1. Introduction
A massive outbreak of the Respiratory syncytial virus is occurring everywhere. It is regarded as the primary cause of newborn pneumonia and bronchiolitis. In addition, it causes pneumonia in the elderly and patients with chronic cardio-pulmonary disorders, as well as otitis media in older children.1 One single-stranded, negatively polarized fragment of RNA and an enveloped virus with a helical nucleocapsid make up its characteristics. In a virion, RNA polymerase is present. Its surface spikes solely contain a fusion protein, unlike other Paramyxoviruses. It is hemagglutinin-free. The serotypes are two.2 Transmission happens when infected hands come into close contact with the nose or mouth or when respiratory droplets come into contact with the hands.3 Unlike many other cold viruses, which re-enter the community every few years, RSV causes respiratory illness epidemics every winter. It affects almost everyone by the age of three and is present everywhere.4 Infection in babies largely affects the lower respiratory system; there is no systemic dissemination.5 Immune response most likely plays a role in pathogenesis.6 RSV also contributes to respiratory infection epidemics in hospitalized babies.7
Enzyme immunoassay (rapid antigen test), which finds RSV antigens in respiratory secretions, is used in the laboratory to make a diagnosis. Cell culture isolation is also an option. Giant cells with several nuclei can be seen with an electronic microscope. Following that, immunofluorescence is used to confirm the RSV infection’s identification. When making a diagnosis of a baby, serology is useless.8 For critically unwell newborns, aerosolized ribavirin is used as a treatment.9 Passive immunization with the monoclonal antibody palivizumab10 or the injection of immune globulins from previously infected infants who were able to overcome this obstacle are two methods of prevention.11 In the nursery for new babies, nosocomial outbreaks may be avoided by hand washing and glove use.12 RSV frequently leads to reinfection throughout life despite having a modest degree of antigenic change.13 Despite more than 50 years of study, there is still no licensed safe and effective RSV vaccine.14 In children under the age of five, RSV is thought to be the cause of roughly 22% of severe respiratory tract infections.15 The current study’s objective was to develop lipid nanoparticles of the mRNA vaccination of the fusion spike protein to combat the fatal RSV that affects newborns worldwide.
2. Methods
The potential open reading frames of mRNA of surface spike fusion protein of RSV virus was identified by bioinformatics using NCBI website; mRNA of surface spike fusion protein was expressed then purified via organic extraction method. The purified mRNA was enclosed with lipid nano-particles bubbles synthesized using hot micro-emulsion technique. The vaccine delivery system was lipid nanoparticles with particle size 90 nanometre. Purified mRNA was administered intraperitoneally to 100 transgenic mice to test the immunogenicity in animal models. During the immunogenicity testing in preclinical trials animal testing and randomized human clinical trials stages 1/2, the pathogenic 2 valent Respiratory syncytial serotypes consisted of A, B serotypes.
Construction of mRNA transcripts of fusion spike protein of RSV:
[ DynabeadsTM mRNA purification kits with Catalog number: 61006 were obtained from Invitrogen Thermo Fisher Scientific, USA]
2.1. Principle of mRNA In-Vitro Transcription
In the present study linearized DNA template of the gene of interest coding fusion spike protein that present on the envelope of respiratory syncytial virus was obtained using restriction endonuclease type II enzymes such as EcoR I and Hind III. This was followed by cloning using polymerase chain reaction[ PCR] technique. PCR cycle: The required reagents and template were added to PCR tubes. Afterwards blended and spined for 3 minutes at 300 rpm. The mineral oil was added to prevent evaporation in a thermal cycler without a heated lid. Then, amplification per thermocycler and primer parameters was achieved. Using agarose gel electrophoresis and ethidium bromide staining, the amplified DNA was examined. PCR cycles included: Initial denaturation at 94°C for 2 minutes: The double-stranded DNA template strand was heated during this initiation stage to the point where the strands began to denaturize and the hydrogen bonds between the nucleotide base pairs ruptured. At 94 to 98 °C, the first denaturation stage was carried out. The primers were then annealed for 30 seconds at 55°C: Within this temperature range, the forward and reverse primers were stable enough to anneal to each of the single-stranded DNA template strands. Additionally stable enough to attach to the primer DNA sequence was the DNA polymerase.
Then, extension of DNA for 1 minute at 72°C was done: The Taq polymerase possess an optimal temperature around 70-75°C so this step enabled the DNA polymerase to synthesize and elongate the new target DNA strand accurately and rapidly. Repeating previous steps was achieved 25-30 times. On the other hand, final Extension for 5 minutes was performed at 72°C to fill-in any protruding ends of the newly synthesized strands. PCR kits which were utilized during the present study included PureLinkRTM PCR Purification kit with Catalog number K310001 which was purchased from ThermoFisher scientific company, USA. RNA polymerase was then, mixed with DNA template and ribonucleotides for in vitro mRNA transcription. The invitro mRNA transcription lasted about 2 hours; then an RNAase inhibitor was added to the mixture to aid in the the purification of mRNA extract. The postinvitro transcription comprised the addition of DNAase for the digestion of DNA template. As well as, proteinase K was added to aid in the cleaning up of any protein contamination.
2.2. Procedure for In Vitro Transcription
Using the following process, more than 10 µg of mRNA transcript could be produced from a 1 µg DNA template. The TranscriptAidTM T7 High Yield Transcription kit was utilized for high-yield transcription, producing up to 200 µg of mRNA. Thawed and combined frozen reagents under 300 rpm centrifugation for three minutes. Nucleotides and enzymes were kept cold. At room temperature, the reaction buffer was retained. At room temperature, the following combination for the reaction was created:
Table 2.
It shows the components of in vitro mRNA transcription reaction mixture:.
Table 2.
It shows the components of in vitro mRNA transcription reaction mixture:.
| Ingredient |
Volume |
| 5X Transcription buffer |
10 µl |
| ATP/GTP/ CTP/ UTP Mix, 10 mM each |
10 µl[ 2mM final concentration] |
| Linearized DNA template |
1 µg |
| RiboLockTM RNAase inhibitor |
1.25 µl[ 50 U] |
| T7/ T3/ SP6 RNA polymerase |
1.5 µl[ 30 U] |
| DEPC-treated water |
Up to 50 µl |
| Total volume |
50µl
|
The mRNA transcription reaction mixture was then incubated at 37 0C for 2 hours. 2 µl[ 2 U] of DNAase I, RNAase- free were added, mixed and incubated at 37 0C for 15 minutes in order to remove DNA template. The reaction was obstructed aside the addition of 2 µl 0.5 M EDTA at PH 8.0 and incubation at 65 0C for 10 minutes. mRNA was subjected to hydrolysis in the absence of a chelating agent such as EDTA.
