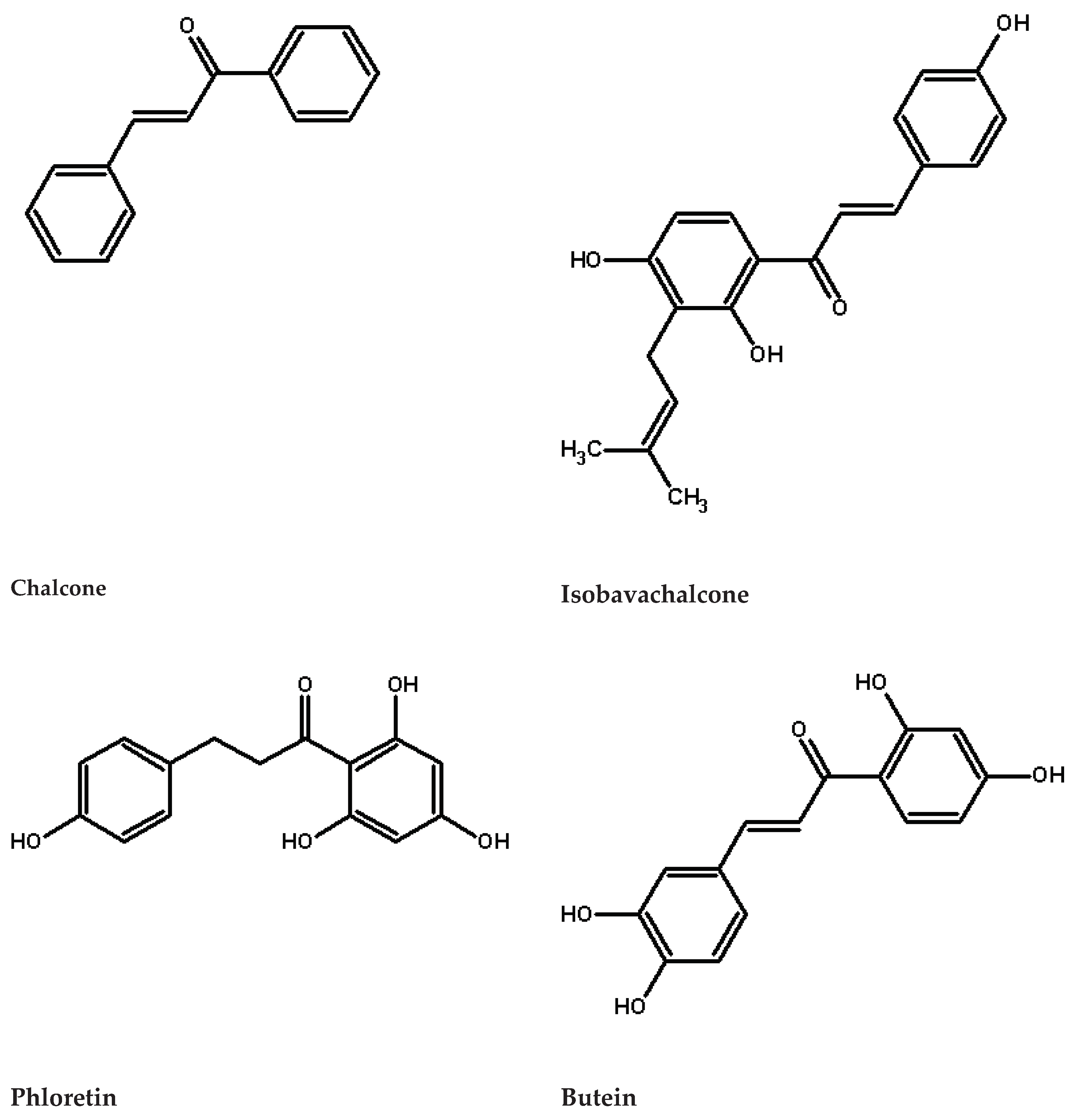
1. Introduction
Chalcones are characterised by the absence of ‘C ring’ of the basic flavonoid skeleton structure (see
Figure 1). Hence, they can also be referred to as open-chain flavonoids, which are well-known specialized (secondary) metabolites occurring ubiquitously in plant kingdom. They can be found in different parts of the plant supplying its growth and defence against pathogens. Flavonoids belong to a class of phenolic compounds and according to the degree of oxidation of the heterocyclic ring and the number of hydroxyl or methyl groups on the benzene ring, flavonoids can be divided into 12 subgroups: anthocyanins, aurones, chalcones, dihydroflavonols, flavanones, flavones, flavanols, isoflavones, leucoanthocyanidins, phlobaphenes, proanthocyanidins and stilbenes [
1].
The term chalcone is originated from the Greek phrase “chalcos” which means bronze. Major examples of chalcones include phloridzin, butein, phloretin and chalco-naringenin. Chalcones can be found in considerable amounts in strawberries, berries, some wheat products, tomatoes, pears, apples, citrus fruit and hop plant. Because of numerous nutritional and biological benefits chalcones and their derivatives have gathered considerable attention [
2,
3,
4,
5]. Natural and synthetic chalcones have been reported to possess anti-inflammatory, antitumoral, antibacterial, antifungal, antimalarial, antitubercular and antipigmentation activities, not rarely with an excellent result [
4,
6,
7,
8]. Moreover, chalcones can be useful for weed control [
9,
10]. Interestingly, a single compound may exibit several activities, for example isobavachalcone is known to have chemopreventive, anti-cancer, anti-bacterial and anti-fungal activities [
11,
12]. Naturally occurring chalcones have been used in traditional medicine for years, only now new applications appear [
13]. Due to increased effort to reduce synthetic pesticides usage within Farm to Fork strategy from the European Union until 2030, biopesticides are gaining popularity not only because of different mode of action but also, and more importantly, they are more environmentally friendly.
