Submitted:
30 March 2024
Posted:
02 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
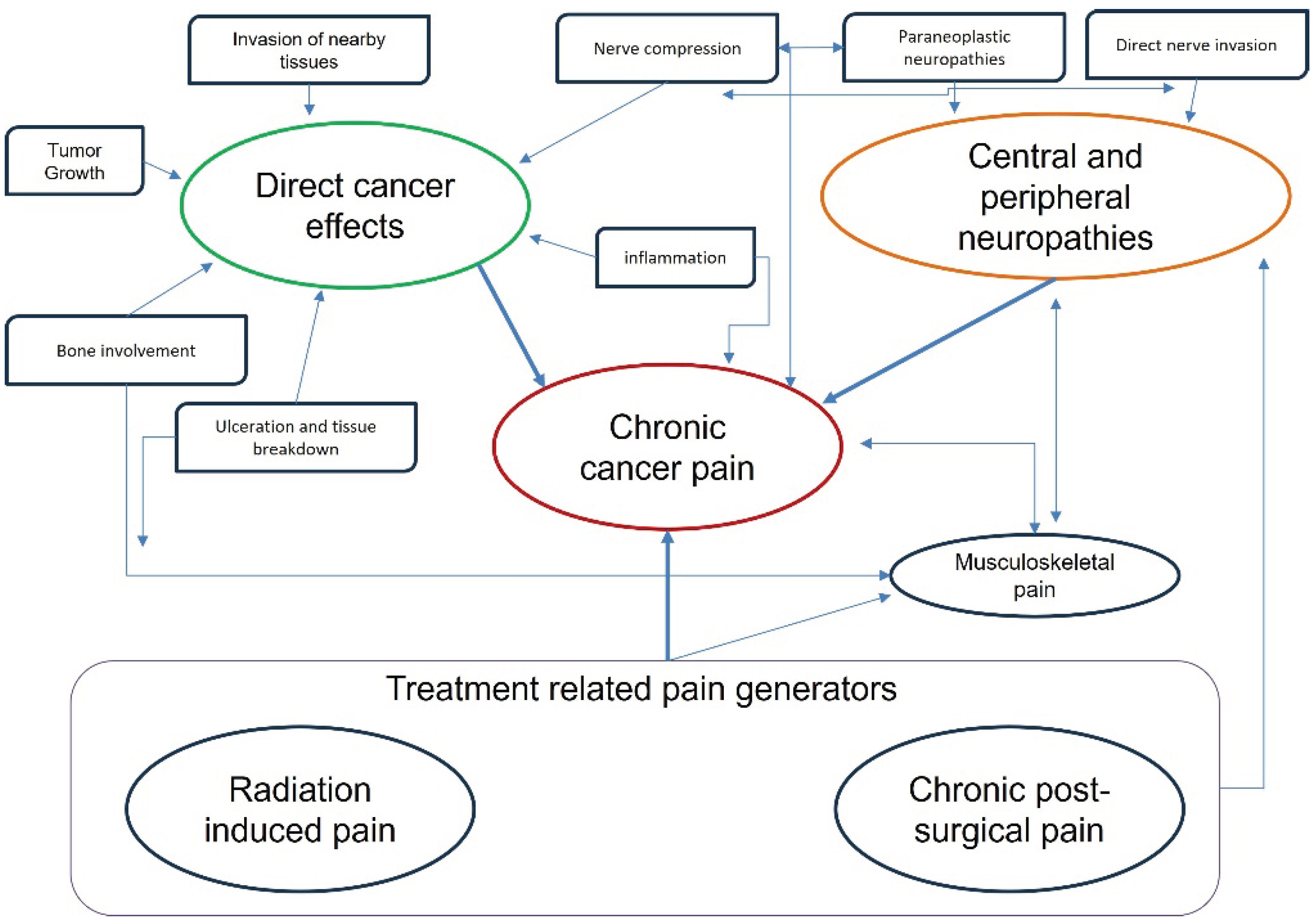
2. Cancer Pain Peculiarities
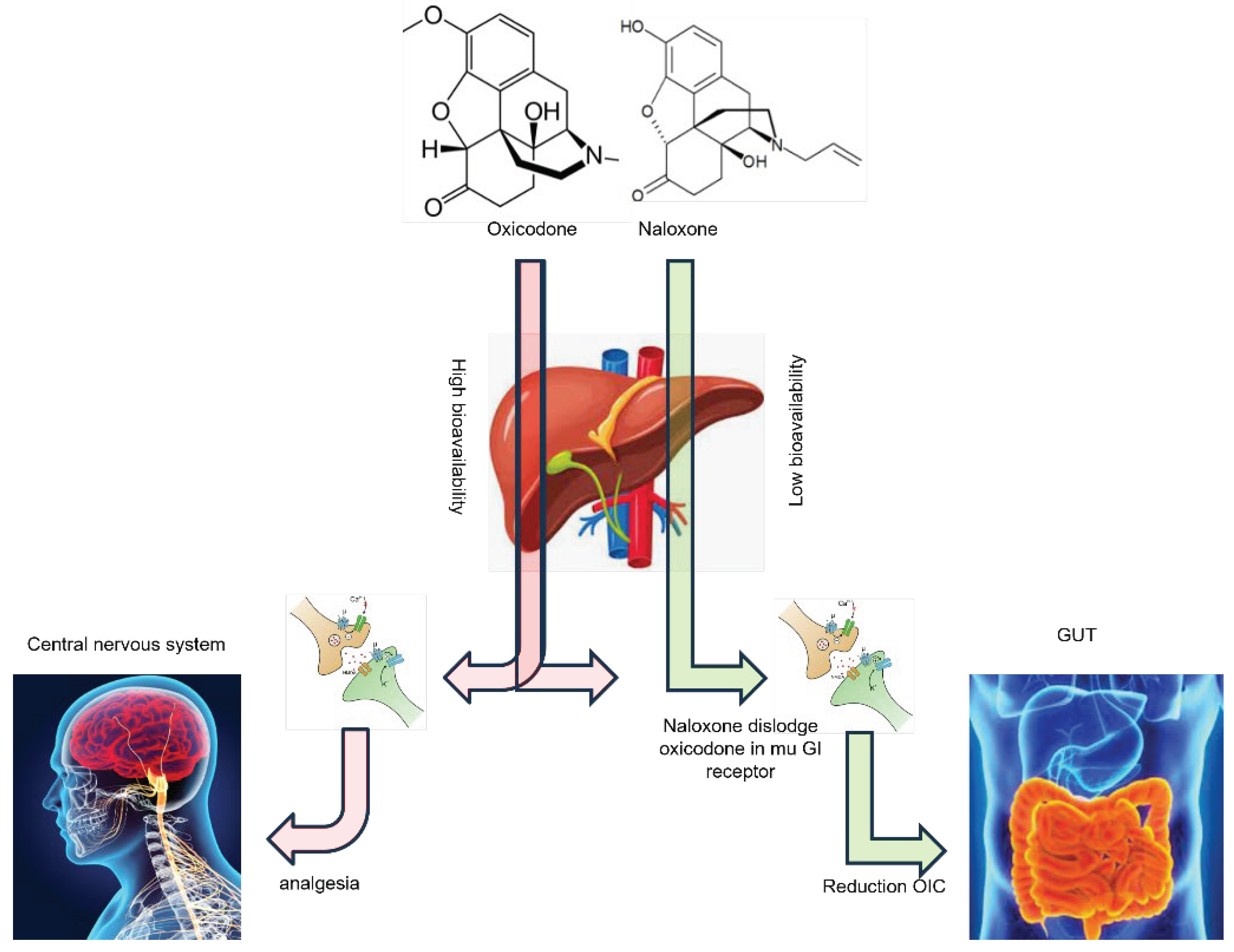
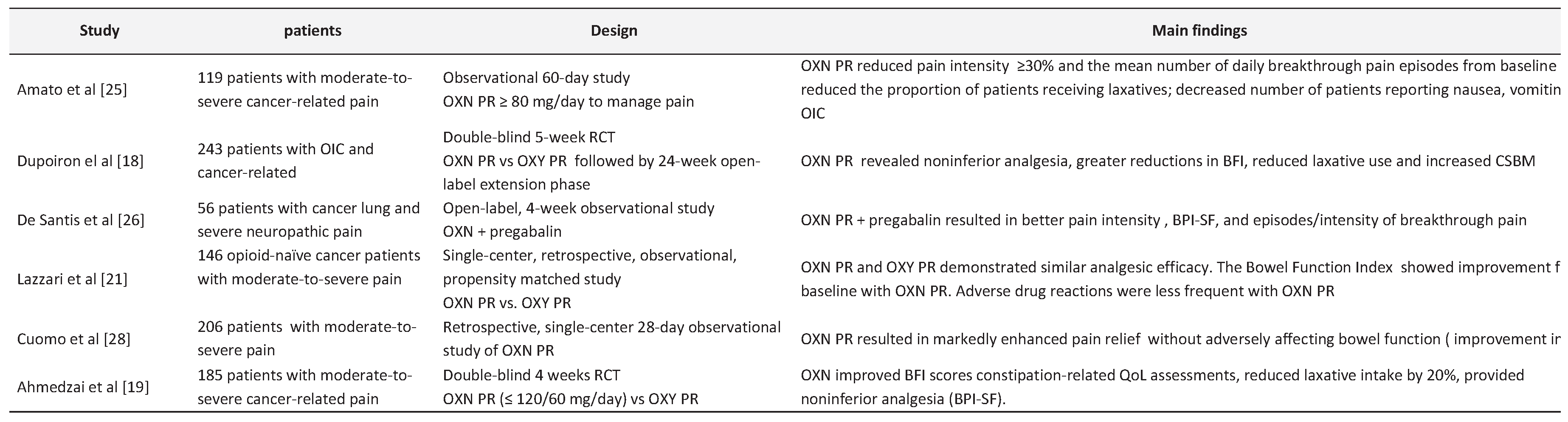
3. The Safety and Efficacy of Oxycodone/Naloxone PR in Cancer Pain
4. Patient Experience and Quality of Life
5. Controversial and Limitations
6. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mestdagh, F.; Steyaert, A.; Lavand’homme, P. Cancer Pain Management: A Narrative Review of Current Concepts, Strategies, and Techniques. Curr Oncol 2023, 30, 6838–6858. [Google Scholar] [CrossRef]
- Kalso, E. Oxycodone. Journal of Pain and Symptom Management 2005, 29 (5, Supplement), 47–56. [Google Scholar] [CrossRef]
- Sizar, O.; Genova, R.; Gupta, M. Opioid-Induced Constipation. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2023. [Google Scholar]
- Morlion, B.J.; Mueller-Lissner, S.A.; Vellucci, R.; Leppert, W.; Coffin, B.C.; Dickerson, S.L.; et al. Oral Prolonged-Release Oxycodone/Naloxone for Managing Pain and Opioid-Induced Constipation: A Review of the Evidence. Pain Pract 2018, 18, 647–665. [Google Scholar] [CrossRef]
- Leppert, W. Oxycodone/naloxone in the management of patients with pain and opioid-induced bowel dysfunction. Curr Drug Targets 2014, 15, 124–135. [Google Scholar] [CrossRef]
- Kim, E.S. Oxycodone/Naloxone Prolonged Release: A Review in Severe Chronic Pain. Clin Drug Investig 2017, 37, 1191–1201. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. Tumor Microenvironment. Current biology : CB 2020, 30, R921. [Google Scholar] [CrossRef]
- Le Bitoux, M.-A.; Stamenkovic, I. Tumor-host interactions: the role of inflammation. Histochem Cell Biol 2008, 130, 1079–1090. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Ngoc, D.T.M.; Choi, J.-H.; Lee, C.-H. Unveiling the Neural Environment in Cancer: Exploring the Role of Neural Circuit Players and Potential Therapeutic Strategies. Cells 2023, 12, 1996. [Google Scholar] [CrossRef]
- Wang, W.; Li, L.; Chen, N.; Niu, C.; Li, Z.; Hu, J.; et al. Nerves in the Tumor Microenvironment: Origin and Effects. Frontiers in Cell and Developmental Biology 2020, 8. [Google Scholar] [CrossRef]