2.2.1. Purification of mRNA Transcripts
This was done using the liquid-liquid extraction method known as AGPC, which stands for Acid Guanidinium Thiocyanate-Phenol-Chloroform. Chloroform reagent solutions were manufactured and consisted of 96% chloroform and 4% isoamyl alcohol. They could be combined with an equivalent volume of phenol to create a 25:24:1 solution. RNases were made inactive by isoamyl alcohol, which also helped to minimize foaming. The phenol utilized in this intervention was delivered as a water-saturated solution with a Tris buffer made up of 50% phenol, 48% chloroform, and 2% isoamyl alcohol solution.
Chloroform was stabilized with small quantities of amylene or ethanol, because exposure of pure chloroform to oxygen and ultraviolet light produces phosgene gas. This method relied on phase separation by centrifuge of a mixture of the aqueous sample and a solution containing water-saturated phenol and chloroform, resulting in an upper aqueous phase and a lower organic phase (mainly phenol). Guanidinium thiocyanate, a chaotropic agent, was added to the organic phase to aid in the denaturation of proteins (such as those that strongly bind nucleic acids or those that degrade RNA). The nucleic acids (RNA and/or DNA) partitioned into the aqueous phase, while protein partitioned into the organic phase. The pH of the mixture determined which nucleic acids got purified.
While RNA stayed in the aqueous phase under acidic conditions (pH 4-6), DNA partitioned into the organic phase. Both DNA and RNA partitioned into the aqueous phase at neutral pH levels (7-8). The nucleic acids were eventually extracted from the aqueous phase using 2-propanol precipitation. After washing the 2-propanol with 70% ethanol, the pellet was quickly air-dried before being dissolved in TE buffer. While phenol, isopropanol, and water had low solubility, 1 ml of 4 M ( 50%) guanidinium thiocyanate denatured proteins, including RNases, and separated rRNA from ribosomal proteins. A clear, upper aqueous phase, which contains the nucleic acids, and a lower phase, which contains the proteins dissolved in phenol and the lipids dissolved in chloroform, completely separated in the presence of chloroform or BCP ( bromochloropropane). PH was maintained at about 4, which preferentially retained mRNA in the aqueous phase for mRNA transcript purification.16
Northern blot technique for the confirmation of the purification of mRNA transcripts coding for the fusion spike protein:
The sizes and amounts of the various mRNA transcripts for the 2 valent serotypes of RSV that code for the fusion spike protein formation were measured using the Northern blot method. Denaturing gel was originally employed in Northern blot to separate mRNA transcripts based on size. Then, with the same distribution as in the gel, mRNA was transferred into a nylon membrane. In order to hybridize the immobilized mRNA, a la-belled probe complementary to the target gene was added after the mRNA transcripts were fixed to the membrane. After that, the loosely bound probes were rinsed away. The solid membrane was then dried, made visible, and was subjected to examination with the probe precisely bound to the target mRNA transcripts. The Northern blot measured the quantities and seizes of the target mRNA transcripts.
2.2.2. Inclusion of mRNA Vaccine with Lipid Nanoparticles
Bubbles made of lipid nanoparticles were added. 45 mcg of dimethyl dioctadecyl ammonium bromide lipid (DDAB) were used to create these lipid nanoparticles. A Quaternary ammonium lipid called DDAB formed vesicles that contained mRNA transcripts when it complexed with mRNA to trigger innate immunity. The delivery system for the vaccine made of lipid nanoparticles included particles that were around 90 nm in size. It was accomplished throughout this investigation to create lipid nanoparticles with various cationic and solid lipid types and to assess their suitability as LNP-mRNA delivery systems. This was accomplished by using a hot micro-emulsion process to combine cationic lipids such stearylamine, DOMTA, or DDAB with solid lipids like Compritol 888 ATO [C] or cetyl palmitate 15[CP 15] to create a sequence of lipid nanoparticles. The created cationic solid nanoparticles systems( CSLNS) were examined using a Malvern NanoZS analyzer[ obtained from Biotechne, USA] to determine their particle size, size distribution, and zeta potential. Comparing and assessing the cytotoxicity of CSLNS systems was done using the Resazurin test. For the examination of the CSLNS systems, which effectively bound mRNA transcripts and shown minimal cytotoxicity, in-vitro cellular uptake [fluorescence microscopy] and gene silencing[ Northern blot] experiments were used. On the other hand, high Performance size exclusion chromatography (HPSEC) was exploited to assess the particle configuration of mRNA transcripts. Later on loading on a CSLNS formulation, the transition temperature [Tm] of the mRNA transcripts was deliberated exploiting differential scanning fluorimetry (DSF).
2.3. Experimental Preclinical Trials[ Animal Testing]
2.3.1. In Vitro Vaccine Immunogenicity Testing on Transgenic Mice
Transgenic mice have their genes changed by tissue culture and recombinant DNA technologies. A transgenic animal has a gene of DNA sequence (a trans-gene) incorporated into the cell’s genome by human intervention. The vaccination was administered to 100 transgenic mice. 28 days separated the two dosages that were given to them. To boost immunity, the initial dosage was cut in half. For boosting viral protein expression and inducing potent humoral and cell-mediated immunity, transgenic mice were made human by lung human cell line [Human lung epithelial BEAS-2B cells, which showed features of mesenchymal stem cells, bought from Accegen, USA]. After being inoculated with Respiratory syncytial virus at an infectious dosage of 170-200 viral units via an intranasal method of administration, transgenic mice, which were adult male mice weighing 40–50 g, might be triggered by lower respiratory illnesses such pneumonia.17
Formulation:
lipid nanoparticle vaccination with mRNA. The dose form was an intramuscular injection of a sterile solution of the mRNA transcripts of the genes encoding the proteins promoting the synthesis of RSV fusion spike protein. Each 1 ml dose contained 5 mcg of the mRNA transcript for each cluster gene of RSV that codes for spike fusion protein of 2 valent RSV pathogenic serotypes, 45 mcg of the lipid dimethyl dioctadecyl ammonium bromide (DDAB), and 0.8 mg of aluminium hydroxide. Additionally, 0.923 mg of sodium dihydrogen phosphate dihydrate and 7 mg of sodium chloride were included in each dose.