Figure 1.
Chalcones structures.
Figure 1.
Chalcones structures.
2. Characteristic
From the chemical point of view, chalcones are
α,
β unsaturated ketones consisting of two aromatic rings (ring A and ring B) linked through a three-carbon alkenone unit [
14]. In higher plants, chalcones are synthesized by the enzyme chalcone synthase (CHS,EC2.3.1.74) from one molecule of
p-coumaroyl-CoA and three molecules of malonyl-CoA. CHS plays role not only in the development process in many plants but also is induced under stress conditions, like UV, wounding, herbivory and microbial pathogens, resulting in the production of secondary metabolites e.g. phenolic compounds [
15,
16]. Chalcone is a common simple scaffold found in many naturally occurring compounds but large number of new chalcone structures have been synthesized over the years [
17]. A collection of bioactive synthetic chalcone derivatives, structurally improved to reduce their toxicity, allowed their use not only in medicine and chemical industry [
14] but also in food production and agriculture sector [
18].
Chalcones can be obtained either from natural sources or by chemical synthesis. From the beginning of 19th century, many researchers have developed synthetic chalcones, not to forget Kostanecki and Tambor experiment where they first obtained synthetic chalcone using o-acetoxychalcone dibromides with alcoholic alkali [
14,
19]. Among synthetic methods, the Claisen-Schmidt condensation, hydrochloric acid usage, chalcone synthesis from phosphonate carbanion, synthesis based on microwave and solvent-free conditions, involving biocatalysts or aldol condensation synthesis using (hetero)aryl methyl ketones and 4(benzyloxy)benzaldehyde are worth highlighting [
12,
20,
21].
Native chalcone glycosides tend to transform to flavanone glycosides during extraction. Chalcones are by itself of restricted occurrence in food [
3]. Mixtures of retrochalcones along with isomeric flavanones and chalcones (eg liquiritigenin and isoliquiritigenin) have been reported in licorice root (Glycyrrhiza spp) and some licorice-based traditional medicines [
22]. Dihydrochalcones (DHC) are distinctive for apples and its products, with phloridzin being the most common [
23]. Peeled fruits contain less DHCs, as they are removed together with the peel. Similarly, DHC content is 5-10 times higher in commercially produced juices and ciders not only because the whole fruit is used but also due to the thermal treatment which inactivates the enzymes that degrade DHC [
24].
3. Chalkones Identification
Sample Preparation
One of the most efficient sample preparation methods before chromatographic analysis (i.e. UPLC-MS/MS) is freeze drying. In details: a mixer mill with a zirconia bead can be used for freeze-dried sample for 1.5 min at 30 Hz, then dissolve 100 mg of lyophilized powder with 1.2 mL 70% methanol solution, vortex for 30 s every 30 min, 6 times in total, and place the sample in a refrigerator at 4 °C overnight. Following centrifugation at 12,000 rpm for 10 min, the extracts should be filtered before UPLC-MS/MS analysis [
25]. In order to purify the crude product, column chromatography can be used and eluted with (petroleum ether/EtOAc = 3:1, V/V) to give compounds of interest [
4]. Krauze-Baranowska et al. [
26] optimized the SPE-HPLC method for chalcones in some species and clones of
Salix used for the pharmaceutical industry. The authors dried and pulverized 1 g of bark sample which was subsequently extracted with methanol (3 x 30 mL) for 45 min in 60°. The methanolic extracts were combined and concentrated under reduced pressure. Eighty μL of the extract was evaporated to dryness and re-dissolved in the same volume of 20% ACN. Next, the sample was subjected to a solid phase extraction (SPE) procedure. Also methanol was used as an extractant by Guvenalp et al. [
27]for mint samples in order to isolate bioactive compounds, among which two new chalcone glycosides were reported. Air-dried and powdered aerial parts of the plant (1000 g) were extracted four times with MeOH at 40°C. After vacuum evaporation the crude extract was dissolved in water and subjected to liquid–liquid partitions successively with petroleum ether, CHCl
3, EtOAc and n-butanol. Thereafter, the solvents were evaporated under reduced pressure in a rotary evaporator oven [
28]. Chen et al. [
28] extracted powdered fruit of
Fructus Psoreleae using hydrochloric acid in methanol as an extraction solvent under ultrasonication. Afterwards, the sample was centrifuged at 3000 g for 20 minutes and the supernatant was collected.
Liquid Chromatography Coupled with Absorbance Detectors
There are two main absorption band in chalcones – band I and band II. Band I usually appears at 340-390 nm and band II usually appears at 220 – 270 nm.
According to Pobłocka-Olech [
29] in order to perform qualitative and quantitative analysis of the mixture of five flavonoids: naringenin, naringenin (+) and (-)-5-
O-glycosides, naringenin 7-
O-glycoside, isosalipurposide and its
p-coumaric ester using HPLC method, the chromatographic separation was carried out on reversed phases’ system on Discovery C18 column, including the use of gradient elution in the mixture of acetonitrile / water+orthophosphoric acid. Identification was completed not only using the detector UV-Vis (λ = 280 nm), but also the diode array detector (DAD). The SPE method was incorporated to speed up the analysis time [
30]. Isosalipurposide also known as phloridzin chalcone is a monosaccharide derivative that is trans-chalcone substituted by hydroxy groups at positions 4, 4' and 6 and a
β-D-glucopyranosyloxy group at position 2' respectively. It has a role as a plant metabolite and an antioxidant [
31].