- Kartikasari, A.E.R.; Huertas, C.S.; Mitchell, A.; Plebanski, M. Tumor-Induced Inflammatory Cytokines and the Emerging Diagnostic Devices for Cancer Detection and Prognosis. Front Oncol 2021, 11, 692142. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther 2021, 6, 263. [Google Scholar]
- Falk, S.; Bannister, K.; Dickenson, A.H. Cancer pain physiology. Br J Pain 2014, 8, 154–162. [Google Scholar] [CrossRef]
- Yam, M.F.; Loh, Y.C.; Tan, C.S.; Khadijah Adam, S.; Abdul Manan, N.; Basir, R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int J Mol Sci 2018, 19, 2164. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central Sensitization: A Generator of Pain Hypersensitivity by Central Neural Plasticity. J Pain 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152(3 Suppl), S2–15. [Google Scholar] [CrossRef]
- He, F.; Jiang, Y.; Li, L. The effect of naloxone treatment on opioid-induced side effects. Medicine (Baltimore) 2016, 95, e4729. [Google Scholar] [CrossRef]
- Dupoiron, D.; Stachowiak, A.; Loewenstein, O.; Ellery, A.; Kremers, W.; Bosse, B.; et al. A phase III randomized controlled study on the efficacy and improved bowel function of prolonged-release (PR) oxycodone-naloxone (up to 160/80 mg daily) vs oxycodone PR. Eur J Pain 2017, 21, 1528–1537. [Google Scholar] [CrossRef]
- Ahmedzai, S.H.; Nauck, F.; Bar-Sela, G.; Bosse, B.; Leyendecker, P.; Hopp, M. A randomized, double-blind, active-controlled, double-dummy, parallel-group study to determine the safety and efficacy of oxycodone/naloxone prolonged-release tablets in patients with moderate/severe, chronic cancer pain. Palliat Med 2012, 26, 50–60. [Google Scholar] [CrossRef]
- Roberto, A.; Greco, M.T.; Legramandi, L.; Galli, F.; Galli, M.; Corli, O. A comparison between the administration of oral prolonged-release oxycodone-naloxone and transdermal fentanyl in patients with moderate-to-severe cancer pain: a propensity score analysis. JPR 2017, 10, 2123–2133. [Google Scholar] [CrossRef]
- Lazzari, M.; Greco, M.T.; Marcassa, C.; Finocchi, S.; Caldarulo, C.; Corli, O. Efficacy and tolerability of oral oxycodone and oxycodone/naloxone combination in opioid-naïve cancer patients: a propensity analysis. DDDT 2015, 9, 5863–5872. [Google Scholar] [CrossRef]
