1. Introduction
Numerous pharmaceutical companies are focused on researching and developing new formulations based on herbal sources, which can help manage chronic diseases. The World Health Organization also supports conventional plant-based treatments due to their accessibility, safety for long-term uses, and relatively low production costs. This shift towards natural remedies is mainly because some synthetic pharmaceutical drugs may have harmful side effects when used for the long-term treatment of chronic diseases [
1]. Therefore, based on traditional medical systems (Ayurvedic and Chinese), phytotherapy in chronic disorders is currently used as an alternative treatment worldwide. Berberis is a significant plant genus with approximately 500 species worldwide. It belongs to the
Berberidaceae family and has considerable potential applications in the food and pharmaceutical industry [
2].
Berberis species are native to central and southern Europe, Asia (with northern zones of Pakistan and Iran), and the north-eastern area of the United States.
Berberis vulgaris (L.), known as European barberry, Common barberry, or Épine-Vinette, has an essential role in herbal therapy; its different parts (fruits, leaves, roots, stem, branches, stem/root bark) have been used in traditional medicine for more than 2500 years. This species was reported helpful in various inflammations, high blood pressure, gastrointestinal diseases, hepatic disorders, and diabetes. Numerous studies show that
B. vulgaris has valuable pharmacological properties such as antioxidants, antihyperglycemic, anticholinergic, hypolipidemic, anti-inflammatory, anticancer, and antimicrobial properties. In homeopathy,
B. vulgaris is mainly used in urinary lithiasis, dermatology, rheumatism, and liver diseases [
3]. Berberine, the specific isoquinoline alkaloid mainly extracted from common barberry root and stem barks, is formulated for oral administration alone or in various combinations. The berberine-based phytotherapeutics’ administration could have a beneficial impact on lipid and carbohydrate metabolism, particularly on glucose homeostasis, being helpful in weight loss, diabetes mellitus [
4] and endocrine disorders, liver diseases [
5], cardiovascular diseases, atherosclerosis, neurodegenerative diseases, rheumatic diseases, infectious diseases [
1]. Several studies reported berberine-induced toxicity in humans and mice [
1]. However, toxic phenomena could be diminished through berberine combination with other phytochemicals or plant extracts. Synergistic effects would also be expected in adequate combinations [
1]. Moreover,
B. vulgaris and berberine display anticancer effects through various cell signaling pathways’ modulation [
6], diminishing tumor cell viability and reducing their multiplication in various neoplasia (lung, breast, ovary, gastric cancer) [
1].
In the present study, we aimed to investigate the hydro-ethanolic dry extract of B. vulgaris stem bark (BVE), obtained using a reflux extraction process in 50% ethanol, rotary evaporation, and freeze-drying. A complex analysis of BVE’s phenolic compounds was performed using ultra-high-performance liquid chromatography coupled with high-resolution mass spectrometry (UHPLC–HRMS/MS). Berberine was quantified through HPLC-DAD. The BVE’s antioxidant potential was in vitro evaluated through free radical scavenging (DPPH and ABTS) and reducing power (FRAP). The acute toxicity of BVE and Berberine - Berberine sulfate hydrate (BS) - was assessed in vivo on two Daphnia species. In contrast, their teratogenic potential was evaluated by applying the embryo test on Daphnia magna embryos. Moreover, the antitumor potential of BVE and BS was investigated on several human cancer cell lines: hepatocellular (HEP G2), colon (LoVo and HT-29), breast (MDA-MB-231), ovary (SK-OV-3), and tongue (PE/CA-PJ49), using classical oncolytic drugs as positive controls. Extensive data analyses support our results, showing significant correlations between the BVE’s concentration, exposure time, phenolic constituents’ content, antioxidant activity, and cytotoxicity.
2. Results and Discussions
2.1. Phenolic Compounds (Polyphenols and Phenolic Acids) Quantification
Berberidis cortex dry hydro-ethanolic extract was obtained with a yield of 16.35%. Other studies reported similar yields: 18,7% for roots and 14.7% for leaf extracts in ethanol [
6]. The TPC and TPA values are presented in
Table 1. BVE is rich in total polyphenols (17.6780 ± 3.9320 mg Eq Tannic acid / 100 g extract); however, it has shown a phenolic acids content of only 3.3886 ± 0.3481 mg Eq Chlorogenic acid / 100 g extract.
The literature data show that TPC in various
B. vulgaris extracts are very different. Our hydro-ethanolic extract of Berberidis cortex has a TPC of 1767.80 mg/g, while El-Zahar et al. [
7] reported much lower TPC levels in ethanol extracts of roots (147.2 mg/g) and leaves (120.7 mg/g). Och et al. [
8] indicated similar TPC values quantified in 80% methanol extracts of various
B. vulgaris parts: 58.5 mg/g for the leaf extract, 57.7 mg/g for the stem one, and 52.8 mg/g for the fruit extract.
2.2. Identification and Quantification of BVE Phytoconstituents by UHPLC–HRMS-MS and HPLC-DAD
Table 2. registers all phytochemicals identified in BVE.
Some constituents were quantified, and gallic acid had the highest amount (540.00 µg/g). It is followed, in decreasing order, by naringenin (90.41 µg/g), berberine (78.95 µg/g), rutin (72.41 µg/g), kaempferol (68.24 µg/g), and galangin (67.21 µg/g).
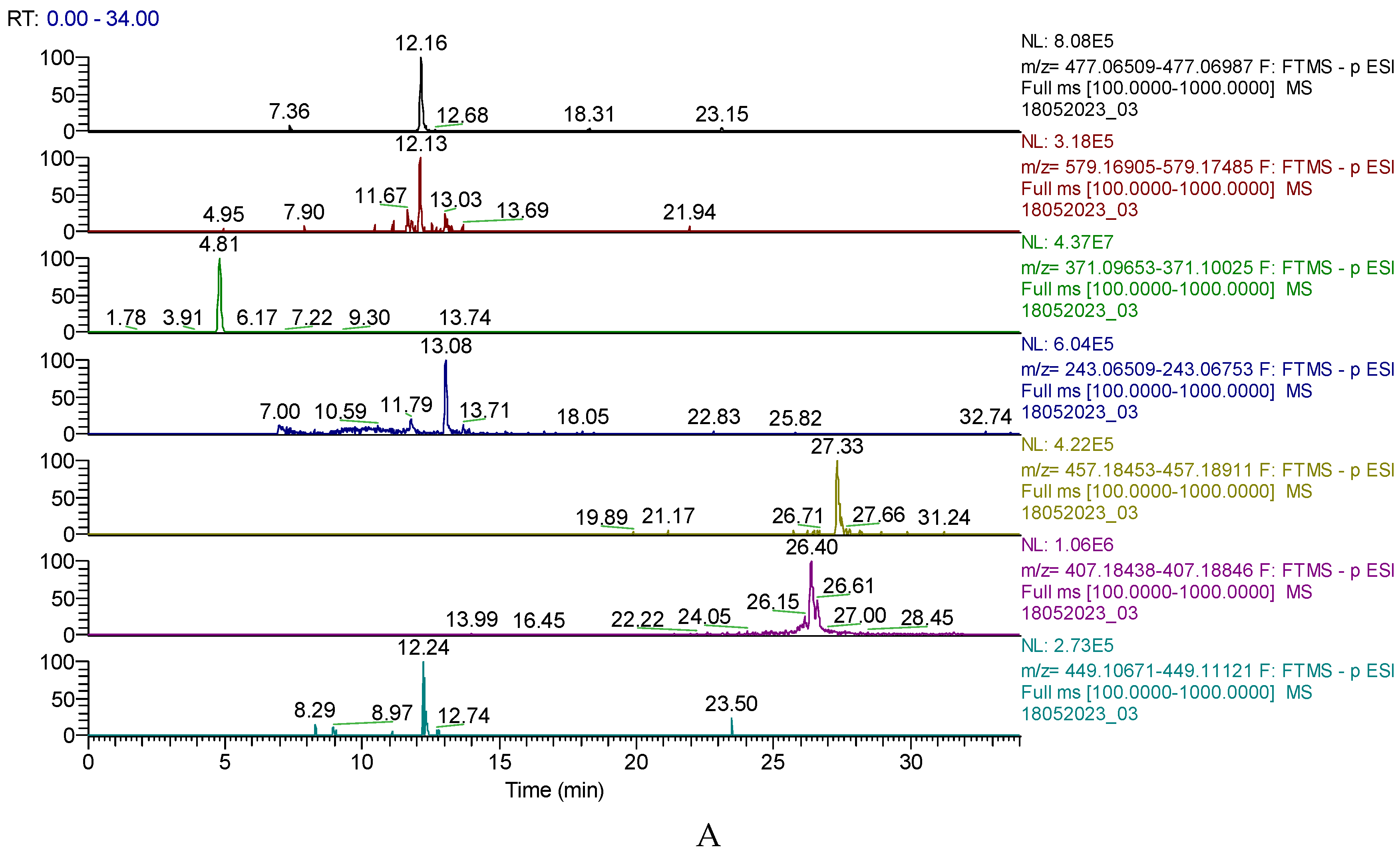
Figure 1. A displays the chromatogram of the primary phytochemicals identified in BVE by
UHPLC-MS, and
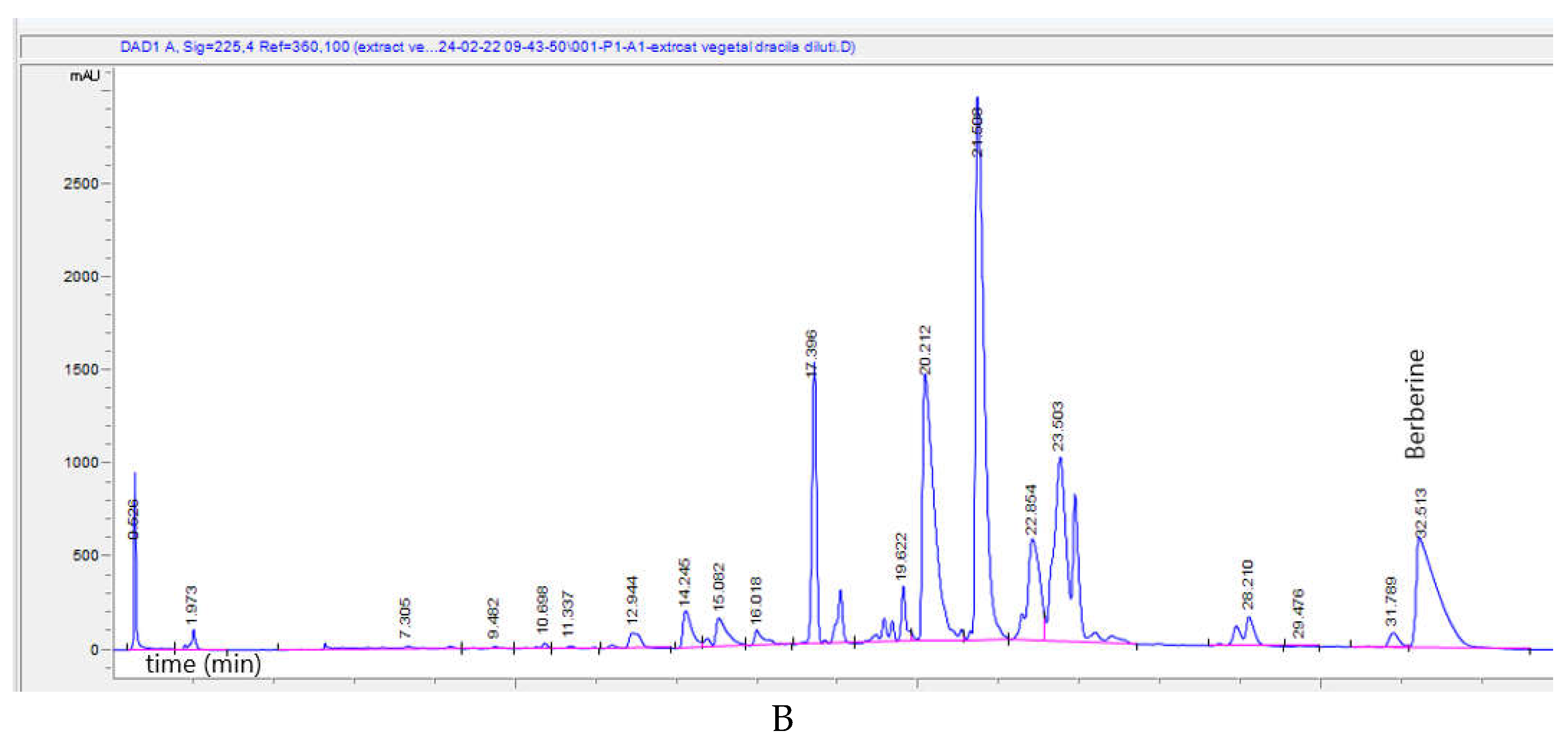
Figure 1B shows the HPLC-DAD chromatogram of BVE, where berberine has an
RT=32.513.
In Argentina/Patagonian barberry fruits (
Berberis microphylla) ethanol extract, Boeri et al. [
9]reported the highest amounts of quercetin (1,134.54 µg/g), caffeic acid (1,092.75 µg/g), and syringic acid (368.55 µg/g). In contrast, gallic acid was 48.17 µg/g. Berberidis cortex is a significant and frequently used crude drug registered in the "Drug Standards of Tibetan Medicines" since 1995. The specific bioactive compounds are alkaloids (berberine, magnoflorine, jatrorrhizine, palmatine), and the most known is berberine, quantified in our BVE (78.95 µg/g). HPLC analysis of methanolic extract of Berberidis cortex from China, harvested from different geographical zones, recorded a range of 21.12 – 37.5 µg/g berberine [
10]. Our results indicated a value twice higher than the first one.