According to Krauze-Baranowska et al. [
26] the separation of chalcones from willow tree bark was performed using a Discovery C18 column (5 μm, 150 ◊ 2.1 mm). The gradient elution was performed according 15 min; flow rate, 0.4 mL/min; Chalcones and flavanones were identified with UV-Vis DAD detection at 280 nm. The content of both compounds was determined by an external standardization with the use of isoliquiritigenin as a reference substance - a commercially available chalcone, and also isosalipurposide and its derivative, 6”-
O-
p-coumaroyl ester [
27]. Chen et al. [
28] performed HPLC - UV analyses using a DL-C
l8 column (5.0 μm, 250 mm 4.6 mm) with a flow rate of 0.5mL/min using acetonitrile (A) and 0.01M formic acid (B) as a mobile phase. Gradient elution was used and the detection wavelength was 246 nm.
Infrared Spectroscopy, FTIR and HNMR
The IR spectra of chalcones asymmetric and symmetric stretching vibrations of the aromatic C–H bonds are seen at 3120 – 3080 cm
−1 and 3060 – 3040 cm
−1 ranges with two low intensity bands. C–H stretching band of the =C–H group is observed at 3030 – 3010 cm
−1. The bands at 1610 - 1570 cm
−1 are assigned to the vibrations of the aromatic ring. The inplane deformation of the=C–H bond appears as broad weak band at 1460 – 1430cm
−1. The carbonyl stretching vibrations for the enones (=C–C=O) can be found between 1650 and 1685cm
−1. [30]
A mixture of chalcone derivatives can also be evaluated via spectroscopic techniques, such as FTIR and 1HNMR [
4,
31]. According to Hassan et al., the FTIR spectrum showed the appearance of compounds by the carbonyl chalcone C=O stretching in 1708 and 1712 cm-1 and the C=C of alkene detected at 1612 and 1622 cm-1. The 1HNMR for derivative C showed the protons of the amine group that were detected at 10.7 ppm and 10.6 ppm, the protons of the aromatic ring detected at a range of 7.5 – 6.6 ppm, and protons of HC-S detected at 5 ppm. In derivative D the protons of the amine group were detected at 10.5 ppm, the aromatic ring 094–101 protons detected at a range of 7.7 – 6.8 ppm, and the protons of HC-S were detected at 4.8 ppm, and protons of the methyl group were detected at 2.2 ppm. [
31].
Liquid Chromatography Coupled with Mass Spectrometry
According to Zou et al. [
25], the
Paeonia delavayi var.
lutea sample extracts were analyzed using an UPLC-ESI-MS/MS Shimadzu system under the above analytical conditions: UPLC: column, Agilent SB-C18 (1.8 µm, 2.1 × 100 mm); the mobile phase consisted of pure water with 0.1% formic acid and acetonitrile with 0.1% formic acid. Sample measurements were performed with a gradient program and the injection volume was 4 μL. The effluent was alternatively connected to an ESI-triple quadrupole-linear ion trap (QTRAP)-MS [
25]. Ma et al. [
32] have developed a simple and selective specific high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for determination of isobavachalcone (IBC) in rat plasma. Neobavaisoflavone was used as an internal standard (IS) and together with the analyte were separated on a 2.6 μm Kinetex C18 column (100 mm×2.1 mm i.d., Phenomenex) by isocratic elution with acetonitrile:water (60:40, v/v) as the mobile phase at a flow rate of 0.2 mL/min. An electrospray ionization (ESI) source was applied and operated in the negative ion mode. Multiple reactions monitoring (MRM) mode was used for quantification, and the target fragment ions m/z 323.0→118.9 for IBC and m/z 321.1→265.0 for the IS were chosen. Good linearity was observed in the concentration range of 3.79-484.5 ng/mL for IBC in rat plasma [
32]. Chen et al. [
28] carried out the detection of four major constituents including bakuchiol, bavachin, bavachinin and isobavachalcone in
Fructus Psoraleae using HPLC coupled with UV, MS and electrochemical detectors (ECD). MS provided a high selectivity and sensitivity for determination of bavachin, isobavachalcone, and bavachin in negative-ion mode using selected ion monitoring (SIM) for the listed compounds in mass range 50 – 1000
m/z.
MALDI Technique
According to Krittanai et al. -[
33] liquid chromatography coupled with UV detection has poor sensitivity to detect licochalcone (LicoA), which is found in the root of Chinese licorice (
Glycyrrhiza inflata Batalin), therefore enzyme-linked immunosorbent assay (ELISA) was developed for the quantitative determination of LicoA using a constructed antibody. The assay validation results were highly specific for the target compound, but minimally cross-reactive with the structure-related substances. After method optimization, the detection limit was 4.32 ng/mL and the quantification limit was 6.84–107.21 ng/mL. The developed technique was applied to determine the concentration of LicoA in raw licorice and marketed samples.
4. Properties
Chalcones Biological Activities and Their Applications in Agriculture
Chalcones are highly bioactive substances that are of great concern for agriculture in terms of controlling weeds and pests. Eco-friendly pesticides and weed control agents have far-reaching biological effects and can be used to combat many organisms [
6].
The structure of chalcones is a key factor that determine their biological activity. In this regard, the number and position of various substituents, mainly hydroxyl groups and the á,â-double bond are important [
13]. Moreover, chalcones can be modified by the addition of specified moieties to obtain the desired activities. Thanks to their specific structure, they have been used as intermediate for the preparations of compounds having therapeutic value [
34]. Chalcones have been found to be effective for controlling weeds and pests by exhibiting phytotoxic, bactericidal, antifungal, antiviral, antihelmintic, insecticidal, and antifeedant activities.
