in connection to their demographic, anthropometric and biochemical indices or parameters as regards Apolipoprotein E gene polymorphism. The results are presented under the following headings.
3.1. Analysis of Demographic and Clinical Data
Demographic and clinical information’s of the study subjects is summarized in Table 4.1
Table 1.
Information regarding the study subjects.
Table 1.
Information regarding the study subjects.
| S. No |
Variables |
Characteristics |
| 1 |
Total Subjects |
260 |
| 2 |
Age |
40-85 |
| 3 |
Gender (M/F) |
90 (34.6%)/170 (65.3%) |
| 4 |
Profession |
Factory workers, Housewives |
| 5 |
Income |
Low income, upto PKR 20,000/month |
| 6 |
Region |
Lahore and its surrounding areas |
| 7 |
Duration of Onset of Diabetes Range: 2-30 Years |
Below 10 years:
M: 52(25%), F:112(53.8%)=78.8 %
Above 10 years:
M: 20(9.6%), F: 24(11.5%)=20.7%. |
| 8 |
Family History of Diabetes |
Positive in M:8(3.8%), F:7(3.3%) = 7.1% |
| 9 |
Physical Activity M/F |
M:15(7.2%), F:10(4.8%) = 12.0% |
| 10 |
Smoking |
30 males (14.4 %), one pack of 10 cigarettes/day.No shisha smokers. No female smokers |
| 11 |
Diet |
Oily parathas/bread and curry 2 times a day, Deep fried snacks once a day and less fibre |
| 12 |
Alcohol |
Nil |
| 13 |
Treatment (Oral/Insulin or Both) |
Oral:
M:30 (14.4%), F:56(26.9%)=41.3%
Insulin +oral: M:42(20.1%), F:80(38.4%)=58.5% |
| 14 |
Hypertension (Systolic and Diastolic) |
Range: (110/ 75)-(202/110)
M:57(27.4 %), F:100(48.0 %)=75.4% |
| 15 |
BMI <25kg/m2 Below
BMI >25kg/m2 Above |
Range: 19.6-36
M:13(6.2%) , F:2(0.9%) = 7%
M: 59 (28.3%), F: 134(64.4%)=92.7% |
3.2. Characteristics of the Study Groups
Table 2 illustrates the basic demographic and clinical characteristics of the study group subjects indicating number of samples, age, BMI, systolic blood pressure and diastolic blood pressure in each group. All the groups consisted of 52 subjects, which were further divided into male and females out of which 18 subjects were males and and 34 subjects were females. According to results there were no significant difference in age of both genders in control and diabetic groups and in between groups where P= 0.7622
Age: No significant and diabetic groups and in between groups where P= 0.7622 (
Table 3)
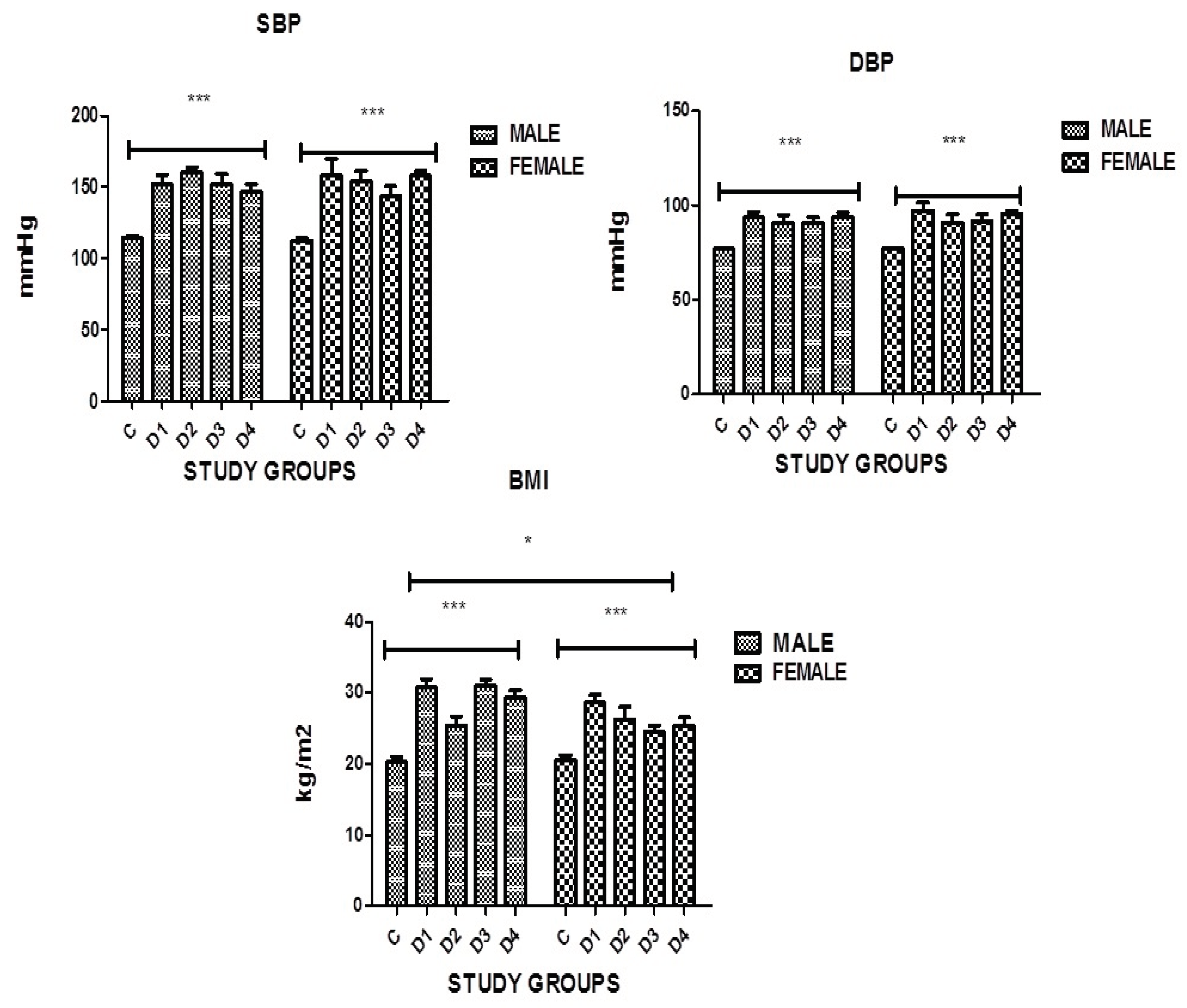
Figure 1.
Graphical presentation of BMI, SBP and DBP in the study groups where p≤0.05, C: CONTROL; D1: DM ONLY; D2: DM+IHD; D3: DM+STROKE; D4: DM+STROKE+IHD; BMI: BODY MASS INDEX. ERROR BARS are SE, *** represent P=0.001,* represents P<0.05.
Figure 1.
Graphical presentation of BMI, SBP and DBP in the study groups where p≤0.05, C: CONTROL; D1: DM ONLY; D2: DM+IHD; D3: DM+STROKE; D4: DM+STROKE+IHD; BMI: BODY MASS INDEX. ERROR BARS are SE, *** represent P=0.001,* represents P<0.05.