2.3. Antioxidant Activity
Table 2 shows significant differences between the IC
50 / EC
50 values determined by all three methods: IC
50DPPH = 0.2610 mg/mL, IC
50ABTS = 0.0442 mg/mL, and EC
50FRAP = 0.1398 mg/mL, compared to ascorbic acid (IC
50 = 0.0165 mg/mL). Similar values were reported on
B. microphylla ethanol extract [
9] ABTS IC
50 = 0.26 mg/mL and DPPH IC
50 = 0.38 mg/mL. The substantial antioxidant potential is underlined by phenolic constituent content and berberine and other alkaloids, known for their protective activity [
11]
2.4. 48-Hours Acute Toxicity Test Using Daphnia Magna and Daphnia Pulex
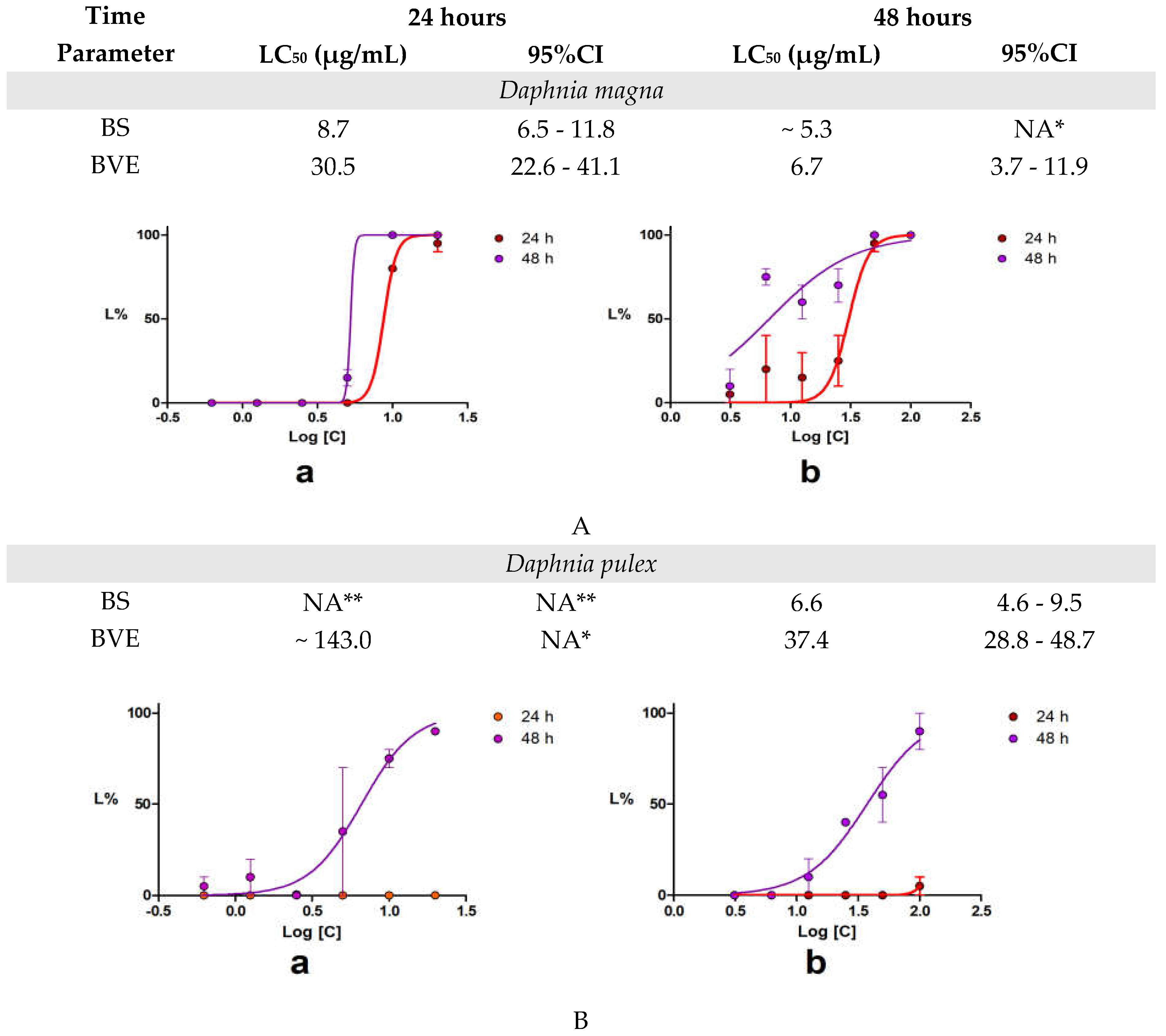
After 24 hours,
D. magna total lethality was recorded at concentrations ≥ 25 µg/mL BVE and ≥ 10 µg/mL BS. Similarly,
D. pulex total lethality occurred at concentrations ≥ 50 µg/mL BVE and ≥ 25 µg/mL BS. Our results revealed that BS toxicity is higher than BVE for both
Daphnia species,
D. magna being more vulnerable than
D. pulex. The time-dependent toxicity is more evident in
D. magna than in
D. pulex. (
Figure 2). The lethality curves’ analysis revealed that, in
D. magna bioassay (
Figure 2A), BS exhibited a lower LC
50 value at 24 hours compared to BVE, suggesting higher toxicity of the pure alkaloid, which was expected. However, at 48 h, the BVE toxicity significantly increases, almost at the same potency as BS. After 48 h of exposure, the concentration-response curves for both tested solutions in
D. pulex displayed similar trends to those recorded in
D. magna but with differences in the magnitude of lethality (
Figure 2B).
2.5. Daphnia Magna Embryonic Development Assay
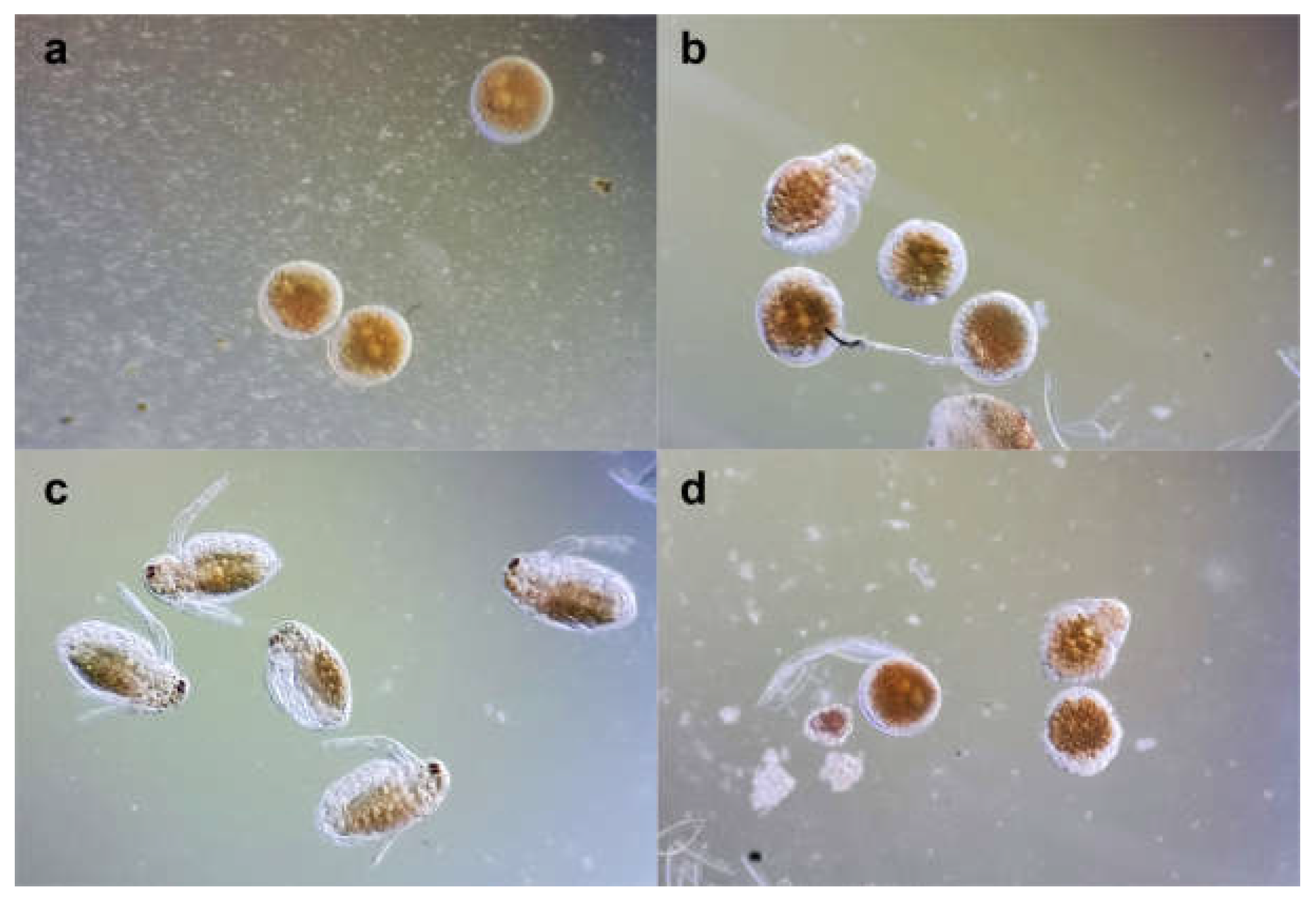
Following the Acute Toxicity Test results, the embryo assay was performed at non-lethal concentrations (2.5 µg/mL BS and 3.125 µg/mL BVE). Minor differences were observed after 24 hours (
Figure 3 a,b). After 48 hours, BS stimulated the development of all embryos (
Figure 3c), while BVE exhibited a significant inhibitory effect (
Figure 3d), which could be due to the extract’s complex composition. Only 20% of the embryos treated with BVE were fully developed, compared to 90% recorded for those exposed to BS. The mobility and viability of neonates developed in BS solution were similar to those of the control. However, they all failed to form the compound eye, even after 48 hours of exposure, suggesting a potential developmental risk.
On
Daphnia magna, Vesela et al. [
12] reported that berberine chloride toxicity recorded an LC
50 of 0.903 µg/mL after 24 h and 0.822 µg/mL at 48 h exposure. Our berberine sulfate hydrate recorded 9.7 µg/mL and 5.3 µg/mL, respectively. In another study on another crustacean [
13], 7 µg/mL berberine chloride induced 100% lethality in
Artemia salina larvae. These differences should be explained by Berberine salt, the animal model species, and provenance.
D. magna embryos failed to form compound eyes after BS and BVS treatment. Natural berberine also affects cardiovascular system morphogenesis and functionality in Zebrafish embryos [
14]. Based on these findings, In Medicinal Plants Monograph volume 4 [
15], WHO mentions berberine’s potential side effects on humans after consuming more than 500 mg.
2.6. In Vitro Anticancer Activity
The antiproliferative activity induced by BVE was evaluated in vitro through several cytotoxic assays by applying different BVE and BS concentrations (6.25—400 µg/mL) on cells derived from six tumor cell lines of different histological origin: HEP G2, LoVo, HT-29, MDA-MB-231, SK-OV-3, PE/CA-PJ49. Furthermore, human umbilical vein endothelial cells (HUVECs) were used as reference normal cells.
The BVE and BS antiproliferative capacities, tested on normal human cells and tumor cell lines, are shown in
Table 3.
The IC
50 values displayed in
Table 3 could be interpreted according to Hidayat et al. [
16], resulting in an overview of BVE and BS cytotoxicity on various cell lines. On normal endothelial cells (HUVEC), they have no cytotoxicity after 24 and 48 hours (IC
50 >> 400 µg/mL). The same interpretation is also available for HT-29 tumor cells, which showed no significant decrease in viability after BVE/BS treatments.
Generally, BVE exhibited moderate cytotoxicity in the other tumor cells. The most substantial effect, with the lowest IC50 values after 24 and 48 hours (IC50 >100 µg/mL, respectively IC50 > 50 µg/mL) on breast cancer cells (MDA-MB-231) and OSCC ones (PE/CA-PJ49). On LoVo cells (colon cancer), the cytotoxicity at 24 hours is appreciably lower (IC50 > 200 µg/mL), but after 48 h of exposure is moderate, similar to the previous ones (IC50 > 50 µg/mL). BVE exhibited the lowest effect on human ovary cancer (SK-OV-3 cells) and hepatocellular carcinoma (HEP G2 cells) after 24 and 48 hours (IC50 > 400 μg/mL and, respectively, > 100 μg/mL).
Globally, the BS antiproliferative effects are more potent than BVE. On MDA-MB-231 cells, moderate to high cytotoxicity was registered (IC50 >25 and, respectively, >12.5 μg/mL after 24 and 48 hours, p < 0.05). BS shows similar activity on PE/CA-PJ49 and LoVo (IC50> 50 μg/mL in both exposure times). Moreover, it recorded low toxicity after 24 h contact with SK-OV-3 and HEP G2 cells and a moderate one after 48 h (IC50 > 200 μg/mL, respectively, > 50 μg/mL).
The results of
in vitro studies are detailed and presented in
Figure S1 and File S1 from Supplementary Material.
After 24 h, BVE cytotoxicity at the selected concentration range (6.25 - 400 µg/mL) on normal and tumor cell lines shows significant differences (at α < 0.05, p-value was established at 0.0024) between PE/CA-PJ49 and HUVEC, and MDA-MB-231 and HUVEC (p = 0.001,
Figure S1a). Substantial differences (p < 0.05) were also observed in the case of HUVEC and all other tumor cells, except HT-29 ones, and MDA-MB-231 and HUVEC compared to HT-29 cells (
Figure S1a from Supplementary Material).
After 48 hours of exposure to BVE, percentual values of cell vitality significantly differ between HUVEC and MDA-MB-231 cells (p < 0.001). Appreciable differences (p < 0.05,
Figure S1b) were reported between HUVEC and LoVo, PE/CA-PJ49, and SK-OV-3. Moreover, there were notable differences between HT-29 and LoVo, MDA-MB-231 and PE/CA-PJ49, and HEP G2 and MDA-MB-231 (p < 0.05).