Herbicides and Plant Growth Regulators
From the agricultural point of view, very important is the chalcones’ phytotoxic activity, which may be used in the process of developing new herbicides. As evidenced by studies, many chalcones are able to exhibit strong herbicidal activity with low toxicity for crops [
6,
9]. The activity depends on the groups added to rings A and B that are part of their structure, the applied concentrations, plant species, and organs. Derivatives containing phenoxyacetic acid, 4-(N,N-dimethylamino)phenyl, N-methylpyrrole, or especially thiophenyl functional groups have shown promising inhibitory activity. [
10,
13].
Chotsaeng, et al. [
10] have shown that flavokawains , which are chalcone-related derivative of xantoxyline, can greatly suppress the growth of Chinese amaranth and barnyardgrass.Among 45 synthesized chalcones, (E)-2-(2-(3-Oxo-3-(thiophen-2-yl)prop-1-enyl)phenoxy)acetic acid was found as the most potent, thanks to the thiophenyl group on ring A and phenoxyacetic acid group on ring B.
Perera et al. [
35] proved that other three chalcones derivatives, salsolol A and B are effective against
Lemna pausicotata with IC50 values of 261, 275, and 251 µM, respectively.
One of the mechanisms of phytotoxicological action of chalcones were presented by Yun et al. [
36]. The authors proved that chalcone efficiently suppress the growth of several annual plant species by inhibition the activity of coenzyme A ligase (4CL), one of the key enzymes in the biosynthesis of lignin monomers. The mechanizm of inhibition of key enzyme is used also by Nguyen et al. [
37], who identified chalcones as selective inhibitors of phosphoenolpyruvate carboxylase (PEPC), a key enzyme for carbon fixation and biomass increase in the C4 photosynthetic pathway of many of the world’s most damaging weeds.
The results of the study of Diaz-Tielas et al. [
38], highlighted the mode of action of transchalcone as programmed cell death (PCD) inducer, through the probable mechanism of action based on the modification of mitochondrial function with the subsequent depolarization of the membrane and the release of factors, which trigger PCD in these cells. These results confirmed the phytotoxic activity of chalcone on
Arabidopsis seedlings and support its potential use as plant-growth regulator.
In addition, Diaz-Tielaz et al. (2014) [
9] evaluated if the phytotoxic effect obtained after the chalcone treatment can be selective and work differently for crops and associated weeds. Moreover, they have investigated two forms of herbicide application – spraying and watering on the growth of the model plant – adult
Arabidopsis and found that trans-chalcone is harmful to the germination and/or early root growth of certain weeds and crops (though apparently beneficial for others), and likewise is detrimental to the development of adult
Arabidopsis plants. These results support the role of chalcone as a plant growth regulator.
In other studies, one of the most known dihydrochalcone, phloretin exhibited significant dose-dependent growth retardation, severe morphological abnormalities and agravitropic behavior in
Arabidopsis seedlings [
39].
Fungicides
Chalcones are well known from their antifungal properties against many fungal pathogens of humans. The mechanizm of action is inhibition of ß(1,3)-glucan and chitin synthases, enzymes, that catalyze the biosynthesis of ß(1,3)-glucan and chitin polymers of the fungal cell wall, respectively [
40].
However, there are also many research results proving the effectiveness of chalcones against plant pathogens responsible economic losses of arable land worldwide.
The study of Svetaz et al. (2004) [
41] have proven significant sensitivity
Phomopsis longicolla to action of chalcones derived from
Zuccagnia punctata. The chloroformic fractrion of ethanolic extract from this plant consisting of 2’,4’-dihydroxy-3’-methoxychalcone and 2’,4’-dihydroxychalcone displayed very good activities against
P. longicolla Hobbs CE117 (MIC = 6.25 and 3.12 mg ml
1, respectively), as well as against
Colletotrichum truncatum CE175 (MIC = 6.25 mg). Both fungi species cause of the most soybean diseases, reduction of seed quality and yields, due to their high incidence and persistence. Badaracco et al. [
42] found that chalcone of plant origin 1,3-difenylo-2propen-1on was characterized by the inhibition of
Alternaria sp.,
P longicolla,
Fusarium proliferatum, and
Fusarium Subglutinans, the causal agents of pathologies in agronomic and food crops of agronomic and food importance. Minimum inhibitory concentrations (MIC) were from 62.5 to 125 μg ml
-1. Regarding fungicidal activity, the compound was effective only on
Alternaria sp. and
P. longicolla at minimum fungicidal concentrations (CFM) of 125 and 250 μg ml
- 1, respectively. Oleszek et al. [
43] tested antifungal activity of methanolic extract from apple pomace. The fraction rich in phloridzin, one of the most known chalcones, exhibited the strongest antifungal properties against
Botrytis sp.,
Fusarium oxysporum,
Petriella setifera, and
Neosartorya fischeri.
Naturally occuring chalcones became the inspiration of scientists for design and synthesis of new synthetic chalcones with antifungal properties. Hence, the latest research largely concerns synthetic compounds. Chen et al. (2023) [
4] tested a series of chalcone derivatives containing pyridazine against nine fungi:
Rhizoctonia solani,
Botrytis cinerea,
Phomopsis sp.,
Colletotrichum acutatum,
Botryosphaeria dothicdea (Bd),
Fusarium graminearum (FG),
Colletotrichum gloeosporioides,
Sclerotinia sclerotiorum (SS) and
Phytophthora capsica . The results showed that most of tested compounds exhibited stronger antifungal activity than azoxystrobin, positive control agent. Therefore, they have the potential to become fungicides, due to the ability to disruption the cell membrane of the mycelium and thus inhibition of the fungus growth.
Other research on the synthetic derivatives of chalcones was conducted by Zhou et al. [
44], who tested chalcone derivatives containing a piperazine against
Rhizoctonia solani and
Colletotrichum gloeosporioides. The mechanism of action was induction irregular and shrivelled growth of mycelium and rupture of the mycelium surface.
