Submitted:
30 March 2024
Posted:
01 April 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
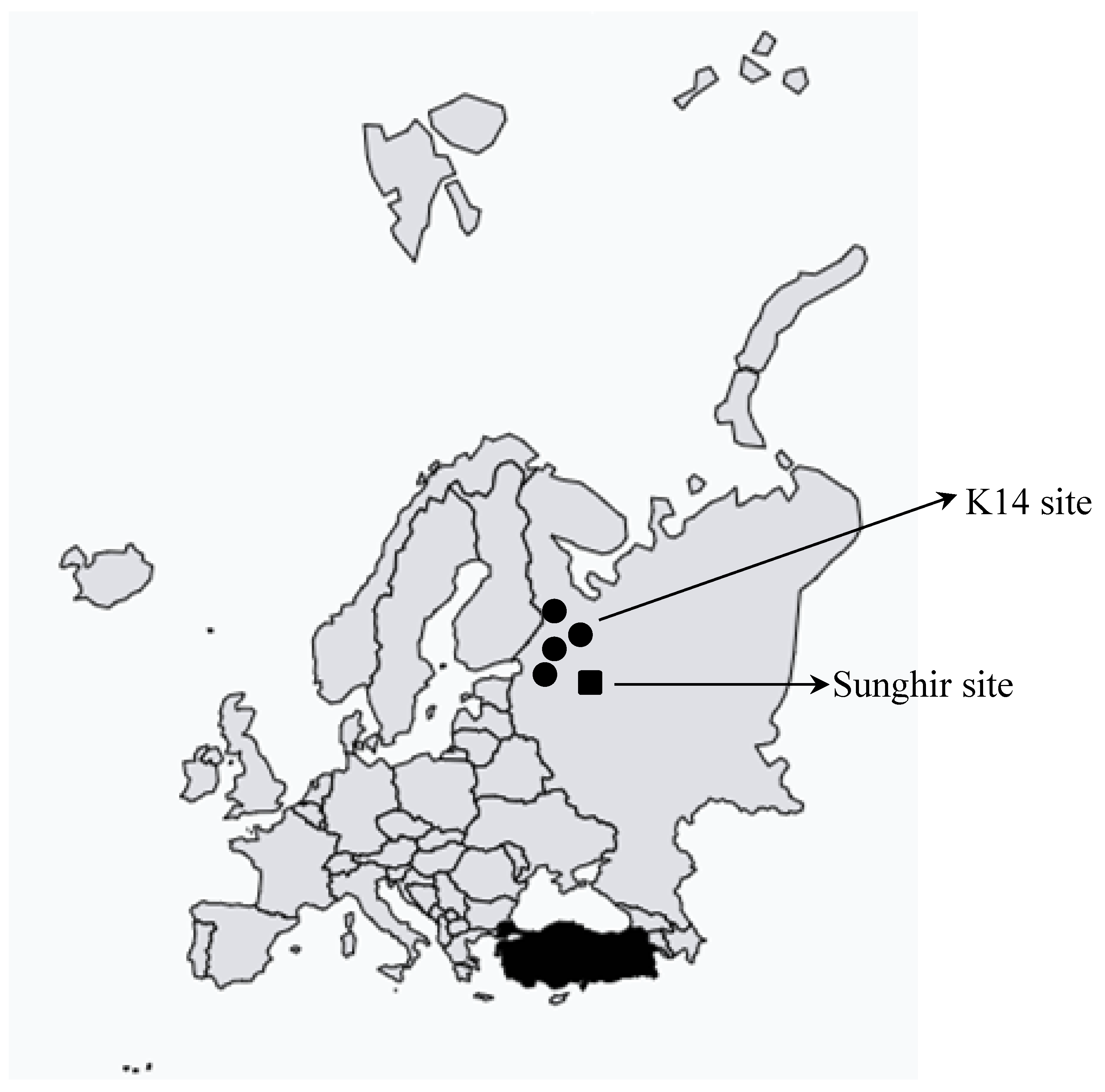
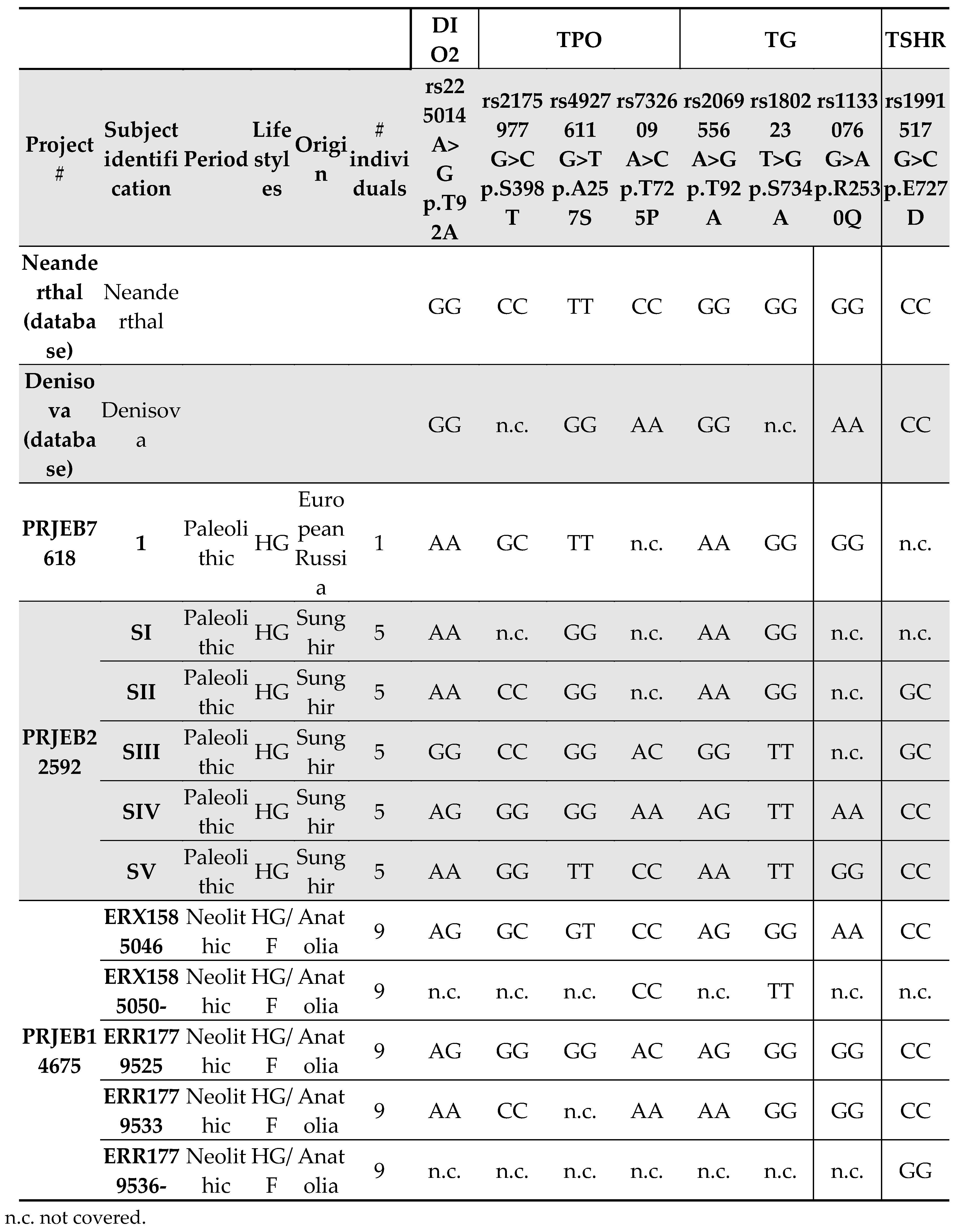
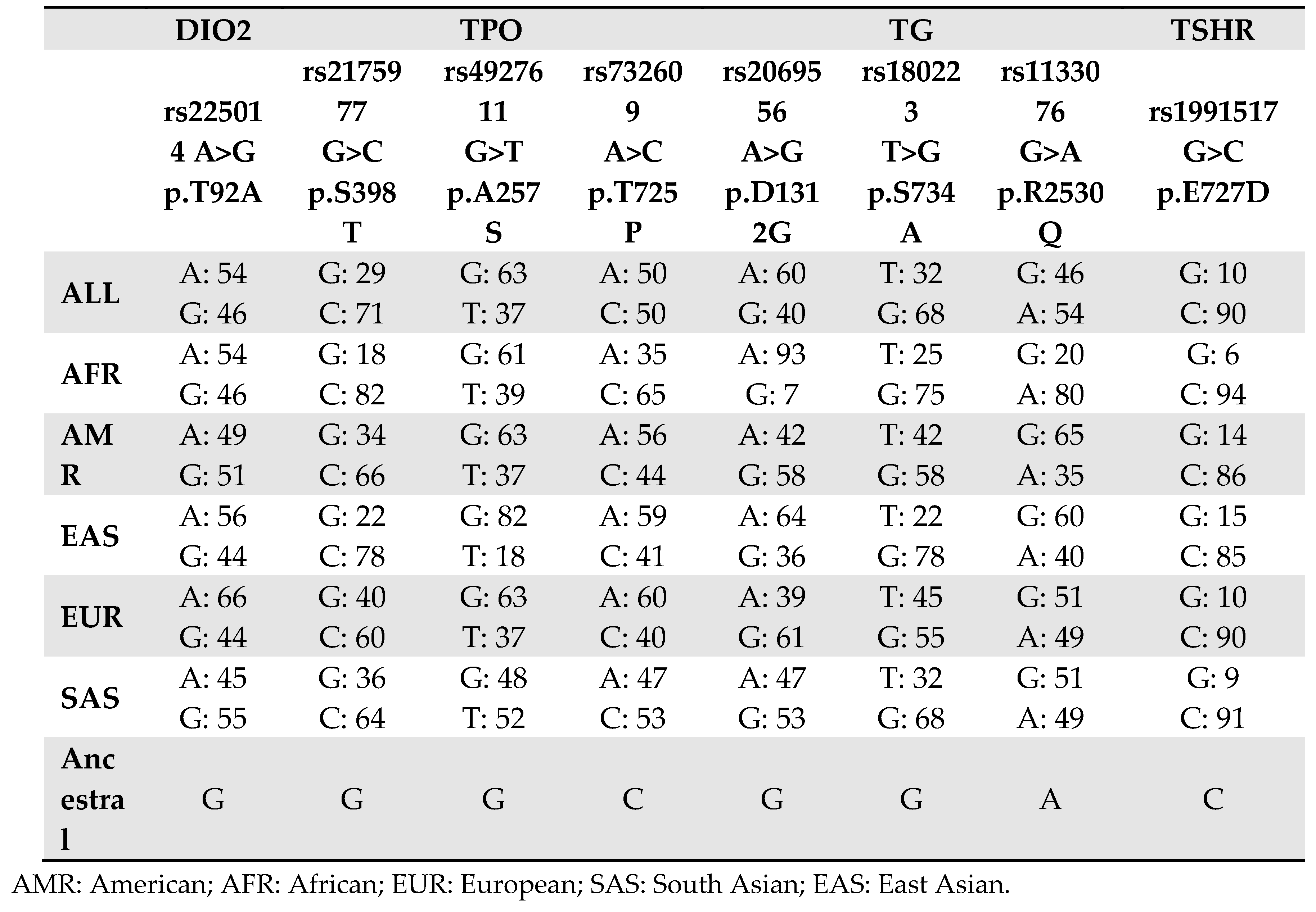
Results
Type II Iodothyronine Deiodinase (DIO2)
Thyroperoxidase (TPO)
Tyreoglobulin (TG)
Thyroid-Stimulating Hormone Receptor (TSHR)
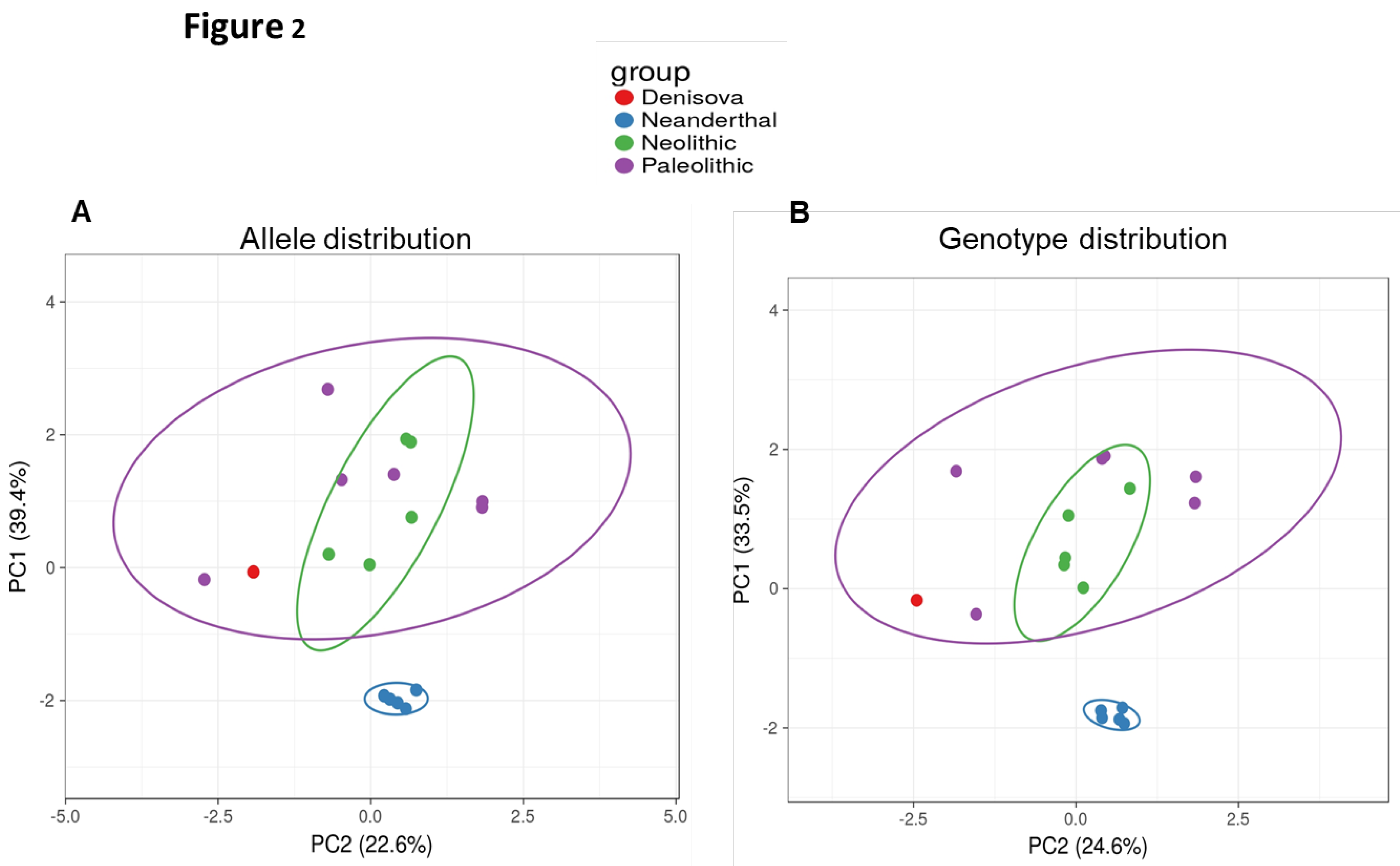
PCA Plot
Discussion
Funding
Conflict of Interest
References
- Brent GA. Mechanisms of thyroid hormone action. J Clin Invest. 2012;122(9):3035-43. [CrossRef]