By administering the pure LNP-mRNA vaccine via intraperitoneal route to 200 male transgenic mice weighing 45–50 gm, immunogenicity in animal models was assessed:
The effectiveness of vaccinations was evaluated using Active Protection testing. 100 transgenic mice were challenged with an increasing quantity of infectious microorganisms by intranasal delivery after receiving the vaccination under test. The infectious dosage of RSV delivered by intranasal delivery varied from 170 to 200 virus units. To assess the immunogenicity and efficacy of the vaccine, the lowest number of microorganisms required to cause 50% of transgenic mice to die ( LD 50%) was established and compared to LD 50% in non-vaccinated subjects. To conduct passive protection testing, 100 transgenic mice were given varying dosages of immunized subjects’ serum intraperitoneally. The mice were subsequently challenged with 180 to 200 viral units of the infectious agent via an intranasal route. The greatest serum dilution that could protect 50% of the animals (i.e., ED 50%) was chosen as a benchmark for the vaccine’s effectiveness.
2.3.2. ELISA
ELISA for detection of neutralizing antibodies to the test vaccine:
[ Invitrogen coated and instant ELISA kits with product number 37581 obtained from Thermo Fisher scientific company, USA].
Each well received 10 µl of the antigen suspension by passive adsorption, which was then given an hour to incubate. Bovine serum albumin served to inhibit the extra binding sites. Three PBS-T washes were applied to the plates to remove unattached molecules. Biotinylated IgG was incubated for 15 minutes after being mixed with 50 µl of horseradish peroxidase (HRP) in each well. The wells were once again washed with PBS-T to remove any unattached molecules. The enzyme and then the antigen could be identified after each well received 50 µl of chromophore substrate( TMB) for 15 minutes.
2.3.3. Flow Cytometry
Flow cytometry for detection of CD+4 and CD+8 T lymphocytes:
The CD+4 and CD+8 T cells that were specific to the mRNA gene cluster vaccination were seen and examined using an Invitrogen Attune Cytpix flow cytometer (obtained from the USA). The patient’s cells were marked in this experiment using a monoclonal antibody. The CD4 protein, which is used to count T helper cells, is one of the proteins that these antibodies were created against. Fluorescent dyes like rhodamine and fluorescein were used to label monoclonal antibodies. Individual cells came into contact with the laser beam and lit up. A fluorescence-activated cell Sorter (FACS) was used to measure the fluorescence.
2.3.4. Randomized Human Clinical Trials Phases 1/2
Vaccine immunogenicity evaluation was carried out through human randomized clinical trials phases 1/2: Three groups of human participants were employed in the current experiment. 100 people were split among each group: Group 1 (the negative control group) received an intramuscular injection of the placebo.
Injections of the standard 2-valent RSV vaccine were given intramuscularly to Group 2 (the positive control group).
Group 3 (the test group) received an intramuscular injection of the RSV test LNP-mRNA vaccine. The three groups were exposed to graded amounts of the infectious 2 serotypes of RSV and after two weeks to enhance the production of protective neutralizing antibodies( this was ethical and approved to determine the effectiveness of the test vaccination). Following a 21-day period, intradermal booster doses were administered to the three groups. The degree of protection provided by the test vaccination was evaluated over a three-year period. In contrast to the protective cell-mediated immunity, which was assessed using a flow cytometry method, the protective antibodies were identified using an enzyme-linked immunosorbent assay (ELISA).
2.3.5. Statistical Analysis
It was done in triplets for every culture. Means and standard deviation were used in their presentation. The statistical analysis was conducted using an excel spreadsheet and a one-way analysis of variance with a p value less than 0.05. The present screening experimental inquiry made use of the F statistical analysis test.
3. Results
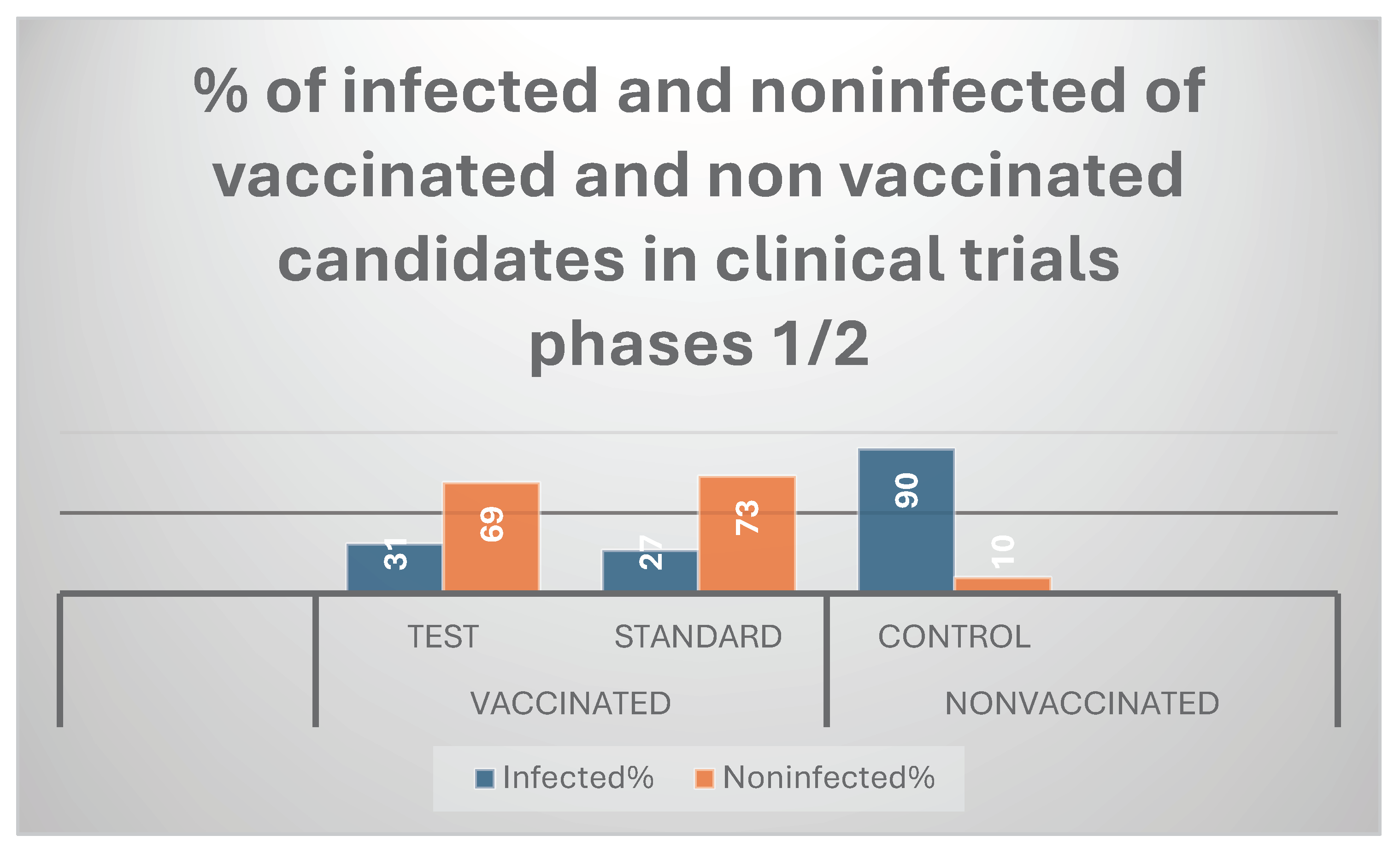
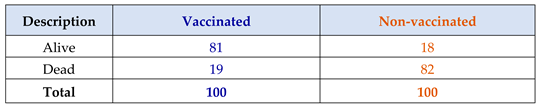
The present mRNA RSV vaccine resulted in 81 % immunogenicity( efficacy) during preclinical trials; while it showed 69% in clinical trials phases 1/2.