Antiviral Agents
Chalcones and their derivatives has been investigated also as antiviral agents. Chalcones show significant antiviral activity against tomato ringspot virus (ToRSV). In one of the first study conducted on
Chenopodium quinoa, 2-hydroxychalcone was found weak inhibitor of ToRSV infection [
45]. Onyilagha et al. [
46] investigated 21 different chalcones activity against ToRSV. This study proved that the antiviral properties was increased by hydroxylation of the A-ring at 2‘,3‘,4‘ po sitions and B-ring at C-4‘, and decreased by hydroxylation at C-5‘ and methoxylation of the B-ring.
In the newest literature related to agriculture, most information refer to the issue of tobacco mosaic virus (TMV), which were diminished by the application of chalcone type-compounds. Substituents and structure moieties as well as their positions in chalcone molecule are most important for the potency of antiviral activity of chalcones.
Dong et al. [
47] synthesized and tested a series of novel chalcone derivatives containing 1,1-dichloropropene moiety, and stated that most of the tested compounds exhibited moderate to good antiviral activity. Particularly one of them possessed excellent inactivation activity against TMV similar to that of ningnanmycin, commercialy available antiviral agent.
Zhou et al. [
48]stated that presence of purine and benzenesulfonamide moieties in chalcone molecule resulted in effective antiviral activity both against TMV, and also cucumber moisaic virus (CMV), which are two significant plant viruses causing serious economic problem in crops production. In particular, one of tested compounds proved to be indubitable effective against both viruses, thanks to electron-donating group at the 2-position of benzenesulfonamide aromatic rings, and with low steric hindrance group. This compound demonstrated the strongest binding capacity and affinity for coat protein (CP) of TMV, which is key functional protein of the TMV, involved in translation of mRNAs, transcription of tRNA, elongation, and self-assembly of TMV. The hydrogen bond plays an important role in the stabilization of interactions of compound with TMV-CP, and their number determines antiviral activity.
One of the newest studies conducted by Zhang et al. [
49] proved anti-TMV activity of chalcone derivatives containing other nitrogenous base: pyrimidine. As regards of curative activity, the EC50 value of four tested chalcones derivatives were much lower than ningnanmycin, commercial agent. The curative and protective activity of the target compounds depended on the type, location and carbon chain length of the substituent.
Nematicides
Plant-parasitic nematodes pose a major threat to crop protection. With the increasing resistance to nematicides and the lack of new modes of action, there is a growing need for novel nematicides.The most publications about nematicidal activity of chalcones concern Meloidogyne genus, the most economically important phytopathogens. To find new nematicidal leads, a series of fused ring compounds were obtained by utilizing a ring closure design strategy based on the structure of chalcone. These compounds were further modified and their nematicidal activity against M. incognita was evaluated.
Silva et al. [
50] stated that (1
E,4
E)-1,5-di(4-nitrophenyl)-2-butylpenta-1,4-dien-3-one, one of twelve tested synthesized chalcone analogues poses greater activity than commercial nematicide Carbofuran
®, exhibiting lower LC
50. This compounds is able to reduce of 51% and 68% of galls and eggs, respectively, when applied to infected tomatoes. The mechanism of action based on the inhibition of P450 enzyme associated with the oxidation of several substances in the nematode.
The bioassay conducted by Cao et al. [
51] revealed that modified chalcones-like compounds containing 2-carbonyl tiophene exhibited excellent nematicidal activity. The most active compound showed significant bioactivity with an LC
50 / 72h value of 3.20 mg/L in vitro and an inhibition rate of 100.00% at 40 mg/L in the matrix.
Attar et al. [
52] reported that polarity as well as planarity of chalcones compounds play important role in their activity of against nematode
Caenorhabditis elegans. Tested organic chalcones were found to be less polar than synthesized ferrocenyl (Fc) analogues, and simultanously, they were much more active. It is associated with chalcone’s ability to pass through the organism’s cell walls.
Insecticides
Many studies proved effectiveness of both natural and synthesized chalcones against insects. Amoung naturally occuring chalcones, xanthohumol and isoxanthohumol isolated from hop (Humulus lupulus L.) should be mentioned as good example of insecticides. Their effective insecticidal activity has been proven against the peach-potato aphid (Myzus persicae) [
53].
Shakil and Saxena [
54] isolated new chalcone cordifolin from woody stem of Giloe (
Tinospora cordifolia), and determined its activity agaist larvae of
Spodoptera litura. The results showed that cordifolin coused pupation delay, prolonged pupal period and decreased pupal weight.
Many studies were conducted on the synthesis and development new chalcones and its derivatives with insecticidal properties. The works consisted of the modification of chalcones structure and selection of substituents.
Hidalgo et al. [
55] conducted study on bis and mono chalcones as insecticides agains
Spodoptera frugiperda. The results showed that two monochalcones containing bromines and hydroxyl groups in ring A and N-N dimethyl group in ring B killed 40 and 60 % larvae, when incorporated to the larval diet at 100 mg per g of diet (40 and 60%, respectively). Bis-chalcones did not exhibit such activity. Contradictory results were obtained by Devi et al. [
56], where bis-chalcones showed more toxicity than mono-chalcones. The study of Kumar et al. [
8] is the first report on the pesticidal activity of chalcones against
Plutella xylostella, wherein series of chalcones were synthesized under microwave irratiation. Electron-withdrawing ring A of chalcone was found crucial for pesticidal activity, meanwhile ring B can bear either electron-withdrawing or electron-releasing substituents. Particularly, Cl substitution and its positions on ring A as well as on ring B were found vital. Compound 1,3-Bis(4-chlorophenyl)prop-2-en-1-one showed the maximum activity with LC50 value of 170.24 µg mL
-1. The results of the study provide the foundations for futher modification of potent units and the design of novel chalcone-based pesticidal agents against
P.xylostella and related insect pests.