Body mass Index (BMI): The present study data (
Table 3, Figure 1 ) shows that BMI was higher in diabetic subjects and significant difference was found in both genders in all groups as compared to controls, having the p value 0.0001 which is statistically significant.
Hypertension (B.P): The present study data (
Table 3, Figure 1) shows that both systolic and diastolic blood pressure was found to be high in diabetic subjects. Significant difference was found in both genders in all groups as compared to controls, having the p value 0.0001 which is statistically significant.
Table 4 illustrates the stages of hypertension. The data indicated that 75.4% subjects (27.4% male and 48.0% female) were hypertensive. The study subjects were assessed for the hypertension stage. A total of 14.9 % subjects (4.8% males and 10.1 % females) had stage 1 hypertension. The number of subjects having stage 2 hypertension was 60.5% (18.75 % males and 41.8% females). About 24.5% (11.0% males and 13.4 % females) subjects were normotensive. The prevalence of Stage 2 hypertension is more as compared to stage 1.
3.3. Effect of Age on Hypertension
It has been documented in the literature that age effects blood pressure which is an important risk factor for developing cardiovascular complications in diabetics. In the present study the blood pressure was estimated to define the stages of hypertension and the subjects were divided in to two groups according to age (
Table 5). In subjects between 40-60 years of age total of 38.5% subjects were hypertensive. 4.8% had stage 1 hypertension 33.6 % subjects had stage 2 hypertension 2.8% were normotensive. In subjects above 60 years of age total of 36.9% subjects were hypertensive 8.5% had stage 1 hypertension and 28.4% had stage 2 hypertension 17.5 % were normotensive. In both groups most patients had stage 2 hypertension.
3.4. Effect of Duration of Diabetes on Hypertension
It has been normally observed that the duration of onset of diabetes effects blood pressure. The present study indicates that subjects with duration of diabetes below 10 years 54.4 % were hypertensive (5.4% stage 1 and 49% stage 2) and the subjects with duration of diabetes above10 years 20.9 % were hypertensive (4.9% stage 1 and 16 % stage 2). The number of hypertensive patients was more when the duration of diabetes was below 10 years and stage 2 hypertension was more prevalent as compared to stage 1 above and below 10 years (
Table 6)
3.5. Onset of Diabetes in Study Subjects
Subjects with duration of diabetes above 10 years were 21.1% (9.6% males and 11.5% females) and subjects with duration below 10 years were 80.2% (26.4% males and 53.8% females). This shows that there were more subjects with duration of diabetes less than 10 years in our study (
Table 7)
3.6. Smoking
As evident by previous studies smoking increases the risk of cardiovascular complications in diabetic subjects. Therefore, the present study also focused on assessing the role of demographic and clinical variables in diabetic smokers and nonsmokers (
Table 8). Smokers and non smokers in all disease groups had significantly higher body mass index, systolic and diastolic blood pressure as compared to healthy controls.
3.7. Biochemical Analysis
In order to see how the biochemical variables like BSF,HbA1c,Fasting lipid profile and creatinine present in the groups effect the diabetic cardiovascular complications, the distribution of biochemical markers was evaluated in study subjects and groups. The results obtained are presented in
Table 9,
Table 10,
Table 11 and
Table 12. In the disease (diabetic) group, the blood sugar fasting (BSF) and Gycated hemoglobin (HbA1c) was high. Lipid profile i.e. Total Cholesterol (TC), Total
. Biochemical variables were significantly raised in all groups when compared with control. However in group D1(DM+IHD) the percentage of HbA1c was noted to be higher in females as compared to males of the same group, however, total cholesterol and triglyceride levels were higher in males of this group. In the present study it was also observed that the levels of total cholesterol and LDL were higher in males than the female subjects in group D4
.(DM+IHD+STROKE). The significance of biochemical data is applicable in the form of p-values therefore it was important to calculate the Mean ± SEM of the biochemical variables in study group as presented in
Table 11.
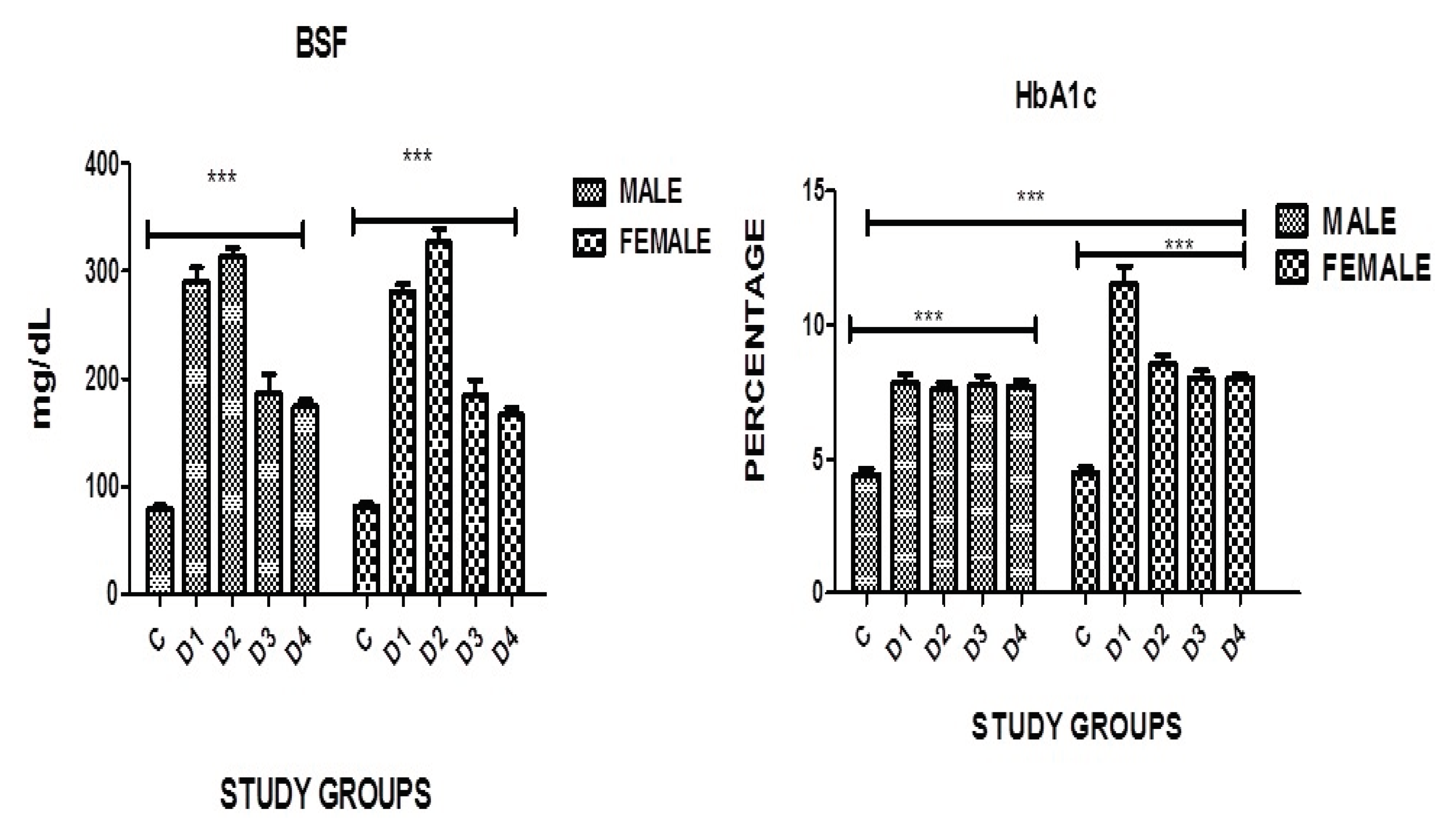
Figure 1.