In the case of BS, significant differences (p = 0.0001 and p = 0.000) are recorded exclusively between HUVEC and MDA-MB-231 cell viability in both periods of exposure (
Figure S1c,d). After 24 hours, remarkable differences were reported between HUVEC and LoVo, PE/CA-PJ49, and SK-OV-3, and HT-29 and MDA-MB-231 and PE/CA-PJ49 (p < 0.05,
Figure S1c). Moreover, after 48 hours, there were notable differences between HUVEC and HEP G2, PE/CA-PJ49, LoVo, and SK-OV-3 (p < 0.05); the same for HT-29 and PE/CA-PJ49 and MDA-MB-231 (
Figure S1d).
No statistically significant differences were reported between BVE and BS cytotoxicity at the same exposure period on the same cell line (
Figure S2 from Supplementary Material).
The cytotoxic activity of BVE was compared to the one induced by several drugs (5-Fluorouracil, Cisplatin, and Doxorubicin) [
17] that are commonly used in oncological treatments and were applied throughout all the experiments as positive controls. The concentration range used for Cisplatin (CisPt) and 5-Fluorouracil (5-FU) was 3.125—200 µM, while for Doxorubicin (DOX) was between 0.625 and 40 µM [
17] and are illustrated in
Figure 4 and
Figure 5.
The effects of BVE and BS compared to anticancer drugs on normal endothelial cells (HUVEC) viability after 24 and 48 hours of exposure are displayed in
Figure 4.
The highest HUVEC cell viability diminution was recorded after 24 h at 200 µM CisPt (85.58%), while, at the corresponding concentrations, BVE, BS, and both other drugs did not significantly affect it. At 48 hours, 200 µM CisPt reduced normal cell viability to 55.80%, followed by BS at 200 µg/mL, with 77.15%. BVE at 200 µg/mL (89.95%) acted similarly with 200 µM 5-FU (88.63%) and 20 µM DOX (91.97%).
Figure 5 shows the BVE and BS influence on cancer cell viability compared to standard oncolytic drugs in the same concentration range (12.5 - 200 µg/mL for BVE and BS, 12.5 – 200 µM for 5-FU and CisPt, and 1.25 – 20 µM for DOX) [
17].
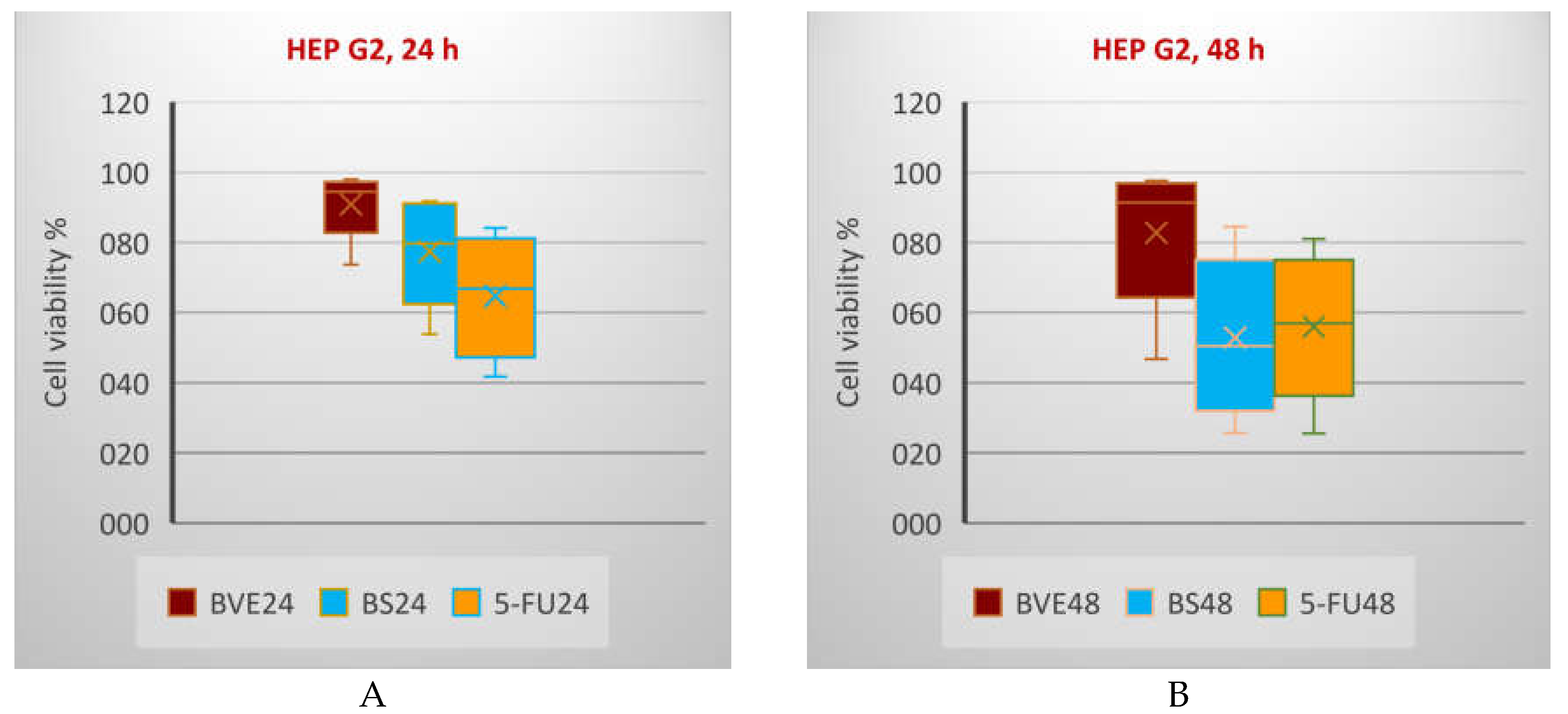
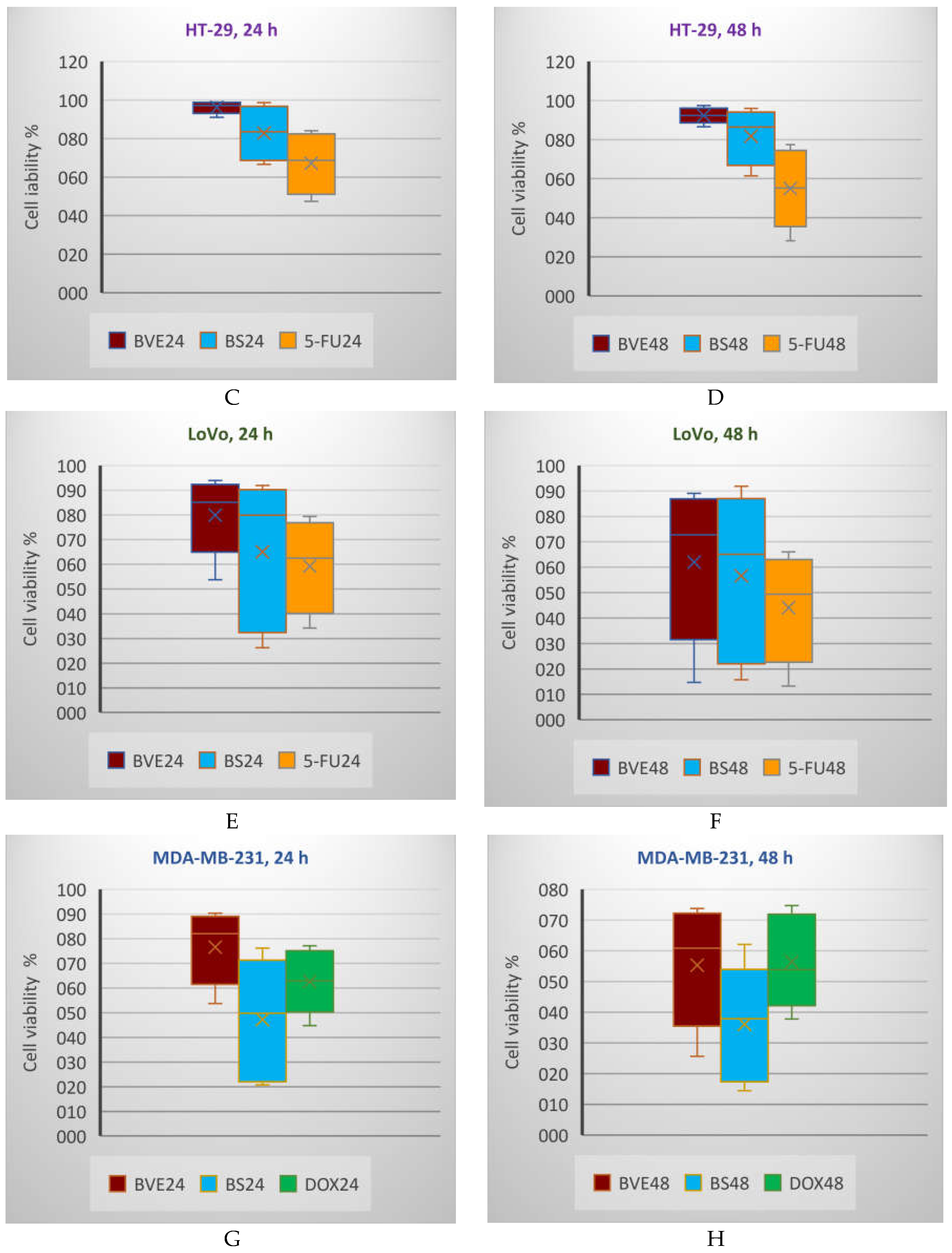
Generally, the tumor cell viability diminution is higher after 48 h than 24 h. In all cases, BS shows a higher cytotoxicity than BVE. On HT-29 and LoVo cells, BS activity is lower than 5-FU after both exposure times, while on HEP G2, BS activity is higher than 5-FU after 24 h and lower after 48 h (
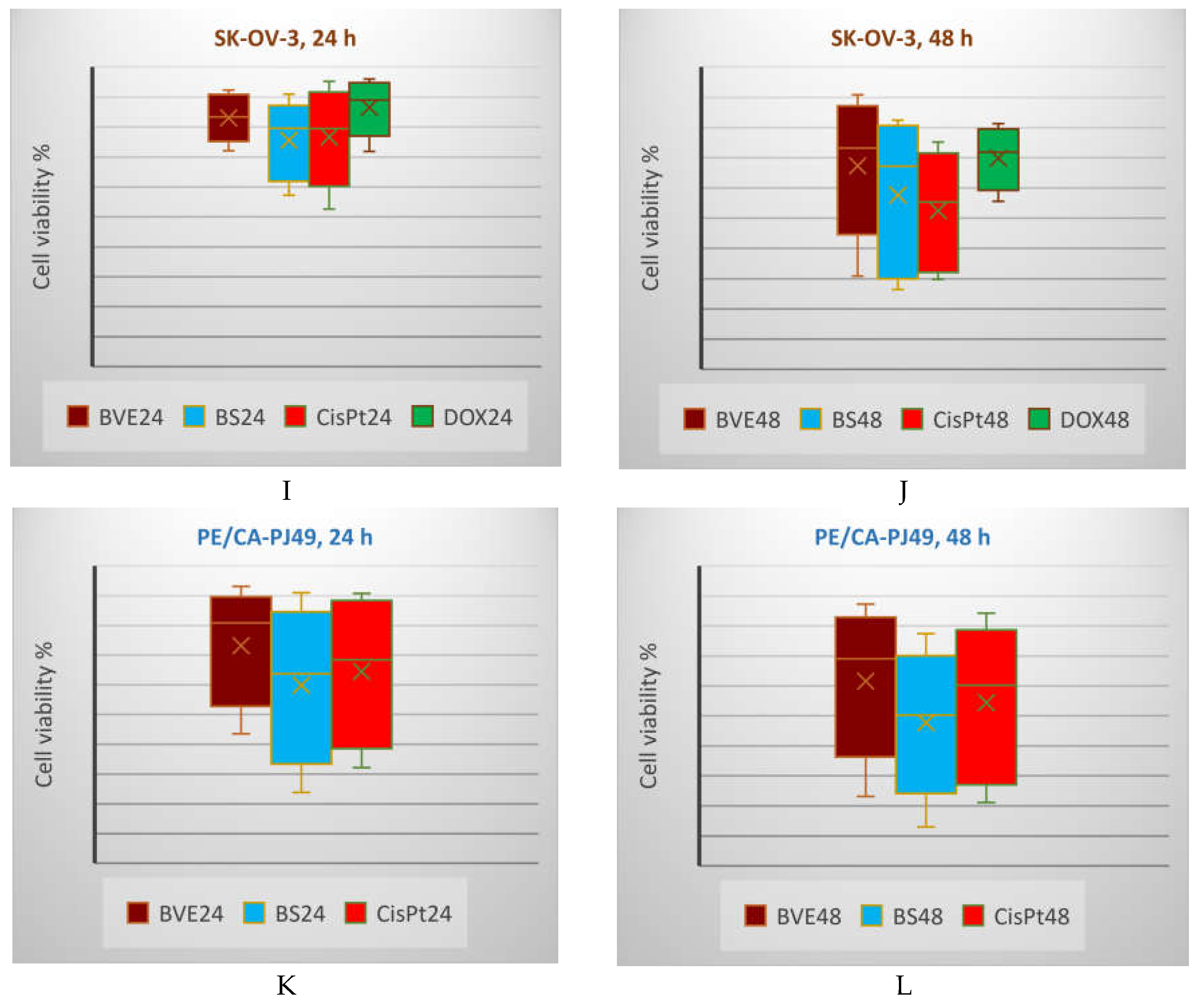
Figure 5, A-F). The PE/CA-PJ49 cell viability decreases in order: BVE> CisPt>BS after 24 and 48 hours of treatment (
Figure 5, K,L). On MDA-MB-231, the cell viability after 24 h decreases in order BVE>DOX>BS; after 48 h, BVE acts slightly higher than DOX (
Figure 5, G,H). After 24 h, the SK-OV-3 cell viability decreases in order DOX>BVE>CisPt>BS, while after 48 h, the previously mentioned order is changed: DOX>BVE>BS>CisPt (
Figure 5, I,J). These effects are due to the potential synergism between phytochemicals in BVE [
18].