- Ortiga-Carvalho TM, Chiamolera MI, Pazos-Moura CC, Wondisford FE. Hypothalamus-Pituitary-Thyroid Axis. Compr Physiol. 2016;6(3):1387-428. [CrossRef]
- Chaker L, Razvi S, Bensenor IM, Azizi F, Pearce EN, Peeters RP. Hypothyroidism. Nat Rev Dis Primers. 2022;8(1):30. Erratum in: Nat Rev Dis Primers. 2022;8(1):39. [CrossRef]
- Rastogi MV, LaFranchi SH. Congenital hypothyroidism. Orphanet J Rare Dis. 2010;5:17. [CrossRef]
- Park SM, Chatterjee VK. Genetics of congenital hypothyroidism. J Med Genet. 2005;42(5):379-89. [CrossRef]
- Grasberger H, Refetoff S. Genetic causes of congenital hypothyroidism due to dyshormonogenesis. Curr Opin Pediatr. 2011;23(4):421-8. [CrossRef]
- Su Y, Wang J, Zhou J, Chen Y, Zhao H, Zeng Y, Lin F, Zhang H, Zhu W, Chen H. Association of thyroperoxidase gene polymorphisms with dyshormonogenesis in congenital hypothyroidism. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2015;32(6):861-5. Chinese. [CrossRef]
- Kollati Y, Akella RRD, Naushad SM, Thalla M, Reddy GB, Dirisala VR. The rs1991517 polymorphism is a genetic risk factor for congenital hypothyroidism. 3 Biotech. 2020;10(6):285. [CrossRef]