Graphical presentation of BSF, and HbA1c in study groups where p≤0.05 C: CONTROL; D1: DM ONLY; D2: DM+IHD; D3: DM+STROKE; D4: DM+STROKE+IHD; HbA1c: GLYCATED HEMOGLOBIN, ERROR BARS are SE, *** represent P=0.001.
Figure 1.
Graphical presentation of BSF, and HbA1c in study groups where p≤0.05 C: CONTROL; D1: DM ONLY; D2: DM+IHD; D3: DM+STROKE; D4: DM+STROKE+IHD; HbA1c: GLYCATED HEMOGLOBIN, ERROR BARS are SE, *** represent P=0.001.
3.8. Blood Sugar Levels
A highly significant difference in blood sugar fasting was found in both genders in all diseased groups when compared with the controls where P=0.0001. However no significance was noticed in between the groups although in diabetics with IHD level of BSF was higher in females as compared to males of the same group (
Table 11, Figure 1)
3.9. HbA1c
A highly significant difference in HbA1c was found in both genders in all disease groups when compared with the controls where P=0.0001. A significance was also noticed in diabetic group without complications where the females had much higher levels as compared to males of the same category (
Table 11, Figure 1).
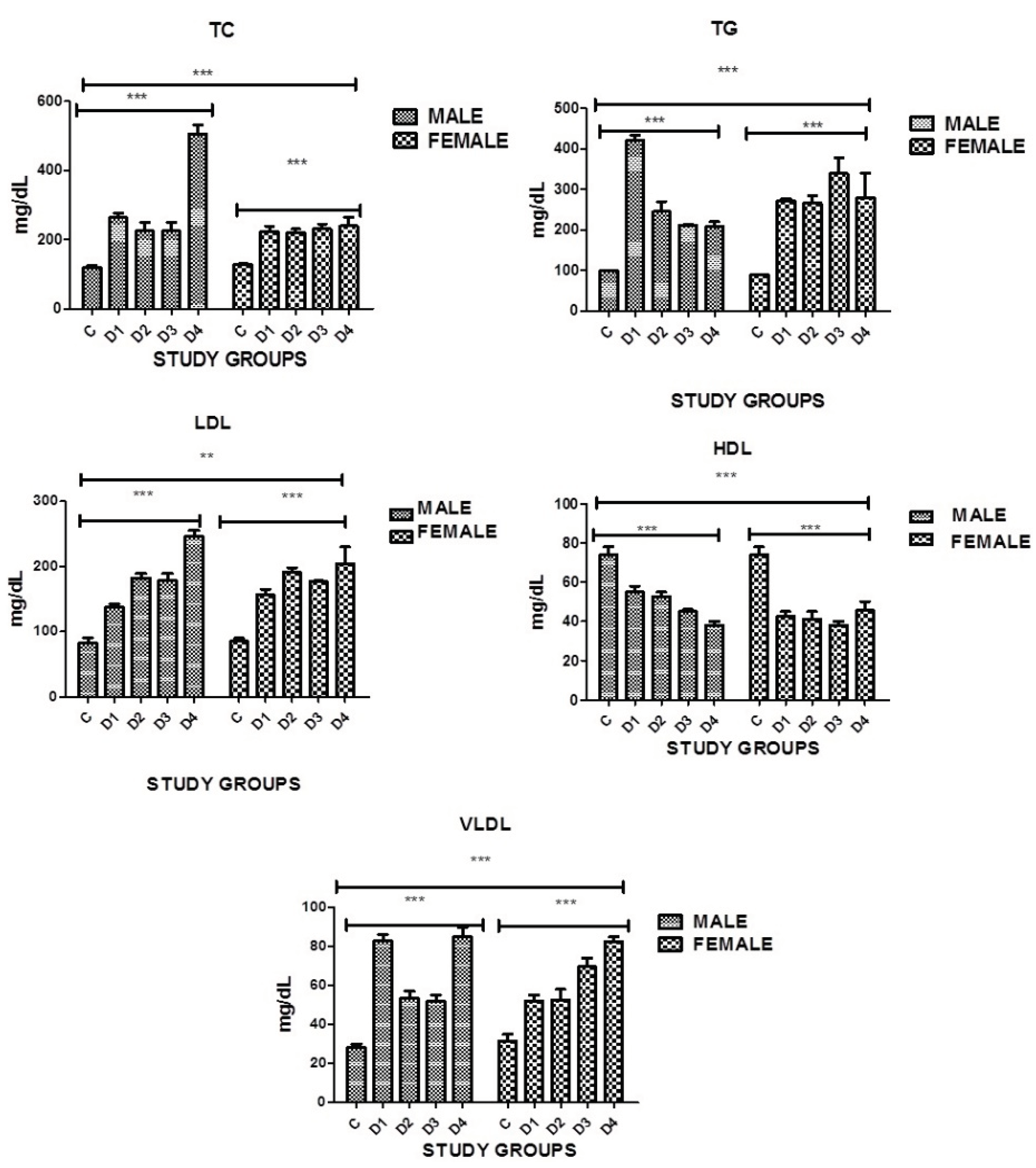
3.10. Lipid Profile: Total Cholesterol
Present study indicated significant difference in blood cholesterol levels was found in both genders in all disease groups when compared with the controls. Serum cholesterol was high in all disease groups. However a significance was noticed in between the groups as well. In diabetics with stroke and IHD and in diabetics with no complications males had higher levels of cholesterol as compared to females where P=0.0001 (
Table 11, Figure 2).
3.11. Total Triglycerides
A highly significant difference in triglyceride levels was found in both genders in all disease groups when compared with the controls. In diabetics with stroke and IHD and diabetics with stroke females had higher levels of triglycerides as compared to males. In diabetics with no complications the levels of triglycerides were high in males as compared to females, where P=0.0001. However significance was also noticed in between the groups as well (
Table 11, Figure 2).
3.12. Low Density Lipoproteins
A highly significant difference in low density lipoproteins was found in both genders in all disease groups when compared with the controls. High levels of LDL proteins was seen in all disease groups as compared to controls. In diabetics with IHD and stroke levels were high in males as compared to females. In diabetics with IHD females showed higher levels of LDL protein as compared to males, where P=0.0001.However a significance was noticed in between the groups as well (
Table 11, Figure 2).
3.13. High Density Lipoproteins
groups when compared with the controls, where P=0.0001 (
Table 11, Figure 2).
3.14. Very Low Density Lipoproteins
Significantly Increased levels of VLDL were seen in both genders and in all disease groups when compared with the controls, where P<0.0001. However a significance was also noticed in between the groups. Diabetic males with no complications had higher levels of VLDL as compared to females and diabetic females with stroke had higher levels as compared to males of the same group (
Table 11, Figure 2).