- Corli, O.; Iorno, V.; Legramandi, L.; Rulli, E.; Roberto, A.; Azzarello, G.; et al. Oral prolonged-release Oxycodone-Naloxone: analgesic response, safety profile, and factors influencing the response in advanced cancer patients. Pain Pract 2019. [CrossRef]
- Ahmedzai, S.H.; Leppert, W.; Janecki, M.; Pakosz, A.; Lomax, M.; Duerr, H.; et al. Long-term safety and efficacy of oxycodone/naloxone prolonged-release tablets in patients with moderate-to-severe chronic cancer pain. Support Care Cancer 2015, 23, 823–830. [Google Scholar] [CrossRef]
- Mucci-LoRusso, P.; Berman, B.S.; Silberstein, P.T.; Citron, M.L.; Bressler, L.; Weinstein, S.M.; et al. Controlled-release oxycodone compared with controlled-release morphine in the treatment of cancer pain: a randomized, double-blind, parallel-group study. Eur J Pain 1998, 2, 239–249. [Google Scholar] [CrossRef]
- Amato, F.; Ceniti, S.; Mameli, S.; Pisanu, G.M.; Vellucci, R.; Palmieri, V.; et al. High dosage of a fixed combination oxycodone/naloxone prolonged release: efficacy and tolerability in patients with chronic cancer pain. Support Care Cancer 2017, 25, 3051–3058. [Google Scholar] [CrossRef]
- De Santis, S.; Borghesi, C.; Ricciardi, S.; Giovannoni, D.; Fulvi, A.; Migliorino, M.R.; et al. Analgesic effectiveness and tolerability of oral oxycodone/naloxone and pregabalin in patients with lung cancer and neuropathic pain: an observational analysis. Onco Targets Ther 2016, 9, 4043–4052. [Google Scholar] [CrossRef]
- Clemens, K.E.; Quednau, I.; Klaschik, E. Bowel function during pain therapy with oxycodone/naloxone prolonged-release tablets in patients with advanced cancer. Int J Clin Pract 2011, 65, 472–478. [Google Scholar] [CrossRef]
- Cuomo, A.; Russo, G.; Esposito, G.; Forte, C.A.; Connola, M.; Marcassa, C. Efficacy and gastrointestinal tolerability of oral oxycodone/naloxone combination for chronic pain in outpatients with cancer: an observational study. Am J Hosp Palliat Care 2014, 31, 867–876. [Google Scholar] [CrossRef]