Nearly 600 viral units were found to be the RSV LD50%. It was discovered that the mRNA RSV vaccine’s ED50% was 5 mcg/ ml. The mRNA vaccine formulation contained 5 mcg of mRNA transcript of cluster gene of each of the 2 valent pathogenic RSV[ A and B] per millilitre. Phases 1 and 2 of randomized human clinical trials involved 90 infected participants in the negative control group, 27 infected participants in the positive control (standard) group, and 31 infected participants in the test group. It demonstrated moderate immunogenicity and fewer adverse reactions than other common vaccinations. The vaccine needs to be updated on a regular basis despite its long-lasting efficacy to avoid the high pathogenicity liability of RSV annually every winter season. The high purity of recombinant mRNA transcripts that were purified using the liquid-liquid extraction technique Acid guanidinium thiocyanate-phenol-chloroform( AGPC) was demonstrated by the ratio of mRNA transcript absorbance at 260 and 280 nm, which was almost 2 when measured with a UV spectrophotometer.
All LNP-mRNA vaccine formulations that fall under the category of CSLNS systems displayed small particle sizes of approximately 90 nm, a narrow size distribution as measured by the polydispersity index (PDI), which ranged from 0.31 to 0.39, and high zeta potentials of between + 19 and + 29 mV. Because of its significant cytotoxicity on typical Vero cell lines, using CSLNS in combination with stearyl amine was deemed unsuitable. Because it displayed the best complexion capacity with mRNA transcripts and the least amount of cytotoxicity on typical Vero cell lines during the Resazurin assay, CSLNS combined cationic lipid DDAB with solid lipid CP 15, was the ideal formulation. Only the DDAB and CP 15 combo was absorbed by PC-3 cells and showed silencing effectiveness. Following loading on the CSLNS formulation, differential scanning fluorimetry( DSF) revealed that the transition temperature [Tm] of the mRNA transcripts augmented for a while between 0.6 and 0.8 0C. No deterioration of the mRNA transcripts’ particle structure was discovered using High Performance size exclusion chromatography [HPSEC] studies after electrostatic-ally loading them onto CSLN. Bioinformatics analysis: Type 1 transmembrane proteins with external and intracellular domains were found in the fusion spike proteins of A and B RSV serotypes.
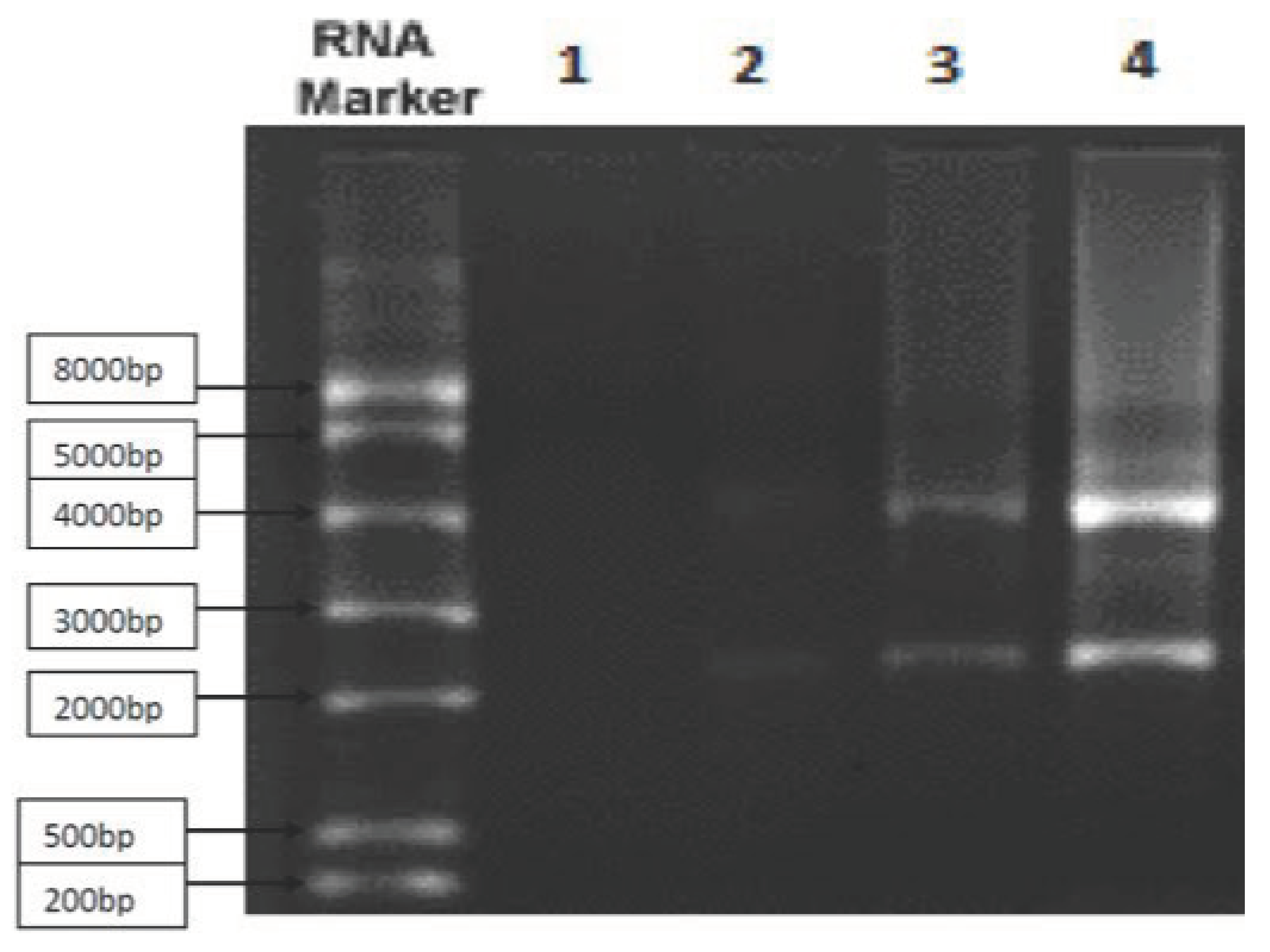
Figure 1.