Figure 2.
Graphical presentation of TC, TG, LDL, HDL and VLDL in study groups where p≤0.05 C: CONTROL; D1: DM ONLY; D2: DM+IHD; D3: DM+STROKE; D4: DM+STROKE+IHD; HbA1c: GLYCATED HEMOGLOBIN, ERROR BARS are SE, *** represent P=0.001 ,** represents P=0.05.
Figure 2.
Graphical presentation of TC, TG, LDL, HDL and VLDL in study groups where p≤0.05 C: CONTROL; D1: DM ONLY; D2: DM+IHD; D3: DM+STROKE; D4: DM+STROKE+IHD; HbA1c: GLYCATED HEMOGLOBIN, ERROR BARS are SE, *** represent P=0.001 ,** represents P=0.05.
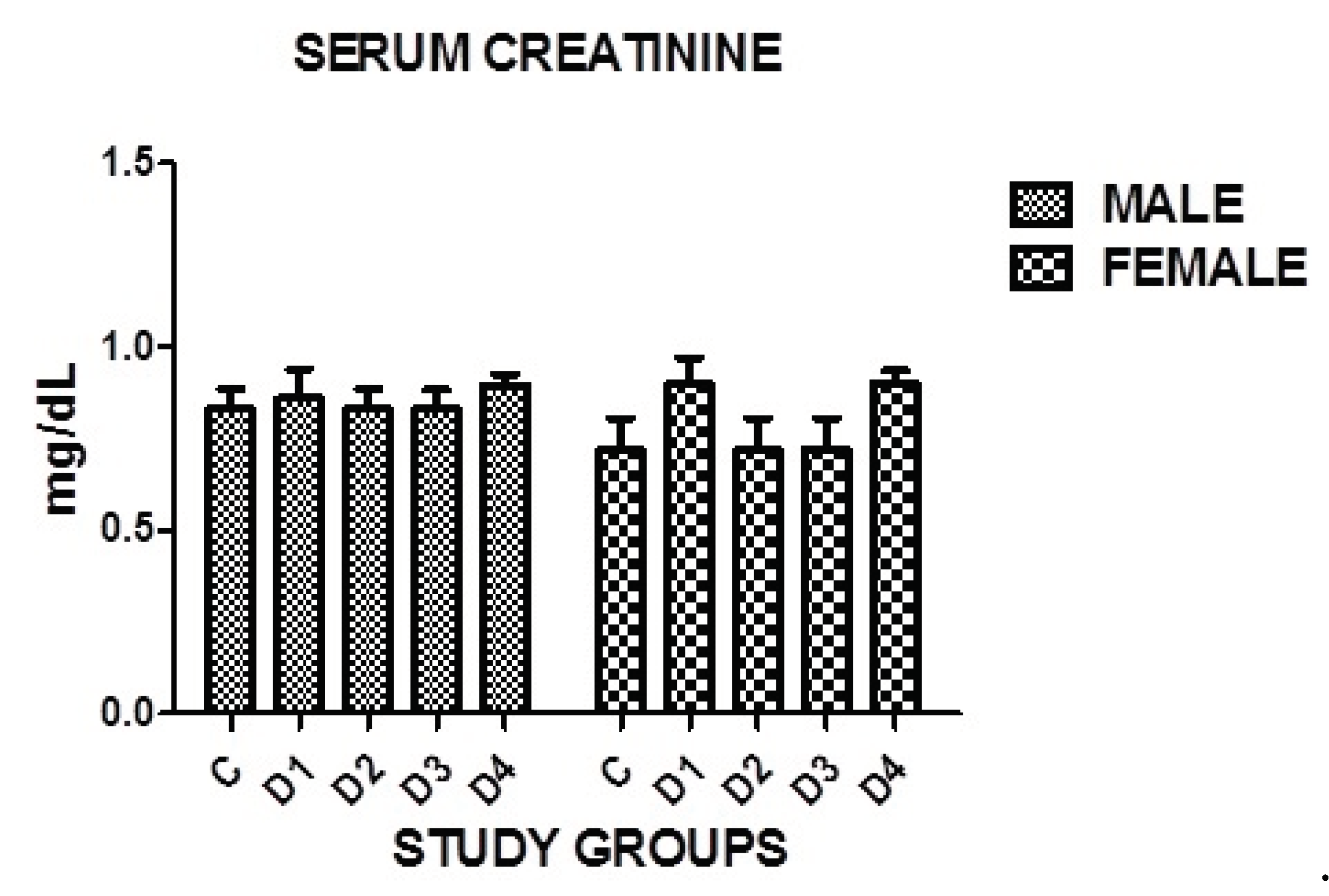
3.15. Serum Creatinine
Insignificant difference in serum creatinine was found in both genders in all disease groups when compared with the controls where P=0.1614. However no significance was noticed in between the groups as well (
Table 11, Figure 3)
Figure 3.
Graphical presentation of serum creatinine in study groups where p=0.1614.
Figure 3.
Graphical presentation of serum creatinine in study groups where p=0.1614.