2.7. Statistical Analysis
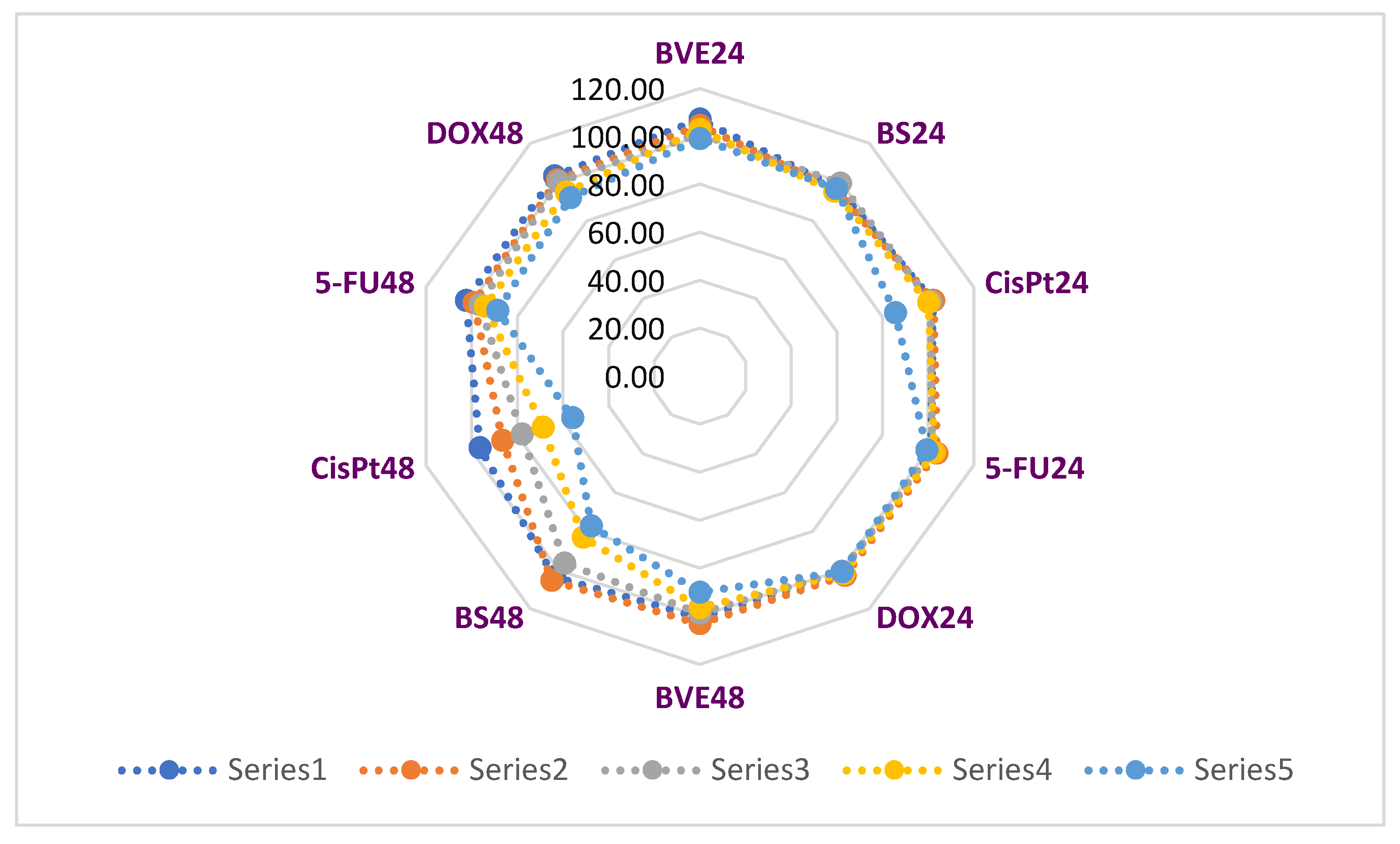
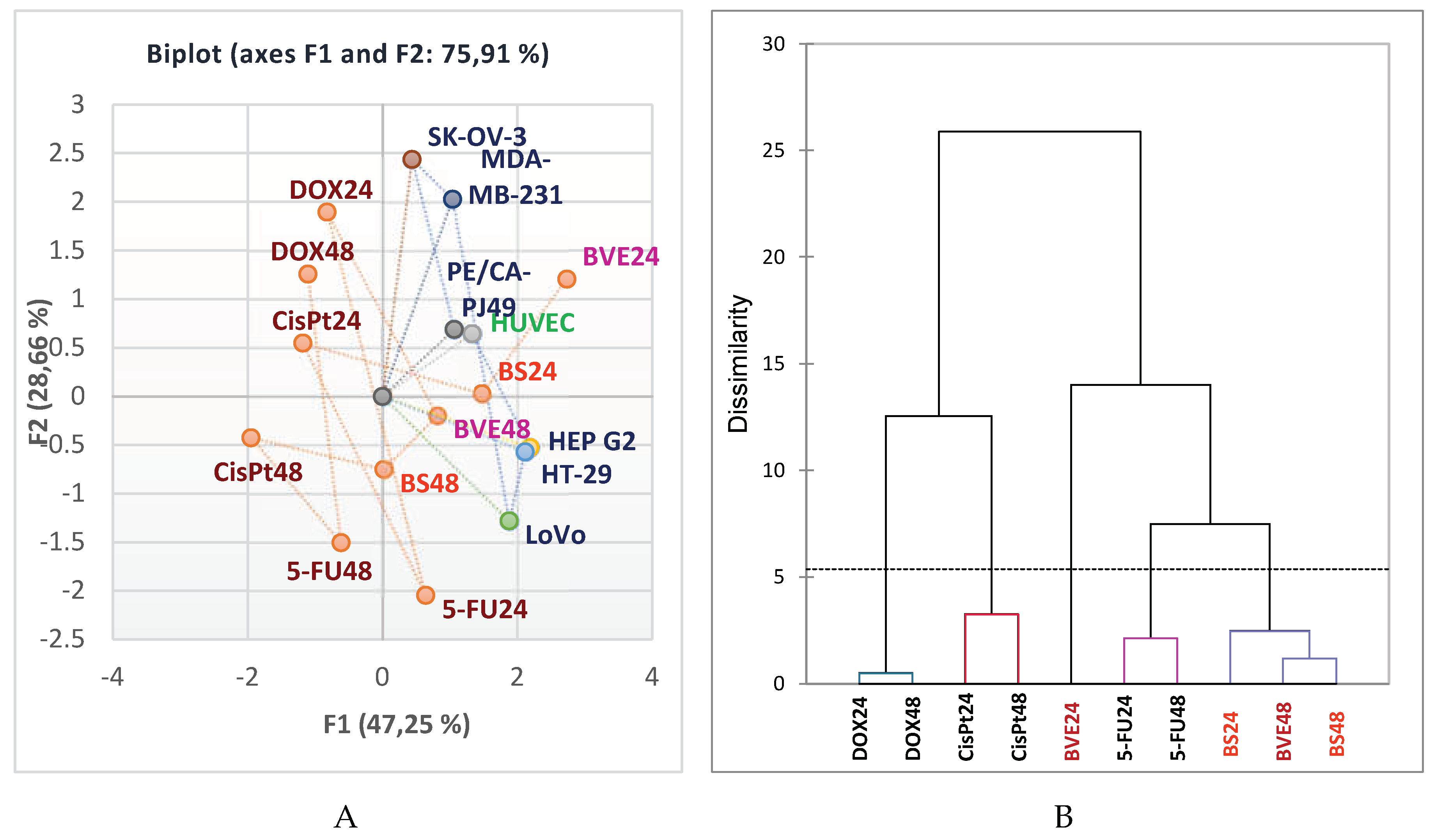
Pearson correlation shows that BVE24 is highly correlated with BS24, BVE48 and BS48 (r = 0.906, r = 0.910, r = 0.866, p < 0.05). BS24 exhibits a strong correlation with 5-FU24, BVE48 and BS48 (r = 0.813, r = 0.935, r = 0.928, p < 0.05). BVE48 reports a considerable correlation with BS48 (r = 0.955) and a moderate one with 5-FU48 (r = 0.788), p < 0.05. Moreover, CisPt24 significantly correlates with DOX24, CisPt48 and DOX48 (r = 0.934, r = 0.997, r = 0.837, p < 0.05), CisPt48 with DOX48 (r = 0.830, p < 0.05), DOX24 with CisPt48 and DOX48 (r = 0.918, r = 0.920, p < 0.05), 5-FU24 with BVE48 and BS48 (r = 0.866, r = 0.853, p < 0.05); 5FU24 also shows a moderate correlation with 5-FU48 (r = 0.788, p < 0.05). The place of each cytotoxic agent linked to the cell type is shown in
Figure 6A, and the similarities between them are displayed in
Figure 6B.
The correlations between the bioactive phytoconstituents - total phenolic content (TPC) and total phenolic acids (TPA) - and their pharmacological potential are detailed in
Table S3 from Supplementary Material. Their dual redox behavior could explain the antiproliferative effect on tumor cells, leading to decreasing viability; the prooxidant effect of phytochemicals is responsible for the BVE cytotoxicity. The antioxidant effect, measured by 3 methods, shows a substantial positive correlation with TPC and TPA (r = 0.972 - 0.994, p < 0.05). Moreover, the variable parameters determined by all 3 methods (DPPH, FRAP, ABTS) are intercorrelated (r = 0.997 - 0.998, p < 0.05) and show a substantial negative correlation with antiproliferative activity (r = ‒[0.951 – 0.999], p < 0.05). Similarly, TPC and TPA display a significant negative correlation with cell viability diminution (r = ‒[0.970 – 0.997], p < 0.05 (
Table S3). The outstanding capacity of
B. vulgaris for scavenging ABTS, hydroxyl radicals, and DPPH is due to berberine and phenolic compounds with dual redox behavior that can act synergistically in the extract [
2]. Recent studies showed that galangin and berberine in a synergic combination might induce esophageal carcinoma cells’ apoptosis through cell cycle arrest in the G2/M phase via oxidative stress.
The accessed literature data regarding the cytotoxic effects of berberine evaluated
in vitro on various tumor cells, potential mechanisms, and IC
50 values are synthesized in
Table 4.
In the present study, BS-IC
50 against HEP G2 was slightly over 50 ug/mL, being around that registered in
Table 4; the same for colon carcinoma (LoVo), colon cancer (SK-OV-3) and tongue squamous cell carcinoma (PE/CA-PJ49) cell lines. Moreover,
Table 4 indicates that the IC
50 of BS against OSCC is 18-136 µM, and our value belongs to this range. For the MDA-MB-231 (breast cancer) cell line, the IC
50 was > 25 µg/mL after 24 h and 12,5 after 24 h. Similar studies investigated the anticancer effects
of B. vulgaris extract and berberine chloride on other cancer cell lines, evaluating cell viability after 24 and 48 hours and reporting various IC
50 values [
32]. On HEP G2 (liver cancer), Caco-2 (colon cancer), and MCF-7 (breast cancer), IC
50 values for barberry extract were 68.02 > 49.96 > 15.61 µg/mL, and for berberine chloride, lower values were recorded: 65.86 > 17.64 > 15.93 µg/mL. After 48 h, the IC
50 values drastically decreased: 5.55, 3.84, and 4.44 µg/mL for berberis extract vs 11.49, 5.1, and 4.43 µg/mL for berberine chloride. Moreover, on HEP G2 and CaCo2, the antitumor activity of berberis extract was stronger than berberine chloride [
32]. In our study, both BE and BS had moderate cytotoxicity.
3. Materials and Methods
3.1. Materials
3.1.1. Chemicals
All chemicals were of analytical grade. Analytical standards of 31 compounds were purchased from Sigma-Aldrich, Germany. Methanol and ethyl alcohol, HPLC grade, were purchased from Merck Romania; formic acid (98%) and ultrapure water (LC-MS grade) were also purchased from Merck (Merck Romania, Romania). The Pierce LTQ Velos ESI positive and negative ion calibration solutions (Thermo Fisher Scientific, Germany) calibrated the Orbitrap Mass Spectrometer.
The standard phenolic compounds (8 phenolic acids, 7 isoflavones, and 15 flavonoids), berberine sulfate hydrate, ethanol, sodium acetate, AlCl3, DPPH, ABTS ammonium salt, trichloroacetic acid, phosphate buffer (pH=6.6), ascorbic acid, K3(FeCN)6 and FeCl3 were purchased from Sigma-Aldrich, Germany. Methanol and ethanol, potassium persulfate, formic acid (98%), and ultrapure water (LC-MS grade) were provided by Merck (Merck Romania SRL, Bucharest, Romania). The Pierce LTQ Velos ESI positive and negative ion calibration solutions (Thermo Fisher Scientific, Germany) calibrated the Orbitrap Mass Spectrometer.
In the
in vitro studies on cell lines, various materials were used: Dulbecco’s Modified Eagle Medium (DMEM, PAN Biotech, Aidenbach, Germany), cell washing medium HBSS (Hanks’ Balanced Buffer Solution), 200 mM L-glutamine, fetal bovine serum (FBS), 100 mM ethylenediaminetetraacetic acid (EDTA), phosphate buffered saline (TFS), dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO, USA), antibiotic mixture (10,000 U/mL penicillin and 10,000 µg/mL streptomycin) (Biochrom GmbH, Berlin, Germany), Trypan Blue and CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) kit (Promega). The anticancer drugs (5-Fluorouracyl, CisPlatin, and Doxorubicin) and berberine sulfate hydrate were purchased from Sigma-Aldrich Chemie GmbH, Schnelldorf, Germany. The cell lines used throughout the
in vitro experiments were obtained from international cell banks "American Type Culture Collection" – ATCC and "European Collection of Authenticated Cell Cultures" - ECACC), as follows: cell lines derived from colon tumors (LoVo and HT-29) [
33], breast adenocarcinoma (MDA-MB-231), ovarian adenocarcinoma (SK-OV-3), hepatocellular carcinoma (HEP G2), and OSCC cell line derived from tongue tumors (PE/CA-PJ49). HUVEC endothelial cells were used as normal cells.