The messenger RNA of the gene cluster that contributes to the synthesis of
RSV fusion spike protein is shown using
the Northern blot method. The purity of the sample was about 84%. Graph 1 shows that throughout phases 1/2 of clinical trials,
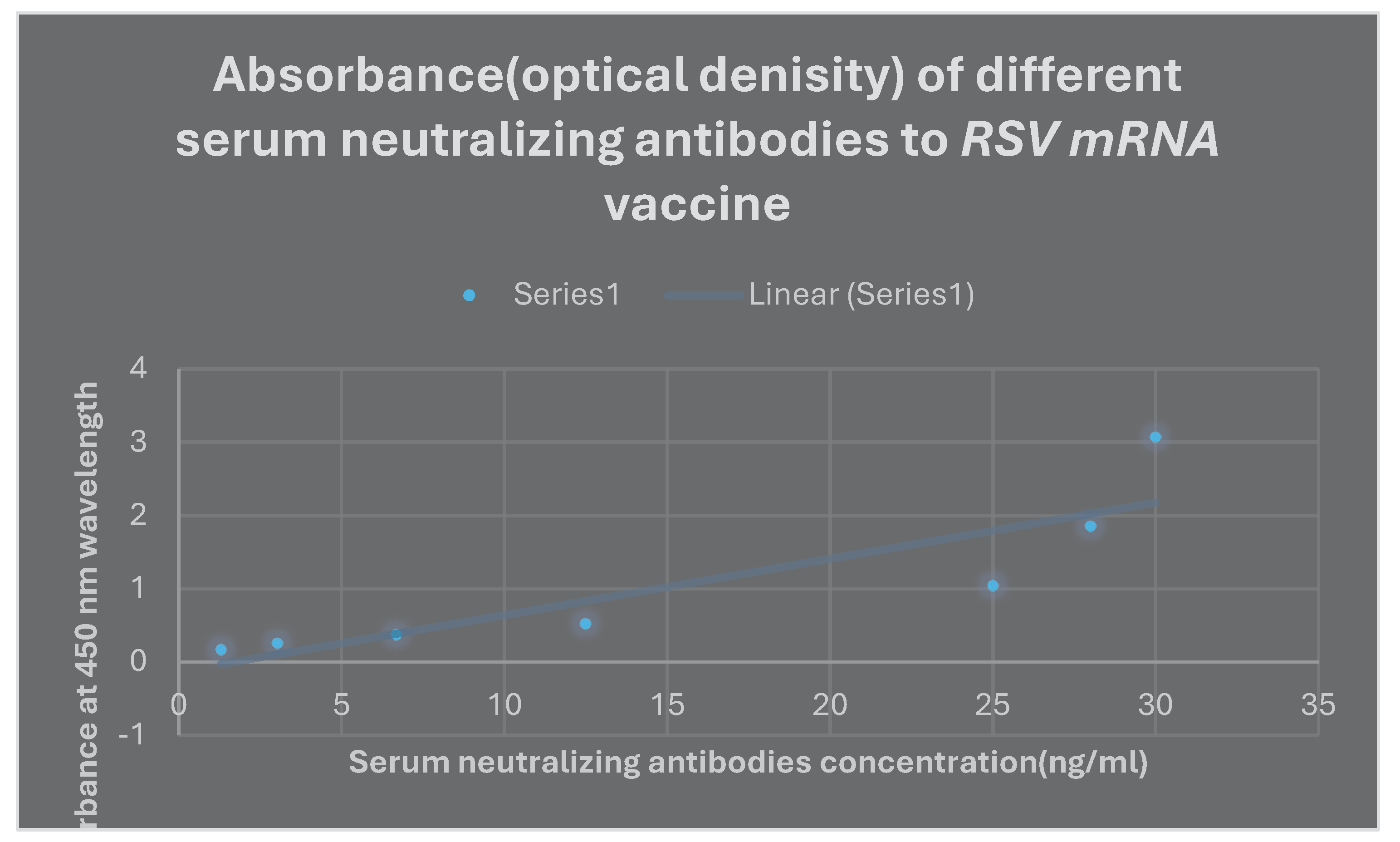
the mRNA RSV vaccine’s protective efficacy was 69%. The absorbance of various serum concentrations of neutralizing antibodies to
the mRNA RSV vaccination as measured by
ELISA is shown in Graph 2.
Table 3 displays the immunogenicity of the LNP-mRNA RSV vaccine administered to transgenic mice.
Table 4 displays the T lymphocyte count following the LNP-mRNA RSV immunization.