3.16. Evaluation of Biochemical Parameters in Smokers and Non-Smokers in Study Groups
There is an evidence from the present research that smoking can contribute to development of IHD and Stroke in diabetic subjects. Biochemical markers like BSF, HbA1c, total cholestrol, triglycerides, LDL, HDL and VLDL in these individuals was noticed to be significantly higher as compared to healthy controls (
Table 12)
3.17. Restriction Analysis and Genotyping of Amplified DNA Product
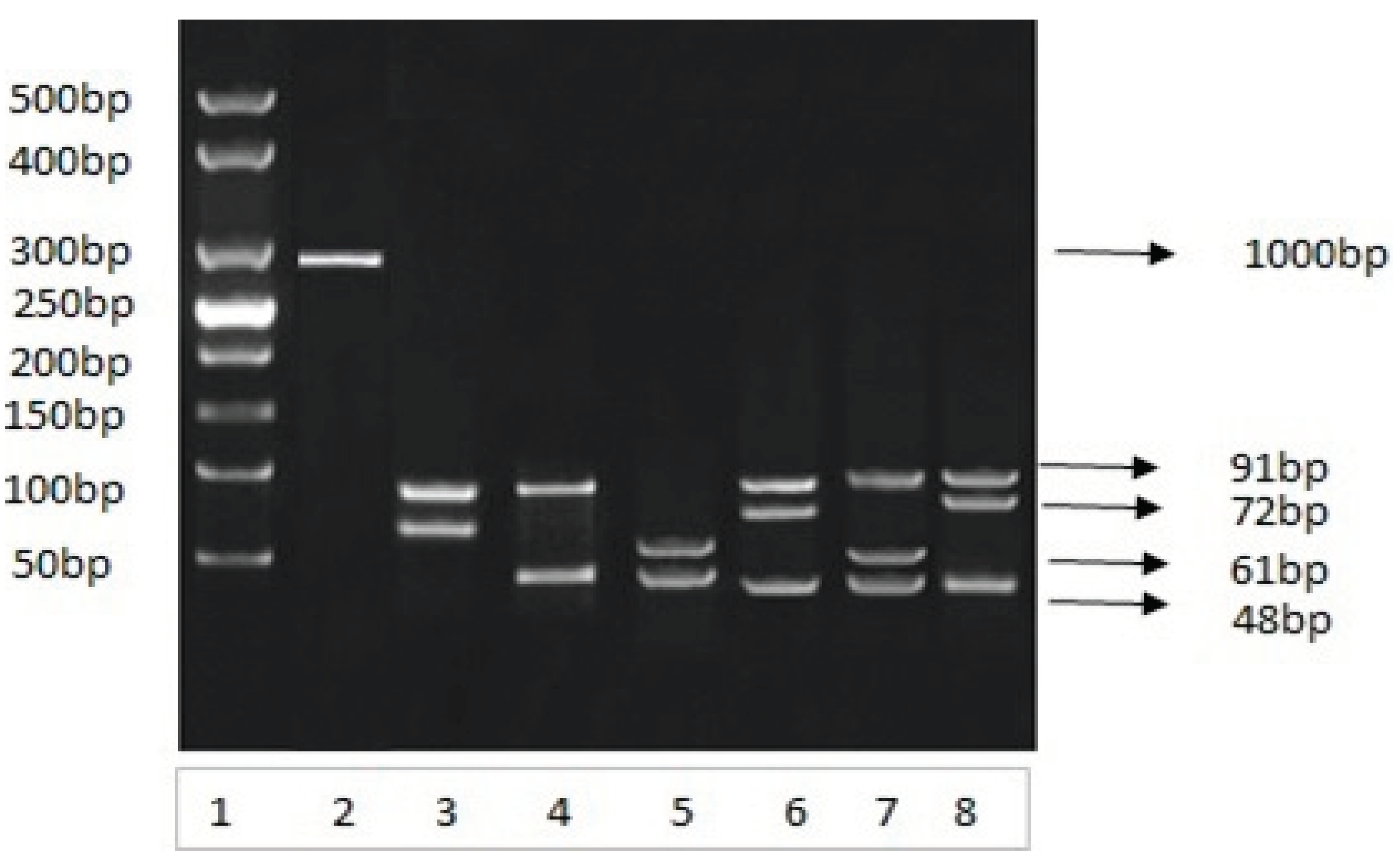
The amplified 292-bp PCR products amplified from different blood samples were subjected to HhaI restriction endonuclease digestion. HhaI restriction endonuclease cleaved the amplified DNA on specific sites, which resulted a specific restriction pattern for each genotype (Figure 4A,B). The restricted DNA bands were compared with 25 bp DNA marker and the different individual genotypes were separated and categorized based on the band size criteria:
Figure 4.
A: Analysis of PCR amplified 292 bp product. Restriction analysis of Apolipoprotein E gene on 03 percent agarose gel under an electrical field Lane 1 is molecular weight DNA marker obtained from study samples. Lane 2, is amplified PCR product; lanes 3, 4, 5, 6, 7 and 8 contain restricted fragments by using Hha1 restriction enzyme. The restricted fragments were visualized by ethidium bromide staining.
Figure 4.
A: Analysis of PCR amplified 292 bp product. Restriction analysis of Apolipoprotein E gene on 03 percent agarose gel under an electrical field Lane 1 is molecular weight DNA marker obtained from study samples. Lane 2, is amplified PCR product; lanes 3, 4, 5, 6, 7 and 8 contain restricted fragments by using Hha1 restriction enzyme. The restricted fragments were visualized by ethidium bromide staining.
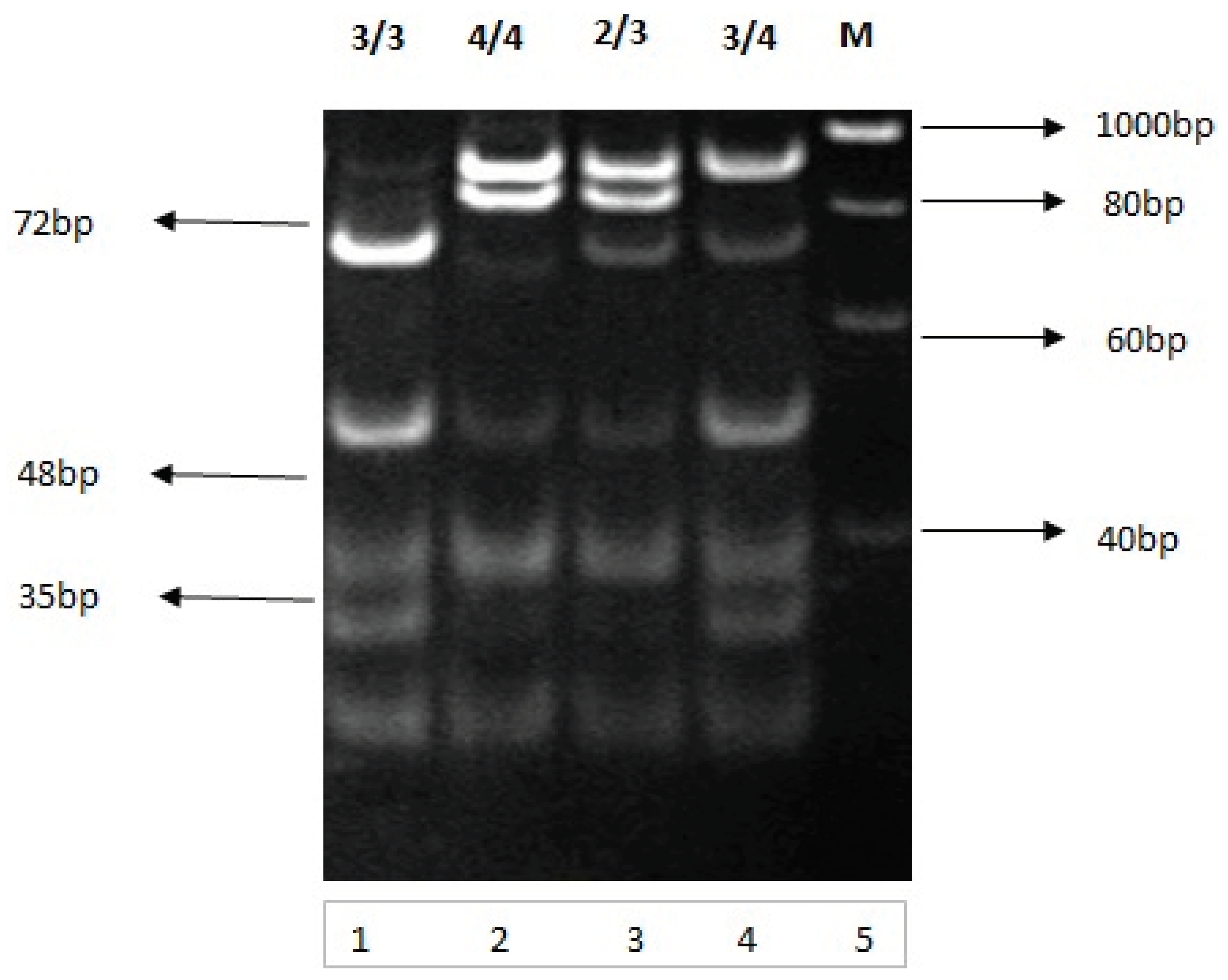
Figure 4.