3.1.2. B. vulgaris Extract Preparation
B. vulgaris (L.) cortex was purchased from a local ecological crop. It was identified by Prof. Octavian Tudorel Olaru, Department of Pharmaceutical Botany and Cell Biology, and Prof. Cerasela Elena Gîrd, Department of Pharmacognosy, Phytochemistry and Phytotherapy, Faculty of Pharmacy, University of Medicine and Pharmacy "Carol Davila," Bucharest. The voucher specimen is also preserved in the Discipline of Pharmacognosy, Phytochemistry, and Phytotherapy collection. Morphological peculiarities: the vegetable product is presented as flat or slightly recurved fragments; the inner face shows a bright yellow-green fluorescence in UV light (due to berberine). Organoleptic characteristics include a brown-gray color on the outside and a golden-yellow on the inside (due to berberine), which becomes brown through preservation (
Figure S3 from Supplementary Material), bitter taste, and no smell. As previously described, 50 g of powdered stem bark was subjected to reflux extraction with 50% ethanol (Sigma-Aldrich, Darmstadt, Germany)[
34]. After filtration, the obtained extract (BVE) was concentrated in a rotary evaporator (Buchi, Vacuum Pump V-700) and lyophilized (Christ Alpha 1-2/B Braun, BiotechInt).
3.2. Total Polyphenols Content (TPC)
The Folin-Ciocalteu reagent was used following a spectrophotometric method described extensively in a previously published article [
35]. The absorbances were measured at 725 nm (Jasco V-530 spectrophotometer, Japan), and tannic acid was the standard for the calibration curve in a linear concentration range of 2–9 µg/mL. The TPC is expressed as mg Eq Tannic acid / 100 g BVE.
3.3. Total Phenolic Acids (TPA)
The quantification method is based on the phenolic acids that form nitro derivatives with nitrous acids. It was detailed in our previously published article [
34]. The absorbance was immediately measured at 525 nm (Jasco spectrophotometer, Japan) and compared to a sample that lacked the Arnow reagent. Chlorogenic acid (Sigma-Aldrich, Germany) was used as a standard for the calibration curve in the linear range of 11–53 μg/mL with R2 = 0.9998. The total phenolic acids content (TPA) was expressed as mg chlorogenic acid equivalents per gram of extract (mg Eq Chlorogenic acid /g BVE).
3.4. Identification and Quantification of Phenolic Constituents and Berberine
3.4.1. Ultra-High-Performance Liquid Chromatography Coupled with High-Resolution Mass Spectrometry (UHPLC–HRMS/MS)
The phenolic profile of BVE was established based on non-targeted tandem mass spectrometry (MS-MS) using the hyphenated technique represented by Ultra-High Performance Liquid Chromatography (UHPLC) coupled with the Q-Exactive High-Resolution Mass Spectrometer (HRMS). The same method was used to quantify selected phenolic compounds for each available analytical standard (Sigma-Aldrich, Germany). All detailed data are described in our previously published study [
17].
3.4.2. High-Performance Liquid Chromatography
The separation was achieved on a reverse-phased analytical column (octadecylsilyl silica gel – C18, [25 x 0.4] mm i.d., 5 µm particle). The mobile phase consisted of a mixture of water and phosphoric acid = 0.1% (v/v) (solvent A) and 0.1% phosphoric acid in the acetonitrile (solvent B). The gradient used was: 90%A/10%B, 0 min.; 90 →78A/10→ 22/B, 0-15 min; 78 → 60A/28 → 40/B, 15-25 min.; 60 →30%A/40→70%B, 15-40 min; 30 →20%A/70 →80%B, 40-55 min. The flow rate was 1.0 mL /min, with an injection volume of 20 μL and a monitoring wavelength of 330 nm.
3.5. Antioxidant Activity
3.5.1. 2,2-Diphenyl-1-Picrylhydrazyl Free Radical Scavenging Assay (DPPH)
Under an antioxidant, the purple free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) forms its corresponding yellow hydrazine. The absorbance value is measured at λ=515 nm. The IC
50 value was determined from inhibition curves and their linear equations [
36].
3.5.2. 2,20-Azinobis-3-Ethylbenzotiazoline-6-Sulfonic Acid Assay (ABTS)
The turquoise-colored ABTS radical resulted from a potent oxidizing agent (potassium persulfate) reaction with the ammonium salt of 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid). Under the action of the antioxidant, the intensity of the color is reduced to colorless. The absorbance was determined by spectrophotometry at λ= 734 nm. The IC
50 value was calculated from inhibition curves and their linear equations [
34].
3.5.3. Ferric Reducing Antioxidant Power Assay (FRAP)
The antioxidant analyte reacts with Fe
3+ reducing to Fe
2+, imprinting blue. The coloration intensity is directly proportional to the antioxidant activity. The absorbance values were measured at λ=700 nm (spectrophotometer Jasco V-530), compared to the control (prepared under the same conditions without sample solution). It is expressed as an EC
50 value; it represents the sample concentration at which the absorbance has a value of 0.5 or half the concentration at which the antioxidant activity is maximum, determined by the trendline equation [
37].
3.6. 48-Hours Acute Toxicity Test Using Daphnia Magna and Daphnia Pulex
The daphnids belonging to species
Daphnia magna and
Daphnia pulex were chosen based on their size from parthenogenetic cultures maintained in an artificial medium for 24 hours before testing [
38]. The assay was performed in 24 wells-culture plates (Greiner Bio-One), each well containing around 10 organisms. The samples were tested in six concentrations ranging from 3.125 μg/mL to 100 μg/mL for BVE; as a positive control, BS was used from 0.625 to 20.0 μg/mL. The tests were duplicated, and lethality was assessed at 24 and 48 hours. The 50% lethal concentrations (LC
50) and the 95% confidence interval (CI95%) of LC
50 values were determined using the least square fit method (GraphPad Prism v 5.1 software) [
17].
3.7. Daphnia Magna Embryonic Development Assay
Based on the results obtained in the viability test, the following concentrations were chosen for testing: BVE at 3.125 µg/mL and BS at 2.5 µg/mL. Embryos were exposed to the tested solutions in the dark, maintaining a constant temperature and humidity of 25°C and 75% RH, respectively. The experiments were carried out on culture plates with 48 wells (Greiner Bio-One). Every 24 hours, the embryos were examined at a magnification of 80× under a microscope (bScope® microscope, Euromex Microscope BV, Arnhem, The Netherlands) to assess developmental stages and detect abnormalities, compared to untreated control [
17].
3.8. In Vitro Anticancer Activity
3.8.1. Cell Cultures and Treatments
The antiproliferative effect of BVE hydro-ethanolic extract and BS standard was evaluated in vitro on six tumor cell lines (SK-OV-3, LoVo, HEP-G2, HT-29, MDA-MB-231, PE/CA-PJ49) and normal HUVEC cells, used as control. Adherent cell lines were routinely maintained in culture in DMEM/F12 medium added with 2 mM L-glutamine and 10% fetal calf serum and antibiotics mixture (100 U/mL penicillin and 100 μg/mL streptomycin) and incubated at 37⁰ C in 5% CO
2 humidified atmosphere. For the cytotoxicity assays, cells were detached from culture flasks and then cultivated in 96-well flat bottom plates for 24 hours until they reached around 70% confluence. Then, cells were treated for various periods (24h and 48h) with different concentrations of BVE, BS, or oncolytic drugs (5-FU, CisPt, DOX) - used as positive controls [
39]. BVE and BS stock solutions were prepared by dissolving them in a minimal amount of DMSO and preserved at 4⁰ C; all working solutions were prepared from the stocks by serial dilutions with culture medium before each treatment assay [
17].
3.8.2. MTS-Assay
The cytotoxic potential of BVE and BS was evaluated by a colorimetric cell viability method, the MTS assay, and it was assessed on both tumor and normal cells and compared with the action of oncolytic drugs: DOX, CisPt, and 5-FU [
40].
All assays were performed in triplicate, using CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) kit (Promega, USA), which contains a reagent mixture of two components: MTS [3-(4,5 dimethylthiazol 2 yl) 5 (3 carboxymethoxy phenyl) 2 (4 sulfophenyl) 2H tetrazolium] and PES (phenazine ethosulphate), a cationic dye with high chemical stability which may be combined with MTS to form a stable solution [
41]. The method’s principle is based on the ability of metabolically active cells to reduce MTS (a yellow tetrazolium salt) to the colored formazan, which is soluble in the culture medium and can be spectrophotometrically quantified at 492 nm wavelength nm. Briefly, 1.5 x 10
4 cells/well were cultured in 100 µL of the medium; after 24h, culture supernatants were discarded, and the cells were treated with increasing concentrations of BVE, BS, or reference drug solutions for 24 h or 48 h. At the expiration of the contact time, 20 µL of reagent mixture was added to each well, and the culture dishes were incubated for an additional 4 h at 37 ◦C, with gentle shaking every 20 min. Absorbance was read at λ = 492 nm with the Dynex ELISA reader (DYNEX Technologies—MRS, USA) [
42].
Cell viability was expressed as a percentage, was reported to the untreated cells (considered 100% viable), and was calculated according to the following formula:
where T = optical density of treated cells, B = optical density of the blank (culture medium, in the absence of cells), and U = optical density of untreated cells.
The obtained results were expressed as mean values from three different experiments (n = 3) ± standard deviation (SD)[
43]. For the assessment of DMSO cytotoxicity, the same experimental determinations were performed in the MTS assay, and no impairment of cell viability was observed at concentrations lower than 1% (data not shown). Also, to observe the possible nonspecific reactions between BVE, BS, or drugs and MTS, their absorbance was determined without cells, and the values were extracted during calculations.
3.9. Data Analysis
The statistically significant differences (at
α < 0.05) between various experimental groups were established by Multiple pairwise comparisons using Dunn’s procedure from XLSTAT 2023.1.4. by Lumivero (Denver, CO, USA) [
44].
The correlations between the bioactive constituents of the extracts and their antioxidant activity and cytotoxicity were determined using Principal Component Analysis [
45] performed with XLSTAT 2023.1.4. by Lumivero (Denver, CO, USA) through Pearson correlation. The probability value
p < 0.05 indicates statistically significant differences [
46].
4. Conclusions
This research investigated the autochthonous Berberidis cortex, obtaining a dry extract in 50% ethanol through successive reflux extraction, then solvent evaporation and freeze-drying. Through complex UHPLC–HRMS/MS and HPLC-DAD analysis of BVE, 40 phenolic constituents, including berberine, were identified. The main classes of phenolic metabolites (polyphenols and flavonoids) and bioactive representatives were also quantified. The BVE’s significant antioxidant potential was revealed by in vitro evaluation of radical scavenging ability and reducing power. Then, the acute toxicity tests highlighted the BVE significant acute toxicity and teratogenicity on Daphnia sp. It also displays moderate antiproliferative activity on various tumor cell lines and does not affect normal cells. Compared to BVE, Berberine showed higher toxicity. It is essential to show that berberine sulfate reduced their viability on several tumor cell lines more than standard anticancer drugs used as positive controls.
Strong and statistically significant correlations were recorded between exposure time, concentration, phenolic metabolites content, antioxidant activity, and cytotoxicity of B. vulgaris stem bark dry hydro-ethanolic extract.
Our results could enrich the scientific database regarding the composition and pharmacological properties of autochthonous Berberidis cortex. Further research could explore the acute toxicity and teratogenicity of BVE and Berberine using other animal models and investigate their anticancer activity mechanisms on various other tumor cell lines.
Supplementary Materials
The following supporting information can be downloaded at paper posted on Preprints.org, File S1 which includes Figures S1-S3 and Tables S1-S3.
Author Contributions
Conceptualization, I.M.I., V.P., and C.E.G.; methodology, C.L.C., L.P., E.A.L., E.I.I., L.I.B., C.M.H., O.T.O., G.M.N., R.B., and C.E.G.; software, V.P., C.L.C., L.P., E.A.L., E.I.I., L.I.B., C.M.H., and R.B.; validation, C.L.C., L.I.B., O.T.O., G.M.N., and R.B.; formal analysis, V.P., C.L.C., L.P., and R.B.; investigation, I.M.I., and E.I.I.; resources, I.M.I.; data curation, I.M.I., V.P., O.T.O., and G.M.N.; writing—original draft preparation, V.P., C.L.C., L.P., E.A.L., E.I.I., L.I.B., C.M.H., O.T.O., and G.M.N.; writing—review and editing, V.P., C.L.C., L.P., E.A.L., E.I.I., L.I.B., C.M.H., O.T.O., G.M.N., R.B. and C.E.G.; visualization, I.M.I., V.P., C.L.C., L.P., E.A.L., E.I.I., L.I.B., C.M.H., O.T.O., G.M.N., R.B. and C.E.G.; supervision, C.E.G.; project administration, C.E.G.; funding acquisition, I.M.I., V.P. and C.E.G. All authors have read and agreed to the published version of the manuscript.
Funding
Publication of this paper was supported by the University of Medicine and Pharmacy "Carol Davila" through the institutional program "Publish not Perish."
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Data Availability Statement
Data are available in the manuscript and Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mana, T.; Devi, O.B.; Singh, Y.D. Therapeutic Application of Berberine: A Consolidated Review. Curr Pharmacol Rep 2023, 9, 329–340. [Google Scholar] [CrossRef]
- Salehi, B.; Selamoglu, Z.; Sener, B.; Kilic, M.; Kumar Jugran, A.; de Tommasi, N.; Sinisgalli, C.; Milella, L.; Rajkovic, J.; Flaviana, B. Morais-Braga, M.; et al. Berberis Plants—Drifting from Farm to Food Applications, Phytotherapy, and Phytopharmacology. Foods 2019, 8, 522. [Google Scholar] [CrossRef] [PubMed]
- https://www.medicament.com/620-berberis-tube-granules-boiron-4ch-5ch-7ch-9ch-12ch-15ch-30ch.html., accessed on 23 March 2024.