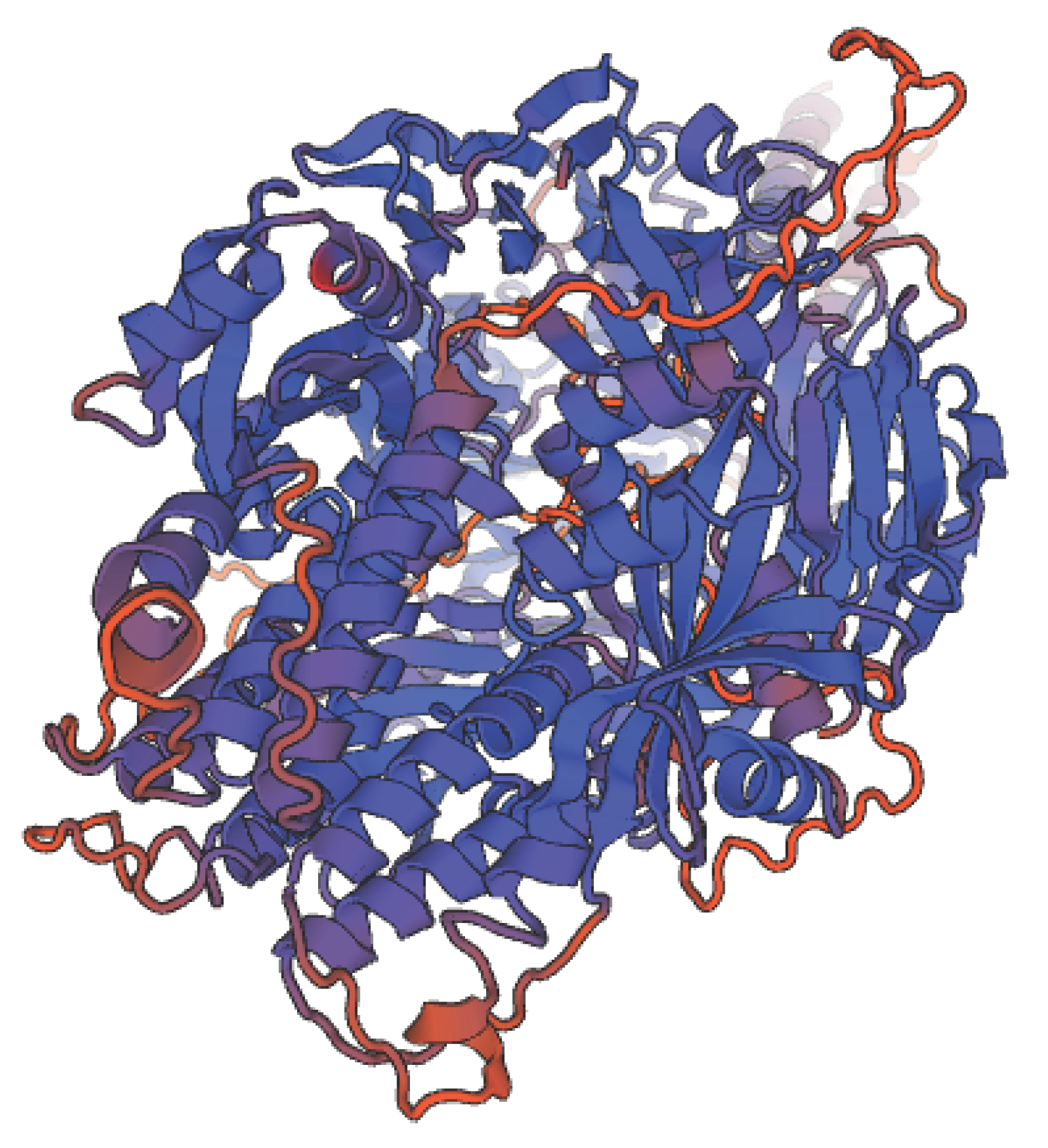
Human Respiratory virus fusion protein is shown in three dimensions in Figure 2.
Graph 1.
It represents that protection power of mRNA RSV vaccine was 69% during clinical trials stages1/2.
Graph 1.
It represents that protection power of mRNA RSV vaccine was 69% during clinical trials stages1/2.
Graph 2.
It represents the absorbance of different serum concentrations of neutralizing antibodies to mRNA RSV vaccine via ELISA.
Graph 2.
It represents the absorbance of different serum concentrations of neutralizing antibodies to mRNA RSV vaccine via ELISA.
Figure 1.
By using the Northern blot technique, it depicts the messenger RNA of gene cluster that helps synthesizes RSV fusion spike protein. Approximately 84% of the sample was pure.
Figure 1.
By using the Northern blot technique, it depicts the messenger RNA of gene cluster that helps synthesizes RSV fusion spike protein. Approximately 84% of the sample was pure.
Figure 2.
It shows 3D structure of Human Respiratory virus fusion protein.
Figure 2.
It shows 3D structure of Human Respiratory virus fusion protein.
4. Discussion
The goal of the current study was to create an LNP-mRNA RSV vaccine to protect newborns against bronchiolitis and pneumonia, both of which can be fatal. The most common cause of viral lower respiratory tract illness (LRI) in newborns and young children across the world is Respiratory syncytial virus( RSV), which also significantly affects the elderly and people with immune system deficiencies. The purpose of the RSV vaccine is to stop significant LRI that is related to RSV. The need to immunize very young infants, who may not respond well to vaccination, the existence of two antigenically distinct RSV groups, A and B, and the history of disease enhancement following the administration of a formalin-inactivated vaccine are some of the challenges facing the development of effective RSV vaccines. Only the pure F protein (PFP) subunit vaccines and live attenuated vaccines have recently undergone clinical trials, even though vector delivery methods, synthetic peptide, and immune-stimulating complex vaccines have all been tested in animal models. While live cold-passaged, temperature-sensitive RSV vaccinations (also known as cpts vaccines) will likely be helpful in early infants, PFP-2 looks to be a beneficial vaccine for the elderly and for children with underlying pulmonary illnesses who are RSV-seropositive. This was according to Dudas RA et al, 1998 study.18
In a phase 2a research, healthy people (18 to 50 years of age) were randomly allocated in a 1:1 ratio to receive either a single intramuscular injection of the bivalent prefusion F( RSVpreF) vaccine or a placebo. The RSV A Memphis 37b challenge virus was administered intravenously to subjects about 28 days after injection, and they had a 12-day observation period. These were the key endpoints that were set for each protocol: RT-qPCR( reverse transcriptase quantitative polymerase chain reaction) confirmed detectable RSV infection on at least two consecutive days with at least one clinical symptom of any grade from two categories or at least one grade 2 symptom from any category, the total symptom score from day one to discharge, and the area under the curve (AUC) for the RSV viral load in nasal-wash samples measured by RT-qPCR from day two after challenge to discharge. The immunogenicity and safety of the treatment were also evaluated. Asymptomatic RSV infection proven by any detectable viral RNA on at least 2 consecutive days was shown to have vaccination effectiveness of 86.7% (95% CI, 53.8 to 96.5) following the challenge virus inoculation of the participants. In the vaccination group, the median AUC for the RSV viral load (hours copies per milliliter) was 0.0 (interquartile range, 0.0 to 19.0), while in the placebo group, it was 96.7( interquartile range, 0.0 to 675.3). 28 days after injection, the geometric mean factor increase in RSV A-neutralizing titers from baseline in the vaccination group was 20.5 (95% CI, 16.6 to 25.3) and in the placebo group, it was 1.1( 95% CI, 0.9 to 1.3). The vaccination group reported more local injection-site discomfort than the placebo group did. In neither group were any severe negative occurrences recorded.19 The rapid creation of a live-attenuated RSV vaccine might significantly lessen the burden of RSV illness worldwide. The Ruth A. Karron et al., 2021 research provided evidence for this.20
The present vaccine resulted in 69% efficacy in clinical trials phases 1/2; while it was 81% in preclinical trials. It showed a moderate biological activity in most participants in the present study; on the other hand it demonstrated a few side effects effects like mild fever and pain at the site of intramuscular or intradermal injection. Its efficacy was long lasting. It did not cause dementia due to absence of amyloid plaque formation in the brain and the spinal cord. This was because it contained small amounts of mRNA. The present LNP-mRNA RSV vaccine was devoid of antibody dependent enhancement which leads to the formation non protective antibodies. By triggering cytokines and the complement cascade, activating immune complexes, or facilitating viral entrance and multiplication inside the host-infected cells, these non-neutralizing antibodies aggravate infection. The current vaccination significantly increased humoral immunity while just mildly increasing cell-mediated immunity. The primary bodily defense system that stopped the virus was humoral immunity. The primary RSV viral surface spike fusion protein-neutralizing antibodies in blood were IgM2 and IgG3. Due to the virus’ antigen not being acquired during a natural infection, few IgA antibodies were generated against it. The current vaccination only slightly stimulated cell-mediated immunity against RSV viral infection.