B: Analysis of PCR amplified 292 bp product. Restriction analysis and genotyping of Apolipoprotein E gene on 10 percent nondenaturing polyacrylamide gel electrophoresis Lanes 1, 2, 3 and 4 contain restricted fragments (genotypes 3/3, 4/4, 2/3 and 3/4 respectively) by using Hha1 restriction enzyme. Lane 5 is a molecular weight DNA obtained from study samples. The restricted fragments were visualized by ethidium bromide staining.
Figure 4.
B: Analysis of PCR amplified 292 bp product. Restriction analysis and genotyping of Apolipoprotein E gene on 10 percent nondenaturing polyacrylamide gel electrophoresis Lanes 1, 2, 3 and 4 contain restricted fragments (genotypes 3/3, 4/4, 2/3 and 3/4 respectively) by using Hha1 restriction enzyme. Lane 5 is a molecular weight DNA obtained from study samples. The restricted fragments were visualized by ethidium bromide staining.
3.18. Genotypic Distribution / Variation
The purified and amplified genomic DNA was amplified by the primer pair. The 292 bp PCR products were subjected to HhaI restriction endonuclease digestion. HhaI restriction endonuclease cleaved specific sites, resulting in a specific restriction pattern for each genotype (Figure 4A). Apolipoprotein E genotypes were determined by analyzing the restricted fragments obtained from restriction (PCR-RFLP) Fragments were separated on non-denaturing polyacrylamide gel electrophoresis. The restricted DNA fragments obtained from PCR-RFLP were 3/3 (91, 48, and 35 bp), E4/4 (72, 48, and 35bp), E2/3 (91, 83, 48, and 35bp), and E 3/4 (91, 72, 48, and 35 bp) (Figure 6B) shows complete restriction pattern for each genotype in blood samples tested from studied groups.
The genotypes of APOE in Pakistani type 2 diabetic population were 3/3, 4/4, 2/3, and 3/4 (
Table 13). In the study population, the predominant genotype was E3/3.
where E3/3 genotype accounted for 3.8% in the control group (C), 19.2% in diabetics with no complications (D1), 26.9 % in the IHD group (D2), 23.0% in diabetics with stroke (D3) and 26.9% in the IHD-Stroke group (D4). In the present study E3/4 genotype, accounted for 5.7% in the control group (C), 15.3% in diabetics without complications (D1), 21.1% in diabetics with IHD (D2), 25 % in diabetics with stroke (D3) and 23% in the IHD-stroke group (D4). The frequency of E2/3 genotype was increased (23%) in diabetics with ischemic stroke (D3) compared to (5.7%) in controls, (11.5%) in diabetics with no complications (D1), (15.3)% in diabetics with IHD (D2) and (19.2)% in IHD-stroke group (D4). Genotype 4/4 was most frequently found (30.7%) in diabetics with IHD (D2) as compared to controls (3.8%) However its frequency was (13.4%) in diabetics with no complications (D1), (19.2%) in diabetics with stroke (D3) and (25%) in IHD-stroke group (D4). In patients with IHD and stroke (D4) the level of triglycerides, LDL total cholesterol and VLDL were increased and HDL levels were found to be decreased significantly
4. Discussion
Diabetes is a metabolic disorder that causes a heavy burden on healthcare systems worldwide. The present study analyzed a representative sample of people from Lahore Pakistan and its surrounding regions to investigate the association of demographic, clinical, biochemical parameters and Apolipoprotein E gene polymorphism with ischemic heart disease and stroke in type 2 diabetics. In the present study, a total of 260 subjects participated. These study subjects were placed equally in to five groups. Two groups consisted of controls (healthy control and type 2 diabetics, whereas, the other three groups were: type 2 diabetics plus stroke; type 2 diabetics with IDH and type 2 diabetics plus IHD and Stroke. (
Table 4.2). Research by American Diabetes Association ADA showed that management of diabetes is directly affected by gender and a person’s age, where females and adults of 60 years and above are affected due to the coexistence of multiple medical conditions involving the heart and the kidney leading to limitation and insufficiencies of medical prescription. Following the agenda both current study was divided into females and males.
According to present cross-sectional study, age of the participants was from 70-85 years. This age group was selected because previous studies indicated that this age group has greater chances of becoming hypertensive and diabetic. Moreover these two diseases are very well-known for increasing the danger of cardiac and vascular disease (CVD) and are considered known causes of death globally (Zafar et al., 2017). Hence, older type 2 diabetic subjects >40 years of age were selected to estimate the threat of cardiovascular complications in these subjects.
The present study found that females were more affected by type 2 diabetes and its complications compared to males. Machado et al., 2013 stated that females are highly affected by type 2 diabetes because they are less muscular which does not support high uptake of fixed glucose load and have relatively high levels of estrogen and progesterone which are involved in the reduction of the whole-body insulin sensitivity (machado et al., 2013; Bommer et al., 2018; Gerdts and Regitz-Zagrosek 2019).
Likewise, it was found that females suffering from cardiovascular complications of diabetes was more than male subjects in all the disease groups (
Table 4.1)). Similar findings were reported by others (Peters
et al., 2015 ; Members
et al., 2016 and Seghieri
et al., 2017). These findings are consistent with previous analysis in which a 46 % increased danger of fatal (IHD) in diabetic women was reported by (Huxley
et al., 2006).
A study by (Zunt
et al., 2016) suggested that BMI is one of the factors that increases the incidence of diabetes in almost all countries and has a strong connection with insulin resistance (Sharma
et al., 2020). BMI is usually defined by excessive amount of body fat and is assessed by BMI (kg/m
2). It is a significant causative factor of uncontrolled glucose levels in blood and diabetic cardiovascular complications (Marie
et al., 2020; Baloch
et al., 2021 and Bhupathiraju
et al., 2016). In the present study 92.7% subjects (28.3%) males and (64.4%) females were obese (
Table 4.1). Significant difference in BMI belonging to both genders was observed in all groups as compared to healthy control.
Hypertension is very frequently seen in diabetic patients and is an important risk factor for diabetic cardiovascular complications. In the present study, 75.4% individuals (27.4% males, 48.0% females) were hypertensive B.P (>140/90) (
Table 4.1). In age group (70-75 years), 38.5% subjects were hypertensive, 4.8% had (stage 1) and 33.6% had (stage 2) hypertension, In subjects above 75 years of age a total of 36.9% subjects were hypertensive. 8.5% had (stage 1) and 28.4 % subjects had (stage 2) hypertension. Stage 2 hypertension was frequently present in both age groups (
Table 4.5). A highly significant difference in (SBP) and (DBP) was found in both genders in all disease groups (Abdelbagi
et al., 2021) (
Table 4.6). It reported that the incidence of hypertension in diabetics was high and males had greater risk of developing hypertension compared to females (Kemche
et al., 2020).