5. Conclusions
To sum up, chalcones, both these of natural origin and chemically synthesized, form a diverse and sophisticated group of molecules with a wide spectrum of biological potential. They are known for centuries but collecting data on their use is still ongoing. Much of the pharmacological potential of chalcones is still not utilized and not clearly understood. For that reason, they are of great interest amongst the scientists. The herbicidal, fungicidal, antiviral, insecticidal and plant growth regulator-type activities of various chalcones are presented in this review together with their overall description and methods of detection for the first time based on the most up to date literature.
Besause plants do not accumulate chalcones in larger quantities, obtaining them from natural sources is difficult. Another challenge could be their short half-life. It is the reason for the intensive studies on their synthesis and development.
Moreover, further intensive investigation is needed to understand the mode of action of reactive chalcones, their effectiveness in the field, as well as the safety of their possible use for the environment and humans.
Author Contributions
Conceptualization, M.D.-B. and M.O; methodology, M.D.-B.; data curation, M.D.-B.; writing—original draft preparation, M.D.-B. and M.O; writing—review and editing, M.D.-B., M.O. and S.Z; visualization, S.Z.; supervision, W.O; project administration, M.D.-B.; funding acquisition, M.D.-B. and W.O. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
This research is supported by European Union’s Horizon Europe research and innovation programme under the grant agreement No 101084163 project RATION.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jash, S.K. Chemistry and Role of Flavonoids in Agriculture: A Recent Update. In Flavonoid Metabolism-Recent Advances and Applications in Crop Breeding. IntechOpen. [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. R. Flavonoids: an overview. J Nutr Sci. 2016, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Clifford, M.N. Flavanones, chalcones and dihydrochalcones - Nature, occurrence and dietary burden. Journal of the Science of Food and Agriculture 2000, 80, 1073–1080. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, M.; Feng, S.; Gong, C.; Zhou, Y.; Xing Li He, B.; Wub, Y.; Xue, W. Design, synthesis and biological activity of chalcone derivatives containing pyridazine. Arabian Journal of Chemistry 2023, 16, 104852. [Google Scholar] [CrossRef]
- Rozmer, Z.; Perjési, P. Naturally occurring chalcones and their biological activities. Phytochemistry Reviews 2014, 15, 87–120. [Google Scholar] [CrossRef]
- Díaz-Tielas, C.; Graña, E.; Reigosa, M.J.; Sánchez-Moreiras, A.M. Biological Activities And Novel Applications Of Chalcones. Planta Daninha, Viçosa-MG 2016, 34, 607–616. [Google Scholar] [CrossRef]
- Zhou, B.; Xing, C. Diverse molecular targets for chalcones with varied bioactivities. Med Chem. 2015, 5, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sharma, P.; Shard, A.; Tewary, D.K.; Nadda, G.; Sinha, A.K. K. Chalcones as promising pesticidal agents against diamondback moth (Plutella xylostella): Microwave assisted synthesis and structure-activity relationship. Medicinal Chemistry Research 2012, 21, 922–931. [Google Scholar] [CrossRef]
- Diaz-Tielas, C.; Sotelo, T.; Grana, E.; Reigosa, M.J.; Sanchez-Moreiras. Phytotoxic potential of trans-chalcone on crop plants and model species. J. Plant Growth Regul. 2014, 33, 181–194. [Google Scholar] [CrossRef]
- Chotsaeng, N.; Laosinwattana, C.; Charoenying, P. Herbicidal Activity of Flavokawains and Related trans-Chalcones against Amaranthus tricolor L. and Echinochloa crus-galli (L.) Beauv. ACS Omega 2019, 4, 20748–20755. [Google Scholar] [CrossRef]
- Batovska, D.I.; Todorova, I.T. Trends in utilization of the pharmacological potential of chalcones. Curr Clin Pharmacol. 2010, 5, 1–29. [Google Scholar] [CrossRef]
- Kuete, V.; Sandjo, L.P. P. Isobavachalcone: An Overview. Chin J Integr Med 2012, 18, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Aljamali, N.M.; Hamzah Daylee, S.; Jaber Kadhium, A. Review On Chemical-Biological Fields Of Chalcone Compounds. Forefront Journal of Engineering & Technology Volume 2020, 2, 33–44. [Google Scholar]
- Jasim, H.A.; Nahar, L.; Jasim, M.A.; Moore, S.A.; Ritchie, K.J.; Sarker, S.D. Chalcones: Synthetic Chemistry Follows Where Nature Leads. Biomolecules 2021, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.T.; Linthorst, H.J.; Verpoorte, R. Chalcone synthase and its functions in plant resistance. Phytochem Rev. 2011, 10, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Jiwrajka, M.; Phillips, A.; Butler, M.; Rossi, M.; Pocock, J.M. The plant-derived chalcone 2, 2′, 5′-trihydroxychalcone provides neuroprotection against toll-like receptor 4 triggered inflammation in microglia. Oxidative Medicine and Cellular Longevity, 2016.
- Zhuang CZhang, W.; Sheng CZhang, W.; Xing, C. Chalcone: A privileged structure in medicinal chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, A. 2023. Biology and Biotechnology of Environmental Stress Tolerance in Plants: Volume 1: Secondary Metabolites in Environmental Stress.