Advantages: No reversion to virulence was possible; on the other hand the immunogenicity was enhanced through the rise in the count of neutralizing antibodies against fusion spike protein of RSV as well as the moderate enhancement of cell mediated immunity. Disadvantages:
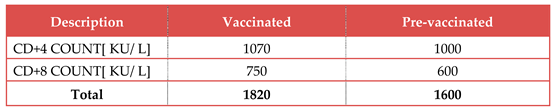
It was impossible for the vaccine virus to be excreted and transmitted to non-immune contacts, which prevented the development of herd immunity against RSV viral infection. It also had a shorter duration of action than the live attenuated vaccine and required refrigeration at -70 0C to prevent contamination and spoilage. The rise in both types of T. lymphocyte counts was the cause of the increased immunogenicity. The number of CD4+ T lymphocytes was greater than 1000 KU/ L; but the number of CD8+ T lymphocytes was greater than 740 KU/ L. On the other hand, following immunisation with mRNA 2 valent RSV vaccine in randomized human clinical trials stages 1/2, there was a significant increase in titters of the powerful neutralizing antibodies to proteins facilitating the attachment of RSV to the host human lung cells.
In the addition of 0.5 mg of aluminium hydroxide as an adjuvant, the immunogenicity increased from 69% to 71%. Infants who are allergic to any of the substances in the test vaccination should not get it. Simple analgesics like paracetamol and ketoprofen were successful in treating a brief duration of moderate fever as well as a minor amount of pain at the site of intramuscular and intradermal injections.
It was reported that the killed RSV vaccine conducted in previous studies before resulted in hypersensitivity reactions through the formation of antigen antibody complex due to the synthesis of non protective antibodies.21 The present LNP-mRNA RSV vaccine was devoid of antibody dependent enhancement which leads to the formation non protective antibodies. In the LNP formulation, the current vaccine showed only modest promise when compared to the criteria outlined in the Yulia E et al., 2022 study.22 The recommended dosage regimen for infants conducted in the present study was intramuscular or the intradermal injection of 2 successive doses of LNP-mRNA RSV vaccine separated by 28 days; then the administration of a booster dose annually to prevent the establishment of lower respiratory infections that are lethal to infants especially older than 6 months of age who lost the protective neutralizing antibodies transmitted to them from their mothers through the placenta during pregnancy. When mRNA LNP-RSV vaccine was compared with RSV killed vaccine, mRNA vaccine in the present study was preferable to to killed vaccine due to the induction of higher titer of neutralizing antibodies against fusion spike protein of RSV and hence longer lasting protection; on the other hand mRNA vaccine moderately activated cytotoxic T lymphocytes which kill RSV infected cells.
Since there aren’t many medications that work well against RSV infections, it’s crucial to avoid infection with the mRNA vaccine.
In the current investigation, all LNP-mRNA RSV pre-F formulations elicited virus-neutralizing titers one month after vaccination that were greater than those linked to palivizumab’s protection of high-risk babies.
Weight loss was reported for different types of RSV vaccines which present in clinical trials evaluation nowadays;23 whereas this condition appeared rarely during the clinical trials phases 1/2 evaluation in the present study comprising LNP-mRNA RSV vaccine establishment. The rare weight loss condition could be managed through the balanced nutrition of the subjected infants during the clinical trials phases 1/2 in the current study. Bioinformatics analysis of RSV fusion protein showed that The fusion protein causes cells to fuse forming multi-nucleated giant cells called syncytia. 39 different types of RSV vaccine were designed. Only 19 of them reached clinical trials stages but until now there is no licensed RSV vaccine.24 During different clinical trials phases in the current time two types of the vaccines against RSV demonstrated a promising consequence in enhancing the immunogenicity. These included live attenuated vaccine type and subunit vaccine one. Live attenuated vaccine was found to be more valid for infants; while the subunit vaccine comprising RSV spike fusion protein which is surface envelope glycoprotein rendered higher titers of of spike fusion protein antibodies that prevented the infection in elderly patients and hindered the development of pneumonia in young children aged 2-12 years of age especially who reached 3 years old.25 The present LNP-mRNA RSV showed similar efficacy to the subunit vaccine type but was more effective to infants; as well as it showed less effectiveness than live attenuated vaccine due to less stimulation of the cell mediated and humoral immunity; but it showed neither hypersensitivity reactions nor antibody dependent enhancement which leads to the formation non protective antibodies.
5. Conclusion
As a defense against RSV infection, the RSV surface spike haemagglutinin protein mRNA vaccination established efficacious. Babies older than six months are advised to use it. Future research into the possibilities of pairing it with other vaccinations such as the Measles, Mumps, and Rubella vaccines is considered.
Ethical statement
For the current investigation, all applicable national, institutional, and/or international rules for using both people and animals were postdated. By the recommendations of the Weather-all report, the local government, the Ethical Committee for Human and Animal Handling at Cairo University (ECAHCU), and the Pharmacy Faculty at, University of Cairo, Egypt, all approved all procedures used in the study, including those involving people and animals, with approval number P-13-2-2021. The number of volunteers and the suffering of the animals used in the study were both minimized.
The type of the study
Screening experimental examination.
Source of animal models
One hundred male transgenic mice, weighing between 40 and 50 g, were bought from Cairo University’s College of pharmacy’s Pharmacology and toxicology department and given the go-ahead for legalization. Human lung cells were injected into these animals.
Inclusion criteria for animal models
Adult male mice weighing 40–50 gm; are susceptible to RSV infection, including transgenic mice. A lung human cell line was used to humanize transgenic mice, boosting viral protein expression and inducing potent humoral and cell-mediated immunity. Human lung epithelial BEAS-2B cells, which displayed features of mesenchymal stem cells, were bought from Accegen, USA. After receiving an intranasal dosage of the respiratory syncytial virus that was infectious at a dose of 170-200 viral units, transgenic mice that were adult males weighing 40–50 g were able to develop lower respiratory illnesses like pneumonia. Three to five days following the beginning of the incubation phase, symptoms started to manifest. During the current investigation, the fatal dosage surpassed 600 virus units.
Exclusion criteria
Young mice; pregnant female mice and male mice weighing less than 40 gm.