- Kollati Y, Akella RRD, Naushad SM, Borkar D, Thalla M, Nagalingam S, Lingappa L, Patel RK, Reddy GB, Dirisala VR. Newborn screening and single nucleotide variation profiling of TSHR, TPO, TG and DUOX2 candidate genes for congenital hypothyroidism. Mol Biol Rep. 2020;47(10):7467-7475. [CrossRef]
- Kollati Y, Akella RRD, Naushad SM, Patel RK, Reddy GB, Dirisala VR. Molecular insights into the role of genetic determinants of congenital hypothyroidism. Genomics Inform. 2021;19(3):e29. [CrossRef]
- Cavalli-Sforza LL. Human evolution and its relevance for genetic epidemiology. Annu Rev Genomics Hum Genet. 2007;8:1-15. PMID: 17408354. [CrossRef]
- Bamshad M, Wooding SP. Signatures of natural selection in the human genome. Nat Rev Genet. 2003;4(2):99-111. [CrossRef]
- Poinar HN (1999) DNA from fossils: the past and the future. Acta Paediatr Suppl 88:133–140. https ://doi.org/10.1111/j.1651-2227.1999.tb144 23.x.
- Scholz M, Bachmann L, Nicholson GJ, Bachmann J, Giddings I, Rüschoff-Thale B, Czarnetzki A, Pusch CM. Genomic differentiation of Neanderthals and anatomically modern man allows a fossil-DNA-based classification of morphologically indistinguishable hominid bones. Am J Hum Genet 2020;66:1927–1932. https ://doi.org/10.1086/302949.