- Nolte, T.; Schutter, U.; Loewenstein, O. Cancer pain therapy with a fixed combination of prolonged-release oxycodone/naloxone: results from a non-interventional study. Pragmat Obs Res 2014, 5, 1–13. [Google Scholar] [CrossRef]
- Wong, A. K.; Grobler, A.; Le, B. ENhANCE trial protocol: A multi-centre, randomised, phase IV trial comparing the efficacy of oxycodone/naloxone prolonged release (OXN PR) versus oxycodone prolonged release (Oxy PR) tablets in patients with advanced cancer. Contemp Clin Trials Commun 2022, 30, 101036. [Google Scholar] [CrossRef]
- Dorn, S.; Lembo, A.; Cremonini, F. Opioid-induced bowel dysfunction: epidemiology, pathophysiology, diagnosis, and initial therapeutic approach. Am J Gastroenterol Suppl 2014, 2, 31–37. [Google Scholar] [CrossRef]
- Bantel, C.; Tripathi, S.S.; Molony, D.; Heffernan, T.; Oomman, S.; Mehta, V.; et al. Prolonged-release oxycodone/naloxone reduces opioid-induced constipation and improves quality of life in laxative-refractory patients: results of an observational study. Clin Exp Gastroenterol 2018, 11, 57–67. [Google Scholar] [CrossRef]
- Kannan, V.D.; Veazie, P.J. US trends in social isolation, social engagement, and companionship ⎯ nationally and by age, sex, race/ethnicity, family income, and work hours, 2003–2020. SSM - Population Health 2023, 21, 101331. [Google Scholar] [CrossRef]
- Alonso, J.; Ferrer, M.; Gandek, B.; Ware, J.E.; Aaronson, N.K.; Mosconi, P.; et al. Health-related quality of life associated with chronic conditions in eight countries: results from the International Quality of Life Assessment (IQOLA) Project. Qual Life Res 2004, 13, 283–298. [Google Scholar] [CrossRef]
- Cleeland, C.S.; Ryan, K.M. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap 1994, 23, 129–138. [Google Scholar]
- Bell, T.J.; Panchal, S.J.; Miaskowski, C.; Bolge, S.C.; Milanova, T.; Williamson, R. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med 2009, 10, 35–42. [Google Scholar] [CrossRef]
- Pappagallo, M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg 2001, 182(5A Suppl), 11S–18S. [Google Scholar] [CrossRef]
- Penning-van Beest, F.J.A.; van den Haak, P.; Klok, R.M.; Prevoo, Y.F.D.M.; van der Peet, D.L.; Herings, R.M.C. Quality of life in relation to constipation among opioid users. J Med Econ 2010, 13, 129–135. [Google Scholar] [CrossRef]