Collection of the samples
100 blood samples were collected from Respiratory syncytial virus infected infants, children and elderly in different locations in Egypt.
Material
All chemical and biological components were sourced from Algomhoria Pharmaceutical Company in Cairo, Egypt, and Alnasr Pharmaceutical Company in Abo zabal Alkhanka, Qalyobia, Egypt. Riboblock RNase inhibitor[ 40 U/µl] with catalog number EO0384 was obtained from ThermoFisher Scientific, USA. T7 RNA Polymerase[ 20 U/µl] with catalog number EP0112 was purchased from ThermoFisher Scientific, USA. Pyrophosphatase, inorganic[ 0.1 U/µl] with catalog number EF0221 was purchased from ThermoFisher Scientific, USA. TrancriptAid T7 High Yield Transcription kit with catalog number K0441 was obtained from ThermoFisher Scientific, USA.
Date and of place study
Between February 2021 and August 2023, this study was conducted at Cairo University’s pharmacy faculty in Egypt.
Equipment
Table 1.
List of instruments:
Table 1.
List of instruments:
References
- Kumar, P. Kumar, Clark’s clinical medicine. Ninth edition, Elsevier Edinburgh London. 2020, 7, 814–819. [Google Scholar]
- Caroline, S.; Zeind Michael, G. Applied therapeutics, the clinical use of drugs. Eleventh edition, Wolters Kluwer, London. 2020, 15, 337–348. [Google Scholar]
- Anthony, T.; Bertram, K.; Marieke, K.-H. Katzung Trevor pharmacology examination board review. Thirteen editions, McGraw Hill Education, New York. 2021, 4, 825–834. [Google Scholar]
- Stan, B.; Jason, W.; Douglas, M. Applied pharmacology. Fourth edition, Elsevier Edinburgh, London. 2020, 13, 1283–1291. [Google Scholar]
- James, O. Clinical pharmacology made ridiculously simple. Seventh edition, MedMaster, Miami, United States of America. 2020, 10, 3011–3027. [Google Scholar]
- Warren, L. Review of medical microbiology and immunology. fifteen editions, McGraw Hill Education, New York. 2021, 11, 1645–1658. [Google Scholar]
- Larry, S.; Paul F, C.; Alan H, M.; Leon, S. Comprehensive Pharmacy Review for NAPLEX. Tenth edition,Wolters Kluwer, London. 2019, 20, 903–913. [Google Scholar]
- Bruce, F.; Pamela, C.; Richard, H. Lippincott illustrated reviews microbiology. Sixth edition, Wolters Kluwer, London. 2021, 12, 1029–1041. [Google Scholar]
- Cecily, D.; Terry, S.; Joseph, D.; Barbara, W. Pharmacotherapy handbook. Eleventh edition, McGraw Hill Education, New York. 2021, 10, 459–468. [Google Scholar]
- Stephen, G. Clinical physiology made it ridiculously simple. Sixth edition, Med Master, Miami, United States of America. 2020, 8, 772–778. [Google Scholar]
- Golder, W. Biochemistry and genetics. Eighth edition, McGraw Hill Education, New York. 2019, 9, 981–987. [Google Scholar]
- Metting Patricia, J. Physiology. Sixteen editions, McGraw Hill Education, New York. 2019, 18, 1257–1269. [Google Scholar]
- Abbas, J. RSV vaccines, finally within reach, could prevent tens of thousands of yearly deaths. JAMA. 2022, 327, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Knudson, C.J.; Hartwig, S.M.; Meyerholz, D.K.; Varga, S.M. RSV Vaccine-Enhanced Disease Is Orchestrated by the Combined Actions of Distinct CD4 T Cell Subsets. PLOS Pathog. 2015, 11, e1004757–e1004757. [Google Scholar] [CrossRef]
- Falsey, A.R.; et al. Efficacy and safety of an Ad26. RSV.preF-RSV preF protein vaccine in older adults. N Eng J Med. 2023, 388, 609–620. [Google Scholar]
- Papi, A.; Ison, M.G.; Langley, J.M.; Lee, D.-G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; Campora, L.; Dezutter, N.; et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. New Engl. J. Med. 2023, 388, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Rawlins, E.L.; et al. The amazing world of lung-specific transgenic mice. Am j Respir Cell Mol Biol. 2012, 46, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Dudas, R.A.; et al. Respiratory syncytial virus vaccines. Journal of clinical microbiology revision. 1998, 11, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Schmoele-Thoma, B.; Zareba, A.M.; Jiang, Q.; Maddur, M.S.; Danaf, R.; Mann, A.; Eze, K.; Fok-Seang, J.; Kabir, G.; Catchpole, A.; et al. Vaccine Efficacy in Adults in a Respiratory Syncytial Virus Challenge Study. New Engl. J. Med. 2022, 386, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Karron, R.A.; et al. Live attenuated vaccines prvent Respiratory syncytial virus associated illness in young children. Am J Respir Crit Care med. 2021, 203, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; et al. Arandomized phase 1/2 study of a respiratory Syncytial virus pre fusion F vaccine. J Infect Dis. 2022, 225, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Eygeris, Y.; Gupta, M.; Kim, J.; Sahay, G. Chemistry of Lipid Nanoparticles for RNA Delivery. Accounts Chem. Res. 2021, 55, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Mejias, A.; Rodríguez-Fernández, R.; Oliva, S.; Peeples, M.E.; Ramilo, O. The journey to a respiratory syncytial virus vaccine. Ann. Allergy, Asthma Immunol. 2020, 125, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Jorquera, P.A.; Anderson, L.; Tripp, R.A. Understanding respiratory syncytial virus (RSV) vaccine development and aspects of disease pathogenesis. Expert Rev. Vaccines 2015, 15, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Lee, Y.-T.; Hwang, H.S.; Kwon, Y.-M.; Kim, M.-C.; Ko, E.-J.; Lee, J.S.; Lee, Y.; Kang, S.-M. Virus-Like Particle Vaccine Containing the F Protein of Respiratory Syncytial Virus Confers Protection without Pulmonary Disease by Modulating Specific Subsets of Dendritic Cells and Effector T Cells. J. Virol. 2015, 89, 11692–11705. [Google Scholar] [CrossRef] [PubMed]
Table 3.
It shows the immunogenicity of vaccination of transgenic mice with LNP-mRNA RSV vaccine in animal testing stage:.
Table 3.
It shows the immunogenicity of vaccination of transgenic mice with LNP-mRNA RSV vaccine in animal testing stage:.
Table 4.
It shows the count of T lymphocytes after the vaccination with LNP-mRNA RSV vaccine:.
Table 4.
It shows the count of T lymphocytes after the vaccination with LNP-mRNA RSV vaccine:.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).