- Noonan JP, Coop G, Kudaravalli S, Smith D, Krause J, Alessi J, Chen F, Platt D, Pääbo S, Pritchard JK, Rubin EM. Sequencing and analysis of Neanderthal genomic DNA. Science 2006;314:1113–1118. https ://doi.org/10.1126/scien ce.11314 12.
- Green RE, Krause J, Ptak SE, Briggs AW, Ronan MT, Simons JF, Du L, Egholm M, Rothberg JM, Paunovic M, Pääbo S. Analysis of one million base pairs of Neanderthal DNA. Nature2006; 444:330–336. https ://doi.org/10.1038/natur e0533 6.
- Wakefield MJ. Genomics from Neanderthals to high throughput sequencing. Genome Biol 2006;7:326. https ://doi.org/10.1186/gb-2006-7-8-326.
- Ricci C, Kakularam KR, Marzocchi C, Capecchi G, Riolo G, Boschin F, Kuhn H, Castagna MG, Cantara S. Thr92Ala polymorphism in the type 2 deiodinase gene: an evolutionary perspective. J Endocrinol Invest. 2020;43(12):1749-1757. [CrossRef]
- Seguin-Orlando A, Korneliussen TS, Sikora M, Malaspinas AS, Manica A, Moltke I, Albrechtsen A, Ko A, Margaryan A, Moiseyev V, Goebel T, Westaway M, Lambert D, Khartanovich V, Wall JD, Nigst PR, Foley RA, Lahr MM, Nielsen R, Orlando L, Willerslev E. Paleogenomics. Genomic structure in Europeans dating back at least 36,200 years. Science. 2014;346(6213):1113-8. [CrossRef]
- Sikora M, Seguin-Orlando A, Sousa VC, Albrechtsen A, Korneliussen T, Ko A, Rasmussen S, Dupanloup I, Nigst PR, Bosch MD, Renaud G, Allentoft ME, Margaryan A, Vasilyev SV, Veselovskaya EV, Borutskaya SB, Deviese T, Comeskey D, Higham T, Manica A, Foley R, Meltzer DJ, Nielsen R, Excoffier L, Mirazon Lahr M, Orlando L, Willerslev E. Ancient genomes show social and reproductive behavior of early Upper Paleolithic foragers. Science. 2017;358(6363):659-662. [CrossRef]
- Kılınç GM, Omrak A, Özer F, Günther T, Büyükkarakaya AM, Bıçakçı E, Baird D, Dönertaş HM, Ghalichi A, Yaka R, Koptekin D, Açan SC, Parvizi P, Krzewińska M, Daskalaki EA, Yüncü E, Dağtaş ND, Fairbairn A, Pearson J, Mustafaoğlu G, Erdal YS, Çakan YG, Togan İ, Somel M, Storå J, Jakobsson M, Götherström A. The Demographic Development of the First Farmers in Anatolia. Curr Biol. 2016;26(19):2659-2666. [CrossRef]
- Leinonen R, Sugawara H, Shumway M; International Nucleotide Sequence Database Collaboration. The sequence read archive. Nucleic Acids Res. 2011;39:D19-21. [CrossRef]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol 1990;21:403–410. [CrossRef]
- Tauno Metsalu, Jaak Vilo, ClustVis: a web tool for visualising clustering of multivariate data using Principal Component Analysis and heatmap, Nucleic Acids Research, Volume 43, Issue W1, 1 July 2015, Pages W566–W570. [CrossRef]
- Hannoush ZC, Weiss RE. Defects of Thyroid Hormone Synthesis and Action. Endocrinol Metab Clin North Am. 2017;46(2):375-388. [CrossRef]
- Wajdi Safi, Faten Hadj Kacem, Hana Charfi, Mouna Mnif Feki, Mohamed Abid and Noura Bougacha-Elleuch. Genetic investigation of thyroid dyshormonogenesis in a Tunisian consanguineous family. Endocrine Abstracts (2021) 73 AEP707 | DOI: 10.1530/endoabs.73.AEP707.