- Han, Y.; Xiang, Y.; Shi, Y.; Tang, X.; Pan, L.; Gao, J.; Bi, R.; Lai, X. Pharmacokinetics and Pharmacological Activities of Berberine in Diabetes Mellitus Treatment. Evidence-Based Complementary and Alternative Medicine 2021, 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Grădinariu, L.; Dediu, L.; Crețu, M.; Grecu, I.R.; Docan, A.; Istrati, D.I.; Dima, F.M.; Stroe, M.D.; Vizireanu, C. The Antioxidant and Hepatoprotective Potential of Berberine and Silymarin on Acetaminophen Induced Toxicity in Cyprinus Carpio L. Animals 2024, 14, 373. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Alsahli, M.A.; Rahmani, A.H. Berberine: An Important Emphasis on Its Anticancer Effects through Modulation of Various Cell Signaling Pathways. Molecules 2022, 27, 5889. [Google Scholar] [CrossRef]
- El-Zahar, K.M.; Al-Jamaan, M.E.; Al-Mutairi, F.R.; Al-Hudiab, A.M.; Al-Einzi, M.S.; Mohamed, A.A.-Z. Antioxidant, Antibacterial, and Antifungal Activities of the Ethanolic Extract Obtained from Berberis Vulgaris Roots and Leaves. Molecules 2022, 27, 6114. [Google Scholar] [CrossRef] [PubMed]
- Och, A.; Olech, M.; Bąk, K.; Kanak, S.; Cwener, A.; Cieśla, M.; Nowak, R. Evaluation of the Antioxidant and Anti-Lipoxygenase Activity of Berberis Vulgaris L. Leaves, Fruits, and Stem and Their LC-MS/MS Polyphenolic Profile. Antioxidants 2023, 12, 1467. [Google Scholar] [CrossRef]
- Boeri, P.; Piñuel, L.; Dalzotto, D.; Monasterio, R.; Fontana, A.; Sharry, S.; Barrio, D.A.; Carrillo, W. Argentine Patagonia Barberry Chemical Composition and Evaluation of Its Antioxidant Capacity. J Food Biochem 2020, 44, 13254. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lv, X.-M.; Tang, C.; Lai, X.-R.; Zhang, Y.; Fan, G. Quality Evaluation of Cortex Berberidis from Different Geographical Origins by Simultaneous High-Performance Liquid Chromatography Combined with Statistical Methods. Tropical Journal of Pharmaceutical Research 2016, 15, 1973. [Google Scholar] [CrossRef]
- Fernández-Poyatos; Ruiz-Medina; Zengin; Llorent-Martínez Phenolic Characterization, Antioxidant Activity, and Enzyme Inhibitory Properties of Berberis Thunbergii DC. Leaves: A Valuable Source of Phenolic Acids. Molecules 2019, 24, 4171. [Google Scholar] [CrossRef]
- Veselá, Š.; Ondruška, V.; Kuča, K.; Patočka, J. Freshwater Microcrustacean Daphnia Magna Straus as an Early Screen Model to Compare Toxicity of Acetylcholinesterase Inhibitors. J. Appl. Biomed 2006, 4, 105–110. [Google Scholar] [CrossRef]
- Paudel, B.; Bhattarai, H.D.; Kim, I.C.; Lee, H.; Sofronov, R.; Ivanova, L.; Poryadina, L.; Yim, J.H. Estimation of Antioxidant, Antimicrobial Activity and Brine Shrimp Toxicity of Plants Collected from Oymyakon Region of the Republic of Sakha (Yakutia), Russia. Biol Res 2014, 47, 10. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.; Pucci, C.; Gabellini, C.; Pellegrino, M.; Andreazzoli, M. Exposure to the Natural Alkaloid Berberine Affects Cardiovascular System Morphogenesis and Functionality during Zebrafish Development. Sci Rep 2020, 10, 17358. [Google Scholar] [CrossRef]
- The World Health Organisation WHO Monographs on Selected Medicinal Plants Volume 4.
- Hidayat, D.; Dwira, S. Phytochemical Analysis and in Vitro Cytotoxicity Test of Black Soybean ( Glycine Soja L.) Ethanolic Extract as a Growth Inhibitor of the HCT-116 Colon Carcinoma Cell Line. J Phys Conf Ser 2018, 1073, 032041. [Google Scholar] [CrossRef]
- Ivan, I.M.; Popovici, V.; Chițescu, C.L.; Popescu, L.; Luță, E.A.; Ilie, E.I.; Brașoveanu, L.I.; Hotnog, C.M.; Olaru, O.T.; Nițulescu, G.M.; et al. Phytochemical Profile, Antioxidant and Cytotoxic Potential of Capsicum Annuum (L.) Dry Hydro-Ethanolic Extract. Pharmaceutics 2024, 16, 245. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Zhang, W.; Wu, G.; Ren, J.; Lu, H.; Li, Z.; Han, X. Synergistic Anticancer Effects of Galangin and Berberine through Apoptosis Induction and Proliferation Inhibition in Oesophageal Carcinoma Cells. Biomedicine & Pharmacotherapy 2016, 84, 1748–1759. [Google Scholar] [CrossRef]
- YANG, X.; HUANG, N. Berberine Induces Selective Apoptosis through the AMPK-Mediated Mitochondrial/Caspase Pathway in Hepatocellular Carcinoma. Mol Med Rep 2013, 8, 505–510. [Google Scholar] [CrossRef]
- Letašiová, S.; Jantová, S.; Miko, M.; Ovádeková, R.; Horváthová, M. Effect of Berberine on Proliferation, Biosynthesis of Macromolecules, Cell Cycle and Induction of Intercalation with DNA, DsDNA Damage and Apoptosis in Ehrlich Ascites Carcinoma Cells. Journal of Pharmacy and Pharmacology 2010, 58, 263–270. [Google Scholar] [CrossRef]
- Anis, K.V.; Kuttan, G.; Kuttan, R. Role of Berberine as an Adjuvant Response Modifier During Tumour Therapy in Mice. Pharmacy and Pharmacology Communications 1999, 5, 697–700. [Google Scholar] [CrossRef]
- REFAAT, A.; ABDELHAMED, S.; YAGITA, H.; INOUE, H.; YOKOYAMA, S.; HAYAKAWA, Y.; SAIKI, I. Berberine Enhances Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand-Mediated Apoptosis in Breast Cancer. Oncol Lett 2013, 6, 840–844. [Google Scholar] [CrossRef]
- Chu, S.-C.; Yu, C.-C.; Hsu, L.-S.; Chen, K.-S.; Su, M.-Y.; Chen, P.-N. Berberine Reverses Epithelial-to-Mesenchymal Transition and Inhibits Metastasis and Tumor-Induced Angiogenesis in Human Cervical Cancer Cells. Mol Pharmacol 2014, 86, 609–623. [Google Scholar] [CrossRef]
- Hong Berberine Inhibits P53-Dependent Cell Growth through Induction of Apoptosis of Prostate Cancer Cells. Int J Oncol 2009, 34, 250. [CrossRef]
- Chung Involvement of Reactive Oxygen Species and Caspase-Dependent Pathway in Berberine-Induced Cell Cycle Arrest and Apoptosis in C6 Rat Glioma Cells. Int J Oncol 2009, 34, 299. [CrossRef]
- Zhao, Y.; Roy, S.; Wang, C.; Goel, A. A Combined Treatment with Berberine and Andrographis Exhibits Enhanced Anticancer Activity through Suppression of DNA Replication in Colorectal Cancer. Pharmaceuticals 2022, 15, 262. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Y.; Gao, X.; Guo, F. Mitochondrial Protein Cyclophilin-D-Mediated Programmed Necrosis Attributes to Berberine-Induced Cytotoxicity in Cultured Prostate Cancer Cells. Biochem Biophys Res Commun 2014, 450, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Kuo, W.-H.; Tseng, H.-C.; Chou, F.-P. Synergistic Tumor-Killing Effect of Radiation and Berberine Combined Treatment in Lung Cancer: The Contribution of Autophagic Cell Death. International Journal of Radiation Oncology*Biology*Physics 2008, 70, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, N.; Miyamoto, K.; Hazama, S.; Yoshino, S.; Yoshimura, K.; Okita, K.; Fukumoto, T.; Yamamoto, S.; Tangoku, A.; Oka, M. Anticachectic Effects of Coptidis Rhizoma, an Anti-Inflammatory Herb, on Esophageal Cancer Cells That Produce Interleukin 6. Cancer Lett 2000, 158, 35–41. [Google Scholar] [CrossRef]
- Kuo, C.L.; Chi, C.W.; Liu, T.Y. The Anti-Inflammatory Potential of Berberine in Vitro and in Vivo. Cancer Lett 2004, 203. [Google Scholar] [CrossRef]
- Pereira, G.C.; Branco, A.F.; Matos, J.A.C.; Pereira, S.L.; Parke, D.; Perkins, E.L.; Serafim, T.L.; Sardão, V.A.; Santos, M.S.; Moreno, A.J.M.; et al. Mitochondrially Targeted Effects of Berberine [Natural Yellow 18, 5,6-Dihydro-9,10-Dimethoxybenzo( g )-1,3-Benzodioxolo(5,6- a ) Quinolizinium] on K1735-M2 Mouse Melanoma Cells: Comparison with Direct Effects on Isolated Mitochondrial Fractions. Journal of Pharmacology and Experimental Therapeutics 2007, 323, 636–649. [Google Scholar] [CrossRef]
- Abd El-Wahab, A.E.; Ghareeb, D.A.; Sarhan, E.E.; Abu-Serie, M.M.; El Demellawy, M.A. In Vitro Biological Assessment of Berberis Vulgaris and Its Active Constituent, Berberine: Antioxidants, Anti-Acetylcholinesterase, Anti-Diabetic and Anticancer Effects. BMC Complement Altern Med 2013, 13, 218. [Google Scholar] [CrossRef]
- Mihaila, M.; Hotnog, C.M.; Bostan, M.; Munteanu, A.C.; Vacaroiu, I.A.; Brasoveanu, L.I.; Uivarosi, V. Anticancer Activity of Some Ruthenium(III) Complexes with Quinolone Antibiotics: In Vitro Cytotoxicity, Cell Cycle Modulation, and Apoptosis-Inducing Properties in LoVo Colon Cancer Cell Line. Applied Sciences 2021, 11, 8594. [Google Scholar] [CrossRef]
- Costea, L.; Chitescu, C.L.; Boscencu, R.; Ghica, M.; Lupuliasa, D.; Mihai, D.P.; Deculescu-Ionita, T.; Dutu, L.E.; Popescu, M.L.; Luta, E.-A.; et al. The Polyphenolic Profile and Antioxidant Activity of Five Vegetal Extracts with Hepatoprotective Potential. Plants 2022, 11, 1680. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, A.R.; Chițescu, C.L.; Luță, E.A.; Moroșan, A.; Mihaiescu, D.E.; Mihai, D.P.; Costea, L.; Ozon, E.A.; Fița, A.C.; Balaci, T.D.; et al. Outlook on Chronic Venous Disease Treatment: Phytochemical Screening, In Vitro Antioxidant Activity and In Silico Studies for Three Vegetal Extracts. Molecules 2023, 28, 3668. [Google Scholar] [CrossRef] [PubMed]
- Luță, E.-A.; Biță, A.; Moroșan, A.; Mihaiescu, D.E.; Mihai, D.P.; Popescu, L.; Bejenaru, L.E.; Bejenaru, C.; Popovici, V.; Olaru, O.T.; and; et al. Implications of the Cultivation of Rosemary and Thyme ( Lamiaceae ) in Plant Communities for the Development of Antioxidant Therapies. Int. J. Mol. Sci. 2023, 24, 11670. [Google Scholar] [CrossRef] [PubMed]
- Luță, E.A.; Biță, A.; Moroșan, A.; Mihaiescu, D.E.; Ghica, M.; Mihai, D.P.; Olaru, O.T.; Deculescu-Ioniță, T.; Duțu, L.E.; Popescu, M.L.; et al. The Influence of Phytosociological Cultivation and Fertilization on Polyphenolic Content of Menthae and Melissae Folium and Evaluation of Antioxidant Properties through In Vitro and In Silico Methods. Plants 2022, 11, 2398. [Google Scholar] [CrossRef] [PubMed]
- Barbuceanu, S.-F.; Rosca, E.-V.; Apostol, T.-V.; Socea, L.-I.; Draghici, C.; Farcasanu, I.C.; Ruta, L.L.; Nitulescu, G.M.; Iscrulescu, L.; Pahontu, E.-M.; et al. New Heterocyclic Compounds from Oxazol-5(4H)-One and 1,2,4-Triazin-6(5H)-One Classes: Synthesis, Characterization and Toxicity Evaluation. Molecules 2023, 28, 4834. [Google Scholar] [CrossRef] [PubMed]
- Ivan, B.-C.; Barbuceanu, S.-F.; Hotnog, C.M.; Olaru, O.T.; Anghel, A.I.; Ancuceanu, R.V.; Mihaila, M.A.; Brasoveanu, L.I.; Shova, S.; Draghici, C.; et al. Synthesis, Characterization and Cytotoxic Evaluation of New Pyrrolo[1,2-b]Pyridazines Obtained via Mesoionic Oxazolo-Pyridazinones. Int J Mol Sci 2023, 24, 11642. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, A.; Badea, M.; Olar, R.; Silvestro, L.; Mihaila, M.; Brasoveanu, L.I.; Musat, M.G.; Andries, A.; Uivarosi, V. Cytotoxicity Studies, DNA Interaction and Protein Binding of New Al (III), Ga (III) and In (III) Complexes with 5-hydroxyflavone. Appl Organomet Chem 2018, 32, e4579. [Google Scholar] [CrossRef]
- Munteanu, A.; Musat, M.G.; Mihaila, M.; Badea, M.; Olar, R.; Nitulescu, G.M.; Rădulescu, F. Ștefan; Brasoveanu, L.I.; Uivarosi, V. New Heteroleptic Lanthanide Complexes as Multimodal Drugs: Cytotoxicity Studies, Apoptosis, Cell Cycle Analysis, DNA Interactions, and Protein Binding. Appl Organomet Chem 2021, 35. [Google Scholar] [CrossRef]
- Ivan, B.-C.; Barbuceanu, S.-F.; Hotnog, C.M.; Anghel, A.I.; Ancuceanu, R.V.; Mihaila, M.A.; Brasoveanu, L.I.; Shova, S.; Draghici, C.; Olaru, O.T.; et al. New Pyrrole Derivatives as Promising Biological Agents: Design, Synthesis, Characterization, In Silico, and Cytotoxicity Evaluation. Int J Mol Sci 2022, 23, 8854. [Google Scholar] [CrossRef]
- Maciuca, A.-M.; Munteanu, A.-C.; Mihaila, M.; Badea, M.; Olar, R.; Nitulescu, G.M.; Munteanu, C.V.A.; Bostan, M.; Uivarosi, V. Rare-Earth Metal Complexes of the Antibacterial Drug Oxolinic Acid: Synthesis, Characterization, DNA/Protein Binding and Cytotoxicity Studies. Molecules 2020, 25, 5418. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, A.R.; Popovici, V.; Oprean, C.; Danciu, C.; Schröder, V.; Olaru, O.T.; Mihai, D.P.; Popescu, L.; Luță, E.-A.; Chițescu, C.L.; et al. Cytotoxicity Analysis and In Silico Studies of Three Plant Extracts with Potential Application in Treatment of Endothelial Dysfunction. Pharmaceutics 2023, 15, 2125. [Google Scholar] [CrossRef] [PubMed]
- Neagu, R.; Popovici, V.; Ionescu, L.E.; Ordeanu, V.; Popescu, D.M.; Ozon, E.A.; Gîrd, C.E. Antibacterial and Antibiofilm Effects of Different Samples of Five Commercially Available Essential Oils. 2023, 12, 1191.