- Candy, B.; Jones, L.; Vickerstaff, V.; Larkin, P.J.; Stone, P. Mu-opioid antagonists for opioid-induced bowel dysfunction in people with cancer and people receiving palliative care. Cochrane Database Syst Rev 2022, 9, CD006332. [Google Scholar] [CrossRef]
- Ueberall, M.A.; Müller-Lissner, S.; Buschmann-Kramm, C.; Bosse, B. The Bowel Function Index for evaluating constipation in pain patients: definition of a reference range for a non-constipated population of pain patients. J Int Med Res 2011, 39, 41–50. [Google Scholar] [CrossRef]
- Vanneste, L.; Lefebvre, T.; Tack, L.; Van Eygen, K.; Cool, L.; Schofield, P.A.; et al. Pain Medication Adherence in Patients with Cancer: A Pragmatic Review. Pain Med 2022, 23, 782–798. [Google Scholar] [CrossRef]
- McNicol, E.; Horowicz-Mehler, N.; Fisk, R.A.; Bennett, K.; Gialeli-Goudas, M.; Chew, P.W.; et al. Management of opioid side effects in cancer-related and chronic noncancer pain: a systematic review. J Pain 2003, 4, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Burness, C.B.; Keating, G.M. Oxycodone/Naloxone prolonged-release: a review of its use in the management of chronic pain while counteracting opioid-induced constipation. Drugs 2014, 74, 353–375. [Google Scholar] [CrossRef] [PubMed]
- Solhaug, V.; Molden, E. Individual variability in clinical effect and tolerability of opioid analgesics - Importance of drug interactions and pharmacogenetics. Scand J Pain 2017, 17, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Noble, M.; Treadwell, J.R.; Tregear, S.J.; Coates, V.H.; Wiffen, P.J.; Akafomo, C.; et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev 2010, 2010, CD006605. [Google Scholar] [CrossRef] [PubMed]
- Roden, D.M.; McLeod, H.L.; Relling, M.V.; Williams, M.S.; Mensah, G.A.; Peterson, J.F.; et al. Pharmacogenomics. Lancet 2019, 394, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Bugada, D.; Lorini, L.F.; Fumagalli, R.; Allegri, M. Genetics and Opioids: Towards More Appropriate Prescription in Cancer Pain. Cancers (Basel) 2020, 12, 1951. [Google Scholar] [CrossRef]