Smoking is considered to speed up the risk of cardiovascular complications by two folds in type 2 diabetes (Yang et al., 2016). In the present study 14.4% subjects (all males) gave the history of smoking almost 10 cigarettes /day. In diabetics with IHD and stroke the smokers had high levels of total cholesterol and diabetics with stroke non-smokers had high levels of triglycerides as compared to smokers (Table 4.1 and 4.12). 0ut of 30 smokers, with IHD, 6 in diabetics with stroke 7.in diabetics with both stroke in the disease groups only one was normotensive others were hypertensive. There were 14 smokers in Diabetics and IHD group and 2 in diabetics with no complications, we can say that maximum number (27) of smokers belonged to the groups with diabetic complications and 2 belonged to the group of diabetic subjects without complications. These results regarding smoking are supported by (Campagna et al., 2019) ,The United Kingdom Prospective Diabetes Study (UKPD) and In the Nurses' Health Study (Chang and journal 2012).
Physical activity can decrease the occurrence of cardiac disease and stroke in type 2 diabetics by improving ischemia, blood sugar levels and insulin sensitivity as observed by (Zheng
et al.,2018) and (Nilsson
et al., 2019). Exercise is considered to be a significant factor in managing diabetes mellitus. In the present study, only 12.0% subjects were practicing healthy routine and physical activity 2.3 times /week. Almost 88% subjects were physically inactive (see
Table 4.1). The results of present study were consistent with observations of (Abushamat
et al., 2019). In his study he observed that 40.8% of diabetic individuals were inactive physically and were exercising less than 10 minutes per week. This shows that majority of people in our society do not give importance to exercise. In a recent metaanalysis by (shah
et al., 2021), it was proved by almost all the studies that there is a significant role of exercise in decreasing HbA1c to the point at which threat of diabetic complications was almost minimum. This is also similar to our results.
Patients with positive family history of diabetes and cardiovascular disease have same risks involved. It increases the danger of developing diabetes three times as observed by (Tsenkova
et al., 2016). In the current study family history was Positive in only 10.4 % subjects (3.8% males and 3.3% females) (
Table 4.1). However, in a study carried out in Lahore Pakistan 64% individuals gave the positive family history of T2DM. Family history was positive in 67% subjects in individuals with uncontrolled blood sugar levels and in patients with controlled blood sugar levels only 55% had positive family history. Data of the research carried out on 137 type 2 diabetic subjects in 2010 reported that, diabetics have more significant (p=0.0001) family history of diabetes when compared with non-diabetics (Rasheed
et al., 2015).
Hyperglycemia and hyperinsulinemia result in increasing amounts of circulating FFA in plasma and atherosclerosis leading to cardiovascular complications as reported by (Wang
et al., 2016). In the present study about 87% of subjects in the disease groups had uncontrolled fasting blood sugar (>126 mg/dL) only 13% had controlled blood sugar fasting (<126 mg/dL) (see Figure 4.5). A highly significant difference in blood sugar fasting was found in both genders in all disease groups when compared with controls, where P <0.0001. This is consistent with a study conducted by (Riaz
et al., 2021). According to his observation majority of subjects (76.9%) had raised fasting blood sugar levels and diabetes was not controlled. Only 24% subjects had controlled sugar levels. Results of the present study are comparable to a study carried out in Mirpur Khas by (Shaikh
et al., 2008) which showed that 73% patients had uncontrolled diabetes). Majority of patients (92%) inducted in a study, who claimed that they were on regular medication had high glucose levels (Memon
et al., 2012). This is comparable to our results (
Table 4.11, Figure 4.5)
Dyslipidemia was also present in (83.5%) of Pakistani population in the current study individuals when compared with controls. Some studies have also reported increased danger of stroke and heart disease in diabetics due to dyslipidemia, especially with elevated levels of LDL-C (Maida et al., 2022). LDL-C increases the levels of FFA in plasma after decreased insulin sensitivity develops. The situation becomes more complicated because of high levels of inflammatory cytokines as reported by (Narindrarangkura et al., 2019). In the current study elevated LDL levels were observed (130 mg/dL) as compared to controls (Figure 4.10). A highly significant difference in low density lipoproteins was found in both genders in all disease groups when compared with the control, where P<0.0001.It was also noticed that 81% individuals in the disease groups with cardiovascular complications were suffering from mixed dyslipidemia.61% females and 20% males. LDL (94.2%), mixed dyslipidemia (91.1%), hypercholesterolemia (82.1%), increased triglycerides (73.7%) and decreased HDL (46.9%). Most prevalent dyslipidemia in both genders was LDL, 65.1% in men and 51.4% in women. Cholesterol levels were also found to be high (>200.mg/dL) in both genders as compared to healthy controls and a significance was observed when cholesterol levels were compared with controls in both genders and in all disease groups where P<0.0001. High levels (>150 mg/dL) of Triglycerides were observed in both genders as compared to healthy controls. These observations were similar to the study carried out by (Mehta et al., 2021) in Eastern Nepal and (Das et al., 2020). In the present study deranged lipid profile due to atherosclerosis was found in majority of the patients which could be due to decreased insulin sensitivity enhanced by hyperglycemia and toxicity due to lipid accumulation.
Genetic and epigenetic factors also influence DM, hypertension, obesity and metabolic syndrome. The genotypes of APOE gene in Pakistani type 2 diabetic population were E3/3, E4/4, E2/3, and E3/4. Frequency of genotype E3/3 was high in disease groups compared to controls. Our results were consistent with previous studies carried out by (Lebedy et al., 2016) in Egyptian population. Similarly frequency of E3/E4 was significantly higher in diabetics with cardiovascular complications when compared to controls. This is similar to results obtained by (Gao et al., 2021 and Lebeidy et al., 2016).
In all these patients studied, the levels of triglycerides, LDL total cholesterol and VLDL were raised. Levels of HDL were decreased compared to healthy controls. The frequency of E2/3 genotype was also observed to be higher in diabetics with IHD and stroke. This is consistent with observations of (Wang et al., 2021 and Ping et al., 2020) in Chinese population. However, the distributions levels (concentration) of genotypes determined in the present study varied in study groups.
From these findings, it could be extracted that genotypes E3/3. E4/4. E2/3 and E 3/4 are responsible for the development of IHD and stroke in type 2 diabetics of Lahore, Pakistan.
5. Conclusion
This study, was conducted in Lahore, Pakistan, found a significant frequency of cardiovascular disorders such as ischemic heart disease (IHD) and stroke among type 2 diabetes patients, particularly females. Obesity, physical inactivity, poor nutrition, and hypertension, as well as uncontrolled blood sugar levels, were prevalent risk factors among these individuals. Dyslipidemia was also prevalent, with increased cholesterol and triglyceride readings and low HDL. Apolipoprotein E gene variants, particularly genotypes 3/3, 4/4, 2/3, and 3/4, have been linked to an increased risk of IHD and stroke in diabetics. Genotypes 2/3 and 3/4 were more common in those who had a stroke or had both IHD and stroke. These findings point to a hereditary susceptibility for cardiovascular problems in type 2 diabetes.