- Popovici, V.; Matei, E.; Cozaru, G.C.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Karampelas, O.; Musuc, A.M.; Atkinson, I.; et al. Evaluation of Usnea Barbata ( L.) Weber Ex F. H. Wigg Extract in Canola Oil Loaded in Bioadhesive Oral Films for Potential Applications in Oral Cavity Infections and Malignancy. Antioxidants 2022, 11, 1601. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
A. UHPLC–HRMS/MS chromatogram of phytochemicals identified in BVE; from top to bottom: quercetin 3-O-glucuronide (m/z=477.06749, RT=12.16), narirutin (naringenin-7-O-rutinoside) (m/z=579.17195, RT=12.13), hidroxyferulic acid (m/z=371.09839, RT=4.81), piceatannol (m/z=243.06631, Rt=13.08), lignan (m/z=457.18682, RT=27.33), lehmannin (m/z=407.18642, RT=26.40), taxifolin 3-O-rhamnoside (m/z=449.10896, RT=12.24). B. HPLC-DAD chromatogram of BVE; Berberine has RT=32.513.
Figure 1.
A. UHPLC–HRMS/MS chromatogram of phytochemicals identified in BVE; from top to bottom: quercetin 3-O-glucuronide (m/z=477.06749, RT=12.16), narirutin (naringenin-7-O-rutinoside) (m/z=579.17195, RT=12.13), hidroxyferulic acid (m/z=371.09839, RT=4.81), piceatannol (m/z=243.06631, Rt=13.08), lignan (m/z=457.18682, RT=27.33), lehmannin (m/z=407.18642, RT=26.40), taxifolin 3-O-rhamnoside (m/z=449.10896, RT=12.24). B. HPLC-DAD chromatogram of BVE; Berberine has RT=32.513.
Figure 2.
The results of the 48-hour Acute Toxicity Test using Daphnia sp. Lethality curves obtained after 48 h exposure of Daphnia sp to BS (a) and BVE (b): D. magna (A) and D. pulex (B). BS – Berberine sulfate hydrate; BVE — B. vulgaris stem bark dry hydro-ethanolic extract; NA— the values could not be calculated: * The interval is vast; ** The values could not be calculated as the maximum L% was 10%.
Figure 2.
The results of the 48-hour Acute Toxicity Test using Daphnia sp. Lethality curves obtained after 48 h exposure of Daphnia sp to BS (a) and BVE (b): D. magna (A) and D. pulex (B). BS – Berberine sulfate hydrate; BVE — B. vulgaris stem bark dry hydro-ethanolic extract; NA— the values could not be calculated: * The interval is vast; ** The values could not be calculated as the maximum L% was 10%.
Figure 3.
Daphnia magna embryonic development assay: (a) embryos before testing; (b) embryos development after 24 h in BS 2.5 μg/mL; (c) embryos development after 48 h in BS 2.5 μg/mL; (d) embryos development after 48 h in BVE 3.125 μg/mL.
Figure 3.
Daphnia magna embryonic development assay: (a) embryos before testing; (b) embryos development after 24 h in BS 2.5 μg/mL; (c) embryos development after 48 h in BS 2.5 μg/mL; (d) embryos development after 48 h in BVE 3.125 μg/mL.
Figure 4.
The effects of BVE and BS on normal endothelial cell (HUVEC) viability (%) compared to oncolytic drugs after 24 and 48 hours. Series 1 — 5 = concentration range: 12.5 - 200 µg/mL for BVE and BS, 12.5 – 200 µM for 5-FU and CisPt, and 1.25 – 20 µM for DOX. BVE — dry hydro-ethanolic extract of Berberis vulgaris stem bark; BS — berberine sulfate hydrate; CisPt — Cisplatin; DOX — Doxorubicin, 5-FU — 5-Fluorouracil; 24 and 48 — treatment time (24 and 48 hours).
Figure 4.
The effects of BVE and BS on normal endothelial cell (HUVEC) viability (%) compared to oncolytic drugs after 24 and 48 hours. Series 1 — 5 = concentration range: 12.5 - 200 µg/mL for BVE and BS, 12.5 – 200 µM for 5-FU and CisPt, and 1.25 – 20 µM for DOX. BVE — dry hydro-ethanolic extract of Berberis vulgaris stem bark; BS — berberine sulfate hydrate; CisPt — Cisplatin; DOX — Doxorubicin, 5-FU — 5-Fluorouracil; 24 and 48 — treatment time (24 and 48 hours).
Figure 5.
Box and Whisker plots displaying tumor cell viability % (F1 axis) after 24 h (A,C,E,G,I,K) and 48 h (B,D,F,J,L) treatments with BVE, BS, and standard anticancer drugs: A,B: HEP G2; C,D: HT-29; E,F: LoVo; G,H: MDA-MB-231; I,J: SK-OV-3; K,L: PE/CA-PJ49. HEP G2—human hepatocellular carcinoma; HT-29 and LoVo—human colon adenocarcinomas; MDA-MB-231—human breast adenocarcinoma; PE/CA-PJ49—human squamous tongue carcinoma; SK-OV-3—human ovary adenocarcinoma; BVE — dry hydro-ethanolic extract of Berberis vulgaris stem bark; BS — berberine sulfate hydrate; CisPt — Cisplatin; DOX — Doxorubicin, 5-FU — 5-Fluorouracil; 24 and 48 — treatment time (24 and 48 hours).
Figure 5.
Box and Whisker plots displaying tumor cell viability % (F1 axis) after 24 h (A,C,E,G,I,K) and 48 h (B,D,F,J,L) treatments with BVE, BS, and standard anticancer drugs: A,B: HEP G2; C,D: HT-29; E,F: LoVo; G,H: MDA-MB-231; I,J: SK-OV-3; K,L: PE/CA-PJ49. HEP G2—human hepatocellular carcinoma; HT-29 and LoVo—human colon adenocarcinomas; MDA-MB-231—human breast adenocarcinoma; PE/CA-PJ49—human squamous tongue carcinoma; SK-OV-3—human ovary adenocarcinoma; BVE — dry hydro-ethanolic extract of Berberis vulgaris stem bark; BS — berberine sulfate hydrate; CisPt — Cisplatin; DOX — Doxorubicin, 5-FU — 5-Fluorouracil; 24 and 48 — treatment time (24 and 48 hours).
Figure 6.
A. Symmetric biplot displays the correlation between the cytotoxic effects of BVE, BS, and anticancer drugs on normal and tumor cells after 24 and 48 ore of treatment. B. AHC-Dendrogram HEP G2—human hepatocellular carcinoma; HT-29 and LoVo—human colon adenocarcinomas; MDA-MB-231—human breast adenocarcinoma; PE/CA-PJ49—human squamous tongue carcinoma; SK-OV-3—human ovary adenocarcinoma; BVE — B. vulgaris dry extract; BS —berberine sulfate hydrate; CisPt — Cisplatin; DOX — Doxorubicin, 5-FU — 5-Fluorouracil; 24 and 48 — treatment time (24 and 48 hours).
Figure 6.
A. Symmetric biplot displays the correlation between the cytotoxic effects of BVE, BS, and anticancer drugs on normal and tumor cells after 24 and 48 ore of treatment. B. AHC-Dendrogram HEP G2—human hepatocellular carcinoma; HT-29 and LoVo—human colon adenocarcinomas; MDA-MB-231—human breast adenocarcinoma; PE/CA-PJ49—human squamous tongue carcinoma; SK-OV-3—human ovary adenocarcinoma; BVE — B. vulgaris dry extract; BS —berberine sulfate hydrate; CisPt — Cisplatin; DOX — Doxorubicin, 5-FU — 5-Fluorouracil; 24 and 48 — treatment time (24 and 48 hours).
Table 1.
Total polyphenols content, total phenolic acids, and antioxidant activity of BVE.
Table 1.
Total polyphenols content, total phenolic acids, and antioxidant activity of BVE.
| Phenolic Compounds |
|---|
Total Polyphenols
(mg Eq Tannic acid / 100 g extract) |
Total Phenolic Acids
(mg Eq Chlorogenic acid / 100 g extract) |
| 17.6780 ± 3.9320 |
3.3886 ± 0.3481 |
| Antioxidant Activity |
| IC50DPPH (mg/mL) |
IC50ABTS (mg/mL) |
EC50FRAP (mg/mL) |
| 0.2610 |
0.0442 |
0.1398 |
Table 2.
The phytochemicals identified in B. vulgaris stem bark dry extract (BVE) by UHPLC HRMS/MS and HPLC-DAD.
Table 2.
The phytochemicals identified in B. vulgaris stem bark dry extract (BVE) by UHPLC HRMS/MS and HPLC-DAD.
| Nr. crt. |
IdentifiedCompound |
PhytochemicalClassification |
ChemicalFormula |
Adduct Ion/
Monitored
Negative Ion (m/z) |
Retention
Time
(min) |
Content
(µg/g)
|
| 1 |
Quercetin |
Flavonoid |
C15H10O7
|
301.0354 |
15.01 |
28.42 |
| 2 |
Rutin (quercetin 3-rutinoside) |
Flavonoid |
C27H30O16
|
609.14613 |
12.39 |
72.41 |
| 3 |
Apigenin |
Flavonoid |
C15H10O5
|
269.04502 |
16.71 |
10.45 |
| 4 |
Kaempferol |
Flavanol |
C15H10O6
|
285.04049 |
16.51 |
68.74 |
| 5 |
6-Methoxyluteolin (Nepetin) |
Flavonoid |
C16H12O7
|
315.05105 |
16.75 |
- |
| 6 |
Naringenin |
Flavanone |
C15H12O5
|
271.06122 |
15.46 |
90.41 |
| 7 |
Hesperitin |
Flavonoid |
C16H14O6
|
301.07179 |
13.71 |
44.00 |
| 8 |
Galangin |
Flavonoid |
C15H10O5
|
269.04557 |
16.71 |
67.21 |
| 9 |
Genistein |
Isoflavone |
C15H10O5
|
269.04502 |
16.73 |
- |
| 10 |
Glycitein |
Isoflavone |
C16H12O5
|
283.06122 |
11.15 |
19.21 |
| 11 |
Gallic acid |
Hydroxybenzoic acid |
C7H6O5
|
169.01427 |
1.70 |
540.00 |
| 12 |
Chlorogenic acid/Neoclorogenic |
Cinnamate ester |
C16H18O9
|
353.08783 |
6.08 |
10.54 |
| 13 |
Ferulic acid |
Hydroxycinnamic acid |
C10H10O4
|
193.05066 |
9.94 |
39.36 |
| 14 |
AbsCisPtic acid |
Terpenoid |
C15H20O4
|
263.12891 |
14.76 |
8.61 |
| 15 |
p-Coumaric acid |
Hydroxycinnamic acid |
C9H8O3
|
163.03954 |
8.80 |
30.33 |
| 16 |
Syringic acid |
Hydroxybenzoic acid |
C9H10O5
|
197.04555 |
8.73 |
3.35 |
| 17 |
Afrormosin |
Isoflavone |
C17H14O5
|
297.07687 |
17.17 |
- |
| 18 |
Kaempferol-3-O-rutinoside |
Flavonol glycoside |
C27H30O15
|
593.15122 |
9.35 |
- |
| 19 |
Kaempferol (luteolin)-O-glucoside/ isomers |
Flavonoid |
C21H20O11
|
447.09331 |
13.56 |
- |
| 20 |
Vitexin (apigenin 8-C-glucoside)/isovitexin |
Flavonol glycoside |
C21H20O10
|
431.09839 |
11.98 |
- |
| 21 |
Azelaic acid |
Dicarboxylic acid |
C9H16O4
|
187.09761 |
13.99 |
- |
| 22 |
Apigenin 7-O-glucosylglucoside |
Flavonoid |
C27H30O15
|
593.15122 |
9.45 |
- |
| 23 |
Rosmarinic acid |
Ester of caffeic acid |
C18H16O8
|
359.07727 |
13.42 |
- |
| 24 |
Carnasol |
Diterpene |
C20H26O4
|
329.17586 |
18.83 |
- |
| 25 |
Rosmadial/Isomeri |
Diterpene lactone |
C20H24O5
|
343.15510 |
20.38 |
- |
| 26 |
Rosmanol methyl ether |
Diterpene |
C21H28O5
|
359.18640 |
22.19 |
- |
| 27 |
Quercetin 3-O-glucuronide |
Flavonol glucuronide |
C21H18O13
|
477.06749 |
12.16 |
23.04 |
| 28 |
Narirutin (naringenin-7-O-rutinoside) |
Flavonol glycoside |
C27H32O14
|
579.17195 |
12.13 |
- |
| 29 |
Apigenin-7-O-glucuronide |
Flavonoid-7-o-glucuronides |
C21H18O11
|
445.07763 |
13.29 |
- |
| 30 |
Procyanidine B1/B2 |
Flavonoid |
C30H26O12
|
577.13515 |
16.24 |
- |
| 31 |
Sinapic acid |
Hydroxycinnamic acid |
C11H12O5
|
223.06122 |
10.33 |
- |
| 32 |
Hidroxyferulic acid/Isomers |
Hydroxycinnamic acid |
C16H20O10
|
371.09839 |
4.81 |
- |
| 33 |
Valerenic acid |
Sesquiterpenoid |
C15H22O2
|
233.15473 |
21.33 |
- |
| 34 |
Lehmannin |
Flavanone |
C25H28O5
|
407.18642 |
26.40 |
- |
| 35 |
Ginkgetin |
Flavone |
C32H22O10
|
565.11404 |
7.25 |
- |
| 36 |
Taxifolin 3-O-rhamnoside |
Flavonoid |
C21H22O11
|
449.10896 |
12.24 |
- |
| 37 |
Piceatannol |
Stilbenoid |
C14H12O4
|
243.06631 |
13.08 |
- |
| 38 |
Lignan |
Polyphenolic compound |
C25H30O8
|
457.18682 |
27.33 |
- |
| 39 |
Cyanidin 3-arabinoside |
Anthocyanidin-3-o-glycoside |
C20H19ClO10
|
453.05942 |
7.17 |
- |
| 40 |
Berberine |
Isoquinoline alkaloid |
C20H18NO4
|
- |
32.51 |
78.95 |
Table 3.