- Tadje, M. Genetic Variants Influencing Patient Response to Opioid Therapy. Oncol Nurs Forum 2015, 42, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Crews, K.R.; Monte, A.A.; Huddart, R.; Caudle, K.E.; Kharasch, E.D.; Gaedigk, A.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2D6, OPRM1, and COMT Genotypes and Select Opioid Therapy. Clin Pharmacol Ther 2021, 110, 888–896. [Google Scholar] [CrossRef]
- Klimas, R.; Witticke, D.; El Fallah, S.; Mikus, G. Contribution of oxycodone and its metabolites to the overall analgesic effect after oxycodone administration. Expert Opin Drug Metab Toxicol 2013, 9, 517–528. [Google Scholar] [CrossRef]
- Andreassen, T.N.; Eftedal, I.; Klepstad, P.; Davies, A.; Bjordal, K.; Lundström, S. Do CYP2D6 genotypes reflect oxycodone requirements for cancer patients treated for cancer pain? A cross-sectional multicentre study. Eur J Clin Pharmacol 2012, 68, 55–64. [Google Scholar] [CrossRef]
- Zwisler, S.T.; Enggaard, T.P.; Mikkelsen, S.; Brosen, K.; Sindrup, S.H. Impact of the CYP2D6 genotype on post-operative intravenous oxycodone analgesia. Acta Anaesthesiol Scand 2010, 54, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Davison, S.N. Clinical Pharmacology Considerations in Pain Management in Patients with Advanced Kidney Failure. Clin J Am Soc Nephrol 2019, 14, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Le, B.H.; Aggarwal, G.; Douglas, C.; Green, M.; Nicoll, A.; Ahmedzai, S. Oxycodone/naloxone prolonged-release tablets in patients with moderate-to-severe, chronic cancer pain: Challenges in the context of hepatic impairment. Asia Pac J Clin Oncol 2022, 18, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Fallon, M.; Giusti, R.; Aielli, F.; Hoskin, P.; Rolke, R.; Sharma, M.; et al. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann Oncol 2018, 29 (Suppl 4), iv166–91. [Google Scholar] [CrossRef] [PubMed]
- Helander, E.M.; Menard, B.L.; Harmon, C.M.; Homra, B.K.; Allain, A.V.; Bordelon, G.J.; et al. Multimodal Analgesia, Current Concepts, and Acute Pain Considerations. Curr Pain Headache Rep 2017, 21, 3. [Google Scholar] [CrossRef]
- Carlson, R.H. Multimodal Pain Management Minimizes Need for Opioids. Oncology Times 2015, 37, 36. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