References
- Abdelbagi, O.; Musa, I.R.; Musa, S.M.; Altigani, S.A.; Adam, I. Prevalence and associated factors of hypertension among adults with diabetes mellitus in northern Sudan: a cross-sectional study. BMC Cardiovasc. Disord. 2021, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Aljulifi, M.Z. Prevalence and reasons of increased type 2 diabetes in Gulf Cooperation Council Countries. SciVee 2021, 42, 481–490. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care 2019, 42 (Supplement_1), S13–S28. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Hu, F.B. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ. Res. 2016, 118, 1723–1735. [Google Scholar] [CrossRef]
- Bommer, C.; Sagalova, V.; Heesemann, E.; Manne-Goehler, J.; Atun, R.; Bärnighausen, T.; Davies, J.; Vollmer, S. Global Economic Burden of Diabetes in Adults: Projections From 2015 to 2030. Diabetes Care 2018, 41, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Campagna, D.; Alamo, A.; Di Pino, A.; Russo, C.; Calogero, A.E.; Purrello, F.; Polosa, R. Smoking and diabetes: dangerous liaisons and confusing relationships. Diabetol. Metab. Syndr. 2019, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.A. Smoking and Type 2 Diabetes Mellitus. Diabetes Metab. J. 2012, 36, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ovbiagele, B.; Feng, W. Diabetes and stroke: epidemiology, pathophysiology, pharmaceuticals and outcomes. The American Journal of the Medical Sciences 2010, 351, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Das, B.K.L.; Prassan, N.; Ansari, M.F.; Agrawal, K.; Tripathi, S.; Niraula, A. Dyslipidemic profile in Type 2 Diabetes Mellitus: A hospital-based study from Eastern Nepal. J. Biomed. Sci. 2020, 7, 64–70. [Google Scholar] [CrossRef]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 1–19. [Google Scholar] [CrossRef]
- Gerdts, E.; Regitz-Zagrosek, V. Sex differences in cardiometabolic disorders. Nat. Med. 2019, 25, 1657–1666. [Google Scholar] [CrossRef]
- Huang, D.; Refaat, M.; Mohammedi, K.; Jayyousi, A.; Al Suwaidi, J.; Khalil, C.A. Macrovascular Complications in Patients with Diabetes and Prediabetes. BioMed Res. Int. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.; Barzi, F.; Woodward, M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 2005, 332, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Javed, R.A.; Bhatti, A.; AMIN, M. Dyslipidemia in type 2 diabetes mellitus in normal and underweight patient. Pak J Med Health Sci 2016, 10, 568–570. [Google Scholar]
- Kemche, B.; Foudjo, B.U.S.; Fokou, E. Risk Factors of Hypertension among Diabetic Patients from Yaoundé Central Hospital and Etoug-Ebe Baptist Health Centre, Cameroon. J. Diabetes Res. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Weng, R.; Gu, X.; Zhong, Z. Apolipoprotein E gene polymorphism and the risk of cardiovascular disease and type 2 diabetes. BMC Cardiovasc. Disord. 2019, 19, 1–6. [Google Scholar] [CrossRef]
- Machado-Alba, J.E.; Machado-Duque, M.E. Cardiovascular risk factors prevalence among patients with dyslipidemia in Colombia. Revista Peruana de Medicina Experimental y Salud Publica 2013, 30, 205–211. [Google Scholar] [PubMed]
- Maqbool, T.; Awan, S.J.; Malik, S.; Hadi, F.; Shehzadi, S.; Tariq, K. In-Vitro Anti-Proliferative, Apoptotic and Antioxidative Activities of Medicinal Herb Kalonji (Nigella sativa). Curr. Pharm. Biotechnol. 2019, 20, 1288–1308. [Google Scholar] [CrossRef]
- Mansoor, G.; Tahir, M.; Maqbool, T.; Abbasi, S.Q.; Hadi, F.; Shakoori, T.A.; Akhtar, S.; Rafiq, M.; Ashraf, M.; Ullah, I. Increased Expression of Circulating Stress Markers, Inflammatory Cytokines and Decreased Antioxidant Level in Diabetic Nephropathy. Medicina 2022, 58, 1604. [Google Scholar] [CrossRef]
- Mehta, R.K.; Koirala, P.; Mallick, R.L.; Parajuli, S.; Jha, R. Dyslipidemia in Patients with Type 2 Diabetes Mellitus in a Tertiary Care Centre: A Descriptive Cross-sectional Study. J. Nepal Med Assoc. 2021, 59, 305–308. [Google Scholar] [CrossRef]
- Nilsson, P.M.; Tuomilehto, J.; Rydén, L. The metabolic syndrome – What is it and how should it be managed? Eur. J. Prev. Cardiol. 2019, 26, 33–46. [Google Scholar] [CrossRef]
- Peters, S.A.E.; Woodward, M. Sex Differences in the Burden and Complications of Diabetes. Curr. Diabetes Rep. 2018, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, M.; Islam, N.; Mahjabeen, W. Factors associated with uncontrolled type 2 diabetes mellitus. Journal of Islamabad Medical & Dental College (JIMDC) 2015, 4, 68–71. [Google Scholar]
- Reed, J.; Bain, S.; Kanamarlapudi, V. A Review of Current Trends with Type 2 Diabetes Epidemiology, Aetiology, Pathogenesis, Treatments and Future Perspectives. Diabetes, Metab. Syndr. Obesity: Targets Ther. 2021, 14, 3567–3602. [Google Scholar] [CrossRef] [PubMed]
- Riaz, F.; Al Shaikh, A.; Anjum, Q.; Alqahtani, Y.M.; Shahid, S. Factors related to the uncontrolled fasting blood sugar among type 2 diabetic patients attending primary health care center, Abha city, Saudi Arabia. Int. J. Clin. Pr. 2021, 75, e14168. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, M.; Sajid, S.; Ashraf, M.A. Prevalence and pattern of dyslipidemia in hyperglycemic patients and its associated factors among Pakistani population. Saudi J. Biol. Sci. 2016, 23, 761–766. [Google Scholar] [CrossRef]
- Seghieri, G.; Policardo, L.; Anichini, R.; Franconi, F.; Campesi, I.; Cherchi, S.; Tonolo, G. The Effect of Sex and Gender on Diabetic Complications. Curr. Diabetes Rev. 2017, 13, 148–160. [Google Scholar] [CrossRef]
- Shah, S.Z.A.; Karam, J.A.; Zeb, A.; Ullah, R.; Shah, A.; Haq, I.U.; Ali, I.; Darain, H.; Chen, H. Movement is Improvement: The Therapeutic Effects of Exercise and General Physical Activity on Glycemic Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetes Ther. 2021, 12, 707–732. [Google Scholar] [CrossRef] [PubMed]
- Tsenkova, V.K.; Karlamangla, A.S.; Ryff, C.D. Parental History of Diabetes, Positive Affect, and Diabetes Risk in Adults: Findings from MIDUS. Ann. Behav. Med. 2016, 50, 836–843. [Google Scholar] [CrossRef]
- Yan, Q.; Sun, D.; Li, X.; Chen, G.; Zheng, Q.; Li, L.; Gu, C.; Feng, B. Association of blood glucose level and hypertension in Elderly Chinese Subjects: a community based study. BMC Endocr. Disord. 2016, 16, 1–8. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, J.; Pu, C.; Zhang, Y. Apolipoprotein status in type 2 diabetes mellitus and its complications. Mol. Med. Rep. 2017, 16, 9279–9286. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).