The cytotoxicity of BVE and BS on normal cell and tumor cell lines (expressed as cell viability %) after 24 and 48 hours of exposure.
Table 3.
The cytotoxicity of BVE and BS on normal cell and tumor cell lines (expressed as cell viability %) after 24 and 48 hours of exposure.
Concentra-
tion
(µg/mL) |
24 h |
48 h |
| BVE |
BS |
BVE |
BS |
V
(%) |
SD
|
IC50
(µg/mL) |
V
(%) |
SD
|
IC50
(µg/mL) |
V
(%) |
SD
|
IC50
(µg/mL) |
V
(%) |
SD
|
IC50
(µg/mL) |
| HUVEC |
| 6.25 |
109.38 |
5.13 |
|
100.60 |
4.15 |
|
104.59 |
5.04 |
|
107.52 |
5.67 |
|
| 12.5 |
107.10 |
5.38 |
97.10 |
5.91 |
100.54 |
4.35 |
103.36 |
6.91 |
| 25 |
104.60 |
1.06 |
96.28 |
4.58 |
103.16 |
3.56 |
105.17 |
3.38 |
| 50 |
101.16 |
0.09 |
>>400 |
99.53 |
4.33 |
>>400 |
98.71 |
5.03 |
>>400 |
96.18 |
2.98 |
>400 |
| 100 |
102.78 |
3.54 |
|
95.35 |
2.21 |
|
96.38 |
3.59 |
|
82.99 |
6.04 |
|
| 200 |
99.10 |
7.94 |
96.72 |
1.86 |
89.95 |
4.76 |
77.15 |
4.99 |
| 400 |
86.53 |
4.69 |
84.60 |
2.30 |
75.48 |
0.09 |
56.10 |
0.09 |
| HEP G2 |
| 6.25 |
99.57 |
6.47 |
|
97.46 |
6.76 |
|
98.31 |
0.33 |
|
96.91 |
5.81 |
|
| 12.5 |
97.94 |
4.25 |
91.71 |
8.04 |
97.60 |
2.06 |
84.48 |
0.54 |
| 25 |
96.43 |
0.12 |
90.43 |
4.08 |
96.03 |
6.67 |
65.45 |
2.39 |
| 50 |
94.49 |
1.98 |
>400 |
79.68 |
5.30 |
>200 |
91.33 |
1.90 |
>100 |
50.48 |
2.28 |
>50 |
| 100 |
91.83 |
2.97 |
|
71.19 |
7.51 |
|
81.87 |
3.20 |
|
38.32 |
0.22 |
|
| 200 |
73.62 |
0.52 |
53.77 |
5.48 |
46.76 |
1.95 |
25.69 |
2.44 |
| 400 |
52.86 |
0.93 |
42.16 |
2.80 |
22.62 |
3.36 |
21.82 |
6.02 |
| HT-29 |
| 6.25 |
100.78 |
2.80 |
|
100.08 |
1.27 |
|
99.90 |
4.08 |
|
98.23 |
5.95 |
|
| 12.5 |
98.86 |
7.60 |
98.63 |
2.90 |
97.39 |
0.00 |
95.90 |
2.72 |
|
| 25 |
98.62 |
4.80 |
94.72 |
3.54 |
94.81 |
1.53 |
92.29 |
1.93 |
|
| 50 |
97.18 |
3.59 |
>>400 |
83.49 |
4.75 |
>400 |
92.31 |
3.34 |
>>400 |
86.48 |
5.16 |
>400 |
| 100 |
95.08 |
1.16 |
|
70.77 |
0.60 |
|
90.62 |
1.25 |
|
72.18 |
2.44 |
|
| 200 |
91.06 |
0.69 |
66.59 |
4.75 |
86.62 |
0.28 |
61.43 |
2.21 |
|
| 400 |
85.04 |
3.69 |
60.80 |
6.76 |
77.31 |
5.45 |
54.02 |
6.56 |
|
| LoVo |
| 6.25 |
98.74 |
4.77 |
|
97.08 |
5.37 |
|
92.46 |
3.67 |
|
95.64 |
4.80 |
|
| 12.5 |
93.91 |
0.74 |
91.98 |
3.52 |
89.06 |
6.32 |
91.86 |
2.73 |
| 25 |
90.85 |
7.54 |
88.50 |
4.96 |
84.68 |
8.47 |
82.13 |
6.31 |
| 50 |
85.13 |
4.94 |
>200 |
79.91 |
0.87 |
>50 |
72.68 |
4.55 |
>50 |
65.02 |
5.69 |
>50 |
| 100 |
75.97 |
2.35 |
|
38.60 |
1.85 |
|
48.39 |
0.63 |
|
28.19 |
1.39 |
|
| 200 |
53.72 |
4.45 |
26.22 |
2.10 |
14.72 |
0.00 |
15.77 |
1.14 |
| 400 |
25.05 |
4.57 |
15.57 |
8.53 |
3.09 |
2.66 |
8.08 |
2.91 |
|
| MDA-MB-231 |
| 6.25 |
96.83 |
4.41 |
|
87.73 |
6.43 |
|
75.86 |
4.48 |
|
71.40 |
3.52 |
|
| 12.5 |
90.33 |
2.57 |
76.14 |
0.55 |
73.74 |
3.67 |
62.02 |
0.95 |
| 25 |
87.71 |
0.37 |
66.43 |
5.51 |
70.81 |
0.15 |
45.93 |
2.57 |
| 50 |
82.02 |
2.20 |
>100 |
49.76 |
1.10 |
>25 |
60.88 |
1.84 |
>50 |
37.83 |
6.68 |
>12.5 |
| 100 |
69.16 |
2.94 |
|
23.19 |
2.02 |
|
45.27 |
3.97 |
|
20.29 |
2.13 |
|
| 200 |
53.71 |
3.67 |
20.67 |
0.37 |
25.65 |
1.47 |
14.39 |
2.06 |
| 400 |
17.21 |
3.49 |
10.43 |
4.04 |
2.99 |
0.44 |
8.54 |
0.81 |
| PE/CA-PJ49 |
| 6.25 |
99.85 |
6.60 |
|
97.37 |
4.32 |
|
96.00 |
2.18 |
|
91.19 |
5.00 |
|
| 12.5 |
93.17 |
6.89 |
91.01 |
8.44 |
87.26 |
0.51 |
77.49 |
6.21 |
| 25 |
86.25 |
7.68 |
78.14 |
5.90 |
78.68 |
7.12 |
62.58 |
0.51 |
| 50 |
80.89 |
2.54 |
>100 |
63.65 |
2.81 |
>50 |
69.09 |
5.95 |
>50 |
50.24 |
4.70 |
>50 |
| 100 |
61.92 |
1.17 |
|
42.84 |
0.34 |
|
49.39 |
5.35 |
|
35.37 |
1.91 |
|
| 200 |
43.51 |
2.40 |
23.86 |
0.34 |
23.20 |
2.06 |
12.96 |
0.88 |
| 400 |
12.25 |
2.54 |
9.75 |
1.71 |
4.70 |
0.51 |
3.95 |
0.66 |
| SK-OV-3 |
| 6.25 |
99.72 |
4.94 |
|
99.03 |
4.61 |
|
93.80 |
6.76 |
|
91.34 |
5.80 |
|
| 12.5 |
92.28 |
5.89 |
90.99 |
4.63 |
90.86 |
7.09 |
82.43 |
5.30 |
| 25 |
89.38 |
6.28 |
83.36 |
5.25 |
83.56 |
0.55 |
78.78 |
6.68 |
| 50 |
83.35 |
5.84 |
>400 |
79.62 |
6.04 |
>200 |
73.17 |
2.56 |
>100 |
67.19 |
7.40 |
>50 |
| 100 |
78.30 |
2.95 |
|
66.62 |
1.87 |
|
58.24 |
3.52 |
|
33.37 |
6.76 |
|
| 200 |
72.10 |
7.07 |
57.21 |
4.81 |
30.87 |
3.38 |
26.46 |
2.47 |
| 400 |
55.83 |
3.83 |
39.90 |
1.25 |
16.29 |
0.37 |
10.05 |
0.80 |
Table 4.
In vitro cytotoxicity of berberine on various tumor cell lines, based on literature data.
Table 4.
In vitro cytotoxicity of berberine on various tumor cell lines, based on literature data.
Cancer cell
Line |
Cytotoxic
responses |
Berberine
Concentration |
IC50
Value |
Reference |
Liver cancer
HEP G2
SMMC-7721
Bel-7402 |
Decreased the cell viability
in a time- and dose-dependent manner.
|
3.125, 6.25,
12.5, 25,
50 and 100 µM
|
HEP G2 - 34.5 µM,
SMMC-7721 - 25.2 µM
Bel-7402 - 53.6 µM |
[19] |
Ehrlich ascites carcinoma
EAC |
Increased apoptotic cells (at 10 µg/ml)
Inhibited DNA synthesis,
changed the morphology of dsDNA, induced cell death (at 50 and 100 µg/mL) |
10, 50
and 100 µg/mL |
< 1 µg/mL |
[20] |
Dalton’s lymphoma
ascites
DLA |
Cytotoxic effect of 44% at 1 mg/mL
At lower concentrations, it caused a dose-dependent cytotoxicity in DLA cells |
100- 1000 mg/mL |
NA |
[21] |
Breast cancer
MCF-7
MDA-MB-231 |
Dose- and time-dependent inhibitory effect
Increased apoptotic ratio,
Stimulates caspase-3 activity and alteration in cell morphology
- Increased ROS generation
- overexpression of p53 |
10– 100 µM
10-100 ug/mL |
NA
MCF7 – 15.93 ug/mL |
[22] |
Ovarian cancer
CsSki,
SiHa,
HeLa |
It inhibited the invasion of CsSki, HeLa, and SiHa cells in a dose-dependent manner.
It inhibited the migration of CsSki, SiHa, and HeLa cells.
Decreased the SiHa cell motility |
20 µM |
NA |
[23] |
Prostate cancer
LNCaP
PC-82 |
Dose-dependently decreased the cell viability and induced programmed necrosis and apoptosis |
1-100 µM |
NA |
[24] |
Rat glioma
C6 |
In a time- and dose-dependent manner,
altered the cell morphology,
promoted the caspase-3, -8, and -9 activity, increased the production of ROS
induced apoptotic cell death |
100 µM |
NA |
[25] |
Colorectal carcinoma
HCT116,
SW480
LoVo |
In a concentration- and time-dependent manner, the cancer cell growth is inhibited via programmed death |
0-100 µM
for 24 - 72 hours |
NA |
[26] |
Human prostate cancer
LNCaP,
PC-3 |
Inhibited cell growth and proliferation in cancer cells in a time- and concentration-dependent manner
Induced apoptotic cell death |
0, 5, 10, 20, 50,
and 100 µM |
LNCap cells: 60 µM
PC-3 cells: ≥100 µM |
[24,27] |
Lung cancer
A549 |
- Did not show a cytotoxic effect on the cells (up to 24 h).
- Showed slight cytotoxicity after 48 hr (20 and 40 µM) |
2.5-40 µM |
NA |
[28] |
Human esophageal cancer
YES-2 |
Reduced cell viability and proliferation,
inhibited production of interleukin-6
dose- and time-dependently |
8-32 µM |
NA |
[29] |
Oral cancer:
OC2
KB |
Inhibited activator protein 1,
reduced the production of cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2)
anti-inflammatory effect |
1, 10, and 100 µM
( 2-12 hours) |
NA |
[30] |
Human OSCC:
HSC-2, HSC-3, HSC-4
Human Promyelocytic
Leukemia:
HL-60 |
Increased apoptotic cells,
DNA fragmentation,
caspase-3, -8 and -9
and pro-apoptotic BAD protein.
In HSC-2 cells, BAD protein increase is not available |
10, 20 and 80 µM |
18 - 136 µM |
[30] |
Mouse
melanoma
K1735-M2 |
Dose- and time-dependent inhibitory effect on cell proliferation
50% of growth inhibition occurred for 72 and 96 h since drug exposure |
0, 10,
25, 50,
75, and 100 µM |
NA |
[31] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).