- Hashemipour M, Soheilipour F, Karimizare S, Khanahmad H, Karimipour M, Aminzadeh S, Kokabee L, Amini M, Hovsepian S, Hadian R. Thyroid peroxidase gene mutation in patients with congenital hypothyroidism in isfahan, iran. Int J Endocrinol. 2012;2012:717283. [CrossRef]
- Guria S, Bankura B, Balmiki N, Pattanayak AK, Das TK, Sinha A, Chakrabarti S, Chowdhury S, Das M. Functional analysis of thyroid peroxidase gene mutations detected in patients with thyroid dyshormonogenesis. Int J Endocrinol. 2014;390121. [CrossRef]
- Begum MN, Islam MT, Hossain SR, Bhuyan GS, Halim MA, Shahriar I, Sarker SK, Haque S, Konika TK, Islam MS, Rahat A, Qadri SK, Sultana R, Begum S, Sultana S, Saha N, Hasan M, Hasanat MA, Banu H, Shekhar HU, Chowdhury EK, Sajib AA, Islam ABMMK, Qadri SS, Qadri F, Akhteruzzaman S, Mannoor K. Mutation Spectrum in TPO Gene of Bangladeshi Patients with Thyroid Dyshormonogenesis and Analysis of the Effects of Different Mutations on the Structural Features and Functions of TPO Protein through In Silico Approach. Biomed Res Int. 2019;2019:9218903. [CrossRef]
- Kuhlwilm M, Gronau I, Hubisz MJ, de Filippo C, Prado-Martinez J, Kircher M, Fu Q, Burbano HA, Lalueza-Fox C, de la Rasilla M, Rosas A, Rudan P, Brajkovic D, Kucan Ž, Gušic I, Marques-Bonet T, Andrés AM, Viola B, Pääbo S, Meyer M, Siepel A, Castellano S. Ancient gene flow from early modern humans into Eastern Neanderthals. Nature. 2016;530(7591):429-33. [CrossRef]
- Slon V, Mafessoni F, Vernot B, de Filippo C, Grote S, Viola B, Hajdinjak M, Peyrégne S, Nagel S, Brown S, Douka K, Higham T, Kozlikin MB, Shunkov MV, Derevianko AP, Kelso J, Meyer M, Prüfer K, Pääbo S. The genome of the offspring of a Neanderthal mother and a Denisovan father. Nature. 2018;561(7721):113-116. [CrossRef]
- Inchley CE, Larbey CD, Shwan NA, Pagani L, Saag L, Antão T, Jacobs G, Hudjashov G, Metspalu E, Mitt M, Eichstaedt CA, Malyarchuk B, Derenko M, Wee J, Abdullah S, Ricaut FX, Mormina M, Mägi R, Villems R, Metspalu M, Jones MK, Armour JA, Kivisild T. Selective sweep on human amylase genes postdates the split with Neanderthals. Sci Rep. 2016;6:37198. [CrossRef]
- Perry GH, Dominy NJ, Claw KG, Lee AS, Fiegler H, Redon R, Werner J, Villanea FA, Mountain JL, Misra R, Carter NP, Lee C, Stone AC. Diet and the evolution of human amylase gene copy number variation. Nat Genet. 2007t;39(10):1256-60. [CrossRef]
- Eaton SB. The ancestral human diet: what was it and should it be a paradigm for contemporary nutrition? Proc Nutr Soc. 2006;65(1):1-6. [CrossRef]
- Jaouen K, Richards MP, Le Cabec A, Welker F, Rendu W, Hublin JJ, Soressi M, Talamo S. Exceptionally high δ15N values in collagen single amino acids confirm Neandertals as high-trophic level carnivores. Proc Natl Acad Sci U S A. 2019;116(11):4928-4933. [CrossRef]
- Singh A, Singh D. The Paleolithic Diet. Cureus. 2023;15(1):e34214. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