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Chalcone scaffolds as anti-infective agents: Structural and molecular target perspectives. Eur. J. Med. Chem. 2015, 101, 496–524. [Google Scholar] [CrossRef] [PubMed]
- Morsy, N.M.; Hassan, A.S. Synthesis, reactions, and applications of chalcones: A review. European Journal of Chemistry. 2022, 13, 241–252. [Google Scholar] [CrossRef]
- Balan-Porcăraşu, M.; Roman, G. Novel chalcone analogs derived from 4-(benzyloxy)benzaldehyde. Ovidius University Annals of Chemistry. 2023, 34, 112–120. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, R.Y.; Meng TLou, Z.C. C. Determination of nine flavonoids and coumarins in licorice root by high-performance liquid chromatography. J Chromatogr. 1990, 513, 247–254. [Google Scholar] [CrossRef]
- Burda, S.; Oleszek, W.; Lee, C.Y. Phenolic compounds and their changes in apples during maturation and cold storage. J Agric Food Chem 1998, 38, 945–948. [Google Scholar] [CrossRef]
- Suaârez, B.; Picinelli, A.; Moreno, J.; Mangas, J.J. J. Changes in phenolic composition of apple juices by HPLC with direct injection. J Sci Food Agric 1998, 78, 461–465. [Google Scholar] [CrossRef]
- Zou, H.; Han, L.; Yuan, M.; Zhang, M.; Zhou, L.; Wang, Y. Sequence Analysis and Functional Verification of the Effects of Three Key Structural Genes, PdTHC2’GT, PdCHS and PdCHI, on the Isosalipurposide Synthesis Pathway in Paeonia delavayi var. lutea. International Journal of Mol. Sci. 2022, 23, 5696. [Google Scholar] [CrossRef] [PubMed]
- Krauze-Baranowska, M.; Pobłocka-Olech, M.; Głód, D.; Wiwart, M.; Zieliński, J.; Migas, P. HPLC of Flavanones and Chalcones in different species and clones of Salix. Acta Poloniae Pharmaceutica - Drug Research 2014, 70, 27–34. [Google Scholar]
- Guvenalp, Z.; Ozbek, H.; Karadayi, M.; Gulluce, M.; Kuruuzum-Uz, A.; Salih, B.; Demirezer, O. Two antigenotoxic chalcone glycosides from Mentha longifolia subsp. Longifolia. Pharm Biol. 2015, 53, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, Y.; Chen, Z. Separation, identification, and quantification of active constituents in Fructus Psoraleae by high-performance liquid chromatography with UV, ion trap mass spectrometry, and electrochemical detection. Journal of Pharmaceutical Analysis. 2012, 2, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Pobłocka-Olech, L. Zastosowanie metod chromatograficznych w badaniach składu chemicznego kory niektórych gatunków i klonów wierzby. Doctoral dissertation. 2016.
- Dhaliwal, J.S.; Moshawih, S.; Goh, K.W.; Loy, M.J.; Hossain, M.S.; Hermansyah, A.; Kotra, V.; Kifli, N.; Goh, H.P.; Dhaliwal, S.K.S.; et al. Pharmacotherapeutics Applications and Chemistry of Chalcone Derivatives. Molecules 2022, 27, 7062. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.M.; Hassan, S.A.; Abbas, A.K.; Najem, M.R.; Jber, N.R. R. Synthesis of heterocyclic and study activities in agriculture as anti-dubas on date palm trees via cholinesterase inhibitors. GSC Advanced Research and Reviews 2023, 16, 094–101. [Google Scholar] [CrossRef]
- Ma, T.; Nie, L.J.; Li, H.M.; Huo, Q.; Zhang, Y.X.; Wu, C.Z. Z. Determination of isobavachalcone in rat plasma by LC-MS/MS and its application to a pharmacokinetic study. J Pharm Biomed Anal. 2015, 107, 50–55. [Google Scholar] [CrossRef]
- Krittanaia, S.; Pichetpongtorn, P.; Sakamoto, S.; Waraporn, P. Monoclonal antibody-based immunoassay for the specific quantification of licochalcone A: an active chalcone in licorice. Food And Agricultural Immunology. 2022, 33, 220–234. [Google Scholar] [CrossRef]
- Yerragunta, V.; Suman, D.; Anusha, V.; Patil, P.; Samhitha, T. A review on chalcones and its importance. PharmaTutor 2013, 1, 54–55. [Google Scholar]
- Perera, H.; Meepagala, K.M.; Fronczek, F.R.; Cook, D.D.; Wedge, D.E.; Duke, S.O. O. Bioassay-Guided Isolation and Structure Elucidation of Fungicidal and Herbicidal Compounds from Ambrosia salsola (Asteraceae). Molecules 2019, 24, 835. [Google Scholar] [CrossRef] [PubMed]
- Yun, M.S.; Chen, W.; Deng, F.; Yogo, Y. Selective growth suppression of five annual plant species by chalcone and naringenin correlates with the total amount of 4coumarate: Coenzyme A ligase. Weed Biology and Management 2009, 9, 27–37. [Google Scholar] [CrossRef]
- Nguyen, G.T.T.; Erlenkamp, G.; Jäck, O.; Küberl, A.; Bott, M.; Fiorani, F.; Gohlke, H.; Groth, G. Chalcone-based selective inhibitors of a C4 plant key enzyme as novel potential herbicides. Scientific reports 2016, 6, 27333. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Tielas, C.; Graña, E.; Sotelo, T.; Reigosa, M.J.; Sánchez-Moreiras, A.M. The natural compound trans-chalcone induces programmed cell death in Arabidopsis thaliana roots. Plant, Cell and Environment 2012, 35, 1500–1517. [Google Scholar] [CrossRef] [PubMed]
- Smailagić, D.; Banjac, N.; Ninković, S.; Savić, J.; Ćosić, T.; Pěnčík, A.; Ćalić, D; Bogdanović, M. ; Trajković, M.; Stanišić, M. New insights into the activity of apple dihydrochalcone phloretin: disturbance of auxin homeostasis as physiological basis of phloretin phytotoxic action. Frontiers in Plant Science 2022, 13, 875528. [Google Scholar] [CrossRef]
- Gupta, D.; Jain, D.K. Chalcone derivatives as potential antifungal agents: Synthesis, and antifungal activity. J. Adv. Pharm. Technol. Res. 2015, 6, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Svetaz, L.; Tapia, A.; López, S.N.; Furlán, R.L.; Petenatti, E.; Pioli, R.; Schmeda-Hirschmann, G.; Zacchino, S.A. A. Antifungal chalcones and new caffeic acid esters from Zuccagnia punctata acting against soybean infecting fungi. Journal of Agricultural and Food chemistry 2004, 52, 3297–3300. [Google Scholar] [CrossRef] [PubMed]
- Badaracco, P.; Sortino, M.; Pioli, R.N. N. Study plant-origin in compounds with potential antifungal action against pathogens of cultivated plants. Chilean journal of agricultural & animal sciences 2020, 36, 244–252. [Google Scholar]
- Oleszek, M.; Pecio, Ł.; Kozachok, S.; Lachowska-Filipiuk, Ż.; Oszust, K.; Frąc, M. Phytochemicals of apple pomace as prospect bio-fungicide agents against mycotoxigenic fungal species—In vitro experiments. Toxins 2019, 11, 361. [Google Scholar] [CrossRef]
- Zhou, Q.; Tang, X.; Chen, S.; Zhan, W.; Hu, D.; Zhou, R.; Sun, N.; YongJun, W.; Xue, W. Design, synthesis, and antifungal activity of novel chalcone derivatives containing a piperazine fragment. Journal of Agricultural and Food Chemistry 2022, 70, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, B.; Onyilagha, J.C.; Bohm, B.A.; Towers GH, N.; James, D.; Harborne, J.B.; French, C.J. J. Inhibition of tomato ringspot virus by flavonoids. Phytochemistry 1996, 43, 1271–1276. [Google Scholar] [CrossRef]
- Onyilagha, J.C.; Malhotra, B.; Elder, M.; French, C.J.; Towers, G.N. N. Comparative studies of inhibitory activities of chalcones on tomato ringspot virus (ToRSV). Canadian Journal of Plant Pathology 1997, 19, 133–137. [Google Scholar] [CrossRef]
- Dong, L.R.; Hu, D.Y.; Wu, Z.X.; Chen, J.X.; Song, B.A. Study of the synthesis, antiviral bioactivity and interaction mechanisms of novel chalcone derivatives that contain the 1, 1-dichloropropene moiety. Chinese Chemical Letters 2017, 28, 1566–1570. [Google Scholar] [CrossRef]
- Zhou, D.; Xie, D.; He, F.; Song, B.; Hu, D. Antiviral properties and interaction of novel chalcone derivatives containing a purine and benzenesulfonamide moiety. Bioorganic & medicinal chemistry letters 2018, 28, 2091–2097. [Google Scholar]
- Zhang, W.; Mao, P.; Yuan Ch Zhang, Y.; Zhang, T.; Liu, Y.; Tian, J.; Xue, W. Design, synthesis and antiviral activities of chalcone derivatives containing pyrimidine. Journal of Saudi Chemical Society 2023, 27, 101590. [Google Scholar] [CrossRef]
- Silva, F.J.; Campos, V.P.; Oliveira, D.F.; Gomes, V.A.; Barros, A.F.; Din, Z.U.; Rodrigues-Filho, E. Chalcone analogues: Synthesis, activity against Meloidogyne incognita, and in silico interaction with cytochrome P450. Journal of Phytopathology 2019, 167, 197–208. [Google Scholar] [CrossRef]
- Cao, X.; Qiu, D.; Zhang, R.; Li, Z.; Xu, X. Synthesis, nematicidal evaluation, and SAR study of benzofuran derivatives containing 2-carbonyl thiophene. Chinese Chemical Letters 2023, 34, 107800. [Google Scholar] [CrossRef]
- Attar, S.; O’Brien, Z.; Alhaddad, H.; Golden, M.L.; Calderón-Urrea, A. Ferrocenyl chalcones versus organic chalcones: a comparative study of their nematocidal activity. Bioorganic & medicinal chemistry 2011, 19, 2055–2073. [Google Scholar]
- Stompor, M.; Dancewicz, K.; Gabrys, B.; Anioł, M. Insect antifeedant potential of xanthohumol, isoxanthohumol, and their derivatives. Journal of agricultural and food chemistry 2015, 63, 6749–6756. [Google Scholar] [CrossRef]
- Shakil, N.A.; Saxena, D.B. Isolation and structure of cordifolin, a novel insecticidal oxygenated chalcone, from the stem of Tinospora cordifolia Miers. Natural Product Communications 2006, 1, 553–556. [Google Scholar] [CrossRef]
- Hidalgo, J.R.; Santillán, M.; Parellada, E.A.; Khyaliya, P.; Neske, A.; Ameta, K.L. Synthetic bis-and mono-chalcones with insecticide effects on Spodoptera frugiperda (Lepidoptera: Noctuidae). International journal of pest management 2020, 66, 116–121. [Google Scholar] [CrossRef]
- Devi, A.P.; Alsulimani, A.; Hidalgo, J.R.; Neske, A.; Sayyed, R.Z.; Hassan, M.; Elshazly, H. Bis-and mono-substituted Chalcones exert anti-feedant and toxic effects on fall armyworm Spodoptera frugiperda. Saudi Journal of Biological Sciences 2021, 28, 5754–5759. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).